Abstract
Functional MRI has demonstrated differences in response to memory performance based on risk for Alzheimer's disease (AD). The current study compared blood oxygen level dependent (BOLD) functional MRI response with arterial spin labeling (ASL) perfusion response during an associative encoding task and resting perfusion signal in different risk groups for AD. Thirteen individuals with a positive family history of AD and at least one copy of the apolipoprotien E ε4 (APOE4) gene (high risk) were compared to ten individuals without these risk factors (low risk). In the medial temporal lobes (MTLs) the high risk group had an elevated level of resting perfusion, and demonstrated decreased fractional BOLD and perfusion responses to the encoding task. However, there was no difference in the absolute cerebral blood flow during the task. These data demonstrate that individuals with increased risk for Alzheimer's disease have elevated MTL resting cerebral blood flow, which significantly influences apparent differences in BOLD activations. BOLD activations should be interpreted with caution, and do not necessarily reflect differences in neuronal activation.
Keywords: Alzheimer, APOE, Aging, MRI, fMRI, Imaging, Perfusion, BOLD, Risk factors, Family history, Arterial spin labeling, Cerebral blood flow (CBF)
1. Introduction
Both the apolipoprotien E ε4 allele (APOE4) and a family history of dementia are associated with increased risk of Alzheimer's disease (AD) and subsequent presence of neurofibrillary tangles and amyloid plaques in the brain (Fratiglioni et al., 1993; Corder et al., 1998, 2004; Ghebremedhin et al., 1998). In fact, having a family history of dementia is additive to the risk of having a copy of the APOE4 allele (Cupples et al., 2004), and has been shown to be independently associated with functional imaging activations in the medial temporal lobe (MTL) (Bassett et al., 2006; Johnson et al., 2006). For this reason, imaging studies attempting to identify differences associated with AD risk often stratify by these factors. Blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI) has demonstrated distinctions in medial temporal lobe activations during memory tasks based on presence or absence of APOE4 (Bookheimer et al., 2000; Bondi et al., 2004; Han et al., 2006) and family history (Fleisher et al., 2005; Johnson et al., 2006). In addition, fluorodeoxyglucose positron emission tomography (FDG PET) has shown decreased glucose metabolism in the medial temporal and parietal lobes of those with the APOE4 gene many decades prior to the typical age of AD onset (Reiman et al., 1996, 2004). fMRI studies looking at differences in the BOLD response based on Alzheimer's disease risk factors have shown varying results. Depending on age and the memory task used, some studies have demonstrated increased BOLD signal in the MTL associated with AD risk (Bookheimer et al., 2000; Fleisher et al., 2005). Yet, others have reported reduced BOLD signal response associated with AD risk during encoding (Bondi et al., 2005; Johnson et al., 2006; Trivedi et al., 2006). However, in people with Alzheimer's disease the BOLD response in the MTL during a memory task is consistently decreased, compared to normal controls (Dickerson et al., 2005). In addition, imaging studies using arterial spin labeling (ASL) show decreased resting perfusion in AD and mild cognitive impairment (MCI) (Alsop et al., 2000; Johnson et al., 2005; Xu et al., 2007), as well as decreased perfusion response to encoding in the MTL in those with MCI. (Xu et al., 2007) Taken together, these findings suggest an underlying pathological process that may be observed with functional imaging prior to the clinical onset of dementia.
The BOLD response is often interpreted as an indirect measure of underlying neuronal activity. In this regard, increases in BOLD signal in the MTL in elderly persons at increased risk for AD and MCI have been frequently interpreted as a neuronal stress response to underlying cholinergic system degeneration at the earliest stages of disease (Bookheimer et al., 2000; Bondi et al., 2005; Dickerson et al., 2005). However, the BOLD response relies on many other factors including the baseline perfusion state, vascular compliance, cerebral blood volume, and the coupling relationships between these measures (Buxton et al., 2004; Iadecola, 2004; Iannetti and Wise, 2007). For example, increases in baseline cerebral blood flow (CBF) by use of vasodilators significantly decreases the amplitude of the BOLD response irrespective of task performance (Brown et al., 2003; Stefanovic et al., 2006). There is evidence that these neurovascular relationships may be altered in AD pathology, with increased vascular resistance (Bateman et al., 2006) and differences in coupling of the vascular response to neuronal activity (Iadecola, 2004; Zlokovic, 2005; Girouard and Iadecola, 2006). Therefore group differences in activation BOLD signal, in many cases, may be entirely due to differences in the cerebral perfusion states, not necessarily representative of increased neuronal activity or oxygen consumption. A better understanding of the underlying vascular response and resting blood flow state is necessary for understanding BOLD fMRI signal in AD risk.
Pulsed arterial spin labeling (pASL) is an MRI technique that can simultaneously measure cerebral blood flow and BOLD changes during a functional task. Coupled with its ability to measure baseline cerebral blood flow, pASL is a powerful tool for evaluating the underlying physiologic changes associated with the BOLD response, and hence aid in interpreting factors related to neuronal oxygen utilization (Wong et al., 1998; Obata et al., 2004; Uludag et al., 2004). A recent report demonstrated the utility of these techniques for evaluating the relationship between BOLD and perfusion responses in the MTL during memory performance (Restom et al., 2007). They concluded that the BOLD response and related oxygen utilization in the MTL during memory acquisition can be better understood when changes in cerebral blood flow are accounted for. Use of combined functional perfusion and BOLD imaging has not been previously reported in the evaluation of AD risk. Therefore, in this study we evaluated middle aged, cognitively normal, subjects with and without the APOE4 allele, and with and without a family history of dementia. We acquired, simultaneous, medial temporal lobe BOLD and perfusion response during associative encoding, as well as resting perfusion levels.
2. Methods
2.1. Study population
Thirty-eight healthy right-handed volunteers, 50–65 years of age, were evaluated. Twenty-five had a significant family history of dementia in a first degree relative, 13 did not. All participants were drawn from a larger group of normal control participants currently active in the University of San Diego (UCSD) Alzheimer's Disease Research Center, from the UCSD student, staff and faculty population, as well as the general San Diego community by means of advertisement.
All potential participants were screened and excluded for a history of significant head trauma with residual cognitive deficits, other neurological or major psychiatric disorders such as schizophrenia, bipolar disorder, developmental learning disorder, and alcohol or substance abuse. The geriatric depression scale (Yesavage et al., 1982) was administered to screen for the presence of affective disturbance. Persons with significant cerebrovascular disease (as indexed by modified Rosen ischemic scores greater than 4) were also excluded, as were individuals with unstable diabetes and respiratory disease. All participants underwent careful screening for contraindications for magnetic resonance imaging (i.e., metal in the body, pregnancy, claustrophobia), as well as complete physical and neurological examinations. All participants received APOE4 genotyping using a polymerase chain reaction based method (Saunders et al., 1993).
2.2. Materials and procedures
2.2.1. Cognitive testing
All participants received the following neuropsychological test battery within 1 month prior to imaging: Boston Naming Test (Kaplan et al., 1983), WMS-R Logical Memory Test (Wechsler, 1987), verbal fluency (Monsch et al., 1992), WAIS-R digit span forward and backward (Wechsler, 1981), WAIS-R Digit Symbol Test (Smith, 1982), CVLT (Delis et al., 1988), clock drawing (Mohs et al., 1997), Trail Making Test (A and B) (Reitan, 1958), and MMSE (Folstein et al., 1975).
2.2.2. Functional MRI behavioral task
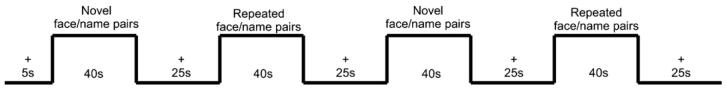
The task was adapted with permission from Dr. Reisa Sperling (Sperling et al., 2001). A face/name encoding task was chosen due to its ability to activate the hippocampal region with BOLD fMRI (Sperling et al., 2001, 2003a). Participants viewed pairs of Novel and previously viewed (Repeated) faces and names in alternating blocks of 40 s each, separated by 25 s of viewing a central fixation crosshair (Fig. 1). Before the scanning session and before each run, subjects were given explicit instructions to concentrate on remembering the name associated with each face. Three task conditions were presented: (1) Novel face-name pairs: each face-name pair was presented for 5 s, followed by a brief (0.8 s) white central fixation crosshair on a black background. Subjects viewed seven Novel face-name pairs during each Novel block. (2) Repeated face-name pairs: subjects examined four repeated face-name pairs (women and men). These Repeated face-name pairs were first shown to the subject in a practice run and assessed for accurate recall, so that the subject was familiar with the face-name pairs at the beginning of the functional scanning runs. As with the Novel face-name pairs, each Repeated face/name pair was shown for 5 s, followed by a brief (0.8 s) central fixation crosshair, and repeated for a total of 40 s. The male and female face-name pairs alternated randomly throughout each presentation block. (3) Visual fixation: subjects examined a white fixation crosshair presented in the center of the visual field on a black background. Four runs, each consisting of the format in Fig. 1, were obtained on each subject in rapid succession, each lasting a total of 4 min and 35 s. A total of 56 Novel face-name pairs and four Repeated face-name pairs were used over the course of the entire experiment. Each face-name pair in the Novel condition was seen only once by each subject, whereas each of the four Repeated face-name pair was seen a total of 14 times over the course of each scanning session. Subjects were also instructed to press one of two buttons on a provided button box to assess whether they felt the name was a good fit or bad fit with the face displayed. This was primarily done to monitor attention to the task.
Fig. 1.
Encoding task block design. Participants viewed pairs of Novel and previously view (Repeated) faces and names in alternating blocks of 40 s each, separated by 25 s of viewing a central fixation crosshair.
2.2.3. Post-scan testing
Immediately following the imaging session, subjects were tested for their recall of all 60 face-name pairs. There were 10 distractor faces in the post-scan testing that were not presented during the experiment. The post-scan testing was a cued recall task. Subjects were shown 70 faces sequentially and were prompted to choose between three name choices for each face. They were then asked to rate their confidence in their face-name choice as “confident” or “not confident”. Recall scores were calculated to verify attention to the scanning task.
2.2.4. Functional neuroimaging procedure
Each session consisted of four functional runs during memory testing with the face-name task. In addition, prior to functional imaging, a pulsed arterial spin labeled scan was obtained during a rest period for 4 min and 35 s with the participant's eyes closed. After resting and functional pASL acquisition, a high-resolution anatomical image was acquired. Those participants requiring vision corrections were fitted with plastic framed glasses with interchangeable lenses closely matching their prescription. Head movement in the receiver head coil was minimized by foam fittings. Stimuli were back-presented via a liquid crystal display projector onto a screen located at the participant's feet that was viewed through a mirror mounted on the receiver head coil. Button pressing responses were recorded using a fiber-optic device designed for use in the magnet. Stimulus presentation and response collection were performed by a personal computer using software designed for administration of cognitive experiments (E-Prime; Psychology Software Tools).
2.3. MRI acquisition
2.3.1. MRI technique
All scans were performed on a General Electric Signa EXCITE 3.0T short bore, twin speed scanner with a body transmit coil and an eight channel receive array. High-resolution structural brain images were acquired with a magnetization prepared three-dimensional fast spoiled gradient sequence acquisition (FSPGR: 124 axial slices, 1 mm×1 mm in-plane resolution, 1.3 mm slice thickness, field of view= 256×256, TR = 7.8 ms, TE = 3.1 ms, flip angle 12°).
Simultaneous perfusion and BOLD data were acquired using PICORE/QUIPSS II pulsed arterial spin labeling technique with a dual echo readout (Wong et al., 1998; Liu and Wong, 2005). A pASL pulse sequence optimized for medial temporal lobe perfusion was used (Restom et al., 2007): TR = 2500 ms, TI1 = 700 ms, TI2 = 1400 ms, tag thickness = 200 mm, 10 mm gap between the edge of the tagging band and the nearest slice, TE1 = 2.8 ms, TE2 = 24, flip angle 90, FOV 240 mm, 64×64 matrix, AND 3.75 mm×3.75 mm in-plane resolution, with 106 repetitions. Five 6-mm slices parallel to the axis of the hippocampus were acquired, with coverage of the entire temporal lobe. Four functional runs and one resting state image, all lasting 4 min and 35 s were acquired. In addition a reference scan of cerebral spinal fluid (CSF) signal was acquired for use in quantifying resting cerebral blood flow. This CSF scan consisted of a single echo, single repetition scan acquired at full relaxation and echo time equal to 2.8 ms. This CSF scan utilized the same inplane parameters as the pASL scan, but the number of slices was increased to cover the lateral ventricles.
2.3.2. Physiologic monitoring
During all arterial spin labeling scan acquisitions pulse and respiratory wave forms were collected using a pulse oxymeter and respiratory effort transducer. Subjects wore a pulse oxymeter probe on their left index finger. A respiratory band was placed around the sternum to measure chest wall movement associated with respiration. Data from these instruments were collected at 40 samples per second using a multi-channel data acquisition board (National Instruments). Scanner TTL pulse data (10 ms duration, 5 V pulse per slice acquisition) were recorded at 1 kHz. The TTL pulse data were used to synchronize the physiologic data to the acquired images. Pulse, respiratory, and TTL data were used to calculate physiologic noise regressors to improve task-specific signal identification (Glover et al., 2000; Restom et al., 2006).
2.4. Data analysis
2.4.1. Post-scanning image processing
The data from the two echos were analyzed separately, with the first and second echo data used to analyze cerebral blood flow and BOLD activity, respectively. For each voxel the MR signal associated with physiologic noise from both pulse rate and respiratory rate data were included in a general linear model (GLM) analysis as regressors to model the physiologic fluctuation of the ASL signal. These physiologic noise component estimates were removed from the data to form corrected first and second echo time series for further analysis of both BOLD and perfusion data. Images were then motion corrected across time points, among all four runs, to the most typical base image in the second run, using a three-dimensional iterated, linearized, weighted least-squares method with Fourier interpolation with the AFNI software (Cox, 1996). Time series were also visually inspected for motion based on the output of a program designed to detect outliers (AFNI-3dToutcount). Time points with isolated head movements not corrected by the registration algorithm were ignored in the statistical analysis.
A general linear model approach was used for statistical analysis of the individual functional datasets. Data from all four functional runs were concatenated for use in the GLM analysis performed by AFNI's 3dDeconvolve program (Cox, 1996). Stimulus-related MR signal was included in the regression model by convolving the block design stimulus pattern with a gamma density function to create reference vectors using the AFNI Waver program (Cox, 1996). In addition motion-corrected signal intensities from translation and rotation indices were used as covariates. Hence, the GLM model included a combination of the following independent variables with MR signal as the dependent variable: reference vectors representing the occurrence of different stimulus types (i.e., Novel face-name pairs, Repeated face-name pairs, or fixation), motion parameters, a linear trend, and a constant. This procedure produced F statistics representing the strength of associations between stimulus presentation and mean peak signal change for each voxel, averaged across each stimulus block for all four functional scans, for each individual participant. Based on the F statistic, mean peak signal changes that satisfied an α level of 0.05 for association with the stimulus were used as the primary outcome variables for between-group analyses. In addition, mean activation changes for each 2500 ms time point within the Novel face/name encoding blocks (averaged over all encoding blocks) were calculated for graphical representation of the time series data. This was done using AFNI's impulse response feature in 3Ddeconvolve.
2.4.2. CBF and BOLD time series analysis
Physiologic noise and motion corrected images were used to calculate both CBF and BOLD-weighted time series. CBF time series were computed by taking the running subtraction of the control and tag image series from the first echo (TE = 2.8), whereas the BOLD weighted time series were computed from the running average (average of each image with the mean of its two nearest neighbor) of the second echo (TE = 24 ms) (Liu and Wong, 2005). Data acquired from the resting scan acquisition were used to calculate resting perfusion flow rates by computing the average difference between the co-registered control and tag images for the entire 4′35″ resting scan. This data were then converted into absolute physiologic units of CBF (ml/(100 mg min)) using the CSF image as a reference signal (Wong et al., 1998; Chalela et al., 2000).
Perfusion and BOLD mean peak signal changes during the stimulus tasks were transformed for further processing. To standardize the MR signal measures task-related percent MR signal changes from baseline were calculated using the average baseline MR signal value for all four functional runs, derived from the constant baseline regressor term in the GLM. These values will be referred to as “fractional changes”: %ΔCBF and %ΔBOLD, for perfusion and BOLD responses, respectively. To approximate absolute physiologic flow changes from the baseline state during the encoding responses, the percent change in perfusion signal (%ΔCBF) was multiplied by the corresponding mean resting perfusion rate in the same voxels, for each participant. This provided an estimate of flow change from baseline in response to the stimulus task in physiologic units (ml/(100 mg min)), and is referred to as change in absolute CBF (ΔCBF). To further assess the perfusion response during the encoding tasks in relation to the resting perfusion state, ΔCBF was added to the resting perfusion rate for functionally activated voxels. This resulted in an estimate of “total absolute CBF' during the encoding tasks.
2.4.3. Region of interest analysis
Region of interest (ROI) analyses for both BOLD and ASL data were performed to avoid structural variance assumptions often made when conforming brain structures to a standardized atlas. This technique improves activation sensitivity in small structures such as the hippocampus which can be lost in atlas based analysis. All time series data were registered to the high-resolution anatomical image using AFNI's 3dVolreg program's two-dimensional registration algorithm (Cox, 1996). Semi-automated ROIs of the hippocampus were drawn for each anatomical image using the FreeSurfer software package (Fig. 2)(Fischl et al., 2002). These ROIs were visually inspected for accurate delineation and registration to the functional datasets, then down-sampled to the resolution of the functional images. From these, binary masks were created to use as search regions for hippocampal region activations. Functional activations that fell within these search regions were further constrained by removing any voxels that did not show a significant activation (α = 0.05) in the generalized linear model of task-related activity (e.g., a significant response to the encoding task) using the F statistic. In addition, to correct for multiple comparisons, voxels were only considered significant if they were found in clusters sizes reaching a α threshold of 0.05. This cluster size threshold was determined for each individual subject ROI by using a Monte Carlo simulation performed by the AFNI program Alphasim (Jinhu Xiong, 1995; Cox, 1996). Voxels were required to be touching on one entire side to be considered part of a cluster. For each search region, data within voxel clusters meeting these criteria were averaged to derive mean signal change measures for Novel encoding and for Novel versus Repeated (NvR) encoding.
Fig. 2.
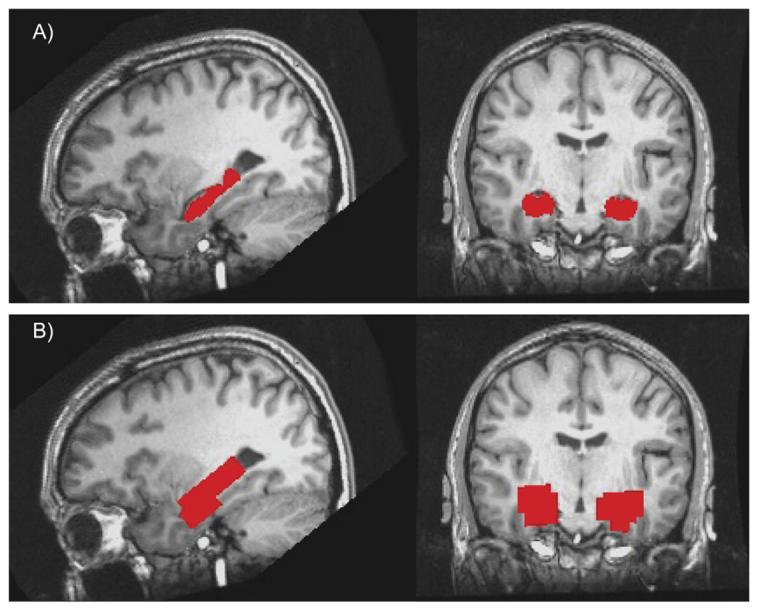
Region of interest. (A) Automated segmentation of the hippocampus; (B) down-sampled ROI to match the resolution of the functional dataset and form the hippocampal search region.
Resting state cerebral blood flow data were calculated from the separate resting perfusion scan, not from periods of fixation during the activation runs. Resting perfusion signal was extracted from those perfusion voxels that were significantly activated during the memory task for each individual. This was done by creating binary masks for each individual from the perfusion activation patterns derived during the encoding runs (as described above). These values were averaged for the hippocampal search regions in each subject.
2.4.4. Volumetric data acquisition
Measurements of total intracranial, whole brain, ventricular and hippocampal volumes were calculated using semi-automated segmentation and volumetric output measures from FreeSurfer ASEG analysis (Fischl et al., 2002). Whole brain, ventricular, and hippocampal volumes were normalized to the total intracranial volumes to adjust for variability due to head size. These normalized volumes were used for between group comparisons to evaluate for significant group differences.
2.4.5. Between group statistics
The 38 participants were separated into two risk groups for the primary statistical analysis. The high risk group consisted of those subjects with both a family history of dementia in a first degree relative and at least one copy of the APOE ε4 gene (excluding those with a copy of the APOE ε2 gene). The low risk group had no family history of dementia and no copies of the APOE ε4 gene. Risk groups were compared by two-tailed Student's t-tests for extent of mean activations during the memory task contrasts for both perfusion and BOLD measures in the hippocampal ROI. If no group differences were found in activation, the ROIs were separated into right and left hippocampal regions to evaluate for lateralized differences. Right and left hemispheres were not directly compared to each other. Between-group comparisons were also made using two-tailed Student's t-test for all demographic, neurocognitive, volumetric measures, and the number of voxels activated during the task. Pearson correlations were performed to assess the relationship between perfusion and BOLD signal that were acquired simultaneously during the encoding tasks.
Group differences in Novel encoding and in Novel versus Repeated face-name pair encoding were evaluated in group analyses. Novel encoding signal was analyzed as the primary statistical outcome measure to optimize task related signal sensitivity. However, Novel versus Repeated face-name pair data was also evaluated to further isolate signal related to pure Novel encoding in the hippocampal region. All analyses were performed from individual native space region of interest data.
3. Results
Twenty-five subjects had a significant family history of dementia in a first degree relative, whereas 13 did not. After enrollment and APOE4 testing, we further stratified the groups into “High” and “Low” risk groups. Thirteen participants had both a positive family history and at least one copy of the APOE ε4 gene (high risk group). Ten had no family history of dementia and no copies of the APOE ε4 gene (low risk group). These groups did not significantly differ by age, education, neurocognitive test scores, post-scan recall task of face-name pairs, or volumetric measures (Table 1). The high risk group had 77.9% females while the low risk group had 50% females.
Table 1.
Demographics, memory test scores, and volumetric measures
| Low risk | High risk | p-Values | |
|---|---|---|---|
| Subjects (no.) | 10 | 13 | |
| Age | 57.7 (±4.39) | 58.2 (±4.27) | 0.75 |
| Sex (% female) | 5M, 5F (50%) | 3M, 10F (77.9%) | n/aa |
| Education (years) | 16.3 | 15.9 | 0.16 |
| MMSE | 29.8 | 29.7 | 0.30 |
| CVLT score | 52.2 | 49.5 | 0.62 |
| Logical memory delayed recallb | 12.7 | 11.6 | 0.20 |
| Post-scan task recall score (% correct) | 74.1 | 71.2 | 0.46 |
| Whole brain volumes (% of TIV) | 60.3 | 60.3 | 0.98 |
| Ventricular volume (% of TIV) | 1.56 | 1.42 | 0.56 |
| Hippocampal volume (% of TIV) | 0.52 | 0.52 | 0.84 |
MMSE: Folstein Mini Mental State Exam (Folstein et al., 1975); CVLT: California Verbal Learning Test (Delis et al., 1988); TIV: total intracranial volume.
No statistics performed due to inadequate sample size of males in the high risk group.
Delayed recall of the WMS-R Logical Memory Test (Wechsler, 1987).
Evaluation of percent signal change during Novel encoding versus fixation (NvF) and Novel versus Repeated encoding revealed significant group differences for both cerebral blood flow and BOLD activations. For NvF activations, both %ΔCBF (p = 0.007) and %ΔBOLD (p = 0.037) were lower in the high risk group by 30.7% and 33.0%, respectively. (Fig. 3 and Table 2). When contrasting Novel encoding to Repeated encoding activations this group difference was only seen in %ΔBOLD (p = 0.027) but not in %ΔCBF (p = 0.100) (Table 2). Further analysis showed that the lack of significant group differences in NvR %ΔCBF were driven by lateralizing effects. Left hippocampal region %ΔCBF was not significantly different between groups (p = 0.30), however right sided NvR activations showed lower %ΔCBF in the high risk group (p = 0.031). %ΔCBF and %ΔBOLD activations were positively correlated to each other for both NvF (r = 0.657, p = 0.001) and NvR (r = 0.605, p = 0.002) encoding contrasts. There were no group differences in the number of voxels activated during the memory tasks.
Fig. 3.
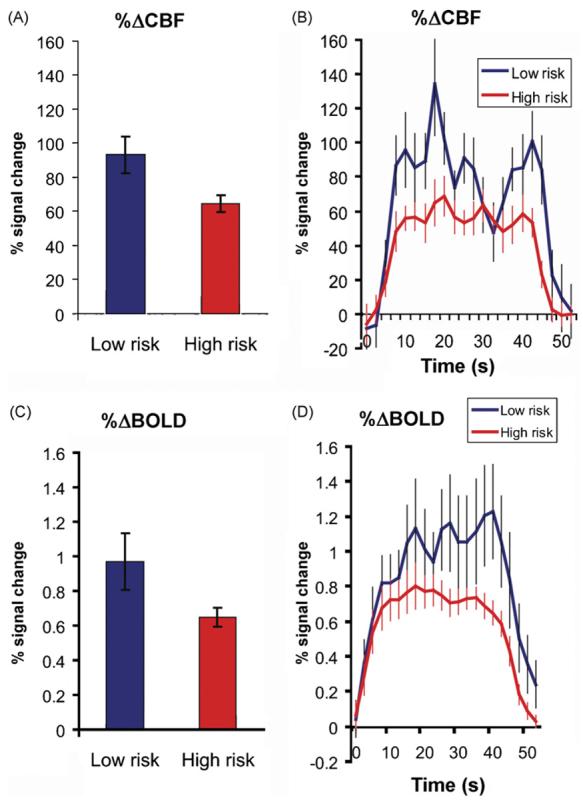
Fractional changes in cerebral blood flow and BOLD signal. Hippocampal region response to Novel face/name pair encoding in percent signal change from baseline. A comparison of high and low risk groups for AD. (A) Mean peak percent changes in cerebral blood flow signal, (B) impulse response time series during the 40 s encoding task in percent change of CBF, (C) mean peak percent changes in BOLD signal, and (D) impulse response time series during the 40 s encoding task in percent change of BOLD signal.
Table 2.
Signal differences between high and low risk groups
| Low risk (n = 10) | High risk (n = 13) | Relative group difference (%) | p-Values | |
|---|---|---|---|---|
| %ΔCBF | ||||
| Novel encoding (%) | 93.1 | 64.5 | −30.7 | 0.007 |
| Novel vs. Repeated encoding (%) | 78.7 | 60.9 | −22.6 | 0.10 |
| Absolute CBF during rest | ||||
| During rest in Novel voxels (mg/(100 ml min)) | 44.6 | 54.7 | +24.0 | 0.029 |
| During rest in Novel vs. Repeated voxels (mg/(100 ml min)) | 43.3 | 57.8 | +25.1 | 0.002 |
| %ΔBOLD | ||||
| Novel encoding (%) | 0.97 | 0.65 | −33.0 | 0.037 |
| Novel vs. Repeated encoding (%) | 0.97 | 0.57 | −41.2 | 0.027 |
Low risk vs. high risk group comparison of mean hippocampal search region fractional signal change for perfusion (%ΔCBF) and BOLD (%ΔBOLD), as well as resting perfusion levels in voxels that had been activated during the task (mg/(100 ml min)). This is presented for both Novel encoding blocks compared to fixation blocks (NvF), and Novel encoding blocks compared to Repeated face/name encoding blocks (NvR).
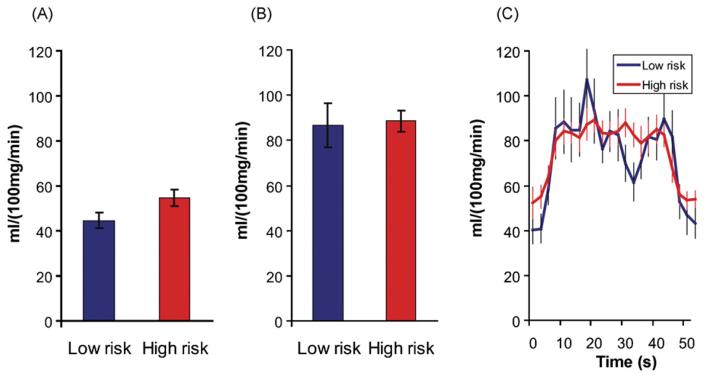
Fig. 4 shows group comparisons of resting state absolute cerebral blood flow rates and estimated absolute cerebral blood flow during Novel encoding. Mean resting absolute CBF in voxels that were significantly activated during the memory tasks showed higher resting state levels of blood flow in the high risk group for both NvF (p = 0.029) and NvR (p = 0.002) contrasts, 24.0% and 25.1%, respectively (Fig. 4A and Table 2). There were no significant group differences in total absolute cerebral blood flow during the encoding task for bilateral, right or left hippocampal regions (Fig. 4B and C). This was true for both the NvF and NvR contrasts.
Fig. 4.
Absolute cerebral blood flow. Estimates of absolute cerebral blood flow in physiologic units in the hippocampal region. A comparison of high and low risk groups for AD. (A) Absolute CBF during rest; (B) total absolute cerebral blood flow during encoding task presentation; (C) impulse response time series during the 40 s encoding task in total absolute CBF units.
4. Discussion
This study demonstrates that functional arterial spin labeling can detect signal differences in the medial temporal lobes of people with differential risk for Alzheimer's disease. In this cognitively normal, middle aged, cohort we found decreased fractional changes in medial temporal lobe cerebral blood flow and BOLD signal during associative encoding in those at increased risk for AD. However, evaluation of resting state cerebral blood perfusion revealed that the high risk group also had higher levels of absolute CBF at rest compared to the low risk group. Estimations of total absolute CBF during encoding showed that, when the resting state was accounted for, both high and low risk groups attained similar levels of perfusion in the activated state. This implies that differences in the encoding-related CBF response associated with AD risk, when assessed as changes from baseline levels (%ΔCBF), is likely driven by differences in the resting state. With %ΔCBF positively associated with %ΔBOLD, fractional changes in the BOLD signal are also likely dependent on the resting state, and may not represent differences in task-related neuronal activity. Not only does this demonstrate the utility of these techniques for identifying risk, but further explores the relationship between BOLD and perfusion signals. BOLD activations should not be directly interpreted as representing neuronal activity, but reflect a more complex relationship between vascular reactivity, cerebral blood flow, oxygen utilization, and the baseline state.
Our finding of a 24% elevation in MTL resting perfusion related to AD risk, and subsequent attenuation of a fractional response during encoding, may have implications for pathology in neurovascular function. There is evidence of neurovascular unit dysfunction in Alzheimer's disease, which may also be present in individuals at increased risk for AD, prior to clinical manifestations. AD is associated with distinct changes in cerbrovascular structure (Farkas and Luiten, 2001; Zlokovic, 2005; Zlokovic et al., 2005) and function (Iadecola, 2004). Amyloid beta (Aβ) aggregation is considered to be an important pathological feature in Alzheimer's disease (Hardy and Selkoe, 2002). And, soluble forms of Aβ may be responsible for vasculotoxic effects (Zlokovic, 2005). Transgenic mouse models have demonstrated that functional hyperemia is impaired with over expression of the amyloid precursor protein (APP), even prior to development of amyloid plaques (Iadecola, 2004). Soluble forms of Aβ can reduce regional CBF by attenuating endothelium-mediated vasodilatation and directly eliciting vasoconstriction (Iadecola, 2004). Production of superoxides in relation to Aβ impairs nitric oxide mediated vasodilitation (Girouard and Iadecola, 2006; Benarroch, 2007). In addition, Aβ has direct toxic and inflammatory effects on vascular endothelium (Folin et al., 2006; Marco and Skaper, 2006). The APOE ε4 gene itself may influence vascular responsiveness in the absence of amyloid plaque deposition by modulating the toxic effects of Aβ1–42 on endothelial cells (Folin et al., 2006). These metabolic effects related to AD risk and AD pathology are consistent with our findings of decreased fractional CBF responsiveness to encoding in the medial temporal lobe, and may be driving the elevation of the resting perfusion state in the high risk individuals.
There are few reports of perfusion differences based on AD risk during rest or memory tasks, and none using ASL MRI techniques. However, the literature on resting perfusion in AD subjects compared to normal subjects is robust. Decreased resting perfusion has been demonstrated in AD subjects compared to normal elderly controls using SPECT (Lojkowska et al., 2002; Varma et al., 2002; Ishiwata et al., 2006), ASL MRI (Alsop et al., 2000; Johnson et al., 2005), and H215O positron emission tomography (15O PET) (Ishii et al., 2000). This has also been demonstrated in people with mild cognitive impairment with resting ASL MRI measures of perfusion (Johnson et al., 2005). These studies did not show specific evidence for perfusion differences in the medial temporal lobe. However, other studies using SPECT have demonstrated MTL decreased resting perfusion in people with MCI (Ishiwata et al., 2006) and early cognitive changes associated with AD (Johnson et al., 1998). In addition, 15O PET has shown activation perfusion differences by APOE ε4 genotype in young normal controls (Scarmeas et al., 2005). Mixed increases and decreases during a non-verbal memory task in various brain regions were found. Importantly, a recent pASL study demonstrated a 22% decreased response in the MTL to encoding in individuals with early amnestic mild cognitive impairment (Xu et al., 2007). This is consistent with our finding of a 30.7% reduction during encoding in individuals at increased risk for dementia, and implies pathologic similarities. Furthermore, FDG-PET studies have demonstrated reduction in MTL glucose metabolism associated with the APOE4 genotype (Reiman et al., 1996, 2001, 2004). It is possible that early manifestations of AD include metabolic derangements resulting in increased baseline blood flow in an effort to compensate for functionally impaired metabolic substrate.
Evidence from published BOLD and perfusion studies can guide our understanding of the relationships between the BOLD response, cerebral blood flow, oxygen metabolism, and neuronal activity. Brown et al. (2003), assessed cortical motor activity with PICORE/QUIPSS II ASL in normal control subjects during a simple finger tapping paradigm before and after administration of acetazolamide (Brown et al., 2003). Acetazolamide is a potent vasodilator thought to modulate CBF without changing the cerebral metabolic rate of oxygen consumption (CMRO2) or neuronal activity. Administration of the drug increased resting CBF, decreased the BOLD response to the motor stimulus, but had no effect on the magnitude of the absolute change of CBF in response to the task. Hence, the change in CBF during task-related neuronal activity, when added to an increased resting CBF level, led to an increased total absolute level of CBF during the task with acetazolamide. The reduced BOLD response during the task is consistent with reduced baseline deoxyhemoglobin content and a smaller fractional CBF increase, both due to the elevated baseline CBF (Buxton et al., 2004). Since the increased CBF in the baseline state is thought to be purely a vascular effect, with no change in baseline neural activity or CMRO2, the subsequent CBF response to the motor task is consistent with a feed-forward mechanism in which neuronal activity itself drives an increase in CBF (Iadecola, 2004).
In another experiment involving baseline state manipulations, a visual stimulus paradigm was used to reduce resting CBF in a setting in which resting neuronal activity may also be reduced (Uludag et al., 2004). In this study, the activation task was viewing a flickering checkerboard, but the baseline state alternated on successive blocks between eyes open with fixation and eyes closed. BOLD signal and CBF levels in the visual cortex were reduced during rest with eyes closed compared to rest with eyes open. Yet, when viewing a visual stimulus, changes in both BOLD and CBF resulted in the same absolute signals, reflecting decreased fractional BOLD and perfusion responses from the eyes-open baseline state compared to the eyes-closed baseline state. Both this and the acetazolamide experiment showed increased resting CBF to be associated with decreased BOLD response and also decreased fractional change in CBF, similar to our current data. And, both of these experiments are consistent with the CBF response being driven by neuronal activity in a feed-forward fashion. What distinguishes the visual stimulus study from the acetazolamide study is that the final absolute CBF levels during the visual stimulus task were similar, whereas with the acetazolamide the final CBF level was increased. Hence, our results are more consistent with the visual stimulus experiment, with comparable task-related total absolute CBF in the two groups. Therefore, our findings more likely represent similar levels of CMRO2 and neural activity during encoding for the two groups, but elevated activity at baseline in the high risk group. Yet, a potential contributory effect of reduced vascular responsiveness during task performance in the high risk group cannot be discounted.
Reports of differences in functional BOLD signal between APOE ε4 carriers and non-carriers is well reported in the literature. However, while some studies have demonstrated higher levels of BOLD reactivity in normal subjects at higher risk for AD (Bookheimer et al., 2000; Bondi et al., 2005; Fleisher et al., 2005; Wishart et al., 2006), others have demonstrated a measurable decrease in BOLD activations (Smith et al., 1999, 2002; Johnson et al., 2006; Trivedi et al., 2006). These finding may appear to be incongruous, however they more likely represent dependence of BOLD signal changes on variables such as cognitive task used, brain region evaluated, and age of cohort. The face-name encoding task used in our study has been used by other groups to demonstrate changes in %ΔBOLD responses during different stages of AD-related memory impairment. Early mild cognitive impairment subjects show increased MTL %ΔBOLD activations (Dickerson et al., 2005; Celone et al., 2006), but decreased signal in more impaired MCI (Dickerson et al., 2005; Celone et al., 2006) and AD (Sperling et al., 2003b; Dickerson et al., 2005). These studies, together with our perfusion data, support the possibility that %ΔBOLD may fluctuate based on age and disease progression, and as argued here, may predominantly represents changes in the baseline state.
Limitations in this study should be noted. Most important is the small sample size investigated here. However, our ability to detect group differences with this small sample size speak towards the possibility of robust effect sizes. In addition, the ratio of female to male participants in the high risk group is a possible concern. We cannot verify that the group differences we report are not potentially influenced by this. Also, areas of BOLD and perfusion activations were not required to be overlapping. Therefore, task activations may represent different regions within the ROI. Since perfusion signal is generated more from the arterial side of the cerebral vasculature and BOLD from the venous side (Luh et al., 2000), there is some expected discrepancy of in signal origination. For this reason we believed that relying on the general linear model to represent true task-associated activation simultaneously for BOLD and perfusion signal was appropriate. Also, we avoided any direct mathematical or statistical comparisons of the BOLD and perfusion signal, other than Pearson correlations. Furthermore, we are unable to directly or indirectly measure CMRO2 due to a lack of understanding the MR signal relationships between APOE ε4 and neurovascular response, unlike the known relationships in young control subjects and aging that allow for mathematical estimations of CMRO2 (Restom et al., 2007). To evaluate the effects of APOE ε4 on oxygen consumption directly, calibration of the BOLD signal for vascular response during hypercapnia can now be done (Davis et al., 1998; Hoge et al., 1999). This would be the next step in investigating the pathophysiologic underpinnings of the BOLD and CBF difference demonstrated by our data. Further development of these techniques may aid in preventative treatment trials for Alzheimer's disease.
Acknowledgement
This research was funded by a National Institute on Aging grant: k23 AG024062.
Footnotes
Disclosure statement
No authors of this manuscript have any actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations within 3 years of beginning the work submitted that could inappropriately influence (bias) their work. The author's institutions do not have contracts relating to this research through which it or any other organization may stand to gain financially now or in the future. Nor does any author have any other agreements of authors or their institutions that could be seen as involving a financial interest in this work. The study was conducted according to Good Clinical Practice, the Declaration of Helsinki and U.S. 21 CFR Part 50-Protection of Human Subjects, and Part 56-Institutional Review Boards. Written informed consent for the study was obtained from all of the participants before protocol-specific procedures were performed.
References
- Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer's disease by spin-labeled magnetic resonance imaging. Ann. Neurol. 2000;47(1):93–100. [PubMed] [Google Scholar]
- Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, Zeger SL, Bassett SS, Kusevic I, Cristinzio C, Yassa MA, Avramopoulos D, Yousem DM, Fallin MD. Familial risk for Alzheimer's disease alters fMRI activation patterns brain activation in offspring of AD cases corresponds to 10q linkage. Brain. 2006;129(Pt 5):1229–1239. doi: 10.1093/brain/awl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman GA, Levi CR, Schofield P, Wang Y, Lovett EC. Quantitative measurement of cerebral haemodynamics in early vascular dementia and Alzheimer's disease. J. Clin. Neurosci. 2006;13(5):563–568. doi: 10.1016/j.jocn.2005.04.017. Epub March 15, 2006. [DOI] [PubMed] [Google Scholar]
- Benarroch E. Neurovascular unit dysfunction: a vascular component of Alzheimer disease? Neurology. 2007;68(20):1730–1732. doi: 10.1212/01.wnl.0000264502.92649.ab. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. Differential BOLD brain response to verbal paired-associate learning by APOE geno-type in nondemented older adults: a functional MRI study. Neurobiol. Aging. 2004;25(S2):506. [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. FMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer's disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. N. Engl. J. Med. 2000;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GG, Eyler Zorrilla LT, Georgy B, Kindermann SS, Wong EC, Buxton RB. BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: differential relationship to global perfusion. J. Cereb. Blood Flow Metab. 2003;23(7):829–837. doi: 10.1097/01.WCB.0000071887.63724.B2. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23(Suppl 1):S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J. Neurosci. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31(3):680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann. NY Acad. Sci. 2004;1019:24–28. doi: 10.1196/annals.1297.005. [DOI] [PubMed] [Google Scholar]
- Corder EH, Lannfelt L, Bogdanovic N, Fratiglioni L, Mori H. The role of APOE polymorphisms in late-onset dementias. Cell Mol. Life Sci. 1998;54(9):928–934. doi: 10.1007/s000180050223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cupples LA, Farrer LA, Sadovnick AD, Relkin N, Whitehouse P, Green RC. Estimating risk curves for first-degree relatives of patients with Alzheimer's disease: the REVEAL study. Genet. Med. 2004;6(4):192–196. doi: 10.1097/01.gim.0000132679.92238.58. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc. Natl. Acad. Sci. U.S.A. 1998;95(4):1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. J. Consult. Clin. Psychol. 1988;56(1):123–130. doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65(3):404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog. Neurobiol. 2001;64(6):575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Houston WS, Eyler LT, Frye S, Jenkins C, Thal LJ, Bondi MW. Identification of Alzheimer disease risk by functional magnetic resonance imaging. Arch. Neurol. 2005;62(12):1881–1888. doi: 10.1001/archneur.62.12.1881. [DOI] [PubMed] [Google Scholar]
- Folin M, Baiguera S, Guidolin D, Di Liddo R, Grandi C, De Carlo E, Nussdorfer GG, Parnigotto PP. Apolipoprotein-E modulates the cytotoxic effect of beta-amyloid on rat brain endothelium in an isoform-dependent specific manner. Int. J. Mol. Med. 2006;17(5):821–826. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Ahlbom A, Viitanen M, Winblad B. Risk factors for late-onset Alzheimer's disease: a population-based, case-control study. Ann. Neurol. 1993;33(3):258–266. doi: 10.1002/ana.410330306. [DOI] [PubMed] [Google Scholar]
- Ghebremedhin E, Schultz C, Braak E, Braak H. High frequency of apolipoprotein E epsilon4 allele in young individuals with very mild Alzheimer's disease-related neurofibrillary changes. Exp. Neurol. 1998;153(1):152–155. doi: 10.1006/exnr.1998.6860. [DOI] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 2006;100(1):328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 2000;44(1):162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, Brown GG, Corey-Bloom J, Salmon DP, Thal LJ, Bondi MW. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol. Aging. 2006 doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn. Reson. Med. 1999;42(5):849–863. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 2004;5(5):347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Wise RG. BOLD functional MRI in disease and pharmacological studies: room for improvement? Magn. Reson. Imaging. 2007;25(6):978–988. doi: 10.1016/j.mri.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Ishii K, Sasaki M, Matsui M, Sakamoto S, Yamaji S, Hayashi N, Mori T, Kitagaki H, Hirono N, Mori E. A diagnostic method for suspected Alzheimer's disease using H(2)15O positron emission tomography perfusion Z score. Neuroradiology. 2000;42(11):787–794. doi: 10.1007/s002340000404. [DOI] [PubMed] [Google Scholar]
- Ishiwata A, Sakayori O, Minoshima S, Mizumura S, Kitamura S, Katayama Y. Preclinical evidence of Alzheimer changes in progressive mild cognitive impairment: a qualitative and quantitative SPECT study. Acta. Neurol. Scand. 2006;114(2):91–96. doi: 10.1111/j.1600-0404.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- Jinhu Xiong J-HGJLLPTF. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum. Brain Mapp. 1995;3(4):287–301. [Google Scholar]
- Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, Albert MS. Preclinical prediction of Alzheimer's disease using SPECT. Neurology. 1998;50(6):1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234(3):851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, Asthana S, Hermann BP, Sager MA. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J. Neurosci. 2006;26(22):6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. Boston Naming Test. Lea & Febiger; Philadelphia, PA: 1983. [Google Scholar]
- Liu TT, Wong EC. A signal processing model for arterial spin labeling functional MRI. Neuroimage. 2005;24(1):207–215. doi: 10.1016/j.neuroimage.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Lojkowska W, Ryglewicz D, Jedrzejczak T, Sienkiewicz-Jarosz H, Minc S, Jakubowska T, Kozlowicz-Gudzinska I. SPECT as a diagnostic test in the investigation of dementia. J. Neurol. Sci. 2002:203–204. doi: 10.1016/s0022-510x(02)00294-0. 215–219. [DOI] [PubMed] [Google Scholar]
- Luh WM, Wong EC, Bandettini PA, Ward BD, Hyde JS. Comparison of simultaneously measured perfusion and BOLD signal increases during brain activation with T(1)-based tissue identification. Magn. Reson. Med. 2000;44(1):137–143. doi: 10.1002/1522-2594(200007)44:1<137::aid-mrm20>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Marco S, Skaper SD. Amyloid beta-peptide1-42 alters tight junction protein distribution and expression in brain microvessel endothelial cells. Neurosci. Lett. 2006;401(3):219–224. doi: 10.1016/j.neulet.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, Sano M, Bieliauskas L, Geldmacher D, Clark C, Thal LJ. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale (ADAS) that broaden its scope. The Alzheimer's Disease Cooperative Study. Alzheimer Dis. Assoc. Dis. 1997;11(Suppl 2):13S–21S. [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch. Neurol. 1992;49(12):1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Obata T, Liu TT, Miller KL, Luh WM, Wong EC, Frank LR, Buxton RB. Discrepancies between BOLD and flow dynamics in primary and supplementary motor areas: application of the balloon model to the interpretation of BOLD transients. Neuroimage. 2004;21(1):144–153. doi: 10.1016/j.neuroimage.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E varepsilon 4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 2001;98(6):3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N. Engl. J. Med. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc. Natl. Acad. Sci. U.S.A. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. Epub December 19, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept. Mot. Skills. 1958;8:271–276. [Google Scholar]
- Restom K, Bangen KJ, Bondi MW, Perthen JE, Liu TT. Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. Neuroimage. 2007;37(2):430–439. doi: 10.1016/j.neuroimage.2007.05.024. Epub May 25, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restom K, Behzadi Y, Liu TT. Physiological noise reduction for arterial spin labeling functional MRI. Neuroimage. 2006;31(3):1104–1115. doi: 10.1016/j.neuroimage.2006.01.026. Epub March 13, 2006. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Habeck CG, Hilton J, Anderson KE, Flynn J, Park A, Stern Y. APOE related alterations in cerebral activation even at college age. J. Neurol. Neurosurg. Psychiatry. 2005;76(10):1440–1444. doi: 10.1136/jnnp.2004.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test Manual—Revised. Western Psychological Services; Los Angeles: 1982. [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison MJ. Altered brain activation in cognitively intact individuals at high risk for Alzheimer's disease. Neurology. 1999;53(7):1391–1396. doi: 10.1212/wnl.53.7.1391. [DOI] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison MJ. Women at risk for AD show increased parietal activation during a fluency task. Neurology. 2002;58(8):1197–1202. doi: 10.1212/wnl.58.8.1197. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003a;20(2):1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson B, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2003b;74(1):44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Cocchiarella AJ, Schacter DL, Rosen BR, Albert MS. Encoding novel face-name associations: a functional MRI study. Hum. Brain Mapp. 2001;14(3):129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Rylander KM, Pike GB. The effect of global cerebral vasodilation on focal activation hemodynamics. Neuroimage. 2006;30(3):726–734. doi: 10.1016/j.neuroimage.2005.10.038. Epub December 5, 2005. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Torgerson BM, Sager MA, Hermann BP, Asthana S, Johnson SC. Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer's disease: a cross-sectional study. BMC Med. 2006;4:1. doi: 10.1186/1741-7015-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uludag K, Dubowitz DJ, Yoder EJ, Restom K, Liu TT, Buxton RB. Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. Neuroimage. 2004;23(1):148–155. doi: 10.1016/j.neuroimage.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Varma AR, Adams W, Lloyd JJ, Carson KJ, Snowden JS, Testa HJ, Jackson A, Neary D. Diagnostic patterns of regional atrophy on MRI and regional cerebral blood flow change on SPECT in young onset patients with Alzheimer's disease, frontotemporal dementia and vascular dementia. Acta Neurol. Scand. 2002;105(4):261–269. doi: 10.1034/j.1600-0404.2002.1o148.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale (rev. ed.) The Pscyhological Corporation, Harcourt Brace Janvanovich Inc.; New York: 1981. [Google Scholar]
- Wechsler D. WMS-R Wechsler Memory Scale—Revised Manual. The Psychological Corporation, Harcourt Brace Jovanovich Inc.; New York: 1987. [Google Scholar]
- Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, Mamourian AC, Belloni DR, Rhodes CH, McAllister TW. Increased brain activation during working memory in cognitively intact adults with the APOE epsilon4 allele. Am. J. Psychiatry. 2006;163(9):1603–1610. doi: 10.1176/ajp.2006.163.9.1603. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn. Reson. Med. 1998;39(5):702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- Xu G, Antuono PG, Jones J, Xu Y, Wu G, Ward D, Li SJ. Perfusion fMRI detects deficits in regional CBF during memory-encoding tasks in MCI subjects. Neurology. 2007;69(17):1650–1656. doi: 10.1212/01.wnl.0000296941.06685.22. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28(4):202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Deane R, Sallstrom J, Chow N, Miano JM. Neurovascular pathways and Alzheimer amyloid beta-peptide. Brain Pathol. 2005;15(1):78–83. doi: 10.1111/j.1750-3639.2005.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]