Abstract
We examined the effects of estradiol on behavioral responses to osmotic challenges in ovariectomized (OVX) rats to test the hypothesis that estradiol enhances sensitivity to gradual changes in plasma osmolality (pOsm) in stimulating water intake. Despite comparably elevated pOsm after a slow infusion of 2 M NaCl, the latency to begin water intake was significantly less in estradiol-treated OVX rats compared to that in oil vehicle-treated rats. Other groups of OVX rats were injected with isoproterenol, which increases circulating angiotensin II. These rats then were given 0.15 M NaCl to drink instead of water, to prevent decreased pOsm associated with water ingestion. Isoproterenol stimulated 0.15 M NaCl intake by both groups; however, estradiol-treated rats consumed less 0.15 M NaCl than did oil-treated rats, findings that are similar to those reported when estradiol-treated rats consumed water. The estradiol enhancement of sensitivity to increased, but not to decreased, pOsm suggests that estradiol has directionally-specific effects on osmoregulatory drinking. Moreover, the estradiol attenuation of 0.15 M NaCl intake after isoproterenol suggests that estradiol effects on osmoregulatory drinking are independent of those on volume regulatory drinking.
Keywords: thirst, osmolality, osmoreceptor, isoproterenol, Angiotensin
1. Introduction
Ovarian hormones affect body fluid regulation in many species, producing fluctuations in plasma osmolality and plasma volume across the reproductive cycle [1-3], during hormone replacement [3-6] and, in humans, during the use of oral contraceptives [7]. The influence of ovarian hormones on body fluid balance is perhaps best illustrated by the effect of estrogens on vasopressin (VP), the antidiuretic hormone, which is critically important in body fluid regulation. Baseline VP levels are increased by estrogen replacement in humans [4] and peak during estrus in rats [2]. In addition, estrogens affect VP release stimulated by increased plasma osmolality (pOsm), though there is disagreement about whether estrogens decrease the threshold [5,8] or shift the curve to the left [3,9]. These findings suggest that estrogens increase the sensitivity to increased pOsm and affect compensatory hormonal responses to this perturbation of body fluid balance. Consistent with this idea, it recently has been reported that estradiol augments activation in the supraoptic nucleus, one of the central sites of VP neurons, in response to a systemic salt load in ovariectomized rats [9]. Moreover, studies by Sladek and colleagues [10,11] point to the importance of estrogen receptors (ERs) within the central nervous system in the estrogen enhancement of osmotically-stimulated VP release.
Importantly, ERs are located throughout the central nervous system [12,13], including many areas involved in body fluid regulation [10,11,13-16]. Thus, an influence of estrogens on other centrally-mediated responses to increased pOsm seems plausible, particularly in regard to compensatory behavioral responses. There is general agreement that, in rats, estradiol reduces water intake stimulated by Angiotensin II (AngII) [17-19], the so-called ‘volume regulatory drinking’. However, most researchers found that estradiol does not affect water intake stimulated by osmotic challenges [17-19], although inhibition of ‘osmoregulatory drinking’ by estradiol treatment has been reported [20]. Differences in dose or duration of estradiol treatment complicate the interpretation of these findings, but a more important issue is that the typical strategy in this type of experiment has been to administer a large systemic salt load, producing substantial hyperosmolality that stimulates copious water drinking. Thus, if estradiol effects on osmotically-stimulated water intake mirror those observed with osmotically-stimulated VP release, increased osmolality well in excess of the threshold may produce maximal drinking regardless of the presence of estrogens and, consequently, obscure more subtle effects of estrogens on osmotic regulation.
The goals of the present studies were to revisit the issue of estradiol effects on osmotically-stimulated water intake and to extend previous studies by investigating estradiol effects on behavioral responses to decreased pOsm. Accordingly, we examined the effect of estradiol replacement on water intake stimulated by gradually increasing pOsm in ovariectomized rats to test the hypothesis that the estrogen enhancement of sensitivity to increased pOsm in stimulating VP release has a behavioral parallel—enhanced sensitivity to increased pOsm in stimulating water intake. We next tested the hypothesis that estrogen enhancement of sensitivity to changes in pOsm also includes behavioral responses to decreased pOsm by assessing the effect of estradiol replacement on stimulated drinking when osmotic dilution produced by water intake was prevented by providing isotonic saline as a drinking fluid. A secondary goal of this latter experiment was to address the possibility that attenuated volume regulatory water intake by estradiol-treated rats [17-19] is, in fact, a consequence of greater inhibition resulting from enhanced sensitivity to the decreased pOsm that accompanies stimulated water intake.
2. Methods
2.1 Animals
Adult female Sprague-Dawley rats weighing 225-325 g were individually housed in plastic cages and given ad libitum access to Purina rodent chow (#5001) and water except as noted. Rats were kept in a temperature-controlled room (21-25°C) on a 12:12 h light/dark cycle with lights on at 7:00 AM. Experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and all procedures were approved by the Oklahoma State University Center for Health Sciences Animal Care and Use Committee.
2.1.1 Ovariectomy
Under sodium pentobarbital anesthesia (50 mg/kg body weight IP; Sigma-Aldrich), rats were bilaterally ovariectomized (OVX) using a ventral approach and given 7-10 days to recover.
2.1.2. Estradiol Replacement
Rats were given subcutaneous injections of 17-β-estradiol-3-benzoate (EB, Fisher Scientific; 10 μg/0.1 ml in sesame oil) or the oil vehicle (OIL; 0.1 ml) on a schedule that mimics patterns of estradiol fluctuations during the estrous cycle. Specifically, rats were given EB or OIL daily for two consecutive days and were tested two days after the second injection (i.e. on Day 4), a protocol we and others have used in behavioral studies [17,21-25]. Rats were weighed daily during this replacement schedule.
2.2. Statistics
All data are shown as group Means ± SEs. Percent change in body weight during the EB/OIL replacement schedule was calculated as 100 * [Day 4 weight (g) - Day 1 weight (g)] / Day 1 weight (g). Statistica software (StatSoft) was used for all statistical analyses.
2.3. Experiment 1. Hypertonic NaCl
2.3.1. Femoral Catheters
After OVX and recovery, rats were anesthetized with sodium pentobarbital (50 mg/kg body weight, IP) and chronic, indwelling femoral venous catheters consisting of PE-50 fused to PE-10 tubing were inserted. Catheters were filled with heparinized 0.15 M NaCl (100 U/ml) and the end was tunneled subcutaneously to exit at the back of the neck. Rats were given one day to recover prior to EB/OIL replacement and testing as described.
2.3.2. Experiment 1a. Water Intake
During the two days of EB/OIL replacement, OVX rats (OIL n = 11; EB n = 10) were adapted to testing procedures. Chow and water were removed from the cages, catheters were connected to tubing attached to an infusion pump, and rats were given water in graduated drinking tubes. Water intake was recorded after 60 min and averaged for the two days of adaptation. On Day 4, chow and water were removed from the cages, catheters were connected to the infusion pump, rats were given water in graduated drinking tubes, and 2.0 M NaCl (HS) was infused intravenously at 35 μl/min for 60 minutes. We recorded latency (min) to begin drinking during HS infusion, duration (min) of the first water intake bout, and water consumed (ml) in the first bout. We defined a bout as ≥15 sec of drinking and the termination of a bout as 30 sec with no drinking. In addition, total intake (ml) was recorded at the end of the 60 min infusion.
2.3.3. Experiment 1b. Plasma Osmolality
A separate group of OVX rats were given EB or OIL replacement and then adapted as described in Experiment 1a, except that water was not available. On Day 4, chow and water were removed from the cages, catheters were connected to the infusion pump and rats were infused intravenously with HS (OIL n = 7, EB n = 6) or 0.15 M NaCl (CON; OIL n = 6, EB n = 6). Water was not available during the infusion. Based on preliminary results from Experiment 1a, we focused on the time at which EB-treated rats began drinking and infused rats with HS or CON at 35 μl/min for 15 min. Immediately thereafter, rats were deeply anesthetized with sodium pentobarbital (0.2 ml, iv) and rapidly decapitated to collect trunk blood. Aliquots of trunk blood were drawn into microcapillary tubes, and then spun in a microcentrifuge (Thermo Electron) for determination of hematocrit (hct) using a hematocrit reader (Thermo Electron) and of plasma protein concentration (pPro) using a refractometer (Reichert). The remaining blood was centrifuged and plasma was collected and stored at -20°C prior to analysis of pOsm (Vapor Pressure Osmometer, Wescor, Inc).
2.3.4. Data Analysis
Independent heteroscedastic t-tests were used to compare % change in body weight, latency to begin water intake, duration of the first water intake bout, and amount of water consumed during the first bout. Total water intake during adaptation and HS infusion were compared using 2-way analysis of variance (ANOVA) with hormone (EB or OIL) and condition (HS or adaptation) as factors, repeated for condition. pOsm, hct and pPro were compared using 2-way ANOVA with hormone (EB or OIL) and infusion (HS or CON) as factors. Pairwise comparisons of statistically significant (p < 0.05) main effects or interactions were made using Student Newman-Keuls tests.
2.4. Experiment 2. Isoproterenol
Another group of rats (n = 11) were OVX and given EB/OIL replacement as described and then tested for intake of 0.15 M NaCl after subcutaneous injection of 0.15 M NaCl (SAL) or isoproterenol (ISOP), a β-adrenergic agonist commonly used in studies of stimulated water intake. All eleven rats were tested in all four conditions and, to control for order effects, rats were randomly assigned to one of two testing sequences (EB-SAL, OIL-ISOP, OIL-SAL, EB-ISOP or EB-ISOP, OIL-SAL, OIL-ISOP, EB-SAL). Tests were conducted at weekly intervals. OVX rats were adapted to testing procedures during the two days of EB/OIL replacement each week. Chow and water were removed from the cages and rats were given 0.15 M NaCl in calibrated drinking tubes for 60 min. On Day 4, chow and water were removed from the cages and rats were injected with ISOP (30 μg/kg body weight) or SAL (1.0 ml/kg body weight). Rats then were given 0.15 M NaCl in graduated drinking tubes and intakes were recorded at 15 minute intervals for 60 min.
2.4.1. Data Analysis
Paired t-tests were used to compare % change in body weight. Comparisons of 0.15 M NaCl intake were made using 3-way ANOVA (hormone: EB or OIL; drug: ISOP or SAL; and time), repeated for all three factors. Student Newman-Keuls tests were used to examine statistically significant (p < 0.05) main effects or interactions.
3. Results
3.1. Body weight
Table 1 shows body weight (g) and % change in body weight during the four day EB/OIL replacement and testing protocols. In both Experiment 1 (top) and Experiment 2 (bottom), EB-treated rats lost weight, whereas OIL-treated rats gained weight. Differences in the % change in body weight between EB- and OIL-treated rats were statistically significant (ps < 0.001) in both experiments. These results are similar to those in our previous studies [17,23,25 see also 26] and demonstrate the effectiveness of EB treatment.
Table 1.
Body weight (g) and % change in body weight during EB/OIL replacement and testing. *** = p < 0.001
| Experiment 1 | OIL | EB |
|---|---|---|
| Day 1 | 278.9 ± 6.6 | 291.8 ± 9.0 |
| Day 2 | 280.5 ± 6.7 | 289.1 ± 8.7 |
| Day 4 | 291.1 ± 5.4 | 284.4 ± 7.7 |
| % change | 4.46 ± 0.54 *** | -2.31 ± 0.48 |
| Experiment 2 | OIL | EB |
| Day 1 | 294.4 ± 4.5 | 302.1 ± 6.3 |
| Day 2 | 295.9 ± 4.6 | 296.8 ± 6.2 |
| Day 4 | 299.8 ± 4.5 | 290.5 ± 5.3 |
| % change | 1.85 ± 0.40 *** | -3.75 ± 0.43 |
3.2. Experiment 1. Hypertonic NaCl
3.2.1. Experiment 1a. Water Intake
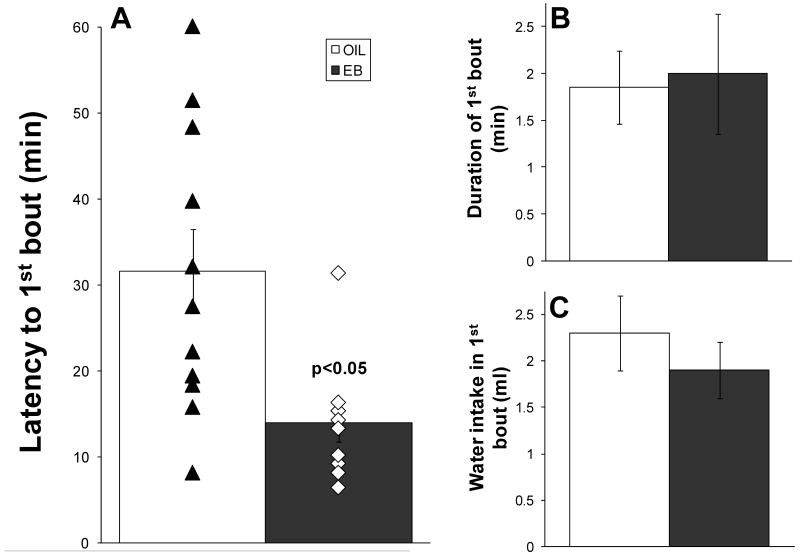
The latency to begin drinking during HS infusion was significantly less in EB-treated rats than in OIL-treated rats (14.0 ± 2.2 min vs. 31.6 ± 4.9 min, p < 0.01; Figure 1A). Latencies to begin drinking for individual rats also are shown in Figure 1A. Individual points from EB-treated rats are tightly clustered, which is consistent with increased sensitivity to NaCl during high estrogen conditions. In contrast, the individual points from OIL-treated rats span a greater range of latencies.
Figure 1.
Latency to begin water intake (min; A), duration of first water intake bout (min; B) and water consumed in the first bout (ml; C) during iv infusion of 2 M NaCl (35 μl/min). In OVX rats, EB treatment (black bars) significantly decreased the latency to begin drinking (p < 0.05). Individual rats are indicated by filled triangles (OIL-treated) or open diamonds (EB-treated). The error bars are obscured by the individual points for the EB-treated rats, which are tightly clustered around ∼15 min, and partially obscured by the individual points for the OIL-treated rats, which span a wider range. EB treatment did not affect the duration of the first bout or the volume of water consumed in the first bout.
Despite group differences in the latency, once rats began to drink, the duration of the first bout (Figure 1B) and the volume of water consumed in the first bout (Figure 1C) were comparable. The total volume of water consumed during the HS infusion was significantly greater than that during adaptation (Figure 2; [F(1,19) = 62.37, p < 0.001]), but there were no differences between EB- and OIL-treated rats.
Figure 2.
Total water intake (ml) by OVX rats given OIL (white bars) or EB (black bars) treatment during 60-min adaptation or 60-min iv infusion of 2 M NaCl (HS) at 35 μl/min. Water intake was significantly increased during HS infusion (p < 0.001), but there were no differences between the hormone conditions.
3.2.1. Experiment 1b. Plasma Osmolality
Table 2 shows the effect of EB on pOsm, hct, and pPro after infusion with HS or ISO. pOsm was significantly increased after HS infusion [F(1,21) = 56.66, p < 0.001], but there were no differences between EB- and OIL-treated rats and no interactions between hormone and infusion. pPro was significantly decreased after HS infusion [F(1,21) = 5.41, p < 0.05] and, again, there were no differences between EB- and OIL-treated rats and no interactions. hct was not affected by EB treatment or by HS infusion.
Table 2.
Plasma osmolality (pOsm; mOsm/L), hematocrit (hct; %) and plasma protein concentration (pPro; g/dL) in OIL- and EB-treated rats after iv infusion (35 μl/min for 15 min) of 0.15 M NaCl or 2.0 M NaCl. * = p < 0.05, 0.15 M NaCl vs 2.0 M NaCl; *** = p < 0.001, 0.15 M NaCl vs 2.0 M NaCl
| 0.15 M NaCl | 2.0 M NaCl | |||
|---|---|---|---|---|
| OIL | EB | OIL | EB | |
| pOsm | 302.0 ± 0.7 | 298.7 ± 1.1 *** | 309.1 ± 1.4 | 310.8 ± 1.6 |
| hct | 32.8 ± 1.5 | 32.2 ± 1.1 | 32.6 ± 0.2 | 32.6 ± 0.4 |
| pPro | 5.7 ± 0.2 | 5.4 ± 0.1 * | 5.3 ± 0.1 | 5.3 ± 0.1 |
3.3. Experiment 2. Isoproterenol
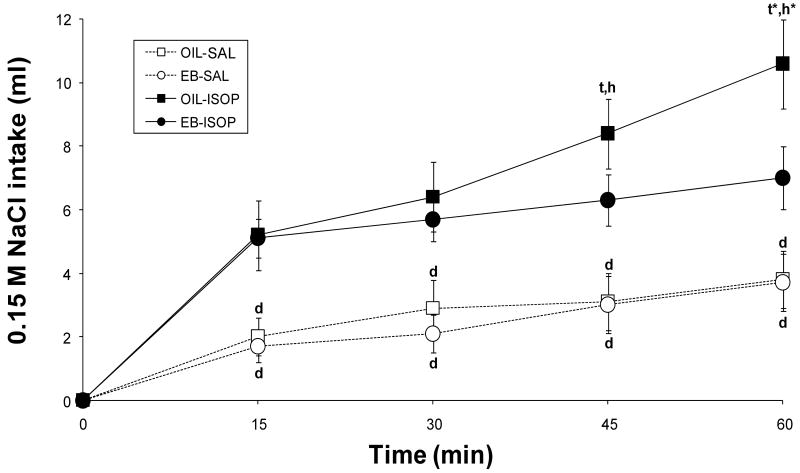
As shown in Figure 3, both EB- and OIL-treated rats consumed more 0.15 M NaCl after ISOP than after SAL injection [F(1,10) = 24.78, p < 0.001], but there were significant differences between the groups as indicated by the hormone, drug, and time interaction [F(3, 30) = 4.32, p < 0.05]. Pairwise comparisons of the interaction revealed that, after SAL injection, EB- and OIL-treated rats drank comparable volumes of 0.15 M NaCl at each time point. Both groups consumed significantly more 0.15 M NaCl at each time point after ISOP compared to intakes after SAL (all ps < 0.001); however, after ISOP injection, EB-treated rats consumed most of the 0.15 M NaCl in the first 15 min of the test and little thereafter (∼1.9 ml; 5.1 ± 0.6 ml/15 min vs. 7.0 ± 1.0 ml/60 min). In contrast, OIL-treated rats consumed 0.15 M NaCl throughout the test, with intake at the end approximately twice that in the first 15 min (∼5.4 ml; 5.2 ± 1.1 ml/15 min vs. 10.6 ± 1.4 ml/60 min). In fact, 0.15 M NaCl intake by OIL-treated rats after 45 min was significantly greater than that after 30 min (p < 0.01) and intake after 60 min was significantly greater than that after 45 min (p < 0.001). Moreover, OIL-treated rats consumed significantly more 0.15 M NaCl than did EB-treated rats both 45 min (p < 0.01) and 60 min (p < 0.001) after ISOP.
Figure 3.
Intake of 0.15 M NaCl (ml) by OVX rats given OIL (squares) or EB (circles) treatment in 60-min tests after administration of Isoproterenol (ISOP; filled symbols, solid lines) or the 0.15 M NaCl vehicle (SAL; open symbols, dotted lines). Intake of 0.15 M NaCl was significantly increased by ISOP (p < 0.001) regardless of hormone condition. However, in the last 30 min of the test, EB-treated rats consumed less 0.15 M NaCl after ISOP than did OIL-treated rats. d = p < 0.001 vs. corresponding time after ISOP within hormone condition; t = p < 0.01 vs. previous time after OIL-ISOP; t* = p < 0.001 vs. previous time after OIL-ISOP; h = p < 0.01 vs. EB-ISOP at corresponding time; h* = p < 0.001 vs. EB-ISOP at corresponding time.
4. Discussion
There is general agreement that estradiol attenuates water intake stimulated by AngII, the hormonal signal of loss of blood pressure or of blood volume. In contrast to the effect of estradiol on volume regulatory drinking, most researchers report that estradiol does not affect water intake stimulated by osmotic challenges [5,8,17-19], findings that are somewhat surprising given estradiol effects on osmotically-stimulated VP release [3,5,8 9]. However, the magnitude of hyperosmolality typically used to stimulate drinking may have limited the ability to detect more subtle effects of estradiol. Accordingly, we tested the hypothesis that estradiol enhances the sensitivity to increased pOsm in stimulating water intake.
EB treatment reduced the latency to begin drinking water during a slow infusion of a highly concentrated NaCl solution (Figure 1A). More specifically, EB-treated OVX rats began drinking in ∼14 min, less than half the time required for OIL-treated OVX rats to begin drinking (∼32 min). Regardless of the differences in latencies, however, the duration of the first bout of water drinking was comparable (Figure 1B) and both groups consumed comparable volumes of water during the first bout (Figure 1C). Moreover, both groups continued to drink after the first bout and consumed comparable volumes of water during the rest of the 60-min drinking test (∼2.5-3.5 ml), so there was no difference between OIL- and EB-treated OVX rats in the total volume of water consumed in response to HS infusion (Figure 2).
Measurements of pOsm at about the time EB-treated OVX rats began to consume water (after 15 min of HS infusion) showed that pOsm had increased to comparable levels in both groups (Table 2). These results suggest that the difference in the latency to begin drinking observed in Experiment 1a was not secondary to a greater degree of hyperosmolality during HS infusion but, instead, may be attributable to enhanced sensitivity to increased pOsm. Consistent with this idea, Stachenfeld and colleagues reported that, in humans, estrogens decrease the osmotic threshold for thirst, as well as for VP release [5,8 27]. Given the antidiuretic actions of VP, one might have expected the increased pOsm after HS infusion to be blunted in EB-treated rats; however, 15 minutes may be insufficient time for the renal effects of enhanced VP release. Alternatively, since estrogens increase the clearance of circulating VP [6,28], the urine-concentrating effects of VP may be of shorter duration, despite the stimulation of VP release at lower levels of hyperosmolality. In any case, pOsm after a slow HS infusion was not higher in EB-treated OVX rats. On the other hand, there was a tendency for lower pOsm after CON infusion in EB-treated rats compared to OIL-treated rats (Table 2). Although pOsm after CON infusion was not statistically different in the two groups, we cannot rule out the possibility that differences in the magnitude of the change from baseline pOsm may have contributed, in part, to differences in the latency to water intake. Additional studies of pOsm in the same rat before HS and at the exact onset of drinking will be necessary to better determine the effect of estradiol on the precise pOsm (and the change in pOsm) at which water intake is stimulated. Finally, it should be noted that pentobarbital was administered immediately after HS or CON infusion through the same catheter to rapidly anesthetize rats for collection of trunk blood. Since all rats were given 0.2 ml of pentobarbital, we did not correct for the additional volume or for the NaCl that otherwise would have remained in the catheter, though this may have contributed to the variability in pOsm.
Together, the results of Experiment 1a and 1b show that EB treatment decreased the latency to drinking during HS infusion, and that pOsm was comparably elevated in EB- and OIL-treated OVX rats at the time when EB-treated rats began to drink water. There was less variability in the latency to begin drinking—and therefore, less variability in the amount of NaCl that had been delivered—which is consistent with enhanced sensitivity to increased pOsm during high estrogen conditions. In contrast, OIL-treated rats required twice as much time (and twice as much NaCl) to begin drinking. Nonetheless, the two groups consumed comparable volumes of water in the first bout. Moreover, both groups continued to consume water after the initial bout, so it would appear that the HS infusion produced a sustained hyperosmolality that was sufficient to stimulate drinking throughout the 60-min test. The testing procedures we used in these initial studies do not allow us to determine whether dynamic changes in pOsm attributable to the absorption and equilibration of the ingested water or to renal excretion of the infused salt load also were influenced by EB. Thus, although it is tempting to speculate that estradiol shifts the concentration-response curve to the left, as has been reported for osmotically-stimulated VP release [3,9], without further studies to measure urinary Na+ excretion, urine osmolality, and/or pOsm throughout the drinking test, we can conclude only that estradiol enhances the sensitivity to increased pOsm in stimulating water intake.
The enhanced sensitivity to increased pOsm in EB-treated rats suggested by these findings raises the possibility that estradiol effects on the sensitivity to changes in pOsm are not unidirectional. That is, estradiol also may increase the sensitivity to decreased pOsm. In terms of hypoosmolality and water intake, the act of consuming water is known to inhibit subsequent fluid ingestion. Numerous factors contribute to this inhibition [for review, see 29], including detection of the ingested water by visceral osmoreceptors and, ultimately, the dilution of body fluids by absorption of the ingested water. We hypothesized that estradiol also enhances the sensitivity to decreased pOsm in inhibiting water intake, which would lead to the prediction that an episode of stimulated drinking would terminate more rapidly in EB-treated rats. This is a straightforward premise; however, the assessment of behavioral responses to decreased pOsm is complicated, not only by the difficulty of establishing and maintaining hypo-osmotic conditions, but also by the necessity of doing so without interfering with the initial stimulus for water ingestion. We took a different approach and substituted an isotonic saline solution for water to prevent decreased pOsm during volume-regulatory drinking stimulated by administration of isoproterenol (ISOP), a β-adrenergic agonist that causes pronounced increases in circulating levels of AngII [e.g., 17,25].
We replicated the testing procedures used in our previous studies, in which we found that estradiol attenuated water intake stimulated by ISOP without affecting the increase in circulating AngII [17,25], except that rats were given 0.15 M NaCl to drink instead of water. ISOP stimulated 0.15 M NaCl intake by both EB- and OIL-treated OVX rats. However, EB-treated rats consumed very little 0.15 M NaCl after the first 15 min of the test, whereas OIL-treated rats continued to drink and consumed significantly greater volumes of 0.15 M NaCl by the end of the test. Given the similarity of these findings to those in our previous study (including volumes consumed: OIL - 9.5 ml water, 10.6 ml 0.15 M NaCl; EB - 5.5 ml water, 7.0 ml 0.15 M NaCl), these observations provide important insights in understanding estradiol effects on both volume- and osmo-regulatory drinking. If estradiol increases sensitivity to inhibitory signals of hypoosmolality arising from activation of osmoreceptors in the small intestine and/or the central nervous system by gastric emptying and/or absorption of the ingested water, then an initial episode of water drinking would be expected to inhibit subsequent intake. Clearly, however, the attenuation of 0.15 M NaCl intake by EB-treated rats cannot be explained by such inhibitory signals. Neither can the early termination of ingestion be attributed to inexperience with the post-ingestive consequences of 0.15 M NaCl intake, as all rats had access to the 0.15 M NaCl solution during the adaptation days that preceded each test, in addition to repeated experience with 0.15 M NaCl intake during testing. Thus, estradiol does not enhance the sensitivity to decreased pOsm in inhibiting water intake. Moreover, enhanced sensitivity to decreased pOsm does not appear to underlie the estradiol-induced reduction of volume regulatory drinking. Alternatively, if estradiol does increase the sensitivity to hypoosmolality, it has, at best, a minor influence on drinking in the face of stimuli for volume regulatory drinking.
In conjunction with previous studies, the present results suggest that there are separate effects of estrogens on compensatory behavioral responses to body fluid challenges: estradiol enhances drinking responses to increased pOsm, but blunts drinking responses to increased AngII. Thus, rather than a global effect of estradiol to inhibit or stimulate both volume and osmo-regulatory drinking via some common mechanism regardless of the stimulus, these observations suggest that two separate mechanisms underlie the effects. In regard to the mechanism of estradiol attenuation of volume regulatory drinking, several studies have shown compelling evidence for a central mechanism. More specifically, estradiol has genomic effects related to AngII receptor density [22], especially in the subfornical organ (SFO) [25], a forebrain circumventricular organ (CVO). Given the role of the SFO in AngII-induced drinking in male rats [e.g., 30], this downregulation of AngII receptors seems a likely candidate mechanism for the estradiol-induced attenuation of drinking responses to ISOP, and possibly to other volume regulatory challenges. The present findings are consistent with the possibility that down-regulation of AngII receptors in the SFO also influences ingestion of isotonic saline stimulated by ISOP.
On the other hand, changes in pOsm or plasma Na+ concentration are detected by osmosensitive neural elements in CVOs [31,32], central nervous system structures with an incomplete blood-brain-barrier. Therefore, estradiol may enhance the sensitivity to increased pOsm in stimulating water intake by affecting the detection of increased pOsm by CVOs, most notably by neurons in the forebrain Organum Vasculosum of the Lamina Terminalis (OVLT), which have been reported to respond robustly to increased pOsm [33,34]. In fact, ERs are localized to neurons in the OVLT that are activated by increased pOsm [11]. Another possibility is that estradiol alters the detection of hyperosmolality by peripheral osmoreceptors located in the liver and/or hepatic portal system [35] that send neural input to the hindbrain Nucleus of the Solitary Tract (NTS) [36,37]. In either case, it is logical to conclude that, if detection is enhanced, then increased sensitivity to hyperosmolality may affect multiple compensatory responses. The present findings that estradiol decreases the latency to drinking, in conjunction with previous reports that estrogens enhance osmotically-stimulated VP release [3,5,8,9] and that both the OVLT and the NTS project to hypothalamic VP neurons [38,39], are consistent with this idea.
Alternatively, estrogens may not affect the detection of increased pOsm by osmoreceptors in CVOs or in the periphery, but instead may alter activity in ‘downstream’ parts of central neural pathway(s) involved in water intake. Very little research has focused on the actions of estrogens in central pathways that influence osmotically-stimulated water intake, a behavioral response that appears to involve acetylcholine [e.g., 40]. In fact, to our knowledge, only a single study examined responses by female rats to central administration of a cholinergic agonist, carbachol [21]. No effect of estradiol was observed; however, only one dose of carbachol was used. Thus, it is possible that estradiol affects the sensitivity to carbachol in stimulating drinking responses in a way that mirrors estradiol effects on water intake in response to increased pOsm. Finally, estradiol may affect both the detection of increased pOsm and the activation of central pathways in osmotically-stimulated water intake, as suggested by parallel studies of VP release by Sladek and colleagues [10,11]. In these studies, estradiol modulation of osmotically-stimulated VP release was shown to require different subtypes of ERs that were differentially located within the central nervous system—ERα on neurons in the OVLT and ERβ on VP neurons in the hypothalamus.
As a steroid hormone, estradiol has genomic effects that may explain its ability to influence the central nervous system. Estrogens have been reported to change neuronal morphology, including dendritic and axonal branching; to up- or down-regulate receptors, such as AngII receptors; and to affect neurotransmitter levels [15,22,24,25,41-43]. These long-term changes may be complemented by recently recognized acute effects of estrogens on neuronal excitability [41,42,44-46]. In either case, the present studies show that estradiol effects on osmoregulatory drinking are independent of those on volume regulatory drinking. The decreased latency to water intake by EB-treated rats during increased pOsm indicates increased sensitivity to hyperosmolality that may be attributable to estradiol-mediated alterations in the detection of the osmotic stimulus, to modulation of activation in central pathways independent of changes in the initial detection, or to both. Ongoing investigations are addressing these issues.
Acknowledgments
This research was supported by an NIH grant from the National Institute on Deafness and Communication Disorders (DC06360). The manuscript was based, in part, on a document submitted by ABJ to the Oklahoma State University in partial fulfillment of the requirements for the Master's degree in Biomedical Sciences. Portions of these data were presented in preliminary form at the annual meeting of the Society for the Study of Ingestive Behavior (Naples, FL, 2006) and of the Society for Neuroscience (San Diego, CA, 2007).
The authors thank Dr. Alexander Rouch for helpful comments and technical expertise and Ms. Liming Fan for assistance with behavioral testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stachenfeld NS, DiPietro L, Kokoszka CA, Silva C, Keefe DL, Nadel ER. Physiological variability of fluid-regulation hormones in young women. J Appl Physiol. 1999;86:1092–6. doi: 10.1152/jappl.1999.86.3.1092. [DOI] [PubMed] [Google Scholar]
- 2.Forsling ML, Peysner K. Pituitary and plasma vasopressin concentrations and fluid balance throughout the oestrous cycle of the rat. J Endocrinol. 1988;117:397–402. doi: 10.1677/joe.0.1170397. [DOI] [PubMed] [Google Scholar]
- 3.Barron WM, Schreiber J, Lindheimer MD. Effect of ovarian sex steroids on osmoregulation and vasopressin secretion in the rat. Am J Physiol. 1986;250:E352–61. doi: 10.1152/ajpendo.1986.250.4.E352. [DOI] [PubMed] [Google Scholar]
- 4.Bossmar T, Forsling M, Akerlund M. Circulating oxytocin and vasopressin is influenced by ovarian steroid replacement in women. Acta Obstet Gynecol Scand. 1995;74:544–8. doi: 10.3109/00016349509024387. [DOI] [PubMed] [Google Scholar]
- 5.Stachenfeld NS, Keefe DL. Estrogen effects on osmotic regulation of AVP and fluid balance. Am J Physiol Endocrinol Metab. 2002;283:E711–21. doi: 10.1152/ajpendo.00192.2002. [DOI] [PubMed] [Google Scholar]
- 6.Stachenfeld NS, Taylor HS, Leone CA, Keefe DL. Oestrogen effects on urine concentrating response in young women. J Physiol. 2003;552:869–80. doi: 10.1113/jphysiol.2003.046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stachenfeld NS, Silva C, Keefe DL, Kokoszka CA, Nadel ER. Effects of oral contraceptives on body fluid regulation. J Appl Physiol. 1999;87:1016–25. doi: 10.1152/jappl.1999.87.3.1016. [DOI] [PubMed] [Google Scholar]
- 8.Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol. 1998;274:R187–95. doi: 10.1152/ajpregu.1998.274.1.R187. [DOI] [PubMed] [Google Scholar]
- 9.Hartley DE, Dickson SL, Forsling ML. Plasma vasopressin concentrations and Fos protein expression in the supraoptic nucleus following osmotic stimulation or hypovolaemia in the ovariectomized rat: effect of oestradiol replacement. J Neuroendocrinol. 2004;16:191–7. doi: 10.1111/j.0953-8194.2004.01150.x. [DOI] [PubMed] [Google Scholar]
- 10.Somponpun SJ. Neuroendocrine regulation of fluid and electrolyte balance by ovarian steroids: contributions from central oestrogen receptors. J Neuroendocrinol. 2007;19:809–18. doi: 10.1111/j.1365-2826.2007.01587.x. [DOI] [PubMed] [Google Scholar]
- 11.Sladek CD, Somponpun SJ. Estrogen receptors: their roles in regulation of vasopressin release for maintenance of fluid and electrolyte homeostasis. Front Neuroendocrinol. 2008;29:114–27. doi: 10.1016/j.yfrne.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 14.Stern JE, Zhang W. Preautonomic neurons in the paraventricular nucleus of the hypothalamus contain estrogen receptor beta. Brain Res. 2003;975:99–109. doi: 10.1016/s0006-8993(03)02594-0. [DOI] [PubMed] [Google Scholar]
- 15.Simonian SX, Herbison AE. Differential expression of estrogen receptor and neuropeptide Y by brainstem A1 and A2 noradrenaline neurons. Neuroscience. 1997;76:517–29. doi: 10.1016/s0306-4522(96)00406-x. [DOI] [PubMed] [Google Scholar]
- 16.Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Co-localization of estrogen and angiotensin receptors within subfornical organ neurons. Brain Res. 1999;837:254–62. doi: 10.1016/s0006-8993(99)01672-8. [DOI] [PubMed] [Google Scholar]
- 17.Krause EG, Curtis KS, Davis LM, Stowe JR, Contreras RJ. Estrogen influences stimulated water intake by ovariectomized female rats. Physiol Behav. 2003;79:267–74. doi: 10.1016/s0031-9384(03)00095-7. [DOI] [PubMed] [Google Scholar]
- 18.Findlay AL, Fitzsimons JT, Kucharczyk J. Dependence of spontaneous and angiotensin-induced drinking in the rat upon the oestrous cycle and ovarian hormones. J Endocrinol. 1979;82:215–25. doi: 10.1677/joe.0.0820215. [DOI] [PubMed] [Google Scholar]
- 19.Kucharczyk J. Localization of central nervous system structures mediating extracellular thirst in the female rat. J Endocrinol. 1984;100:183–8. doi: 10.1677/joe.0.1000183. [DOI] [PubMed] [Google Scholar]
- 20.Thrasher TN, Fregly MJ. Responsiveness to various dipsogenic stimuli in rats treated chronically with norethynodrel, ethinyl estradiol and both combined. J Pharmacol Exp Ther. 1977;201:84–91. [PubMed] [Google Scholar]
- 21.Kisley LR, Sakai RR, Ma LY, Fluharty SJ. Ovarian steroid regulation of angiotensin II-induced water intake in the rat. Am J Physiol. 1999;276:R90–6. doi: 10.1152/ajpregu.1999.276.1.R90. [DOI] [PubMed] [Google Scholar]
- 22.Kisley LR, Sakai RR, Fluharty SJ. Estrogen decreases hypothalamic angiotensin II AT1 receptor binding and mRNA in the female rat. Brain Res. 1999;844:34–42. doi: 10.1016/s0006-8993(99)01815-6. [DOI] [PubMed] [Google Scholar]
- 23.Curtis KS, Davis LM, Johnson AL, Therrien KL, Contreras RJ. Sex differences in behavioral taste responses to and ingestion of sucrose and NaCl solutions by rats. Physiol Behav. 2004;80:657–64. doi: 10.1016/j.physbeh.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 25.Krause EG, Curtis KS, Stincic TL, Markle JP, Contreras RJ. Oestrogen and weight loss decrease isoproterenol-induced Fos immunoreactivity and angiotensin type 1 mRNA in the subfornical organ of female rats. J Physiol. 2006;573:251–62. doi: 10.1113/jphysiol.2006.106740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- 27.Somponpun SJ, Sladek CD. Osmotic regulation of estrogen receptor-beta in rat vasopressin and oxytocin neurons. J Neurosci. 2003;23:4261–9. doi: 10.1523/JNEUROSCI.23-10-04261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YX, Crofton JT, Liu H, Sato K, Brooks DP, Share L. Estradiol attenuates the antidiuretic action of vasopressin in ovariectomized rats. Am J Physiol. 1995;268:R951–7. doi: 10.1152/ajpregu.1995.268.4.R951. [DOI] [PubMed] [Google Scholar]
- 29.Stricker EM, Huang W, Sved AF. Early osmoregulatory signals in the control of water intake and neurohypophyseal hormone secretion. Physiol Behav. 2002;76:415–21. doi: 10.1016/s0031-9384(02)00752-7. [DOI] [PubMed] [Google Scholar]
- 30.Fitts DA. Angiotensin II receptors in SFO but not in OVLT mediate isoproterenol-induced thirst. Am J Physiol. 1994;267:R7–15. doi: 10.1152/ajpregu.1994.267.1.R7. [DOI] [PubMed] [Google Scholar]
- 31.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- 32.Simon E. Interface Properties of Circumventricular Organs in Salt and Fluid Balance. News Physiol Sci. 2000;15:61–67. doi: 10.1152/physiologyonline.2000.15.2.61. [DOI] [PubMed] [Google Scholar]
- 33.Bisley JW, Rees SM, McKinley MJ, Hards DK, Oldfield BJ. Identification of osmoresponsive neurons in the forebrain of the rat: a Fos study at the ultrastructural level. Brain Research. 1996;720:25–34. doi: 10.1016/0006-8993(96)00079-0. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JW, Washburn DL, Ferguson AV. Intrinsic osmosensitivity of subfornical organ neurons. Neuroscience. 2000;100:539–47. doi: 10.1016/s0306-4522(00)00313-4. [DOI] [PubMed] [Google Scholar]
- 35.Adachi A, Niijima A, Jacobs HL. An hepatic osmoreceptor mechanism in the rat: electrophysiological and behavioral studies. Am J Physiol. 1976;231:1043–9. doi: 10.1152/ajplegacy.1976.231.4.1043. [DOI] [PubMed] [Google Scholar]
- 36.Rogers RC, Novin D, Butcher LL. Electrophysiological and neuroanatomical studies of hepatic portal osmo- and sodium-receptive afferent projections within the brain. J Auton Nerv Syst. 1979;1:183–202. doi: 10.1016/0165-1838(79)90016-x. [DOI] [PubMed] [Google Scholar]
- 37.Kahrilas PJ, Rogers RC. Rat brainstem neurons responsive to changes in portal blood sodium concentration. Am J Physiol. 1984;247:R792–9. doi: 10.1152/ajpregu.1984.247.5.R792. [DOI] [PubMed] [Google Scholar]
- 38.Renaud LP. CNS pathways mediating cardiovascular regulation of vasopressin. Clin Exp Pharmacol Physiol. 1996;23:157–60. doi: 10.1111/j.1440-1681.1996.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 39.Sawchenko PE, Swanson LW. The organization and biochemical specificity of afferent projections to the paraventricular and supraoptic nuclei. Prog Brain Res. 1983;60:19–29. doi: 10.1016/S0079-6123(08)64371-X. [DOI] [PubMed] [Google Scholar]
- 40.Khavari KA. Effects of intraventricular administration of carbachol, atropine, and scopolamine on water intake of the rat. Life Sci. 1968;7:971–7. doi: 10.1016/0024-3205(68)90104-5. [DOI] [PubMed] [Google Scholar]
- 41.Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–56. [PubMed] [Google Scholar]
- 42.Fannon SA, Vidaver RM, Marts SA. An abridged history of sex steroid hormone receptor action. J Appl Physiol. 2001;91:1854–9. doi: 10.1152/jappl.2001.91.4.1854. [DOI] [PubMed] [Google Scholar]
- 43.Parducz A, Zsarnovszky A, Naftolin F, Horvath TL. Estradiol affects axo-somatic contacts of neuroendocrine cells in the arcuate nucleus of adult rats. Neuroscience. 2003;117:791–4. doi: 10.1016/s0306-4522(02)00967-3. [DOI] [PubMed] [Google Scholar]
- 44.Womble MD, Andrew JA, Crook JJ. 17beta-Estradiol reduces excitatory postsynaptic potential (EPSP) amplitude in rat basolateral amygdala neurons. Neurosci Lett. 2002;331:83–6. doi: 10.1016/s0304-3940(02)00871-6. [DOI] [PubMed] [Google Scholar]
- 45.Lagrange AH, Wagner EJ, Ronnekleiv OK, Kelly MJ. Estrogen rapidly attenuates a GABAB response in hypothalamic neurons. Neuroendocrinol. 1996;64:114–23. doi: 10.1159/000127106. [DOI] [PubMed] [Google Scholar]
- 46.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid Signaling of Estrogen in Hypothalamic Neurons Involves a Novel G-Protein-Coupled Estrogen Receptor that Activates Protein Kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]