Abstract
This study was done to determine whether social and environmental factors alter cocaine reward and proteins implicated in mediating drug reward in rats during early adolescence. On postnatal day (PND) 23, rats were housed under conditions where both social (number of rats per cage) and environmental (availability of toys) factors were manipulated. Socially isolated rats were housed alone impoverished with no toys (II) or enriched with toys (IE). Social rats were housed 2 rats/cage with no toys (SI2) or with toys (SE2), or 3/cage with (SE3) or without (SI3) toys. On PND 43, cocaine conditioned place preference (CPP) sessions began with the post-test done on PND 47. Cocaine CPP was established in response to 5 or 10 mg/kg cocaine in II rats, and CPP was decreased with the addition of cagemates or toys. No CPP was seen to any dose in SI3 or SE3 rats. Enriched housing (SE3) increased dopamine transporter (DAT) protein in the nucleus accumbens compared to II. There also were differential effects of cocaine on tyrosine hydroxylase and DAT depending on housing, with both increased by cocaine in II but not SE3 rats. DARPP-32 was unchanged by housing or cocaine, while phospho-Thr34-DARPP-32 was increased by cocaine treatment across conditions. Thus, both social and environmental enrichment decrease cocaine CPP during adolescence and different housing alters proteins that regulate dopaminergic neurotransmission in a manner that may account for the observed differences in cocaine-induced reward.
Keywords: enrichment, adolescent, cocaine, conditioned place preference
Introduction
Environmental factors can modulate the behavioral and neurochemical effects of drugs of abuse in adult rats. Isolation or crowding affect food and water consumption by adult male rats (Brown and Grunberg, 1996) and juvenile male rats housed in socially and environmentally enriched conditions eat less and gain weight at a slower pace than rats housed alone without enrichment (Zaias et al., 2008). Adult rats in an impoverished environment (singly housed rats with no toys) exhibited increased activity and rearing (Boyle et al., 1991, Heidbreder et al., 2000), while those in an enriched environment (group housing plus toys) decreased exploration and basal activity (Bowling et al., 1993, Varty et al., 2000, Neugebauer et al., 2004). In addition, an enriched environment has been associated with lower levels of stress-response hormones in rats (Belz et al., 2003). A recent study showed that social activity itself is rewarding in that adolescent rats developed a conditioned place preference to an environment paired with another rat compared to an isolated environment, and that the social environment interacted with cocaine to produce greater reward than either factor alone (Thiel et al., 2008).
Environmental enrichment has been shown to alter systems that mediate the effects of drugs of abuse in adult rats. For example, enrichment increased glucose utilization in the nucleus accumbens (Gonzalez-Lima et al., 1994). In addition, rats in an enriched environment had decreased dopamine uptake in the medial prefrontal cortex, but not striatum or accumbens (Zhu et al., 2004, Zhu et al., 2005) and no change in dopamine transporter binding (Zhu et al., 2005). In contrast to rats, in mice enrichment decreased both dopamine transporter binding and mRNA in striatum (Bezard et al., 2003), but did not alter dopamine levels (Solinas et al., 2008). After two months of housing during adolescence through adulthood, environmental enrichment in mice regulated gene expression of proteins involved in signal transduction, cell proliferation, and cell structure and metabolism (Thiriet et al., 2008), and increased levels of Delta-Fos B (Solinas et al., 2009). In addition, repeated administration of cocaine had multiple effects on Delta-Fos B with increases compared to saline in standard housed mice, but decreases in mice in standard housing vs enriched mice (Solinas et al., 2009).
Most studies investigating the role of environmental conditions used adult animals, or housed the animals under different conditions when they were young but tested during adulthood. Rats housed in groups during adolescence are more sensitive than isolated rats to a conditioned place preference to cocaine in adulthood (Schenk et al., 1986). On the other hand, rats isolated on PND 21 exhibit enhanced cocaine self-administration as adults compared to group housed animals (Schenk et al., 1987, Ding et al., 2005). In contrast, cocaine self-administration is not different in rats that were housed under social or isolated conditions once they were adults (Bozarth et al., 1989). Thus, the environment may play a more important role in influencing behavioral effects of psychostimulants when conditions are implemented while the animal is young. It is not clear, however, if housing effects are immediate or if rats need to be housed differentially for many weeks until they are adults before changes are observed. Adolescence is a critical period for initiation of drug use, thus it is important to understand factors that regulate this behavior. Adolescents under age 18 accounted for 34% of new cocaine initiates, thus approximately 918 adolescents per day tried cocaine for the first time during 2006 (NSDUH, 2007) and there are numerous studies showing that drug effects differ during this period compared to adulthood (for review see Izenwasser, 2005). For example, it has been shown that adolescent male rats exhibit a conditioned place preference to cocaine at lower doses than adult male rats (Badanich et al., 2006, Zakharova et al., 2009b), and that cocaine produces a greater increase in dopamine levels in the nucleus accumbens, compared to adults (Badanich et al., 2006, Walker and Kuhn, 2008). The purpose of this study was to investigate the individual and combined roles that environmental and social housing play in mediating cocaine reward and neurochemistry during adolescence.
Experimental Procedures
Subjects
The animals used in this study were maintained and the studies were conducted in accordance with the guidelines of the Guide for Care and Use of Laboratory Animals, National Research Council, Department of Health, Education and Welfare, NIH Publication 85-23, revised 1996 and all studies were approved by the University of Miami Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Charles River, Wilmington, MA) were used in all studies. Rats were housed in a temperature and humidity-controlled environment under a 12 h light/dark schedule with lights on at 7 a.m. and off at 7 p.m. All behavioral testing was done during the light schedule between 9 a.m. and 4 p.m. with each group tested at the same hour each day and the conditions randomized over the course of the day. Food and water were available ad libitum.
Housing
Rats were received on postnatal day 23 (PND 23) and immediately housed in standard large shoebox cages measuring 46cm long × 29 cm wide × 20.5cm high in one of several conditions. Both social (number of rats per cage) and environmental (availability of toys) factors were manipulated (Table 1). Socially isolated/environmentally impoverished (II) rats were housed alone (1 rat/cage) and had no toys available. Socially isolated/environmentally enriched (IE) rats were housed alone (1 rat/cage) with toys available. Social/environmentally impoverished rats were housed either 2 rats/cage (SI2) or 3/cage (SI3) with no toys, while social/environmentally enriched rats were housed 2/cage (SE2) or 3/cage (SE3) with toys. For the environmentally enriched conditions, toys were placed in the cages and different toys were rotated in and out of the cages each time the cages were changed (twice/week). Plastic tunnels and hollow balls (that the animals could move in and out of or climb over), in addition to toys that the rats could chew (non-toxic dog bones and plastic toys) and scratch paper were used. On PND 43 (20 days after housing conditions began) cocaine CPP studies were begun.
Table 1.
Housing conditions
| Environmental conditions | Social conditions (# rats/cage) | ||
|---|---|---|---|
| Isolated (1 rat/cage) | Social2 (2 rats/cage) | Social3 (3 rats/cage) | |
| Impoverished (no toys) | II | SI2 | SI3 |
| Enriched (toys) | IE | SE2 | SE3 |
Cocaine Conditioned Place Preference (CPP)
The CPP apparatus consisted of an acrylic box (40.64 × 40.64 cm) with a removable center barrier. On one side, the walls were white, the lid was black and white striped, and the floor was smooth. On the other side, the walls were black and white striped, the lid was white, and the bottom was textured. On day 1 (PND 43), a 30 min pretest was done on each rat. During this test, the rats were placed in the chamber with the center barrier removed and were able to move freely to both sides. The amount of time spent on each side of the chamber was recorded for 30 min. During the conditioning phase (days 2–4), the rats were injected twice daily – in the morning (between 9 am and 12 pm) and in the afternoon (1 pm-5 pm) with saline or cocaine (3 – 10 mg/kg, ip). Control groups of rats that received saline on both sides of the chamber also were run. Morning and afternoon sessions were separated by at least 4 hours. As described in numerous other studies of CPP during adolescence (Badanich et al., 2006, Brenhouse and Andersen, 2008, Kota et al., 2008, Solinas et al., 2009), this twice-daily schedule was used instead of conditioning with saline and cocaine on separate days because of the constraints involved in doing developmental studies. To ensure that the entire experiment could be completed within the adolescent period, it was important to have the CPP conditioning period be as short as possible. On day 5, CPP testing was done in the middle of the day. The doses of cocaine were selected based upon our previous studies showing that 5 mg/kg cocaine produced the maximal CPP in male adolescent rats under these conditions (Zakharova et al., 2009a, Zakharova et al., 2009b).
Measurement of Tyrosine Hydroxylase, Dopamine Transporters, Cdk5 and DARPP-32 by Western Blot Analysis
Brains from rats housed either II or SE3 and treated with 5 mg/kg/day of cocaine or saline for three days were collected 22 hours after the last injection. The nucleus accumbens (NA) was dissected as described in our earlier publications (Izenwasser and Cox, 1990, Izenwasser et al., 1990, Izenwasser et al., 1996, Muller and Unterwald, 2005, Perrine et al., 2008). Briefly, to dissect the NA, a slice was taken between Bregma + 2.20 and Bregma + 1.00, according to the rat brain atlas (Paxinos and Watson, 1982), the slice was laid flat and the NA was punched out. Tissues were sonicated in boiling 1% SDS, boiled for 5 minutes, and aliquots were stored at −80°C until assayed. Tyrosine hydroxylase, which is the rate-limiting enzyme in the synthesis of dopamine, the dopamine transporter (DAT), which is the direct target of cocaine, and dopamine, cAMP regulated phosphoprotein - mw 32 (DARPP-32), which is a key intracellular dopamine receptor signaling molecule, and Cdk5, which is purported to increase the phosphorylation of Thr75-DARPP-32 (Bibb et al., 2001), were measured. These molecules have been shown to play important roles in regulating dopaminergic neurotransmission and the response to cocaine (Ritz et al., 1987, Svenningsson et al., 2005).
Protein concentrations were determined using the Lowry assay (Lowry et al., 1951). Protein extracts (25–40µg) were subjected to SDS-polyacrylamide gel electrophoresis (10% Tris-HCl BioRad Ready-gels) and transferred for 95 minutes to nitrocellulose membranes. Membranes subsequently were blocked for 1 hour in blocking solution consisting of 5% nonfat dry milk and Tween-TBS and incubated overnight at 4°C in antibodies to the following proteins: phospho-tyrosine hydroxylase (pTH, 1:1000; Cell Signaling), total tyrosine hydroxylase, (TH, 1:1000; Chemicon), cyclin-dependent kinase-5 (Cdk5, 1:1000; Cell Signaling), phospho-Thr34-Dopamine and cAmp Regulated PhosphoProtein-Mr 32 kDa (p-Thr34-DARPP-32, 1:1000 PhosphoSolutions), total DARPP-32 (1:5000, PhosphoSolutions), dopamine transporter (DAT, 1:1000; Chemicon) and anti-tubulin antibody (1:20000–30000; Sigma). All blots were incubated in the anti-tubulin antibody to correct for differences in protein loading and transfer. Following overnight incubation in primary antibodies, membranes were washed in Tween-TBS and incubated in either anti-mouse or anti-rabbit antibody conjugated to horseradish peroxidase (Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. Immunoreactivity was visualized by chemiluminescence following incubation in Supersignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) with bands being quantified using densitometry (FujiFilm Image System). Proteins of interest are expressed as a ratio to tubulin as measured from the same blot.
Chemicals
Cocaine HCl was obtained from NIDA, (Rockville, MD) and dissolved in saline.
Data Analysis
For the CPP tests, preference for the cocaine-paired side was determined and two different analyses were done. T-tests were done for each dose in each condition to compare the preference data (time spent in cocaine-paired side minus time in saline-paired side) to 0 to determine whether a significant preference (or aversion) had occurred in each group. This test showed whether or not each individual dose produced a significant preference for the cocaine-paired side. In addition, dose-effect data were analyzed using a two-way analysis of variance (training dose x living condition) to compare conditions. Post-hoc comparisons were done, where appropriate, using Fisher’s Protected Least Significant Difference (PLSD). P values less than 0.05 were considered significant for all tests.
Data from western blots were analyzed by a two-way (housing x drug treatment) ANOVA. Where appropriate, ANOVAs were followed by post hoc analyses with Fisher's Protected Least Significant Difference (PLSD). P values less than 0.05 were considered significant.
Results
Cocaine CPP
None of the groups developed a significant CPP to saline (Fig. 1). In these rats, saline was paired with both sides of the chamber on the conditioning days. The II (n=7), IE (n=8) and SI2 (n=9) groups all developed a significant preference for the cocaine-paired side over the saline-paired side after training with 5 mg/kg cocaine, as shown by positive preference values significantly different from 0 (Fig. 1, P<0.05). Data from the SE2 (n=8), SI3 (n=8) and SE3 (n=9) groups were variable with some rats preferring the cocaine-paired side and others preferring the saline-paired side. Thus, overall, none of these groups (SE2, SI3, or SE3) exhibited either a significant preference or aversion to the cocaine-paired side after conditioning with the 5 mg/kg dose of cocaine. A significant CPP also was established in response to 10 mg/kg cocaine in the II rats (n=7; P≤0.0256). In contrast, no CPP was seen in the IE (n=10), SI2 (n=28), SE2 (n=10), SI3 (n=7) or SE3 rats (n=9). A dose of 3 mg/kg cocaine (II (n=7), IE (n=8), SI2 (n=10), SE2 (n=8), SI3 (n=6), and SE3 (n=9)) did not produce a significant preference or aversion in any of the six groups of rats.
Fig. 1.
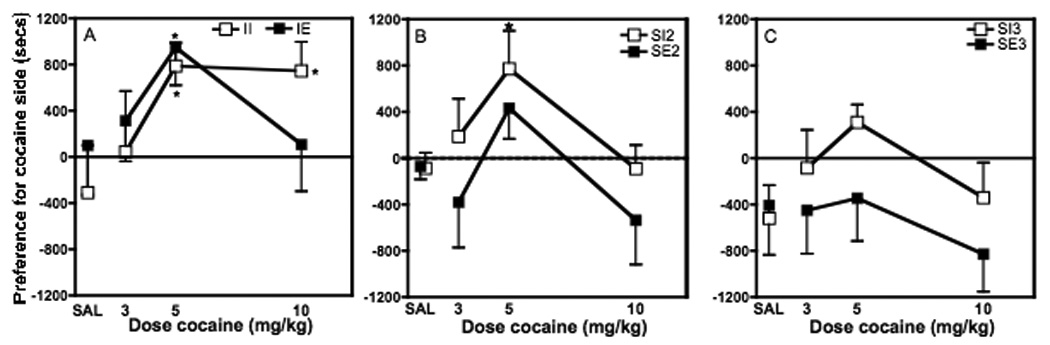
Effects of 3, 5 and 10 mg/kg cocaine on conditioned place preference in groups of rats housed under different social and environmental conditions. SAL refers to groups trained with saline on both sides of the chamber. Data are presented as mean ± SEM preference values (time spent in cocaine-paired chamber minus time spent in saline-pared chamber during posttest expressed in seconds). CPP decreased as either social or environmental enrichment (rats and/or toys) was added to the cages (compare A, B, C). Both 5 and 10 mg/kg cocaine led to significant preferences in the II rats, whereas only 5 mg/kg cocaine produced a significant preference in the IE and SI2 rats. The SE2, SI3, and SE3 rats did not exhibit a significant preference in response to any of the doses tested. *significantly different from 0 (p<0.05).
An overall ANOVA of the CPP data for all three doses showed that there was a significant effect of group (F(5,132)=3.398; P≤0.0064) and of dose (F(2,132)=4.282; P≤0.0158), but no significant interaction. Post hoc tests showed that the SE3 group was significantly different from all other groups.
Since the 3 mg/kg dose of cocaine did not produce CPP in any of the six groups, this may artificially have made the groups look more similar than if only active doses were included in the analysis, since theoretically an unlimited number of ineffective doses could have been tested. If only the doses that produced a significant CPP in any of the groups were included in the ANOVA (i.e. 5 and 10 mg/kg cocaine) there was a significant effect of group (F(5,90)=3.926; P≤0.0029), as had been seen in the overall ANOVA with all three doses). In this case, post hoc tests showed that II, IE, and SE3 were significantly different from all other groups and that SI3 was significantly different from II, IE, and SE3. Thus, the analyses showed that additional rats and/or environmental enrichment to the home cage decreased cocaine-induced CPP. Isolated rats were the most sensitive to conditioned rewarding effects of cocaine whereas neither the SE2, SI3, nor SE3 rats (the three most enriched groups) developed a significant CPP to any of the doses of cocaine tested.
Regulation of Tyrosine Hydroxylase, Dopamine transporters, and DARPP-32
Representative immunoblots for all of the markers are shown in Fig. 2. To investigate potential molecular mechanisms underlying the effects of housing conditions on the rewarding properties of cocaine, the levels of three proteins that are critical to dopaminergic neurotransmission, and hence cocaine reward, were measured in the brains from the two extreme groups, II and SE3. There was a significant interaction between housing condition (II vs SE3) and drug treatment (vehicle vs cocaine) on total TH:tubulin in the NA (F(1,26)=4.27, P ≤ 0.049) but neither main effect was significant. Post-hoc tests showed that total TH was increased significantly in the NA of II rats in response to three injections of 5 mg/kg cocaine (administered during the CPP training sessions) compared to control saline-injected II animals (P ≤ 0.05; Fig. 3A). In contrast, there was no effect of cocaine on total TH in the SE3 rats. Similarly, there was a significant interaction between housing and drug treatment on pTH:Tubulin (F(1,25)=4.805, P ≤ 0.0372), and post-hoc comparisons showed that there was a significant increase in pTH:tubulin in the II rats after cocaine treatment, while there was no difference between cocaine- and saline-injected rats in the levels of pTH in the SE3 group (Fig. 3B). The ratio of pTH to total TH was not significantly altered in response to either housing condition or cocaine administration.
Fig. 2.
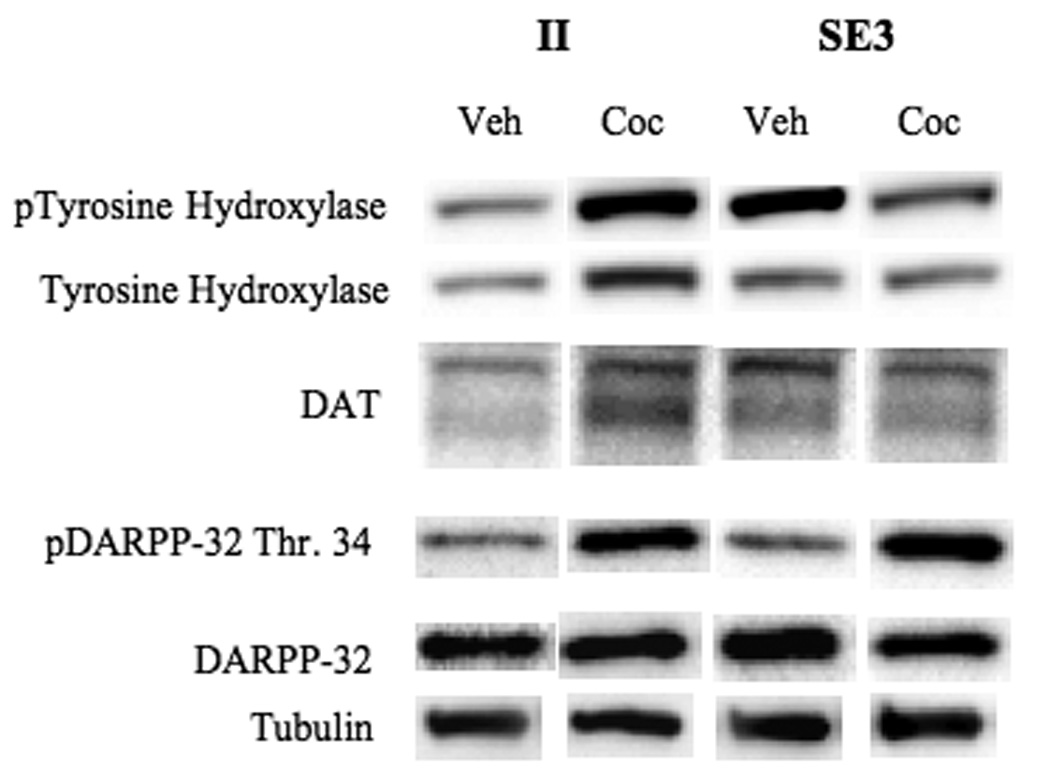
Representative immunoblots of nucleus accumbens tissue from II and SE3 rats following vehicle (veh) or cocaine (coc) administration. Bands represent pTH, total TH, DAT, pDARPP-32 (Thr. 34), DARPP-32, and tubulin (from top to bottom).
Fig. 3.
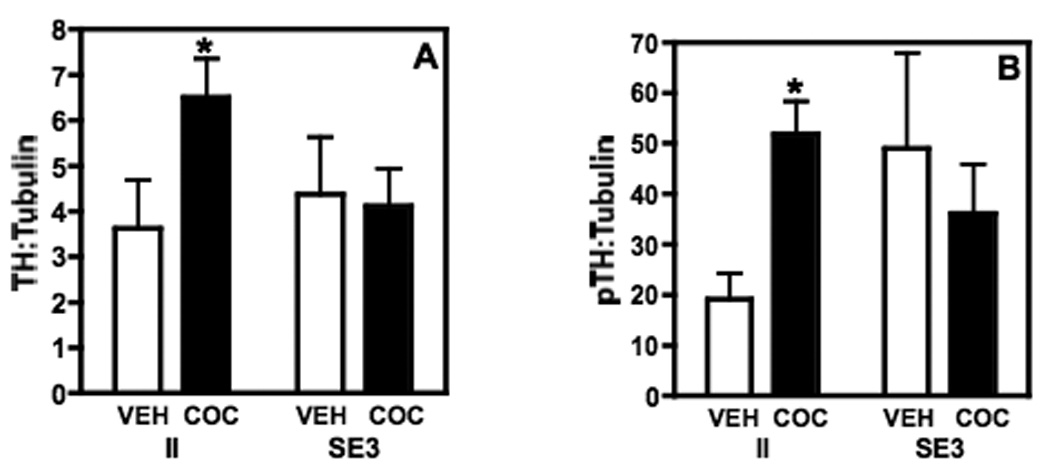
TH, as measured by Western blot in the NA of rats housed in either II or SE3 conditions. (A) Total TH:tubulin is increased following 3 daily injections of cocaine in the II rats, but is unchanged in the SE3 rats. (B) In rats housed in the II condition, cocaine led to a significant increase in phosphorylated TH (pTH) in the NA. There was no effect of cocaine on pTH in the SE3 rats. Data shown are mean ± SEM; n=7–9/group; *P < 0.05 compared to II vehicle (VEH) treated rats.
A two-way ANOVA showed that there was a significant interaction between housing and drug treatment on DAT protein levels (F(1,28)= 6.61, P ≤ 0.016). DAT was significantly higher in the nucleus accumbens of saline-injected SE3 rats than in saline-injected II rats (P ≤ 0.05; Fig. 4). In addition, cocaine significantly increased DAT in the nucleus accumbens of II rats (P ≤ 0.05), but had no effect in the SE3 rats.
Fig. 4.
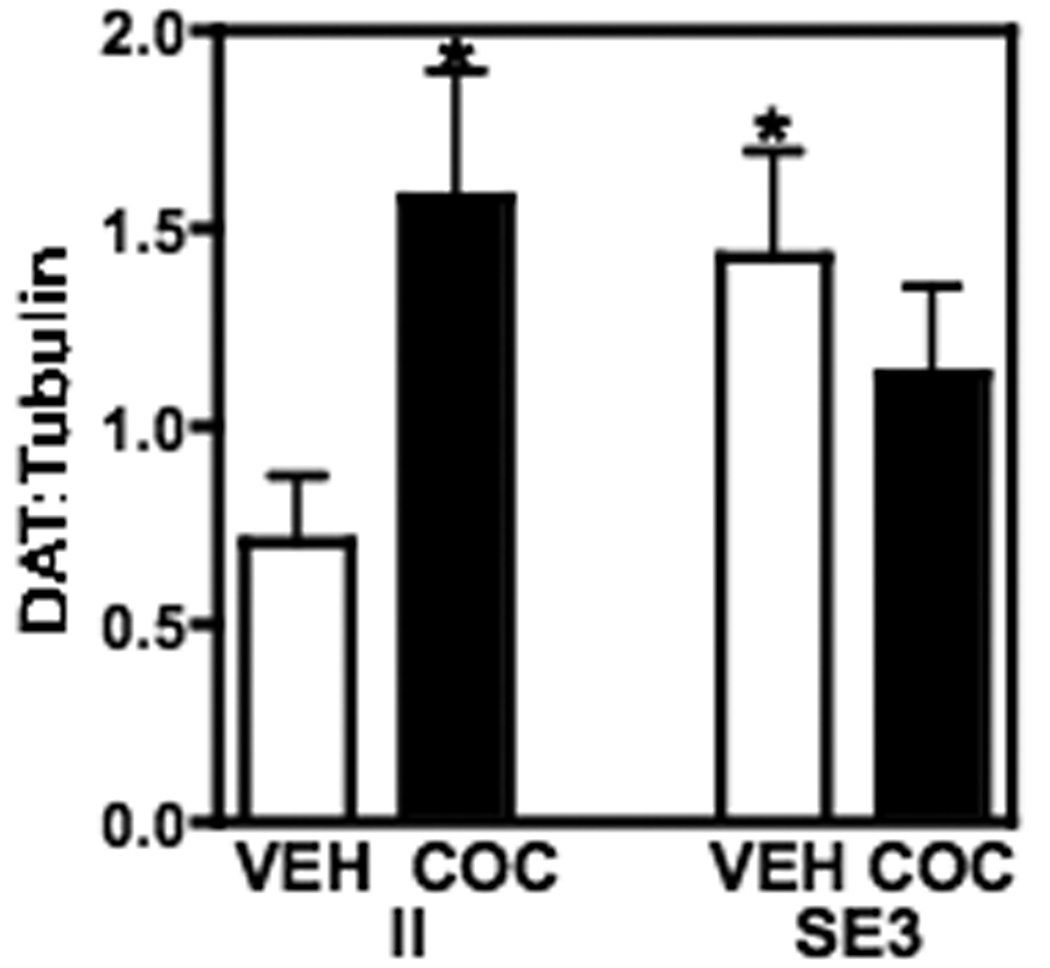
DAT protein, measured by Western blot, in the NA of rats housed in either II or SE3 conditions. DAT is increased significantly in response to housing in the enriched environment (SE3) compared to the isolated environment (II). In addition, 3 daily injections of cocaine significantly increased DAT only in the rats housed in an impoverished condition. There was no effect of cocaine on DAT protein in the SE3 rats. Data shown are mean ± SEM; n=7–9/group; *P < 0.05 compared to vehicle (VEH).
There were no significant effects of either housing or cocaine treatment on total DARPP-32 levels in the nucleus accumbens (Fig. 5A). However, there was a significant overall effect of cocaine treatment on p-Thr34-DARPP-32 levels (F(1,28)=4.333, P ≤ 0.047), such that overall p-Thr34-DARPP-32 was increased across housing conditions in response to cocaine (Fig. 5B). Cdk5 was not altered significantly by either housing or cocaine treatment (data not shown).
Fig. 5.
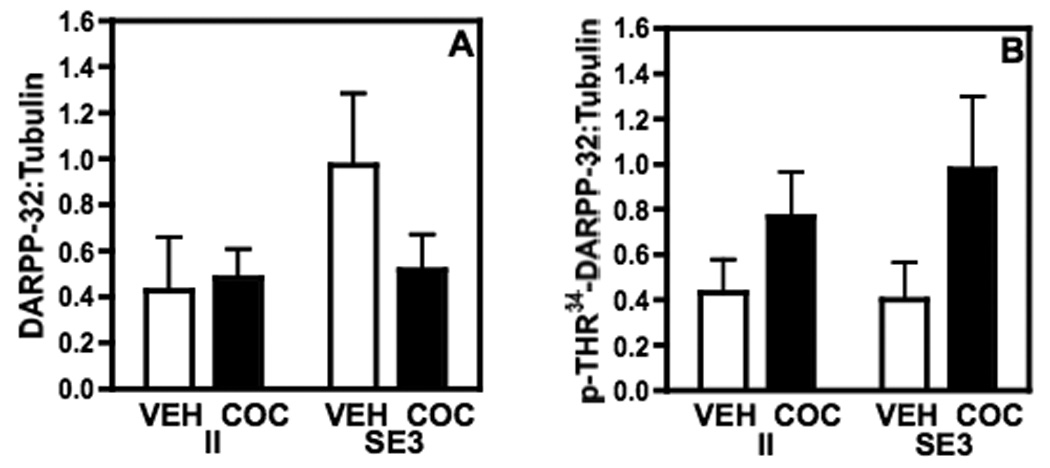
DARPP-32, as measured by Western blot in the NA of rats housed in either II or SE3 conditions. (A) Total DARPP-32:tubulin is not significantly altered by either housing condition or by 3 daily injections of cocaine in either the II rats or the SE3 rats. (B) p-Thr34-DARPP-32 is increased across housing conditions in response to cocaine administration such that overall the cocaine-treated rats had higher levels of p-Thr34-DARPP-32 regardless of housing condition. Data shown are mean ± SEM; n=7–9/group.
Discussion
The present study shows that adolescent rats are affected by alterations in social and environmental housing conditions. These data extend the existing literature of studies done primarily in adult rats by showing that social and environmental factors independently alter the behavioral response to cocaine and that these alterations can be observed soon after housing rats in different conditions.
Cocaine Reward
These results demonstrate that during adolescence the conditioned rewarding effects of cocaine are inversely related to the degree of enrichment. Thus, isolated impoverished adolescent (PND 43) rats established preference for the cocaine-paired side after training with 5 or 10 mg/kg cocaine, while isolated enriched rats (IE) and rats housed two per cage without toys (SI2) demonstrated sensitivity to the conditioned rewarding effects of only 5 mg/kg cocaine. In contrast, rats living 2/cage with toys (SE2), or 3/cage without (SI3) or with (SE3) toys showed no conditioned place preference at any of the doses of cocaine tested. Previously, it was shown that exposure to an enriched environment during adolescence attenuated the behavioral and neurochemical effects of prenatal cocaine during adulthood (Neugebauer et al., 2004). In addition, while isolated adult rats (PND 63) developed a significant CPP to methamphetamine, enriched rats failed to do so (Gehrke et al., 2006). In contrast, it has been reported that animals housed post-weaning in isolation were less sensitive to the conditioned reward effects of amphetamine (Wongwitdecha and Marsden, 1995) or cocaine (Schenk et al., 1986) than were grouped housed animals when tested as adults on PND 63. Thus, the effects of housing appear to change as the animal becomes an adult. One consideration in comparing these studies is that the housing conditions and the ages vary across studies. It is possible that under some conditions, the conditions induce greater levels of stress on the animals than in other studies. For example, once the animals become adults, they are larger and it is possible that this creates a greater stress in the group housed animals, that may become a bit more crowded as they grow. In addition, it is likely that dopaminergic neurochemistry will continue to change as the animals age in response to housing conditions. These factors likely would contribute to differences in cocaine reward.
Other studies have examined drug self-administration in response to environmental enrichment. It was shown that environmental enrichment (8–10 rats/cage with toys) or social enrichment (2 rats/cage) decreased amphetamine self-administration compared to isolated rats housed without toys (Bardo et al., 2001). In contrast, there were no differences in acquisition or levels of administration of cocaine in rats housed as adults (PND 63–91 upon arrival) either in groups (10/cage) or individually, however, rats housed in isolation as adults did acquire heroin self-administration more rapidly than group housed rats (Bozarth et al., 1989). Thus, it may be that social and environmental factors differentially alter drug reward depending upon which drug is being tested. However, the differences in housing conditions and age of the rats both at initial housing and during testing could be factors in mediating these different results. In addition, although psychostimulants ultimately increase dopaminergic transmission, different drugs do so in different ways. Thus, changes in the function of dopamine and serotonin transporters and receptors as a function of housing would not necessarily be expected to alter the effects of these drugs in the same way. Additional studies examining alterations in dopamine transporter activity across time will help to elucidate differences in drug effects. In spite of these caveats, the existing data do show that, in addition, to the differences observed in both the behavioral (e.g. Caster et al., 2005, Badanich et al., 2006, Zakharova et al., 2009b) and neurochemical (Philpot and Kirstein, 1999, Badanich et al., 2006, Walker and Kuhn, 2008) effects of cocaine in adolescent males compared to adults, the behaviors can be altered further by social and environmental manipulations.
Dopaminergic Neurotransmission
There were a number of differences in components of the dopamine system in response to social and environmental enrichment and to cocaine exposure. Tyrosine hydroxylase is the rate-limiting enzyme in the synthesis of dopamine, and its activity is regulated by its phosphorylation state (Haycock and Haycock, 1991), hence the levels of both phosphorylated tyrosine hydroxylase and total tyrosine hydroxylase were measured. Three injections of 5 mg/kg cocaine significantly increased the levels of phosphorylated tyrosine hydroxylase and total tyrosine hydroxylase in the nucleus accumbens of II rats only. In contrast, cocaine exposure had no effect on the abundance of phosphorylated tyrosine hydroxylase or total tyrosine hydroxylase in rats housed in an enriched environment. Increases in phosphorylated tyrosine hydroxylase suggest that dopamine synthesis was increased in response to cocaine selectively in the isolated and impoverished rats.
The level of DAT in the nucleus accumbens was significantly altered in response to housing conditions. Environmentally and socially enriched rats had significantly more DAT in the accumbens than isolated and impoverished rats in the absence of drug administration. In addition, cocaine administration produced a significant increase in DAT only in the II rats. At face value, these findings seem consistent with the CPP data showing that only the II rats developed a significant CPP to multiple doses of cocaine, while the SE3 rats did not exhibit a significant CPP to any of the doses tested. The elevated levels of DAT in the II rats in response to cocaine is interesting because our previous studies showed that while repeated cocaine administration led to an increase in DAT densities in multiple regions of the limbic system in adult rats, there was no effect in adolescent rats housed under standard conditions (2/cage without toys) (Collins and Izenwasser, 2002). Of note, in adult rats living in an enriched environment there is a decrease in dopamine uptake in the medial prefrontal cortex, but not in the striatum or accumbens (Zhu et al., 2004, Zhu et al., 2005), and there are no changes in binding to the dopamine transporter (Zhu et al., 2005). Additional studies are necessary in the adolescent rats to determine whether the changes in DAT protein shown here are accompanied by changes in binding to the dopamine transporter or the function of the transporter.
It has been shown that isolation housing alters a number of dopaminergic markers compared to group housing (4 rats/cage) measured 12 weeks after differential housing beginning on PND 21. Basal dopamine turnover is increased in the amygdala and decreased in the infralimbic prefrontal cortex in response to isolation, and whereas the nucleus accumbens is unchanged (Heidbreder et al., 2000). It also has been shown using in vivo electrochemistry that baseline dopamine signal amplitude and dopamine clearance rates in the medial prefrontal cortex are increased in enriched versus impoverished rats (Neugebauer et al., 2004). In rats housed in an enriched environment for 84 days beginning on PND 84, the density of dopamine D1 receptors is significantly reduced in the prefrontal cortex compared to rats living in an isolated condition (Del Arco et al., 2007). Although these measures were taken in older rats, the findings are consistent with the present behavioral findings showing that cocaine produces little or no reward in rats housed in an enriched environment. Metabolic activity, measured as changes in 2-deoxyglucose utilization, is 40% higher in the nucleus accumbens after only four days of daily exposure to enrichment compared to individually housed impoverished rats (Gonzalez-Lima et al., 1994).
DARPP-32 is a phosphoprotein that is enriched in medium spiny neurons of the striatum. It is a critical mediator of dopaminergic signaling and, as such, regulates the behavioral effects of dopamine and drugs that act on dopaminergic receptors (Svenningsson et al., 2004). When DARPP-32 is phosphorylated at the Thr-34 site by protein kinase A (PKA), DARPP-32 becomes a potent inhibitor of protein phosphatase 1 (Hemmings et al., 1984). In contrast, phosphorylation of DARPP-32 at the Thr-75 site by Cdk5 results in its conversion to an inhibitor of PKA (Bibb et al., 1999). It has been reported that mice containing a mutation of Thr34 in DARPP-32 but not Thr75, exhibit a reduction in cocaine conditioned place preference and acute locomotor responses (Zachariou et al., 2006). The results presented herein show that neither total DARPP-32 nor p-Thr34-DARPP-32 was altered by housing conditions alone. However, p-Thr34-DARPP-32 was increased by three days of cocaine administration under both the II and SE3 housing conditions.
In adult rats, it has been shown previously that five daily injections of cocaine (15 mg/kg/day) increase Cdk5, which is purported to increase the phosphorylation of Thr75-DARPP-32 (Bibb et al., 2001). In the present study, there were no changes in Cdk5 in response to either the differential housing conditions or the three daily injections of 5 mg/kg cocaine in the adolescent rats. The difference in the regulation of Cdk5 could be due to the age of the rats in the present study or to the different dosing regimen of cocaine. Given the increases in p-Thr34-DARPP-32 found after cocaine exposure in the present study, it is not surprising that Cdk5 levels were not increased. This finding also supports previous reports of a lack of correlation between Cdk5 signaling and cocaine-regulated behaviors (Hiroi et al., 1999, Zachariou et al., 2006).
It is interesting to note that a previous study showed that methylphenidate increased p-Thr34-DARPP-32 in brain slices from adult mice but did not alter p-Thr34-DARPP-32 levels in young (PND14–15 or 21–22) mice (Fukui et al., 2003). Further, differences in the levels of p-Thr34-DARPP-32 in the striatum of adult and young (PND24) mice both at baseline and following cocaine administration have been demonstrated (Niculescu et al., 2008). Thus, the regulation of DARPP-32 phosphorylation by psychostimulants appears to be age-dependent and may play an important role in the age-specific responses to these drugs.
Summary
In summary, there are significant effects of both social and environmental enrichment on the conditioned rewarding responses of adolescent males to cocaine. These behavioral changes likely are mediated by neuroadaptations in dopaminergic neurotransmission and possibly other neurochemical systems that underlie the responses to psychostimulants. The present findings demonstrate that isolated impoverished rats are more sensitive to the rewarding properties of cocaine during adolescence, and this may be due to an upregulation of tyrosine hydroxylase activity and DAT levels in the accumbens compared to rats housed under enriched conditions. Social and environmental enrichment may protect against cocaine reward by preventing these changes in dopamine neurotransmission. Therefore, factors including social and environmental conditions should be taken into consideration when ascertaining risk or developing prevention or treatment strategies for psychostimulant abuse in adolescents.
Acknowledgments
This work was supported by the National Institute on Drug Abuse and the NIH Office of Research on Women’s Health (grants DA 015119 and DA 024584 to SI and DA 018326 and DA 009580 to EMU).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Belz EE, Kennell JS, Czambel RK, Rubin RT, Rhodes ME. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav. 2003;76:481–486. doi: 10.1016/j.pbb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Bezard E, Dovero S, Belin D, Duconger S, Jackson-Lewis V, Przedborski S, Piazza PV, Gross CE, Jaber M. Enriched environment confers resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and cocaine: involvement of dopamine transporter and trophic factors. J Neurosci. 2003;23:10999–11007. doi: 10.1523/JNEUROSCI.23-35-10999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Gill K, Smith BR, Amit Z. Differential effects of an early housing manipulation on cocaine-induced activity and self-administration in laboratory rats. Pharmacol Biochem Behav. 1991;39:269–274. doi: 10.1016/0091-3057(91)90178-5. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Murray A, Wise RA. Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacol Biochem Behav. 1989;33:903–907. doi: 10.1016/0091-3057(89)90490-5. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KJ, Grunberg NE. Effects of environmental conditions on food consumption in female and male rats. Physiol Behav. 1996;60:293–297. doi: 10.1016/0031-9384(96)00020-0. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology (Berl) 2005;183:218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Developmental Brain Research. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, Garcia-Verdugo JM, Mora F. Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J Neural Transm. 2007;114:43–48. doi: 10.1007/s00702-006-0565-8. [DOI] [PubMed] [Google Scholar]
- Ding Y, Kang L, Li B, Ma L. Enhanced cocaine self-administration in adult rats with adolescent isolation experience. Pharmacology Biochemistry & Behavior. 2005;82:673–677. doi: 10.1016/j.pbb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Fukui R, Svenningsson P, Matsuishi T, Higashi H, Nairn AC, Greengard P, Nishi A. Effect of methylphenidate on dopamine/DARPP signalling in adult, but not young, mice. J Neurochem. 2003;87:1391–1401. doi: 10.1046/j.1471-4159.2003.02101.x. [DOI] [PubMed] [Google Scholar]
- Gehrke BJ, Cass WA, Bardo MT. Monoamine-depleting doses of methamphetamine in enriched and isolated rats: consequences for subsequent methamphetamine-induced hyperactivity and reward. Behav Pharmacol. 2006;17:499–508. doi: 10.1097/00008877-200609000-00016. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Ferchmin PA, Eterovic VA, Gonzalez-Lima EM. Metabolic activation of the brain of young rats after exposure to environmental complexity. Dev Psychobiol. 1994;27:343–351. doi: 10.1002/dev.420270603. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Haycock DA. Tyrosine hydroxylase in rat brain dopaminergic nerve terminals. Multiple-site phosphorylation in vivo and in synaptosomes. Journal of Biological Chemistry. 1991;266:5650–5657. [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P, Tung HY, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Fienberg AA, Haile CN, Alburges M, Hanson GR, Greengard P, Nestler EJ. Neuronal and behavioural abnormalities in striatal function in DARPP-32-mutant mice. European Journal of Neuroscience. 1999;11:1114–1118. doi: 10.1046/j.1460-9568.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- Izenwasser S. Differential effects of psychoactive drugs in adolescents and adults. Crit Rev Neurobiol. 2005;17:51–68. doi: 10.1615/critrevneurobiol.v17.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izenwasser S, Cox BM. Daily cocaine treatment produces a persistent reduction of [3H]dopamine uptake in vitro in rat nucleus accumbens but not in striatum. Brain Research. 1990;531:338–341. doi: 10.1016/0006-8993(90)90797-f. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Heller B, Cox BM. Continuous cocaine administration enhances ⌈- but not TM-opioid receptor-mediated inhibition of adenylyl cyclase activity in nucleus accumbens. European Journal of Pharmacology. 1996;297:187–191. doi: 10.1016/0014-2999(95)00828-4. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Werling LL, Cox BM. Comparison of the effects of cocaine and other inhibitors of dopamine uptake in rat striatum, nucleus accumbens, olfactory tubercle, and medial prefrontal cortex. Brain Research. 1990;520:303–309. doi: 10.1016/0006-8993(90)91719-w. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Damaj MI. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology (Berl) 2008;198:201–210. doi: 10.1007/s00213-008-1117-8. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Muller DL, Unterwald EM. D1 dopamine receptors modulate deltaFosB induction in rat striatum after intermittent morphine administration. J Pharmacol Exp Ther. 2005;314:148–154. doi: 10.1124/jpet.105.083410. [DOI] [PubMed] [Google Scholar]
- Neugebauer NM, Cunningham ST, Zhu J, Bryant RI, Middleton LS, Dwoskin LP. Effects of environmental enrichment on behavior and dopamine transporter function in medial prefrontal cortex in adult rats prenatally treated with cocaine. Brain Res Dev Brain Res. 2004;153:213–223. doi: 10.1016/j.devbrainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Niculescu M, Perrine SA, Miller JS, Ehrlich ME, Unterwald EM. Trk: a neuromodulator of age-specific behavioral and neurochemical responses to cocaine in mice. J Neurosci. 2008;28:1198–1207. doi: 10.1523/JNEUROSCI.0988-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSDUH, 2007. Substance Abuse and Mental Health Services Administration. Rockville, MD: Results from the 2006 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-30, DHHS Publication No. SMA 06-4194) 2007
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1982. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Miller JS, Unterwald EM. Cocaine regulates protein kinase B and glycogen synthase kinase-3 activity in selective regions of rat brain. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot RM, Kirstein CL. Repeated cocaine exposure: effects on catecholamines in the nucleus accumbens septi of periadolescent animals. Pharmacology Biochemistry and Behavior. 1999;62:465–472. doi: 10.1016/s0091-3057(98)00198-1. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Malovechko R, Robertson A, Klukowski G, Amit Z. Differential effects of isolation housing on the conditioned place preference produced by cocaine and amphetamine. Pharmacol Biochem Behav. 1986;24:1793–1796. doi: 10.1016/0091-3057(86)90523-x. [DOI] [PubMed] [Google Scholar]
- Schenk S, Lacelle G, Gorman K, Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett. 1987;81:227–231. doi: 10.1016/0304-3940(87)91003-2. [DOI] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Rawas RE, Lardeux V, Jaber M. Environmental Enrichment During Early Stages of Life Reduces the Behavioral, Neurochemical, and Molecular Effects of Cocaine. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.51. [DOI] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Rawas RE, Lardeux V, Jaber M. Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology. 2009;34:1102–1111. doi: 10.1038/npp.2008.51. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. Aaps J. 2005;7:E353–E360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008 doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiriet N, Amar L, Toussay X, Lardeux V, Ladenheim B, Becker KG, Cadet JL, Solinas M, Jaber M. Environmental enrichment during adolescence regulates gene expression in the striatum of mice. Brain Res. 2008;1222:31–41. doi: 10.1016/j.brainres.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varty GB, Paulus MP, Braff DL, Geyer MA. Environmental enrichment and isolation rearing in the rat: effects on locomotor behavior and startle response plasticity. Biological Psychiatry. 2000;47:864–873. doi: 10.1016/s0006-3223(99)00269-3. [DOI] [PubMed] [Google Scholar]
- Walker QD, Kuhn CM. Cocaine increases stimulated dopamine release more in periadolescent than adult rats. Neurotoxicol Teratol. 2008;30:412–418. doi: 10.1016/j.ntt.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongwitdecha N, Marsden CA. Isolation rearing prevents the reinforcing properties of amphetamine in a conditioned place preference paradigm. Eur J Pharmacol. 1995;279:99–103. doi: 10.1016/0014-2999(95)00212-4. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Sgambato-Faure V, Sasaki T, Svenningsson P, Berton O, Fienberg AA, Nairn AC, Greengard P, Nestler EJ. Phosphorylation of DARPP-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacology. 2006;31:555–562. doi: 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]
- Zaias J, Queeney TJ, Kelley JB, Zakharova ES, Izenwasser S. Social and physical environmental enrichment differentially affect growth and activity of preadolescent and adolescent male rats. J Am Assoc Lab Anim Sci. 2008;47:30–34. [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent rats. Behavioural Brain Research. 2009a;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009b;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]


