Abstract
Apoptosis proceeds through a set of evolutionarily conserved processes that coordinate the elimination of damaged or unneeded cells. This program of cell death is carried out by organelle-directed regulators, including the Bcl-2 proteins, and ultimately executed by proteases of the caspase family. While the biochemical mechanisms of apoptosis are increasingly understood, the underlying cell biology orchestrating programmed cell death remains enigmatic. In this review we summarize the current understanding of Bcl-2 protein regulation and caspase activation while examining cell biological mechanisms and consequences of apoptotic induction. Organellar contributions to apoptotic induction include death receptor endocytosis, mitochondrial and lysosomal permeabilization, endoplasmic reticulum calcium release and fragmentation of the Golgi apparatus. These early apoptotic events are accompanied by a stabilization of the microtubule cytoskeleton and a translocation of organelles to the microtubule organizing center. Together, these phenomena establish a model of apoptotic induction whereby a cytoskeletal-dependent coalescence and “scrambling” of organelles in the paranuclear region coordinates apoptotic communication, caspase activation and cell death.
Keywords: 14-3-3, Bad, Bax, Bid, cathepsin, ceramide, cytochrome c, Drp1, dynein, GD3, mitochondria, PACS-2, SUMO
Apoptosis, the process whereby cells die through an orchestrated self-destruction, occurs in response to environmental or developmental cues, cellular stresses, and specific cell death signals. This self-inflicted death, named for a characteristic rounding and “falling off” of cells, involves a number of evolutionarily conserved biochemical pathways that have been intensively studied for over two decades (reviewed in (1)). Apoptotic cell death is generally characterized by an inward collapse of organelles, a “blebbing” of the plasma membrane into vesicular apoptotic bodies, and the destruction of genetic material. The molecular events that drive such apoptotic processes were uncovered through genetic studies of the nematode C. elegans, which demonstrated the central importance of the ced-3, ced-4 and ced-9 genes in the control of an efficient cell death program. A search for the mammalian counterparts of ced-3, -9 and -4 identified ced-9 as a homologue of the “B-cell Lymphoma” Bcl-2 oncogene and the more than 20 related Bcl-2 family members. Investigations of ced-3 unveiled a family of 18 cysteinyl aspartate proteases, coined “caspases,” that regulate and execute apoptosis through the cleavage of over 400 identified substrates with essential roles in cellular, metabolic and developmental processes, as well as inflammation, degenerative diseases and cancer (1–3).
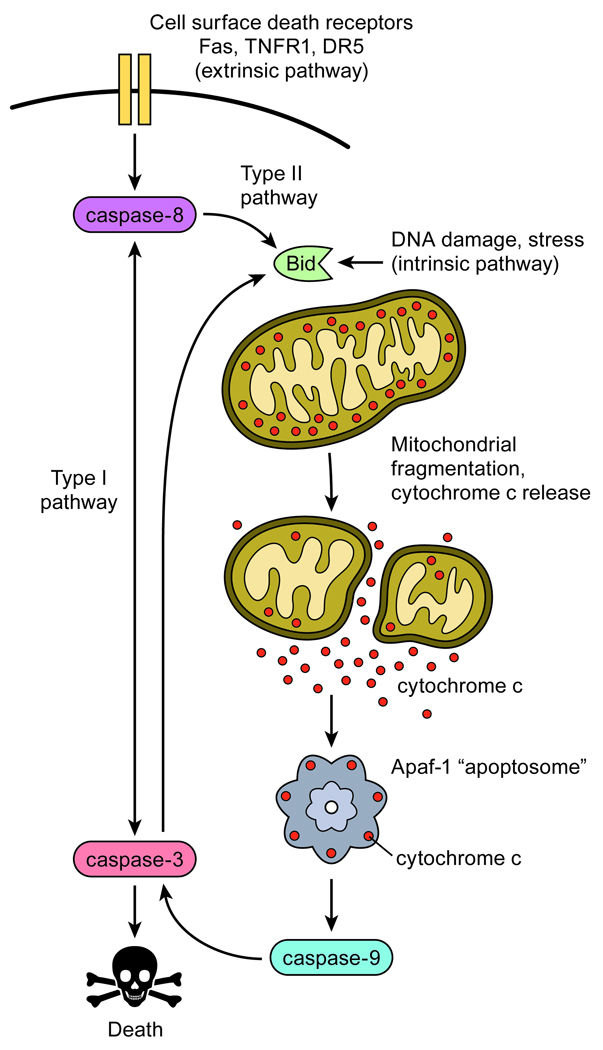
The molecular events regulating apoptosis are dependent on cell type as well as the context of death induction. Nonetheless, key molecular milestones are common to many of modes of cell death (Figure 1). “Executioner” caspases such as caspase-3 carry out the final, committed steps of the apoptotic program after activation of upstream, apical “initiator” caspases, such as caspase-8, which activate apoptosis through two generalized pathways. In cells that employ a type I pathway, initiator caspases directly cleave and activate executioner caspases independent of actions at the mitochondria (4). In type II cells, however, initiator caspases trigger the activation of executioner caspases through Bcl-2 proteins such as Bid, which promote the release of cytochrome c from mitochondria into the cytosol via mitochondria membrane permeabilization (MMP) (4). Upon release, cytosolic cytochrome c binds to the apoptotic protease activating factor Apaf-1 to establish a multimeric “apoptosome” complex that activates caspase-9 to amplify the activation of executioner caspases.
Figure 1. Essential pathways to caspase activation and cell death.
Apoptosis is initiated by internal cellular stress or extracellularly through the binding of ligands to cell surface death receptors. Type I pathways directly activate executioner caspases through initiator caspases to result in death. In Type II pathways, death signals are routed through the Bcl-2 proteins such as Bid and the mitochondria to control the release of cytochrome c. Cytosolic cytochrome c binds Apaf-1 to activate the apoptosome and caspase-9 to result in executioner caspase-3 activation and cell death.
The signaling cascades that ultimately activate cell death through caspases are initiated and regulated by organelle-specific events (5). Death pathways, however, have only recently begun to be integrated with basic cell biological paradigms of intracellular protein and organellar trafficking. In this review, we describe the emerging links between the intracellular signaling events of apoptosis and trafficking at the molecular and organellar level. On the basis of classic as well as more recent data, we present a model of death receptor- and stress-induced apoptosis in which apoptotic events promote a coalescence of organelles and proteins en route to the paranuclear region to coordinate apoptotic interorganellar communication among lysosomes, mitochondria, the endoplasmic reticulum (ER), Golgi and nucleus.
Bcl-2 proteins regulate organellar “life or death” decisions
The Bcl-2 proteins regulate pro- and anti-apoptotic signaling processes at distinct organellar centers to modulate apoptotic communication, caspase activation and the ultimate decision to carry out cellular suicide (Figure 2). While the members of this diverse protein family are primarily noted for roles in regulating the release of apoptogenic factors such as cytochrome c from mitochondria (reviewed in (6)), they also perform a number of daily regulatory functions in healthy cells. This includes roles at the endoplasmic reticulum where Bcl-2 proteins maintain ER homeostasis (7), in the nucleus to control genetic integrity (8), at synapses to modulate neurotransmission (9) as well as at the mitochondria where they regulate mitochondrial division and cellular metabolism (10, 11).
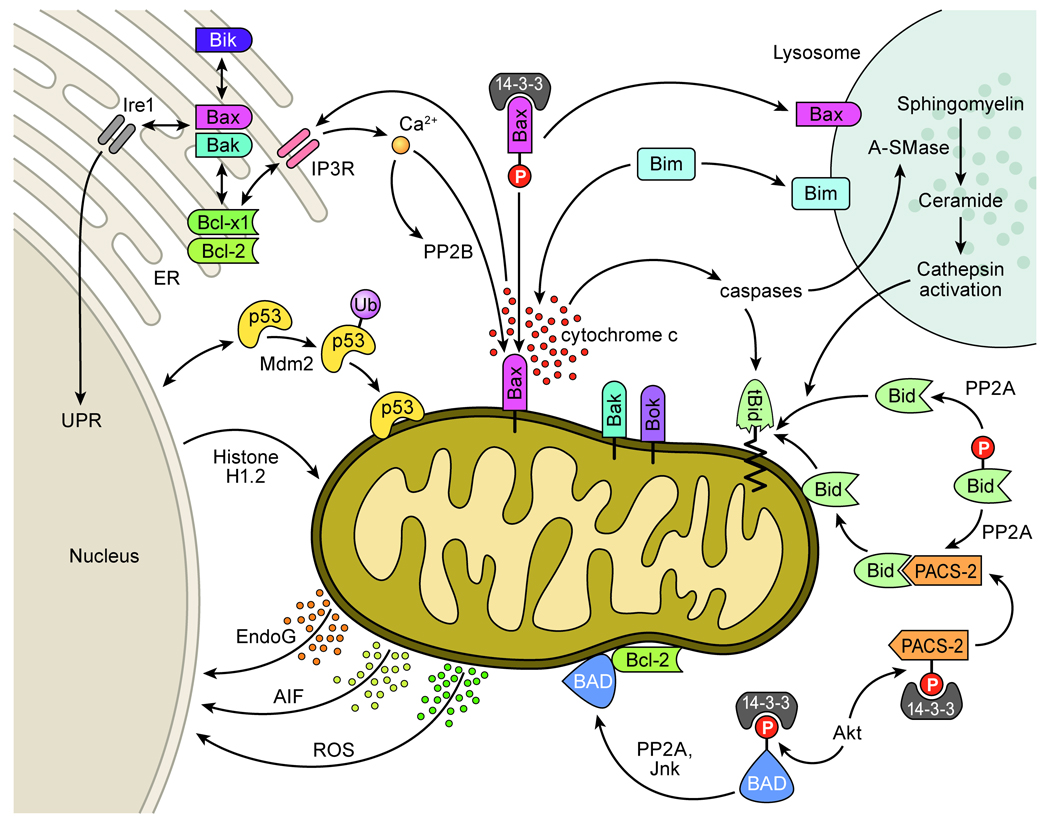
Figure 2. Bcl-2 proteins modulate apoptosis at multiple organellar sites.
Bcl-2 proteins converge on the mitochondria to control the release of apoptogenic factors such as cytochrome c and SMAC/DIABLO to enhance caspase activation (6). Factors such as Endonuclease G, AIF and reactive oxygen species are also released from the mitochondria to the nucleus to promote genome destruction (5, 87). Mitochondrial Bax/Bak release events are activated by Bid translocation to mitochondria and cleavage of Bid by caspases or lysosomal cathepsins (33, 43). Full-length Bid is trafficked to the mitochondria though associations with PACS-2 (59). Active cathepsins are released from lysosomes upon the translocation of Bax and Bim to lysosomes and a caspase activation o f A-SMase to produce ceramide which activates cathepsins (42, 48, 49). Apoptotic proteins such as Bad, Bax and PACS-2 are sequestered by 14-3-3 proteins and become active upon dephosphorylation and 14-3-3 release (28). Factors such as p53 and Histone H1.2 also apoptotically target the mitochondria to modulate Bax activation and apoptosis (94, 95). Bcl-2 proteins additionally regulate apoptosis via ER calcium release through IP3Rs to modulate ER-mitochondria crosstalk (73, 75, 77) and influence the UPR through interactions with Ire1 (74).
Bcl-2 protein family members are categorized on the basis of containing up to four functional Bcl-2 homology “BH domains” (reviewed in (12)). “Multi-BH domain” proteins can participate in both pro- and anti- apoptotic functions while to date, single “BH3-domain only” proteins are strictly pro-apoptotic. The multi-BH domain members Bax, Bak and Bok localize in part to the mitochondrial outer membrane and are requisite for the formation of pores in the mitochondria to permit the release of proapoptotic factors such as cytochrome c, the second mitochondrial activator of caspases SMAC/DIABLO, the apoptosis initiating factor AIF, which activates caspases in the nucleus and Endonuclease G, an apoptotic DNase that degrades nuclear DNA (6) (Figure 2). Pro-apoptotic BH3-domain only proteins such as Bid, Bim and PUMA, induce Bax and Bak activation upon apoptotic induction to promote MMP. Such permeabilization is regulated by anti-apoptotic multi-BH domain proteins such as the Bcl-2 protein itself, Bcl-xl, Bcl-w, Mcl-1 and A1 that prevent Bax and Bak activation by BH3-only pro-apoptotic relatives. While it is presumed that BH3-only proteins such as Bid and Bim directly interact with Bax and Bak to promote their activation and MMP, recent studies suggest that BH3-only proteins only act indirectly to activate MMP by binding to and interfering with anti-apoptotic Bcl-2 family members (13).
Activation and sequestration of Bax and Bad
Bax activation and translocation to mitochondria is regulated by Bax dimerization/oligomerization and dephosphorylation (6) and amplified by a feed-forward wave of caspase activation (14). Non-apoptotic Bax is sequestered in a monomeric state in the cytosol or loosely attached to mitochondria or the ER (15) (Figure 2). Upon apoptotic activation, Bax oligomerizes and exposes its C-terminal segment to form a hydrophobic protrusion that inserts into the outer mitochondrial membrane in a requisite step to induce cytochrome c release (6). Bax is phosphorylated at serine 184 by pro-survival kinases such as Akt/PKB which block Bax activation and translocation to mitochondria (16). This phosphorylation of Bax is influenced through sphingolipid signaling pathways that coordinate inter-organellar communication amongst the Golgi, ER and mitochondria. A key sphingolipid in apoptotic signaling is ceramide which is synthesized de novo at the ER as well as at apoptotic mitochondria (17). Ceramide is also enzymatically formed through an apoptotic upregulation of sphingomyelinase activity at lysosomes (18) (Figure 2). Ceramide directly binds to and activates diverse apoptotic enzymes including protein phosphatase PP2A (17), which dephosphorylates Bax at serine 184 to increase its association with mitochondria in vitro and in vivo (19, 20).
In healthy cells, Bax is held in a non-apoptotic, soluble, monomeric state by cytosolic retention factors. This is in contrast to some Bcl-2 family members such as Bim and Bmf which are sequestered to distinct compartments and cytoskeletal structures through associations with motor protein subunits (21, 22). Bax cytosolic retention factors include humanin, a mammalian anti-apoptotic peptide (23); Ku70, a Bax deubiquitinylation protein and subunit of the Ku DNA-repair complex (24); and the 14-3-3 proteins (25). The 14-3-3 proteins bind more than 200 “client” phosphoproteins in vivo to mediate cell survival as well as cell cycle and cell division events (26). 14-3-3 proteins sequester Bax in an unconventional Bax-phosphorylation independent manner (25). More typically, 14-3-3 proteins bind clients at specific phosphorylated sites to block client pro-apoptotic activities. This includes Bad, which is phosphorylated by Akt/PKB at serine 136 to establish a 14-3-3 binding site (27). Upon loss of Akt survival signals or apoptotic activation of PP2A, Bad is dephosphorylated and releases bound 14-3-3 proteins. Dephosphorylated Bad subsequently translocates to mitochondria to interact with and inhibit the anti-apoptotic effect of Bcl-2, thus activating Bax and MMP (27, 28). In addition to dephosphorylation, clients such as Bax, Bad and c-Abl are released from 14-3-3 proteins upon apoptotic Jnk phosphorylation of 14-3-3 itself which allows for client translocation to mitochondria or the nucleus upon apoptotic induction (29, 30).
Bid translocates to mitochondria to activate Bax and MMP
Unlike Bax and Bad, activation of the pro-apoptotic “BH3 interacting domain death agonist” Bid occurs through its cleavage and myristoylation, which drive an association with mitochondria where Bid is required for Bax activation and MMP (31, 32). Upon death receptor ligation, Bid is cleaved at aspartate 59 by both initiator and executioner caspases, yielding a potently apoptotic truncated “tBid” (31–33). While both the full-length and truncated forms of Bid localize to mitochondria, tBid displays higher affinity for mitochondria and releases cytochrome c from isolated mitochondria in vitro. These observations of Bid regulation initially grounded a caspase cleavage-based model to explain Bid mitochondrial translocation (33). In this classic model, cytosolic, full-length Bid is cleaved by caspase-8, N-terminally myristoylated at the newly exposed amino terminus of glycine 60 (34) and then inserted into mitochondrial membranes to activate Bax and drive apoptosis. Recent studies describing a mitochondrial localization of active caspase 8, however, suggest Bid cleavage and activation occur on mitochondrial lipid microdomains which compartmentalize tBid production in close proximity to the mitochondria (35, 36). Such a model of Bid trafficking in which Bid is cleaved after delivery to the mitochondria is in accordance with reports describing an apoptotic translocation of full-length Bid to the mitochondria (37–39) as well as FRET studies which demonstrate that tBid formation occurs coincidentally with or following the translocation of Bid to mitochondria (40).
Bid cleavage follows lysosomal permeablization and cathepsin release
Proteases other than caspases also cleave and activate Bid, including granzyme B, a protease released from cytotoxic lymphocytes which cleaves Bid at aspartate 75 (41). Lysosomal cathepsins also promote Bid cleavage and apoptosis (42). Cathepsins cleave Bid within an unstructured loop between two alpha helicies at tyrosine 47, glutamine 57, arginine 65 and arginine 71, generating tBid species that induce apoptosis despite the lack of a myristoylatable glycine (43). This suggests that myristoylation is not absolutely required to target Bid to the mitochondria. The activation and release of lysosomal cathepsins has a substantial role in MMP and cell death as cathepsin inhibitors and gene knock-outs prevent apoptosis at the level of Bid cleavage (42, 43). This is evident in cultured fibroblasts from inclusion-cell disease (ICD) patients deficient in lysosomal hydrolase activity which are unresponsive to TNFα death pathways, failing to cleave Bid and activate caspases (44). Likewise, mannose-6-phosphate receptor null mice which fail to deliver cathepsins to lysosomes have an ICD phenotype and do not respond to TNFα and CD95/FasL (44). Interestingly, the requirement for cathepsins in death pathways may be specific to diseased cells which become sensitive to type II pathways involving both mitochondrial and lysosomal permeabilization upon transformation (45–47). The mechanisms by which apoptotic pathways target cathepsins and lysosomes are not well understood but involve initiator caspase activation of lysosomal acidic sphingomyelinase (A-SMase) and its production of ceramide, which directly activates lysosomal cathepsins (48) (Figure 2). Active cathepsins are released to the cytosol upon an apoptotic Jnk-dependent translocation of Bax and Bim to lysosomes, a step inhibited by the Bax sequestering protein Mcl-1 (49). As death receptor-mediated apoptosis promotes a co-localization of cathepsins and Bid (42), as well as an association of lysosomes with mitochondria (50), it has been suggested that cathepsin release and Bid cleavage take place at an interface between mitochondria and lysosomes (51).
PACS-2 mediates the translocation of full-length Bid to mitochondria
The hypothesis that cleavage of Bid occurs in proximity to the mitochondria or along a lysosome-mitochondria axis suggests that Bid localizes to an interface between these organelles prior to cleavage (52). Consistent with such a model, FRET-based assays demonstrate that full-length Bid interacts with Bax upon treatment of HeLa cells with TNFα and that Bid is not cleaved until late into the apoptotic program after the activation of a caspase feedback amplification loop (53). Bid is kept inactive through CK1 and CK2 phosphorylation of serine and threonine residues proximal to Bid’s caspase cleavage site which block protease access and cleavage (54, 55). Accordingly, CK1 phosphorylation of Bid is upregulated in pre-cancerous models of liver disease that block hepatocyte apoptosis (56). CK2 phosphorylation of Bid also regulates the binding of full-length Bid to the multifunctional sorting protein PACS-2, an acidic-cluster binding protein that integrates membrane traffic with ER-mitochondrial communication and apoptosis (57–60). Upon the induction of apoptosis through death receptors or stress, PACS-2 associates with full-length Bid and translocates from the cytosol to the mitochondria (59). In diseased or transformed cells, loss of PACS-2 inhibits apoptotic Bid cleavage and executioner caspase activation but does not inhibit caspase-8, suggesting that Bid cleavage occurs after a PACS-2 mediated delivery of full-length Bid to the mitochondria (59, 61). A functional switch in the homeostatic membrane trafficking and apoptotic activities of PACS-2 is regulated by Akt phosphorylation at PACS-2 serine 437, which binds 14-3-3 proteins (61). Upon the induction of apoptosis by death ligands such as TRAIL, PACS-2 is dephosphorylated at serine 437 to release 14-3-3 proteins in a step prior to Bid translocation to mitochondria and cell death (61). Notably, this TRAIL-induced dephosphorylation of PACS-2 at serine 437 or the expression of a non-phosphorylatable Ser437Ala PACS-2 mutant block the PACS-2-mediated retrieval of acidic cluster-containing cargo to the ER (61). Dephosphorylation of PACS-2 serine 437 may therefore serve as a homeostatic switch that triggers PACS-2, a mediator of membrane traffic, to become an effector of cell death which translocates full-length Bid to mitochondria or to a lysosome/mitochondria interface where Bid is cleaved to tBid to drive MMP, cytochrome c release and cell death.
Drp1-mediated mitochondrial fission recruits Bax and Bid to mitochondria
In respiring cells, mitochondria continuously fuse and divide to organize into reticular networks, exchange metabolites and facilitate mtDNA mixing (62). These processes of mitochondrial fusion and fission are also essential to the apoptotic program as apoptotic changes in mitochondrial size and shape regulate MMP and cytochrome c release. Fission and fusion events are regulated by GTPase proteins which alter the morphology of mitochondrial membranes and cristae to fragment mitochondria and promote the release of cytochrome c and other factors from internal mitochondrial stores (63). One key GTPase, the dynamin related protein Drp1 (64), accumulates at fission sites of the outer mitochondrial membrane with a mitochondrial localized binding partner, hFis1, forming chain-like spirals at membrane scission sites (65).
The apoptotic translocation of Drp1, Bax and Bid to mitochondria appears to be interdependent, as Bax and Bid specifically concentrate at Drp1 generated mitochondrial scission sites (64) (Figure 4). Likewise, mitochondrial fission and Drp1 recruitment require a Bax/Bak dependent release of the Drp1 binding protein DDP/TIMM8a from inner mitochondrial stores which targets Drp1 to the mitochondria (66). Modification of Drp1 by the small ubiquitin related modifier SUMO via the SUMO conjugating enzyme Ubc9 also directs Drp1 to the mitochondria (67) while desumoylation by SENP5 limits Drp1 recruitment (68). Treatment of cells with Mdivi-1, a specific chemical inhibitor of Drp1 GTPase activity, demonstrates that Drp1 is required to drive both mitochondrial fission and cytochrome c release (69). Interestingly, Mdivi-1 treatment of isolated mitochondria prevents the release of cytochrome c following tBid treatment in vitro, implicating Drp1 GTPase activity as a requirement for MMP and cell death (69).
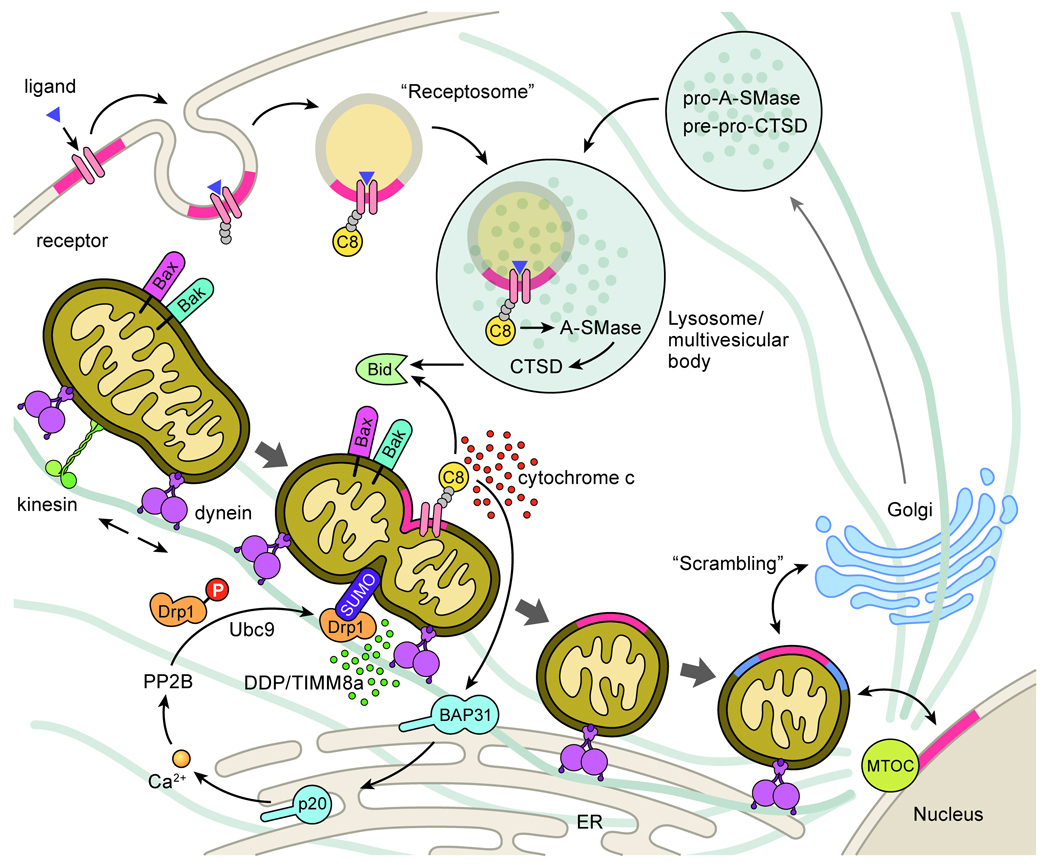
Figure 4. Death receptor ligation promotes mitochondrial fragmentation, mitochondrial clustering, and membrane “scrambling”.
Cell surface death receptors concentrate in GD3-containing lipid rafts (in red) (146). Upon death ligand engagement, receptors recruit death adaptors and are internalized with rafts to specific endosomal compartments such as “TNF receptosomes” to promote caspase-8 (C8) activation (150, 151). Receptosomes fuse with precursor hydrolase-containing Golgi-derived vesicles to form lysosomal multivesicular bodies that activate A-SMase and cathepsin D (CTSD). Caspases and cathepsins cleave Bid to promote Bax/Bak activation, cytochrome c release and caspase activation (33, 42, 43). Mitochondrial-localized caspase-8 cleaves ER localized BAP31 to a p20 fragment which promotes ER calcium release (35, 71), PP2B activation and Drp1 dephosphorylation (70) and mitochondrial fragmentation. Mitochondrial DDP/TIMM8a release (66) and Drp1 sumoylation (67, 68) promote the translocation of Drp1 to mitochondrial scission sites as mitochondria travel in the (-)-end direction (111). Bax and Bid are also recruited to these scission sites that are rich in GD3 (in red). The accumulation of fragmented mitochondria at the MTOC in proximity to the Golgi results in a caspase dependent fragmentation of the Golgi (139) and a “scrambling” of Golgi membranes (in blue) with mitochondria (50). As GD3-contatining rafts (in red) internalize to endosomes and later localize to mitochondria, it is hypothesized that mitochondria act as “cargo boats” to carry GD3 from the cell surface to the nucleus and adjacent organelles (145).
Drp1 recruitment and mitochondrial fission are additionally under the control of apoptotic ER calcium release. Calcium activates PP2B/calcineurin to dephosphorylate Drp1 at serine 656 which activates mitochondrial fission, cytochrome c release and apoptosis (70) (Figure 4). Apoptotic ER calcium release, mitochondrial fission and mitochondrial fragmentation are controlled by caspases which cleave the integral resident ER 31 kD “B-cell Receptor Associated Protein” BAP31 (71). In non-apoptotic cells, BAP31 associates with class I MHC molecules at the ER to regulate their ultimate delivery to the plasma membrane (72). Upon apoptotic induction however, caspase-8 cleaves BAP31 to a p20BAP31 fragment that promotes release of calcium from the ER (35, 71).
Bcl-2 proteins regulate ER calcium release
The p20BAP31-evoked release of calcium from the ER and subsequent fragmentation of mitochondria are under the control of ER-localized Bcl-2 proteins (73). ER-based Bcl-2 proteins also modulate the unfolded protein response (UPR) through interactions with Ire1 which control ER communication with the nucleus (74). The mechanisms by which Bcl-2 proteins localize to the ER remain uncharacterized, though ER associated Bcl-2 proteins are anchored through single transmembrane helical domains. ER calcium is released over the course of apoptotic induction through IP3 receptors which are regulated by direct interactions with Bcl-2 and Bcl-xl (75– 77). Phosphorylation of Bcl-2 likely promotes an interaction with the IP3 receptor that blocks receptor phosphorylation (78). Upon the induction of apoptosis, Bcl-2 is dephosphorylated and loses interactions with IP3Rs in favor of association with ER-localized Bax and Bak (78). Loss of the Bcl-2 – IP3R interaction promotes IP3R phosphorylation, calcium release, mitochondrial fragmentation and apoptosis. The mechanism by which p20BAP31 communicates with Bcl-2 proteins and IP3Rs is not yet understood; however, the ER-localized Bcl-2 family member Bik is required for the process (73). Upon mitochondrial fragmentation, released cytochrome c translocates to the ER to bind IP3Rs and increase calcium release through a feed-forward mechanism requiring PACS-2 (58, 59, 79).
Communication between the ER and mitochondria has a role in metabolic and apoptotic processes involving the exchange of ATP, lipids and calcium. Such communication is facilitated not only by the proximity of these two organelles, but by direct membranous contacts between the ER and mitochondria at mitochondria-associated membranes (MAMs) (80–82). Such contacts facilitate the direct transfer of calcium from the ER through IP3Rs to the mitochondria (82). Upon the induction of apoptosis, calcium signaling between the ER and mitochondria via MAMs is further enhanced by an increased co-localization of mitochondria and ER at the paranuclear region to facilitate calcium transfer between these organelles (83). MAMs also serve as sites for the synthesis of sphingolipids and their transfer between the ER and mitochondria (18, 84). The apposition of the ER and mitochondria through MAMs is maintained by PACS-2, which mediates ER homeostasis in part by localizing the calcium-dependent chaperone Calnexin to the ER (58, 59, 80). PACS-2 may also modulate apoptotic ER calcium communication through the trafficking of TRPP2 (61, 85), an anti-apoptotic ion-channel that limits the concentration of ER calcium available for apoptotic signaling processes between the ER and mitochondria (86). The induction of apoptosis by death ligands such as TRAIL dephosphorylates PACS-2 at serine 437 to not only drive Bid translocation to mitochondria, but also block the PACS-2 mediated retrieval of TRPP2 to the ER (61). Taken together, these studies suggest a model in which PACS-2 serine 437 dephosphorylation regulates a functional switch in PACS-2 from a membrane traffic regulator to an inducer of cell death, coordinating a net increase in apoptotic ER calcium signaling with the translocation Bid to the mitochondria.
Apoptotic communication between the mitochondria and the nucleus
An apoptotic apposition of the mitochondria to the nucleus facilitates the transfer of AIF, Endonuclease G and reactive oxygen species (ROS) directly from the mitochondria to the nucleus to promote genomic destruction (5, 87). Mitochondria and nuclear communication may also be mediated by the shuttling of Bcl-2 proteins to and from these organelles to control cell cycle events (reviewed in (8)). These include Bcl-xl and Bcl-2 which promote cell cycle arrest; Bax, which increases S-phase progression and Bad, which regulates cell cycle transitions upon phosphorylation by Cdc2 (8). Bid also functions in the nucleus to maintain genomic stability. Bid-null mice display chromosomal abnormalities and develop a chronic myelomonocytic leukemia (CMML)-like phenotype (88). Phosphorylation of Bid at serine 78 by the DNA-damage sensing kinase ATM regulates an S-phase checkpoint, suggesting that Bid regulates genomic integrity following DNA damage (89, 90), though these results are controversial (91). Nonetheless, disruption of the MRE11 complex, a key ATM activator, blocks phosphorylation of Bid at serine 78 in response to ionizing radiation (92). Furthermore, there is growing precedence for cytosolic apoptotic regulators participating in genomic maintenance regimens. A recently described example includes, Apaf-1, which translocates from the cytosol to the nucleus in response to DNA damage to promote checkpoint kinase Chk1 activation and cell cycle arrest in response to genotoxic stress (93).
Nucleus-to-mitochondria apoptotic communication is mediated by Histone H1.2 (94) and p53 (95), the tumor suppressor that upregulates apoptotic genes in response to cell cycle arrest and stress induced apoptosis. At the onset of apoptosis, p53 is trafficked to the mitochondria where it binds Bcl-2 family members to activate Bax and release cytochrome c (95). Monoubiquitinylation of p53 by cytosolic Mdm2, an E3 ligase typically considered responsible for tagging p53 for degradation, directs p53 to the mitochondria, which is subsequently deubiquitinylated by mitochondrial-localized HAUSP (96). The mechanism by which Histone H1.2 travels from the nucleus to the mitochondria is not understood. However, p53 is required to release Histone H1.2 from the nucleus upon damage induced by ionizing radiation in a process requiring Chk2 phosphorylation and stabilization of p53 (97).
Paranuclear organellar clustering mediates apoptotic communication and cell death
Bcl-2 family members regulate caspase activation and apoptosis in an organelle-specific manner (5). However, it remains unknown how the distribution of apoptotic organelles modulates the cell death program. While the proximity and direct contacts of the endoplasmic reticulum and mitochondria have an essential role in apoptotic communication (81, 98) it is enigmatic how other systems such as the nucleus, lysosomes, and Golgi apparatus come together to communicate with one another during apoptosis. Interestingly, in a process reminiscent of mitosis (99), fragmented apoptotic organelles collect near the Golgi apparatus and microtubule organizing center (MTOC) through the action of mircrotubule-associated motor proteins. This co-localization may represent a functional coalescence of organelles at the paranuclear region to control a number of steps at the induction of apoptosis (50). This may include the direct, vesicle-independent transfer of sphingolipids such as ceramide amongst mitochondria, lysosomes and the Golgi (17, 100) or perhaps the shuttling of apoptogenic molecules between the mitochondria and nucleus (8, 87). An early apoptotic, microtubule-dependent redistribution of mitochondria to the Golgi-proximal MTOC serves as the best characterized example of this apoptotic paranuclear relocalization, and is observed upon exposure to TNFα (101), the TNFα Related Apotosis Inducing Ligand TRAIL/Apo2L (102), FasL/CD95/Apo1L (50), ceramide (83), oxidative stress (103) and viral infection (104).
Molecular motors drive apoptotic paranuclear clustering
In non-apoptotic cells, microtubule associated (+)-end directed kinesin motors traffic mitochondria away from the paranuclear region (105, 106). Knock-out deletions of kinesin genes such as KIF5B result in an abnormal paranuclear clustering of mitochondria in embryonic knockout cells (106) similar to clustering observed in apoptotic cells (101). If paranuclear trafficking of mitochondria were to serve a pro-apoptotic function, inhibition of kinesin activity would likely enhance apoptosis. Indeed, immunoinhibition of (+)-end directed kinesin motors blocks the basal dispersal of mitochondria in healthy cells, resulting in a paranuclear clustering of mitochondria and a synergistic increase in the apoptotic effects of TNFα (107). While kinesin knockout or siRNA knock-down cells have not yet been explored in studies of mitochondrial clustering and apoptosis, conditional knock-out deletions of KIF3A in photoreceptor cells and renal cells result in apoptotic cell death (108, 109). Apoptotic paranuclear clustering of mitochondria appears to be a mechanistic consequence of apoptotic induction as stress activated MAP kinases p38 and Jnk phosphorylate and inactivate the kinesin light chain (107) and release KIF5B from microtubules to halt mitochondrial dispersal (110) (Figure 3).
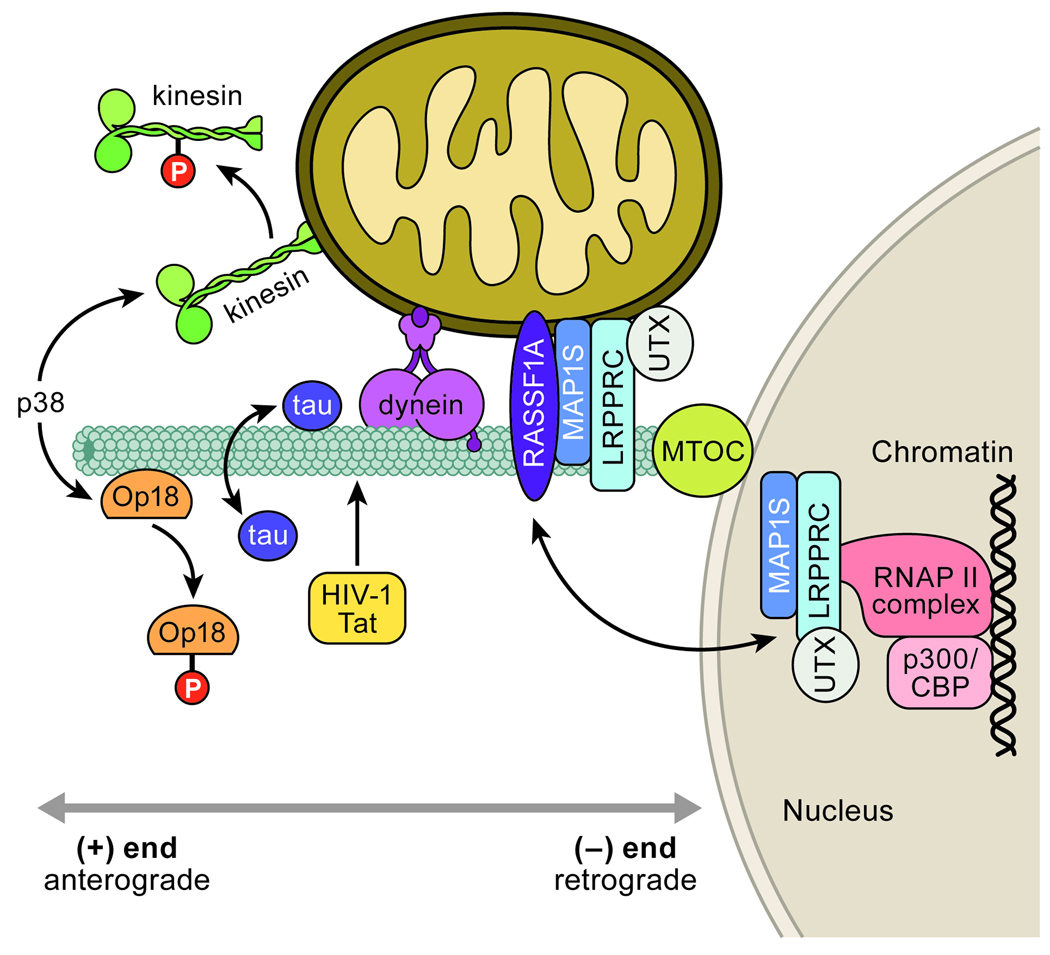
Figure 3. Microtubule associated motors and stabilizing proteins regulate paranuclear mitochondrial clustering and apoptosis.
Apoptotic p38 phosphorylation of kinesin subunits halts anterograde traffic of mitochondria to promote dynein driven (-)-end accumulation (107). p38 phosphorylation also releases Op18 from microtubules to result in microtubule stabilization and mitochondrial clustering (116, 117). Mitochondrial aggregation and apoptosis are also influenced through microtubules by tau (83, 125) and HIV-1 Tat (126). MAP1S – LRPPRC – UXT complexes also stabilize microtubules and localize to apoptotic paranuclear mitochondria as well as the nucleus in complex with RNAPII and p300/CBP (109, 121–124). It has been proposed that these microtubule and nucleus associated proteins coordinate both apoptotic mitochondrial clustering and chromatin remodeling events (123, 124).
As the inactivation of kinesin promotes the trafficking of mitochondria to the paranuclear region, retrograde clustering of organelles is likely driven by a net increase in (-)-end motor activity of dynein and dynactin. Dynein and dynactin complexes associate with mitochondria on microtubules, and disruption of dynein function disperses mitochondria away from the MTOC (111). Mitochondrial paranuclear clustering is intimately coordinated with mitochondrial fission, as inhibition of fission prevents mitochondrial clustering and apoptosis (112). The Drp1 GTPase, together with Bax and Bid, are recruited to dynein complexes at mitochondrial fission sites as mitochondria travel in the (-)-end direction (111) (Figure 4). Targeting of Drp1 to sites of mitochondrial scission is in part mediated by sumoylation of Drp1, as sumoylated proteins generally cluster at mitochondrial fission sites (67). Sumoylation of Drp1 may direct Drp1 specifically to dynein motor complexes as sumoylation promotes the interaction of cargo molecules with dynein to mediate their retrograde transport (113). Like other apoptotic effectors, dynein and dynactin complex proteins such as the cytoplasmic dynein intermediate chain CD-IC and p150glued are regulated by caspase cleavage and myristoylation (114, 115). While it remains to be determined if cleavage and lipid modification anchors a functional, truncated CD-IC fragment to mitochondria, GFP-tagged truncated CD-IC localizes in vesicular mitochondrial-like structures to the paranuclear region (115).
Organelles cluster at the paranuclear region via stabilized microtubules
In proliferating cells, microtubules radiate from the MTOC as a web-like network that acts as a scaffold for mitochondria and other organelles to disperse throughout the cell (105). Cellular stress, as well as specific cell cycle events, stabilizes microtubules such that they become more rigid and rod-like and collapse towards the MTOC, forming concentric rings around the nucleus that may act as a net to bring mitochondria and other associated organelles to the paranuclear region. Death-receptor induced apoptosis triggers microtubule stabilization, mitochondrial clustering and apoptosis through p38 phosphorylation and inactivation of the microtubule destabilizing oncoprotein family member Op18/Stathmin (116, 117) (Figure 3). This p38-mediated stabilization of microtubules phenocopies the stabilization seen in cells treated with chemotherapeutic agents such as Taxol, which inhibit tumor cell growth by stabilizing microtubules to disrupt mitosis (118). Interestingly, Taxol treatment results in the co-localization of mitochondria with microtubule bundles at the paranuclear region (119). This redistribution of mitochondria upon the addition of microtubule stabilizing agents may explain how these drugs synergize the apoptotic effects of death receptor ligands such as TNFα and TRAIL.
Microtubule stability is regulated by microtubule associated proteins such as MAP1S/C19ORF5, which bind to both mitochondria and stabilized microtubules in stressed or Taxol-treated cells (120) (Figure 3). MAP1S associates with other microtubule binding proteins such as the tumor suppressor RASSF1A (120, 121), and UXT (Ubiquitously Expressed Transcript), a γ-tubulin/centrosome associated protein that induces paranuclear clustering of mitochondria (122). UXT, RASSF1A and MAP1S all interact with the leucine-rich protein LRPPRC in microtubule-associated complexes at an interface between paranuclear mitochondria and the nucleus (123). As LRPPRC and UXT both interact with several components of the RNA polymerase II complex and CBP/p300 (124), it has been hypothesized that LRPPRC and UXT link microtubule stabilization and mitochondrial apoptosis to transcriptional as well chromatin remodeling events in the nucleus (124).
Microtubule-associated proteins with pathological functions such as tau, a prime suspect in Alzheimer’s disease, also cause paranuclear mitochondrial clustering. This occurs in part through an association of tau with motor proteins and microtubules (125) (Figure 3). The mechanism by which tau contributes to clustering is not straightforward, however, as apoptotic CDK5 phosphorylation of tau conversely promotes paranuclear co-localization of mitochondria and the ER through a tau microtubule dissociation (83). Other pathogenic factors, such as the pro-apoptotic Tat protein encoded by HIV-1, bind to microtubules to result in a Taxol-like stabilization and cell death (126). Microtubule proteins implicated in tumorigenesis such as the adenomatous polyposis coli (APC) protein and the Von Hippel-Lindau VHL tumor suppressor also coordinate nuclear as well as apoptotic events (127–129). As APC and VHL both localize in part to mitochondria (127, 130), it is enticing to speculate that these tumor suppressors will participate in the apoptotic paranuclear clustering of microtubules and organelles; however, this has not yet been addressed.
Apoptotic signaling proteins cluster at the paranuclear region upon death induction
A number of apoptotic signaling proteins collect in the paranuclear region during the course of apoptosis. Such proteins include p53, which localizes predominantly to the cytosol in non-apoptotic cells through an association with microtubules (131). Non-apoptotic p53 is sequestered by a recently identified Parkin-like ubiquitin ligase, Parc, which binds to but does not apparently ubiquitinylate cytosolic p53 (132). The mechanism by which p53 is released from Parc and the physiological role of Parc as a ubiquitin ligase are, however, not yet understood. Upon DNA damage, p53 is released from Parc and driven to the paranuclear region by microtubule-associated dynein motors (131). p53 associates with microtubules and the dynein motor complex through the dynein light chain LC8, which binds to the p53 binding protein 53BP1, a key cytosolic mediator of the DNA damage response (133). A p53 – 53BP1 interaction is required for the paranuclear clustering of p53 upon DNA damage and subsequently apoptosis (133). This LC8-driven relocalization of p53 to the paranuclear region may be part of a mechanism to import p53 into the nucleus from the cytosol (134), and may explain how p53 is recruited to mitochondria during apoptosis (95). LC8 additionally binds the Bcl-2 family member Bim and is also a target of the p21 activated kinase PAK1 to promote cell survival and tumorigenesis (135). Whether LC8 or other dynein regulatory factors have roles in bringing additional proteins or organelles to the paranuclear region to coordinate apoptotic signaling events will be an important line of investigation in uncovering the relation of cytoskeletal motor activity to apoptosis.
Apoptotic fragmentation and membrane scrambling of the Golgi apparatus
The Golgi apparatus neighbors the MTOC at the paranuclear region in an ideal locale to influence microtubule-based apoptotic processes (136, 137). The cisternal stacks of Golgi membranes are maintained by microtubule and actin cytoskeletal structures (137), as well as Golgi-specific structural proteins such as the Golgins and GRASPs (Golgi Reassembly Stacking Proteins) (137). Like other membranous organellar systems, the Golgi apparatus undergoes a characteristic fragmentation early in the induction of apoptosis that precedes or coincides with mitochondrial cytochrome c release (138, 139). Golgi fragmentation is triggered by the Golgi-localized initiator caspase-2 cleavage of Golgin-160 followed by the executioner caspase-3 cleavage of GRASP65 (138, 140) and the Golgi vesicle tethering protein p115 (141). The C-terminal cleavage product of p115 alone is capable of fragmenting the Golgi and relocates to the nucleus to serve a pro-apoptotic role (141). In contrast, cleaved Golgin-160 enters the nucleus to block apoptosis (136). It was originally hypothesized that Golgi fragmentation was the result of an apoptotic breakdown of the cytoskeleton. Golgi fragmentation, however, occurs early in apoptosis and precedes major apoptotic changes in cytoskeletal structures (139). This suggests that an intact cytoskeleton and MTOC are required to traffic factors to the Golgi prior to fragmentation.
As mitochondria and other organelles cluster at the Golgi/MTOC in a microtubule-dependent fashion at the induction of apoptosis (50, 142, 143), the Golgi may serve as a site for organellar convergence at the induction of apoptosis. Such a stress-induced co-localization of organelles may promote organellar cross-talk processes, including the transfer of apoptogenic lipids such as ceramide between lysosomes, Golgi and mitochondria to prime mitochondrial membranes for Bcl-2 protein-mediated permeabilization. Organellar co-localization is an early consequence of FasL treatment of CEM cells which results in a net redistribution of endosomes, lysosomes and mitochondria to overlapping sucrose density fractions (143). This convergence may represent a physical coalescence and “scrambling” of organelles as FasL treatment also increases the levels of Golgi, endosomal and lysosomal membrane markers that co-purify with apoptotic paranuclear mitochondria in a step prior to caspase activation, Golgi fragmentation and cytoskeleton breakdown (50). Interestingly, the global caspase inhibitor zVAD-fmk blocks this stress-induced mixing of Golgi and mitochondrial markers but increases the presence of endo-lysosomal markers associated with apoptotic mitochondria (50, 143). Together, these experiments suggest a multi-step model in which mitochondria and endo-lysosomes first physically associate to release cathepsins and cleave Bid prior to Golgi-mitochondrial scrambling, Golgi fragmentation, mitochondrial cytochrome c release and ultimately caspase activation (Figure 4).
GD3 lipid rafts link plasma membrane-to-nucleus signaling and paranuclear trafficking
A mixing of the Golgi apparatus, endosomes, lysosomes, mitochondria and plasma membrane is also evident in the interorganellar apoptotic trafficking of the glycosphingolipid GD3 (144–146) (Figure 4). This ganglioside is produced in the Golgi from ceramide by GD3-synthase and is capable of permeabilizing mitochondria to promote cytochrome c release in vitro (147). In healthy hepatocytes and T-cells, GD3 localizes predominantly to the plasma membrane in death receptor-rich lipid rafts. TNFα treatment of hepatocytes, however, redistributes GD3 from the plasma membrane to mitochondria via internalization through endosomal compartments in a process requiring an intact cytoskeleton (148). Upon Fas and TNFR1 death-receptor engagement, cell-surface-localized death induced signaling complexes (DISCs) internalize through receptor-mediated endocytosis (149, 150) to specific endosomal compartments such as “TNF Receptosomes,” which subsequently associate with and activate caspase-8 (52, 151). Receptosomes may represent DISC-rich GD3 raft-containing endosomes that co-localize with mitochondria to promote cell death (152). It has been hypothesized that this co-localization represents a mixing of endosomes with mitochondria, as GD3-containing lipid rafts are found on mitochondria after FasL treatment of CEM cells (144). These GD3 rafts contain mitochondrial signaling complexes that include the voltage dependent anion channel VDAC-1 and hFis1 and act as recruitment sites for Bax, Bid and presumably Drp1 (144). The integrity of these rafts appears to have a role in MMP, as their chemical disruption prevents mitochondrial permeablization in response to exogenous tBid treatment (144). Like ceramide, GD3 has pleiotropic effects on apoptotic activation at the mitochondria, as well as the Golgi, ER and nucleus (146). As GD3 raft-containing mitochondria and other vesicular structures collect at the paranuclear region upon the induction of apoptosis, such fissured mitochondria may act as “cargo boats” that carry DISC-containing GD3-rafts to multiple organelles at the paranuclear region (145) (Figure 4). This hypothesis may explain how GD3 traffics to the nucleus upon apoptotic induction by CD95/FasL to modulate histone structures and transcription (153).
Perspectives and Conclusion
The past decade has brought immense progress in identifying key proteins and signaling pathways that initiate and regulate cell death through organelle-specific processes. Although many of these players first gained attention for their roles in cell death, it is now recognized that several organelle-directed apoptotic decision-makers have “day jobs” that relate their apoptotic functions to physiological roles. Future work will undoubtedly aim to describe how the trafficking of such multi-functional players occurs in apoptotic and non-apoptotic contexts. A recent and noteworthy example is provided by the Bcl-2 protein Bad. In addition to promoting cell death, Bad also translocates to mitochondria in a non-apoptotic role to shuttle glucokinase to respiring mitochondria during high-fat feeding to metabolize glucose and stimulate the release of insulin (11). Physiological and apoptotic cross-functionality is, of course, not limited to Bcl-2 family members. The Drp1 GTPase functions in both apoptosis and mitosis to divide mitochondria and also modulates neurotransmission at synapses (9). Multi-functionality is also seen with the PACS-2 sorting protein which regulates cellular homeostasis, ER-mitochondrial communication and apoptosis (57–61). Likewise, overlapping functionality is also seen in signaling systems that regulate DNA quality control such as ATM/ATR as well as those that signal cell cycle transitions and cell division events such as Cdc2 and the checkpoint kinases. These and other genetic regulatory kinases phosphorylate apoptotic proteins such as Bad and Bid to control cell cycle and cell division events. Such examples of cross-functionality hint that mitotic cell birth and apoptotic cell death may share fundamental machinery and regulatory mechanisms.
Individual organellar systems such as lysosomes and mitochondria initiate and regulate apoptosis (5); however, the relation of organellar trafficking and apoptotic signaling pathways is only now coming to light. An apoptosis-induced co-localization of organelles at the MTOC / Golgi / paranuclear region potentially serves a number of functions in cell death to help package and redistribute fragmented organelles, to facilitate nuclear destruction and also to promote a general shrinkage of dying cells. Organellar coalescence also takes place in autophagic organellar destruction as part of a mechanism to traffic degrading mitochondria to lysosomes (51). Likewise, autophagosomes as well as misfolded protein aggregates in “aggresomes” are targeted to paranuclear-localized lysosomes through dynein/dynactin motor activity (154, 155). If paranuclear clustering and organellar mixing is indeed a bona fide component of the highly regulated apoptotic program, a number of questions regarding the energetics and specificity of this process demand exploration. Interestingly, a subset of Bcl-2 family members, the BNIPs, associate with components of the membrane fusion machinery such as the SNARE protein syntaxin 18 to modulate apoptosis in an αSNAP-dependent manner (156). Future studies relating microtubule structure and motor activity to organellar clustering and the transfer of organellar markers (157) will prove insightful and clarify the role of scrambling in the induction of cell death.
Acknowledgements
We are greatly indebted to Lori Vaskalis for illustrations. We thank Thomas Simmen, Robert Youker, Matthew Brush, Mauro Degli Esposti and the late Dennis Shields for critically reading the manuscript and providing editorial suggestions. This work was supported by National Institute of Health grants DK37274 and AI49793 (to G.T.) and NRSA T32 AI07472 (to J.E.A.) and Oregon Clinical and Translational Research Institute (OCTRI) grant RR0241 (to G.T.).
References
- 1.Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008 doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- 2.Eckhart L, Ballaun C, Hermann M, Vandeberg JL, Sipos W, Uthman A, Fischer H, Tschachler E. Identification of novel mammalian caspases reveals an important role of gene loss in shaping the human caspase repertoire. Mol Biol Evol. 2008 doi: 10.1093/molbev/msn012. [DOI] [PubMed] [Google Scholar]
- 3.Luthi AU, Martin SJ. The CASBAH: a searchable database of caspase substrates. Cell Death Differ. 2007;14(4):641–650. doi: 10.1038/sj.cdd.4402103. [DOI] [PubMed] [Google Scholar]
- 4.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10(1):26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 5.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3(11):E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 6.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 7.Hetz C, Glimcher L. The daily job of night killers: alternative roles of the BCL-2 family in organelle physiology. Trends Cell Biol. 2008;18(1):38–44. doi: 10.1016/j.tcb.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Zinkel S, Gross A, Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 2006;13(8):1351–1359. doi: 10.1038/sj.cdd.4401987. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Chen Y, Jones AF, Sanger RH, Collis LP, Flannery R, McNay EC, Yu T, Schwarzenbacher R, Bossy B, Bossy-Wetzel E, Bennett MV, Pypaert M, Hickman JA, Smith PJ, et al. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105(6):2169–2174. doi: 10.1073/pnas.0711647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443(7112):658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- 11.Danial NN, Walensky LD, Zhang CY, Choi CS, Fisher JK, Molina AJ, Datta SR, Pitter KL, Bird GH, Wikstrom JD, Deeney JT, Robertson K, Morash J, Kulkarni A, Neschen S, et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14(2):144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 13.Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19(5):488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakhani SA, Masud A, Kuida K, Porter GA, Jr, Mehal WZ, Inayat I, Flavell RA. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311(5762):847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300(5616):135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi H, Wang HG. The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene. 2001;20(53):7779–7786. doi: 10.1038/sj.onc.1204984. [DOI] [PubMed] [Google Scholar]
- 17.Morales A, Lee H, Goni FM, Kolesnick R, Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis. 2007;12(5):923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 18.Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J. 2004;382(Pt 2):527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem J. 2005;386(Pt 3):445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xin M, Deng X. Protein phosphatase 2A enhances the proapoptotic function of Bax through dephosphorylation. J Biol Chem. 2006;281(27):18859–18867. doi: 10.1074/jbc.M512543200. [DOI] [PubMed] [Google Scholar]
- 21.Puthalakath H, Huang DC, O'Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3(3):287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 22.Puthalakath H, Villunger A, O'Reilly LA, Beaumont JG, Coultas L, Cheney RE, Huang DC, Strasser A. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293(5536):1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- 23.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423(6938):456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 24.Amsel AD, Rathaus M, Kronman N, Cohen HY. Regulation of the proapoptotic factor Bax by Ku70-dependent deubiquitylation. Proc Natl Acad Sci U S A. 2008;105(13):5117–5122. doi: 10.1073/pnas.0706700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nomura M, Shimizu S, Sugiyama T, Narita M, Ito T, Matsuda H, Tsujimoto Y. 14-3-3 Interacts directly with and negatively regulates pro-apoptotic Bax. J Biol Chem. 2003;278(3):2058–2065. doi: 10.1074/jbc.M207880200. [DOI] [PubMed] [Google Scholar]
- 26.Pozuelo Rubio M, Geraghty KM, Wong BH, Wood NT, Campbell DG, Morrice N, Mackintosh C. 14-3-3-affinity purification of over 200 human phosphoproteins reveals new links to regulation of cellular metabolism, proliferation and trafficking. Biochem J. 2004;379(Pt 2):395–408. doi: 10.1042/BJ20031797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Datta SR, Ranger AM, Lin MZ, Sturgill JF, Ma YC, Cowan CW, Dikkes P, Korsmeyer SJ, Greenberg ME. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev Cell. 2002;3(5):631–643. doi: 10.1016/s1534-5807(02)00326-x. [DOI] [PubMed] [Google Scholar]
- 28.Chiang CW, Harris G, Ellig C, Masters SC, Subramanian R, Shenolikar S, Wadzinski BE, Yang E. Protein phosphatase 2A activates the proapoptotic function of BAD in interleukin- 3-dependent lymphoid cells by a mechanism requiring 14-3-3 dissociation. Blood. 2001;97(5):1289–1297. doi: 10.1182/blood.v97.5.1289. [DOI] [PubMed] [Google Scholar]
- 29.Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170(2):295–304. doi: 10.1083/jcb.200409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida K, Yamaguchi T, Natsume T, Kufe D, Miki Y. JNK phosphorylation of 14-3-3 proteins regulates nuclear targeting of c-Abl in the apoptotic response to DNA damage. Nat Cell Biol. 2005;7(3):278–285. doi: 10.1038/ncb1228. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94(4):491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 32.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94(4):481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 33.Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274(2):1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 34.Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290(5497):1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- 35.Chandra D, Choy G, Deng X, Bhatia B, Daniel P, Tang DG. Association of active caspase 8 with the mitochondrial membrane during apoptosis: potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. Mol Cell Biol. 2004;24(15):6592–6607. doi: 10.1128/MCB.24.15.6592-6607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJ, Petit PX, Vaz FM, Gottlieb E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183(4):681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esposti MD, Erler JT, Hickman JA, Dive C. Bid, a widely expressed proapoptotic protein of the Bcl-2 family, displays lipid transfer activity. Mol Cell Biol. 2001;21(21):7268–7276. doi: 10.1128/MCB.21.21.7268-7276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarig R, Zaltsman Y, Marcellus RC, Flavell R, Mak TW, Gross A. BID-D59A is a potent inducer of apoptosis in primary embryonic fibroblasts. J Biol Chem. 2003;278(12):10707–10715. doi: 10.1074/jbc.M210296200. [DOI] [PubMed] [Google Scholar]
- 39.Tafani M, Karpinich NO, Hurster KA, Pastorino JG, Schneider T, Russo MA, Farber JL. Cytochrome c release upon Fas receptor activation depends on translocation of full-length bid and the induction of the mitochondrial permeability transition. J Biol Chem. 2002;277(12):10073–10082. doi: 10.1074/jbc.M111350200. [DOI] [PubMed] [Google Scholar]
- 40.Ward MW, Rehm M, Duessmann H, Kacmar S, Concannon CG, Prehn JH. Real time single cell analysis of Bid cleavage and Bid translocation during caspase-dependent and neuronal caspase-independent apoptosis. J Biol Chem. 2006;281(9):5837–5844. doi: 10.1074/jbc.M511562200. [DOI] [PubMed] [Google Scholar]
- 41.Waterhouse NJ, Sedelies KA, Browne KA, Wowk ME, Newbold A, Sutton VR, Clarke CJ, Oliaro J, Lindemann RK, Bird PI, Johnstone RW, Trapani JA. A central role for Bid in granzyme B-induced apoptosis. J Biol Chem. 2005;280(6):4476–4482. doi: 10.1074/jbc.M410985200. [DOI] [PubMed] [Google Scholar]
- 42.Heinrich M, Neumeyer J, Jakob M, Hallas C, Tchikov V, Winoto-Morbach S, Wickel M, Schneider-Brachert W, Trauzold A, Hethke A, Schutze S, Cathepsin D. links TNF-induced acid sphingomyelinase to Bidmediated caspase-9 and -3 activation. Cell Death Differ. 2004;11(5):550–563. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- 43.Cirman T, Oresic K, Mazovec GD, Turk V, Reed JC, Myers RM, Salvesen GS, Turk B. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J Biol Chem. 2004;279(5):3578–3587. doi: 10.1074/jbc.M308347200. [DOI] [PubMed] [Google Scholar]
- 44.Tardy C, Autefage H, Garcia V, Levade T, Andrieu-Abadie N. Mannose 6-phosphorylated proteins are required for tumor necrosis factor-induced apoptosis: defective response in I-cell disease fibroblasts. J Biol Chem. 2004;279(51):52914–52923. doi: 10.1074/jbc.M408261200. [DOI] [PubMed] [Google Scholar]
- 45.Fehrenbacher N, Bastholm L, Kirkegaard-Sorensen T, Rafn B, Bottzauw T, Nielsen C, Weber E, Shirasawa S, Kallunki T, Jaattela M. Sensitization to the lysosomal cell death pathway by oncogene-induced down-regulation of lysosome-associated membrane proteins 1 and 2. Cancer Res. 2008;68(16):6623–6633. doi: 10.1158/0008-5472.CAN-08-0463. [DOI] [PubMed] [Google Scholar]
- 46.Fehrenbacher N, Gyrd-Hansen M, Poulsen B, Felbor U, Kallunki T, Boes M, Weber E, Leist M, Jaattela M. Sensitization to the lysosomal cell death pathway upon immortalization and transformation. Cancer Res. 2004;64(15):5301–5310. doi: 10.1158/0008-5472.CAN-04-1427. [DOI] [PubMed] [Google Scholar]
- 47.Peacock JW, Palmer J, Fink D, Ip S, Pietras EM, Mui AL, Chung SW, Gleave ME, Cox ME, Parsons R, Peter ME, Ong CJ. PTEN loss promotes mitochondrially dependent type II Fas-induced apoptosis via PEA-15. Mol Cell Biol. 2009;29(5):1222–1234. doi: 10.1128/MCB.01660-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rotolo JA, Zhang J, Donepudi M, Lee H, Fuks Z, Kolesnick R. Caspase-dependent and -independent activation of acid sphingomyelinase signaling. J Biol Chem. 2005;280(28):26425–26434. doi: 10.1074/jbc.M414569200. [DOI] [PubMed] [Google Scholar]
- 49.Werneburg NW, Guicciardi ME, Bronk SF, Kaufmann SH, Gores GJ. Tumor necrosis factor-related apoptosis-inducing ligand activates a lysosomal pathway of apoptosis that is regulated by Bcl-2 proteins. J Biol Chem. 2007;282(39):28960–28970. doi: 10.1074/jbc.M705671200. [DOI] [PubMed] [Google Scholar]
- 50.Ouasti S, Matarrese P, Paddon R, Khosravi-Far R, Sorice M, Tinari A, Malorni W, Degli Esposti M. Death receptor ligation triggers membrane scrambling between Golgi and mitochondria. Cell Death Differ. 2007;14(3):453–461. doi: 10.1038/sj.cdd.4402043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terman A, Gustafsson B, Brunk UT. The lysosomal-mitochondrial axis theory of postmitotic aging and cell death. Chem Biol Interact. 2006;163(1–2):29–37. doi: 10.1016/j.cbi.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 52.Schutze S, Tchikov V, Schneider-Brachert W. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat Rev Mol Cell Biol. 2008;9(8):655–662. doi: 10.1038/nrm2430. [DOI] [PubMed] [Google Scholar]
- 53.Pei Y, Xing D, Gao X, Liu L, Chen T. Real-time monitoring full length bid interacting with Bax during TNF-alpha-induced apoptosis. Apoptosis. 2007;12(9):1681–1690. doi: 10.1007/s10495-007-0091-7. [DOI] [PubMed] [Google Scholar]
- 54.Degli Esposti M, Ferry G, Masdehors P, Boutin JA, Hickman JA, Dive C. Post-translational modification of Bid has differential effects on its susceptibility to cleavage by caspase 8 or caspase 3. J Biol Chem. 2003;278(18):15749–15757. doi: 10.1074/jbc.M209208200. [DOI] [PubMed] [Google Scholar]
- 55.Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, Antonsson B, Martinou JC. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8(3):601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- 56.Vogel A, Aslan JE, Willenbring H, Klein C, Finegold M, Mount H, Thomas G, Grompe M. Sustained phosphorylation of Bid is a marker for resistance to Fas-induced apoptosis during chronic liver diseases. Gastroenterology. 2006;130(1):104–119. doi: 10.1053/j.gastro.2005.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atkins KM, Thomas L, Youker RT, Harriff MJ, Pissani F, You H, Thomas G. HIV-1 Nef Binds PACS-2 to Assemble a Multikinase Cascade That Triggers Major Histocompatibility Complex Class I (MHC-I) Down-regulation: ANALYSIS USING SHORT INTERFERING RNA AND KNOCK-OUT MICE. J Biol Chem. 2008;283(17):11772–11784. doi: 10.1074/jbc.M707572200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Myhill N, Lynes EM, Nanji JA, Blagoveshchenskaya AD, Fei H, Carmine Simmen K, Cooper TJ, Thomas G, Simmen T. The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell. 2008;19(7):2777–2788. doi: 10.1091/mbc.E07-10-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, Hung CH, Crump CM, Thomas G. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. Embo J. 2005;24(4):717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Youker RT, Shinde U, Day R, Thomas G. At the Crossroads of Homeostasis and Disease: Roles of the PACS proteins in Membrane Traffic and Apoptosis. Biochem J. 2009 doi: 10.1042/BJ20081016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aslan JE, You H, Williamson DM, Endig J, Youker RT, Thomas L, Shu H, Du Y, Milewski RL, Brush MH, Possemato A, Sprott K, Fu H, Greis KD, Runckel DN, et al. Akt and 14-3-3 control a PACS-2 homeostatic switch that integrates membrane traffic with TRAIL-induced apoptosis. Mol Cell. 2009 doi: 10.1016/j.molcel.2009.04.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160(2):189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cerveny KL, Tamura Y, Zhang Z, Jensen RE, Sesaki H. Regulation of mitochondrial fusion and division. Trends Cell Biol. 2007;17(11):563–569. doi: 10.1016/j.tcb.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159(6):931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170(7):1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arnoult D, Rismanchi N, Grodet A, Roberts RG, Seeburg DP, Estaquier J, Sheng M, Blackstone C. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr Biol. 2005;15(23):2112–2118. doi: 10.1016/j.cub.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 67.Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol. 2004;14(4):340–345. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci. 2007;120(Pt 7):1178–1188. doi: 10.1242/jcs.03418. [DOI] [PubMed] [Google Scholar]
- 69.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14(2):193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8(10):939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003;160(7):1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ladasky JJ, Boyle S, Seth M, Li H, Pentcheva T, Abe F, Steinberg SJ, Edidin M. Bap31 enhances the endoplasmic reticulum export and quality control of human class I MHC molecules. J Immunol. 2006;177(9):6172–6181. doi: 10.4049/jimmunol.177.9.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mathai JP, Germain M, Shore GC. BH3-only BIK regulates BAX,BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J Biol Chem. 2005;280(25):23829–23836. doi: 10.1074/jbc.M500800200. [DOI] [PubMed] [Google Scholar]
- 74.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312(5773):572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 75.Bassik MC, Scorrano L, Oakes SA, Pozzan T, Korsmeyer SJ. Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. Embo J. 2004;23(5):1207–1216. doi: 10.1038/sj.emboj.7600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Joseph SK, Hajnoczky G. IP3 receptors in cell survival and apoptosis: Ca2+ release and beyondq. Apoptosis. 2007;12(5):951–968. doi: 10.1007/s10495-007-0719-7. [DOI] [PubMed] [Google Scholar]
- 77.Li C, Wang X, Vais H, Thompson CB, Foskett JK, White C. Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci U S A. 2007;104(30):12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oakes SA, Scorrano L, Opferman JT, Bassik MC, Reed JC, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102(1):105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5(12):1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 80.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19(2):81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levine T, Loewen C. Inter-organelle membrane contact sites: through a glass, darkly. Curr Opin Cell Biol. 2006;18(4):371–378. doi: 10.1016/j.ceb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 82.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280(5370):1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 83.Darios F, Muriel MP, Khondiker ME, Brice A, Ruberg M. Neurotoxic calcium transfer from endoplasmic reticulum to mitochondria is regulated by cyclin-dependent kinase 5-dependent phosphorylation of tau. J Neurosci. 2005;25(16):4159–4168. doi: 10.1523/JNEUROSCI.0060-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ardail D, Popa I, Bodennec J, Louisot P, Schmitt D, Portoukalian J. The mitochondria-associated endoplasmic-reticulum subcompartment (MAM fraction) of rat liver contains highly active sphingolipid-specific glycosyltransferases. Biochem J. 2003;371(Pt 3):1013–1019. doi: 10.1042/BJ20021834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kottgen M, Benzing T, Simmen T, Tauber R, Buchholz B, Feliciangeli S, Huber TB, Schermer B, Kramer-Zucker A, Hopker K, Simmen KC, Tschucke CC, Sandford R, Kim E, Thomas G, et al. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. Embo J. 2005;24(4):705–716. doi: 10.1038/sj.emboj.7600566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wegierski T, Steffl D, Kopp C, Tauber R, Buchholz B, Nitschke R, Kuehn EW, Walz G, Kottgen M. TRPP2 channels regulate apoptosis through the Ca2+ concentration in the endoplasmic reticulum. Embo J. 2009;28(5):490–499. doi: 10.1038/emboj.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li J, Li Q, Xie C, Zhou H, Wang Y, Zhang N, Shao H, Chan SC, Peng X, Lin SC, Han J. Beta-actin is required for mitochondria clustering and ROS generation in TNF-induced, caspase-independent cell death. J Cell Sci. 2004;117(Pt 20):4673–4680. doi: 10.1242/jcs.01339. [DOI] [PubMed] [Google Scholar]
- 88.Zinkel SS, Ong CC, Ferguson DO, Iwasaki H, Akashi K, Bronson RT, Kutok JL, Alt FW, Korsmeyer SJ. Proapoptotic BID is required for myeloid homeostasis and tumor suppression. Genes Dev. 2003;17(2):229–239. doi: 10.1101/gad.1045603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kamer I, Sarig R, Zaltsman Y, Niv H, Oberkovitz G, Regev L, Haimovich G, Lerenthal Y, Marcellus RC, Gross A. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell. 2005;122(4):593–603. doi: 10.1016/j.cell.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 90.Zinkel SS, Hurov KE, Ong C, Abtahi FM, Gross A, Korsmeyer SJ. A role for proapoptotic BID in the DNA-damage response. Cell. 2005;122(4):579–591. doi: 10.1016/j.cell.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 91.Kaufmann T, Tai L, Ekert PG, Huang DC, Norris F, Lindemann RK, Johnstone RW, Dixit VM, Strasser A. The BH3-only protein bid is dispensable for DNA damage- and replicative stress-induced apoptosis or cell-cycle arrest. Cell. 2007;129(2):423–433. doi: 10.1016/j.cell.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 92.Stracker TH, Morales M, Couto SS, Hussein H, Petrini JH. The carboxy terminus of NBS1 is required for induction of apoptosis by the MRE11 complex. Nature. 2007;447(7141):218–221. doi: 10.1038/nature05740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zermati Y, Mouhamad S, Stergiou L, Besse B, Galluzzi L, Boehrer S, Pauleau AL, Rosselli F, D'Amelio M, Amendola R, Castedo M, Hengartner M, Soria JC, Cecconi F, Kroemer G. Nonapoptotic role for Apaf-1 in the DNA damage checkpoint. Mol Cell. 2007;28(4):624–637. doi: 10.1016/j.molcel.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 94.Konishi A, Shimizu S, Hirota J, Takao T, Fan Y, Matsuoka Y, Zhang L, Yoneda Y, Fujii Y, Skoultchi AI, Tsujimoto Y. Involvement of histone H1.2 in apoptosis induced by DNA double-strand breaks. Cell. 2003;114(6):673–688. doi: 10.1016/s0092-8674(03)00719-0. [DOI] [PubMed] [Google Scholar]
- 95.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11(3):577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 96.Marchenko ND, Wolff S, Erster S, Becker K, Moll UM. Monoubiquitylation promotes mitochondrial p53 translocation. Embo J. 2007;26(4):923–934. doi: 10.1038/sj.emboj.7601560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen C, Shimizu S, Tsujimoto Y, Motoyama N. Chk2 regulates transcription-independent p53-mediated apoptosis in response to DNA damage. Biochem Biophys Res Commun. 2005;333(2):427–431. doi: 10.1016/j.bbrc.2005.05.126. [DOI] [PubMed] [Google Scholar]
- 98.Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol. 2007;17(10):511–517. doi: 10.1016/j.tcb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 99.Sesso A, Fujiwara DT, Jaeger M, Jaeger R, Li TC, Monteiro MM, Correa H, Ferreira MA, Schumacher RI, Belisario J, Kachar B, Chen EJ. Structural elements common to mitosis and apoptosis. Tissue Cell. 1999;31(3):357–371. doi: 10.1054/tice.1999.0042. [DOI] [PubMed] [Google Scholar]
- 100.Hu W, Xu R, Zhang G, Jin J, Szulc ZM, Bielawski J, Hannun YA, Obeid LM, Mao C. Golgi fragmentation is associated with ceramide-induced cellular effects. Mol Biol Cell. 2005;16(3):1555–1567. doi: 10.1091/mbc.E04-07-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Vos K, Goossens V, Boone E, Vercammen D, Vancompernolle K, Vandenabeele P, Haegeman G, Fiers W, Grooten J. The 55-kDa tumor necrosis factor receptor induces clustering of mitochondria through its membrane-proximal region. J Biol Chem. 1998;273(16):9673–9680. doi: 10.1074/jbc.273.16.9673. [DOI] [PubMed] [Google Scholar]
- 102.Thomas WD, Zhang XD, Franco AV, Nguyen T, Hersey P. TNF-related apoptosis-inducing ligand-induced apoptosis of melanoma is associated with changes in mitochondrial membrane potential and perinuclear clustering of mitochondria. J Immunol. 2000;165(10):5612–5620. doi: 10.4049/jimmunol.165.10.5612. [DOI] [PubMed] [Google Scholar]
- 103.Dewitt DA, Hurd JA, Fox N, Townsend BE, Griffioen KJ, Ghribi O, Savory J. Peri-nuclear clustering of mitochondria is triggered during aluminum maltolate induced apoptosis. J Alzheimers Dis. 2006;9(2):195–205. doi: 10.3233/jad-2006-9211. [DOI] [PubMed] [Google Scholar]
- 104.Schepis A, Schramm B, de Haan CA, Locker JK. Vaccinia virus-induced microtubule-dependent cellular rearrangements. Traffic. 2006;7(3):308–323. doi: 10.1111/j.1600-0854.2005.00381.x. [DOI] [PubMed] [Google Scholar]
- 105.Frederick RL, Shaw JM. Moving mitochondria: establishing distribution of an essential organelle. Traffic. 2007;8(12):1668–1675. doi: 10.1111/j.1600-0854.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93(7):1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- 107.De Vos K, Severin F, Van Herreweghe F, Vancompernolle K, Goossens V, Hyman A, Grooten J. Tumor necrosis factor induces hyperphosphorylation of kinesin light chain and inhibits kinesin-mediated transport of mitochondria. J Cell Biol. 2000;149(6):1207–1214. doi: 10.1083/jcb.149.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102(2):175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- 109.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A. 2003;100(9):5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stagi M, Gorlovoy P, Larionov S, Takahashi K, Neumann H. Unloading kinesin transported cargoes from the tubulin track via the inflammatory c-Jun Nterminal kinase pathway. Faseb J. 2006;20(14):2573–1575. doi: 10.1096/fj.06-6679fje. [DOI] [PubMed] [Google Scholar]
- 111.Varadi A, Johnson-Cadwell LI, Cirulli V, Yoon Y, Allan VJ, Rutter GA. Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J Cell Sci. 2004;117(Pt 19):4389–4400. doi: 10.1242/jcs.01299. [DOI] [PubMed] [Google Scholar]
- 112.Huang P, Yu T, Yoon Y. Mitochondrial clustering induced by overexpression of the mitochondrial fusion protein Mfn2 causes mitochondrial dysfunction and cell death. Eur J Cell Biol. 2007;86(6):289–302. doi: 10.1016/j.ejcb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 113.van Niekerk EA, Willis DE, Chang JH, Reumann K, Heise T, Twiss JL. Sumoylation in axons triggers retrograde transport of the RNA-binding protein La. Proc Natl Acad Sci U S A. 2007;104(31):12913–12918. doi: 10.1073/pnas.0611562104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lane JD, Vergnolle MA, Woodman PG, Allan VJ. Apoptotic cleavage of cytoplasmic dynein intermediate chain and p150(Glued) stops dynein-dependent membrane motility. J Cell Biol. 2001;153(7):1415–1426. doi: 10.1083/jcb.153.7.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martin DD, Vilas GL, Prescher JA, Rajaiah G, Falck JR, Bertozzi CR, Berthiaume LG. Rapid detection, discovery, and identification of post-translationally myristoylated proteins during apoptosis using a bio-orthogonal azidomyristate analog. Faseb J. 2008;22(3):797–806. doi: 10.1096/fj.07-9198com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mizumura K, Takeda K, Hashimoto S, Horie T, Ichijo H. Identification of Op18/stathmin as a potential target of ASK1-p38 MAP kinase cascade. J Cell Physiol. 2006;206(2):363–370. doi: 10.1002/jcp.20465. [DOI] [PubMed] [Google Scholar]
- 117.Vancompernolle K, Boonefaes T, Mann M, Fiers W, Grooten J. Tumor necrosis factor-induced microtubule stabilization mediated by hyperphosphorylated oncoprotein 18 promotes cell death. J Biol Chem. 2000;275(43):33876–33882. doi: 10.1074/jbc.M004785200. [DOI] [PubMed] [Google Scholar]
- 118.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 119.Karbowski M, Spodnik JH, Teranishi M, Wozniak M, Nishizawa Y, Usukura J, Wakabayashi T. Opposite effects of microtubule-stabilizing and microtubule-destabilizing drugs on biogenesis of mitochondria in mammalian cells. J Cell Sci. 2001;114(Pt 2):281–291. doi: 10.1242/jcs.114.2.281. [DOI] [PubMed] [Google Scholar]
- 120.Liu L, Vo A, Liu G, McKeehan WL. Distinct structural domains within C19ORF5 support association with stabilized microtubules and mitochondrial aggregation and genome destruction. Cancer Res. 2005;65(10):4191–4201. doi: 10.1158/0008-5472.CAN-04-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dallol A, Agathanggelou A, Fenton SL, Ahmed-Choudhury J, Hesson L, Vos MD, Clark GJ, Downward J, Maher ER, Latif F. RASSF1A interactsc with microtubule-associated proteins and modulates microtubule dynamics. Cancer Res. 2004;64(12):4112–4116. doi: 10.1158/0008-5472.CAN-04-0267. [DOI] [PubMed] [Google Scholar]
- 122.Moss TN, Vo A, McKeehan WL, Liu L. UXT (Ubiquitously Expressed Transcript) causes mitochondrial aggregation. In Vitro Cell Dev Biol Anim. 2007;43(3–4):139–146. doi: 10.1007/s11626-007-9016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu L, McKeehan WL. Sequence analysis of LRPPRC and its SEC1 domain interaction partners suggests roles in cytoskeletal organization, vesicular trafficking, ucleocytosolic shuttling, and chromosome activity. Genomics. 2002;79(1):124–136. doi: 10.1006/geno.2001.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu L, Amy V, Liu G, McKeehan WL. Novel complex integrating mitochondria and the microtubular cytoskeleton with chromosome remodeling and tumor suppressor RASSF1 deduced by in silico homology analysis, interaction cloning in yeast, and colocalization in cultured cells. In Vitro Cell Dev Biol Anim. 2002;38(10):582–594. doi: 10.1290/1543-706x(2002)38<582:ncimat>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. J Cell Biol. 1998;143(3):777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen D, Wang M, Zhou S, Zhou Q. HIV-1 Tat targets microtubules to induce apoptosis, a process promoted by the pro-apoptotic Bcl-2 relative Bim. Embo J. 2002;21(24):6801–6810. doi: 10.1093/emboj/cdf683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brocardo M, Lei Y, Tighe A, Taylor SS, Mok MT, Henderson BR. Mitochondrial Targeting of Adenomatous Polyposis Coli Protein Is Stimulated by Truncating Cancer Mutations: REGULATION OF Bcl-2 AND IMPLICATIONS FOR CELL SURVIVAL. J Biol Chem. 2008;283(9):5950–5959. doi: 10.1074/jbc.M708775200. [DOI] [PubMed] [Google Scholar]
- 128.Dikovskaya D, Schiffmann D, Newton IP, Oakley A, Kroboth K, Sansom O, Jamieson TJ, Meniel V, Clarke A, Nathke IS. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J Cell Biol. 2007;176(2):183–195. doi: 10.1083/jcb.200610099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat Cell Biol. 2003;5(1):64–70. doi: 10.1038/ncb899. [DOI] [PubMed] [Google Scholar]
- 130.Shiao YH, Resau JH, Nagashima K, Anderson LM, Ramakrishna G. The von Hippel-Lindau tumor suppressor targets to mitochondria. Cancer Res. 2000;60(11):2816–2819. [PubMed] [Google Scholar]
- 131.Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV, Fojo T. p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat Cell Biol. 2000;2(10):709–717. doi: 10.1038/35036335. [DOI] [PubMed] [Google Scholar]
- 132.Nikolaev AY, Li M, Puskas N, Qin J, Gu W. Parc: a cytoplasmic anchor for p53. Cell. 2003;112(1):29–40. doi: 10.1016/s0092-8674(02)01255-2. [DOI] [PubMed] [Google Scholar]
- 133.Lo KW, Kan HM, Chan LN, Xu WG, Wang KP, Wu Z, Sheng M, Zhang M. The 8-kDa dynein light chain binds to p53-binding protein 1 and mediates DNA damage-induced p53 nuclear accumulation. J Biol Chem. 2005;280(9):8172–8179. doi: 10.1074/jbc.M411408200. [DOI] [PubMed] [Google Scholar]
- 134.Moseley GW, Roth DM, DeJesus MA, Leyton DL, Filmer RP, Pouton CW, Jans DA. Dynein light chain association sequences can facilitate nuclear protein import. Mol Biol Cell. 2007;18(8):3204–3213. doi: 10.1091/mbc.E07-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vadlamudi RK, Bagheri-Yarmand R, Yang Z, Balasenthil S, Nguyen D, Sahin AA, den Hollander P, Kumar R. Dynein light chain 1, a p21-activated kinase 1-interacting substrate, promotes cancerous phenotypes. Cancer Cell. 2004;5(6):575–585. doi: 10.1016/j.ccr.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 136.Hicks SW, Machamer CE. Golgi structure in stress sensing and apoptosis. Biochim Biophys Acta. 2005;1744(3):406–414. doi: 10.1016/j.bbamcr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 137.Rios RM, Bornens M. The Golgi apparatus at the cell centre. Curr Opin Cell Biol. 2003;15(1):60–66. doi: 10.1016/s0955-0674(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 138.Lane JD, Lucocq J, Pryde J, Barr FA, Woodman PG, Allan VJ, Lowe M. Caspase-mediated cleavage of the stacking protein GRASP65 is required for Golgi fragmentation during apoptosis. J Cell Biol. 2002;156(3):495–509. doi: 10.1083/jcb.200110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mukherjee S, Chiu R, Leung SM, Shields D. Fragmentation of the Golgi apparatus: an early apoptotic event independent of the cytoskeleton. Traffic. 2007;8(4):369–378. doi: 10.1111/j.1600-0854.2007.00542.x. [DOI] [PubMed] [Google Scholar]
- 140.Mancini M, Machamer CE, Roy S, Nicholson DW, Thornberry NA, Casciola-Rosen LA, Rosen A. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J Cell Biol. 2000;149(3):603–612. doi: 10.1083/jcb.149.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chiu R, Novikov L, Mukherjee S, Shields D. A caspase cleavage fragment of p115 induces fragmentation of the Golgi apparatus and apoptosis. J Cell Biol. 2002;159(4):637–648. doi: 10.1083/jcb.200208013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Degli Esposti M, Tour J, Ouasti S, Ivanova S, Matarrese P, Malorni W, Khosravi-Far R. Fas death receptor enhances endocytic membrane traffic converging into the Golgi region. Mol Biol Cell. 2009;20(2):600–615. doi: 10.1091/mbc.E08-09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Matarrese P, Manganelli V, Garofalo T, Tinari A, Gambardella L, Ndebele K, Khosravi-Far R, Sorice M, Esposti MD, Malorni W. Endosomal compartment contributes to the propagation of CD95/Fas-mediated signals in type II cells. Biochem J. 2008;413(3):467–478. doi: 10.1042/BJ20071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garofalo T, Giammarioli AM, Misasi R, Tinari A, Manganelli V, Gambardella L, Pavan A, Malorni W, Sorice M. Lipid microdomains contribute to apoptosis-associated modifications of mitochondria in T cells. Cell Death Differ. 2005;12(11):1378–1389. doi: 10.1038/sj.cdd.4401672. [DOI] [PubMed] [Google Scholar]
- 145.Garofalo T, Tinari A, Matarrese P, Giammarioli AM, Manganelli V, Ciarlo L, Misasi R, Sorice M, Malorni W. Do mitochondria act as "cargo boats" in the journey of GD3 to the nucleus during apoptosis? FEBS Lett. 2007;581(21):3899–3903. doi: 10.1016/j.febslet.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 146.Malorni W, Giammarioli AM, Garofalo T, Sorice M. Dynamics of lipid raft components during lymphocyte apoptosis: the paradigmatic role of GD3. Apoptosis. 2007;12(5):941–949. doi: 10.1007/s10495-007-0757-1. [DOI] [PubMed] [Google Scholar]
- 147.Rippo MR, Malisan F, Ravagnan L, Tomassini B, Condo I, Costantini P, Susin SA, Rufini A, Todaro M, Kroemer G, Testi R. GD3 ganglioside directly targets mitochondria in a bcl-2-controlled fashion. Faseb J. 2000;14(13):2047–2054. doi: 10.1096/fj.99-1028com. [DOI] [PubMed] [Google Scholar]
- 148.Garcia-Ruiz C, Colell A, Morales A, Calvo M, Enrich C, Fernandez-Checa JC. Trafficking of ganglioside GD3 to mitochondria by tumor necrosis factor-alpha. J Biol Chem. 2002;277(39):36443–36448. doi: 10.1074/jbc.M206021200. [DOI] [PubMed] [Google Scholar]
- 149.Lee KH, Feig C, Tchikov V, Schickel R, Hallas C, Schutze S, Peter ME, Chan AC. The role of receptor internalization in CD95 signaling. Embo J. 2006;25(5):1009–1023. doi: 10.1038/sj.emboj.7601016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 151.Schneider-Brachert W, Tchikov V, Neumeyer J, Jakob M, Winoto-Morbach S, Held-Feindt J, Heinrich M, Merkel O, Ehrenschwender M, Adam D, Mentlein R, Kabelitz D, Schutze S. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21(3):415–428. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 152.Giammarioli AM, Garofalo T, Sorice M, Misasi R, Gambardella L, Gradini R, Fais S, Pavan A, Malorni W. GD3 glycosphingolipid contributes to Fas-mediated apoptosis via association with ezrin cytoskeletal protein. FEBS Lett. 2001;506(1):45–50. doi: 10.1016/s0014-5793(01)02776-4. [DOI] [PubMed] [Google Scholar]
- 153.Tempera I, Buchetti B, Lococo E, Gradini R, Mastronardi A, Mascellino MT, Sale P, Mosca L, d'Erme M, Lenti L. GD3 nuclear localization after apoptosis induction in HUT-78 cells. Biochem Biophys Res Commun. 2008;368(8):495–500. doi: 10.1016/j.bbrc.2007.12.196. [DOI] [PubMed] [Google Scholar]
- 154.Kimura S, Noda T, Yoshimori T. Dynein-dependent movement of autophagosomes mediates efficient encounters with lysosomes. Cell Struct Funct. 2008;33(1):109–122. doi: 10.1247/csf.08005. [DOI] [PubMed] [Google Scholar]
- 155.Rodriguez-Gonzalez A, Lin T, Ikeda AK, Simms-Waldrip T, Fu C, Sakamoto KM. Role of the aggresome pathway in cancer: targeting histone deacetylase 6-dependent protein degradation. Cancer Res. 2008;68(8):2557–2560. doi: 10.1158/0008-5472.CAN-07-5989. [DOI] [PubMed] [Google Scholar]
- 156.Nakajima K, Hirose H, Taniguchi M, Kurashina H, Arasaki K, Nagahama M, Tani K, Yamamoto A, Tagaya M. Involvement of BNIP1 in apoptosis and endoplasmic reticulum membrane fusion. Embo J. 2004;23(16):3216–3226. doi: 10.1038/sj.emboj.7600333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Degli Esposti M. Organelle intermixing and membrane scrambling in cell death. Methods Enzymol. 2008;442:421–438. doi: 10.1016/S0076-6879(08)01421-3. [DOI] [PubMed] [Google Scholar]