Abstract
Activity-dependent modulation of NMDA receptors containing selective NR2 subunits has been implicated in plastic processes in developing and adult sensory cortex. Aiming to reveal differential sensitivity of NR2 subunits to sustained changes in sensory activity, we utilized four paradigms that blocked, reinstated, or initiated sensory visual activity. Laminar prevalence of NR2A- and NR2B-containing synapses in visual cortex of postnatal and adult ferrets was assessed using quantitative electron microscopy. Light-deprivation at all ages resulted in a downregulation of NR2A, while recovery from deprivation resulted in an upregulation. Furthermore, premature eyelid opening caused a precocious increase of NR2A. Thus, transitions between periods of dark and light rapidly and bidirectionally regulate NR2A, regardless of cortical layer or age. In contrast, NR2B regulation is layer- and age-dependent. Only in layer IV, NR2B prevalence displays a one-time decline about three weeks after the initiation of sensory activity upon normal or premature eyelid opening, or upon termination of dark-rearing. Incongruity in patterns of NR2A and NR2B modulation by activity is consistent with involvement of these subunits in two distinct, yet partially co-occurring processes: developmental plasticity with a critical period, and life-long plasticity that is established in early developmental ages.
Keywords: ferret, glutamate receptors, immuno electron microscopy, ocular dominance plasticity, NMDAR
Activity dependent modulation of NMDA-type glutamate receptors (NMDAR) has been proposed to underlie a wide range of cortical processes, including critical period plasticity of thalamocortical innervation, elicitation of LTP/LTD, metaplasticity in both developing and adult brains, and the development and maintenance of orientation selectivity (Kleinschmidt et al. 1987; Bear et al. 1990; Abraham and Bear 1996; Roberts et al. 1998; Bear and Rittenhouse 1999; Quinlan et al. 1999a; Quinlan et al. 1999b; Philpot et al. 2001; Ramoa et al. 2001; Rivadulla et al. 2001; Fagiolini et al. 2003; Sawtell et al. 2003; Cao et al. 2007; Philpot et al. 2007). Notable functional heterogeneity of NMDAR at different ages, brain structures, cortical layers, origin of terminals, and pre-, post- or extra-synaptic localization is attributed to the diversity of NR2A or NR2B subunit-containing receptors (Carmignoto and Vicini 1992; Yoshimura et al. 2003; Daw et al. 2004; Malenka and Bear 2004; Rao and Daw 2004; van Zundert et al. 2004; Bellone and Nicoll 2007; Corlew et al. 2007). Furthermore, activity dependent changes in relative levels of these two subunits has been the essence of theories on adaptive processes that change the modification threshold of cortical synapses (Bienenstock et al., 1982; Scheetz and Constantin-Paton, 1994; Bear, 2003; Smith et al., 2009). Therefore, how sensory activity modulates the NR2A- or NR2B-containing NMDAR at different levels of cortical circuitry is crucially relevant for understanding the roles of NMDAR in sensory cortex development and function.
Accumulating evidence has demonstrated that in postnatally developing visual cortex, the modulation of NMDAR by sensory deprivation is not uniform across distinct yet spatially overlapping circuits. For example, the thalamocortical input to layer IV is a site of robust NMDAR-mediated plasticity during a critical period in development (Daw 1994; Daw et al. 1995; Bear and Rittenhouse 1999; Rittenhouse et al. 1999; Frenkel and Bear 2004; Rittenhouse et al. 2006). This process is independent from another NMDAR-mediated plasticity between layer IV axons and layer II–III cells, which may precede, enable (Trachtenberg et al. 2000), and outlast (Daw et al. 1992) thalamocortical plasticity. Furthermore, plastic reorganization during monocular deprivation involves two molecularly distinct NMDAR-mediated processes; one mediating a rapid depression through the deprived eye and a subsequent one mediating a progressive potentiation through the non-deprived eye (Sawtell et al. 2003; Frenkel and Bear 2004; Smith et al., 2009). Interestingly, this ipsilateral potentiation, but not the contralateral depression, is also observed after unilateral inactivation of the retina in mature animals (Frenkel and Bear 2004). Therefore, at least two distinct NMDAR-mediated, activity-dependent processes may overlap during early development, and one of these may be maintained beyond developmental ages. The diversity of NMDAR-mediated processes suggests not only the presence of equally diverse NMDAR subtypes at different circuits, but also that different NMDAR subtypes may have different sensitivities to sustained changes in sensory activity.
Based on this rationale, the present study examines the effects of four sensory deprivation paradigms on the prevalence of NR2A- or NR2B-containing NMDAR in layers IV and II–III of postnatal ferret visual cortex. Quantitative immuno-electron microscopy is used in all experiments with four rearing paradigms that modulate activity: Dark-rearing, recovery from dark-rearing, adult light-deprivation, and early eyelid opening. Dark-rearing suppresses all sensory-evoked activity as well as presumably induces a delay in the critical period and the factors associated with it (Cynader and Mitchell, 1980; Daw, 1994). Monitoring the time-course of recovery after dark-rearing allows for the differentiation of a developmental delay versus an acute suppression of responsiveness to sensory stimulus. Light-deprivation distinguishes between exclusively developmental processes and those that persist for life. In contrast to deprivation paradigms, early eyelid opening reveals the inductive influences of sensory activity. Our data reveal the fundamentally different ways that NR2A and NR2B subunits are modulated by sensory activity, and provide support to the notion that developmental and life-long plasticities are two independent mechanisms that transiently overlap in early postnatal ages.
Methods
Animals
A total of 38 ferrets (Marshall Farms, PA) were used for this study. The use of the ferret as a model is advantageous as there is approximately 32 days between birth and eye opening. This allows for a longer time period in which activity may be modulated than other species previously examined (e.g. cat). All procedures were conducted in accordance with National Institutes of Health guidelines for the care and use of laboratory animals and were approved by University of Virginia Animal Care and Use Committee. Sixteen of the animals were used as age-matched controls for the early eye opening, dark rearing, dark rearing with recovery, and adult light-deprivation paradigms. Unless the animals were dark reared, they were kept on a 16:8 light-dark cycle. Figure 1 illustrates the ages of ferrets at each experimental manipulation at the time of perfusion, as described below.
Figure 1.
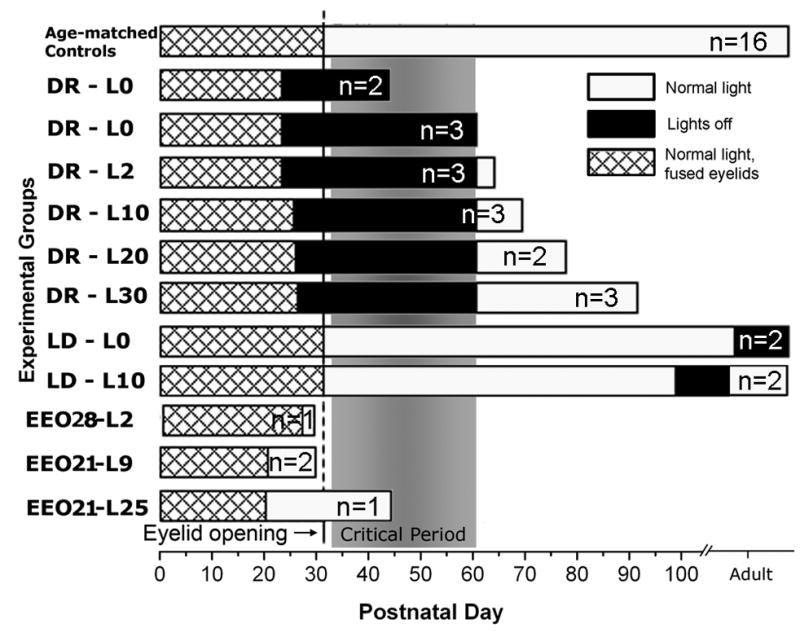
Rearing conditions for ferrets in dark-rearing, recovery after dark-rearing, light deprivation and early eyelid opening paradigms. Each bar represents a rearing condition. Number of animals at each rearing condition is marked within each bar. The end point of bars denote the age at perfusion, except for age-matched controls, which consisted 16 ferrets that were perfused at P30, P43–46, P55–65 or older than P90. Two additional early eyelid opening littermate controls are not depicted here. L0–L30 suffixes denote the number of days of normal light exposure after dark-rearing (DR), light deprivation (LD) or early eyelid opening (EEO). Hatched area is the duration of normal rearing while eyelids are closed. Black area is the duration of dark-rearing. White area is the duration of normal (16/8hrs) light exposure before the perfusion. Normal eye opening occurs at P32 (vertical line). Critical period of layer IV plasticity, as described in earlier studies (Issa et al. 1999) is marked with gray shading. All dark-rearing/recovery animals came from three litters. Except for littermate controls for EEO cases and some adult controls that mothered the experimental litters, genetic relations of normal controls to experimental animals are not available.
Activity manipulation paradigms
Dark-Rearing/Recovery/Light Deprivation
Pregnant jills were received in the facilities more than two weeks prior to their due date, and they were kept in a room equipped with a revolving dark-room door to ensure light-tightness. When the kits reached postnatal day 20 (P20) or P24, the lights were switched to 24 hours off, leaving the room in complete darkness. The integrity of the light proofing in the room was controlled by monitoring the exposure of a photographic film periodically. Daily cleaning and feeding visits were done using night vision goggles (Dipol; Vitebsk-GSP, Belarus). When the kits reached P60–P62, the light setting was restored to 16:8 day/light cycle. Recovery periods after dark-rearing were 2, 10, 20, 30 or 40 days, after which the animals were perfused. Light-deprivation was imposed by placing adult animals in the dark-rearing room for designated durations.
Early Eye Opening
The kits at P21 or P28 were anesthetized with Ketamine (3.0 mg/kg) and Xylazine (1.0 mg/kg), and the area around the eyes was cleaned with betadine, and covered with sterile drapes. The eyelids were parted using sterile forceps, surgical blades and scissors. The wound margins were cleaned and treated with antibiotic ointments before the animals were revived and returned to cage. The eyelids were checked daily, and any effusion was cleaned with warm saline to prevent re-fusing of the eyelids, and to ensure light exposure through open eyelids.
Perfusion and Immunocytochemistry
All animals at designated ages (Fig 1) were given an overdose of Nembutal (100mg/kg) and monitored until withdrawal and corneal reflexes were completely diminished. Then they were intra-aortically perfused with 50–100 mls of Tyrode’s solution (137mM NaCl, 5.5mM Dextrose/Glucose, 1.2mM MgCl2, 2mM KCl, 0.4mM NaH2PO4, 0.9mM CaCl2, 11.9mM NaHCO3, in 1L dH20) at room temperature containing 0.5% heparine for 2 minutes, followed by 4% paraformaldehyde with 0.05% glutaraldehyde in phosphate buffer (PB; ph 7.4) at room temperature for 15–30 minutes. The brains were removed and post-fixed in 4% paraformaldehyde for 24–48 hours at 4°C. A vibratome (OTX-5000, Electron Microscopy Sciences; Hatfield, PA) was used to section the brains coronally at 60μm. The sections were immediately treated with 1% sodium borohydrate in PB to terminate cross-linking function of infused fixatives. Immunocytochemistry procedure was started in less than 2 days after perfusion. Remaining brain sections were stored in 0.05% NaN3/Phosphate Buffer Saline (PBS) at 4°C to be used in future experiments.
Sections of the primary visual cortex were incubated in a 10μg/ml concentration of polyclonal anti-NR2A (Upstate; Cat. #06-313) or in a 5μg/ml polyclonal anti-NR2B (Upstate; Cat. #06-600) in 1% albumin bovine serum (BSA)/PBS with 0.05% NaN3 for 2–3 days at room temperature. Anti-NR2A was prepared in a rabbit antiserum against the last 200 amino acids of the C-terminus of mouse NR2A. Anti-NR2B was prepared in a rabbit antiserum against the 1437–1456 amino acid region from the mouse NR2B. Both antibodies have displayed reactivity on for the subunit specified using western blotting. It should be noted that the western blot characterization of the anibodies were performed using rodent tissue by the manufacturer, and thus it does not eliminate possibility of non-specific binding on ferret tissue. However, the differences in staining patterns by each antibody, and differences non-linear changes in staining patterns in developing animals, as described in results, suggest no, or negligible, cross reaction between two antibodies. The sections were then rinsed and transferred into biotinylated anti-rabbit (1:100; Vector Laboratories; Burlingame, CA) in PBS for 2 hours. Then the tissue was incubated in a 1:100 avidin biotin complex/PBS solution for 2 hours. Visualization of the antibodies was achieved by a diaminobenzidine reaction (0.05% in PBS with 0.001% H2O2) for 5–7 minutes. Sections were then processed for electron microscopy.
Embedding for Electron Microscopy
Immunolabeled sections were embedded in plastic resin following routine procedures (Chan et al. 1990; Erisir and Harris 2003). Briefly, sections were post-fixed in a 1% OsO4/PB solution for one hour. Following several rinses in PB, sections were sequentially dehydrated in a 50% for one minute, 70% with 4% Uranyl Acetate for two hours, and 90% and 100% ethanol for 5 minutes each followed by EM grade acetone (Electron Microscopy Sciences; Hatfield, PA) three times for 2 minutes each. The tissue was then incubated in a 1:1 Acetone-resin (EmBed812, Electron Microscopy Sciences; Hatfield, PA) solution followed by pure resin, 2 hours each. Sections were then flat embedded between two pieces of clear acetate (Aclar, Ted Pella; Redding, CA) and the resin was polymerized at 60°C for two days. Dorsal portions of occipital pole sections were excised and repolimerized at the bottom of Beem capsules filled with resin. Then, capsule embedded sections were trimmed to a trapezoid shape that encompassed all 6 cortical layers, pial surface and a portion of the white matter underlying the cortex, and then were then cut on an ultramicrotome (Ultracut UCT, Leica; Bannockburn, Il) at 70nm thickness.
Immunolabeling on thick vibratome sections is generally confined to 5–10 μm from the surface. In order to sample from a uniformly stained tissue region, the trapezoids are tilted so that initial en face ultrathin sections produce a thin strip of tissue along the entire cortical length. Subsequent ultrathin sections advances the leading edge of the tissue, which always contains the surface of the vibratome sections; regions farther away from this leading edge come from progressively deeper portions of the vibratome section. This approach maximizes the area of tissue re-cut from the surface of the vibratome sections, and allows reliable detection of laminar borders, as described below. In ultrathin sections such prepared, it is possible to obtain uniform labeling intensity within a strip that is 100–200 μm wide from the leading edge of the tissue. Ultrathin sections were placed on copper grids for examination on a JEOL 1010 transmission electron microscope (Peabody, MA).
Analysis
In order to sample areas with both ideal ultrastructural preservation and antibody penetration, the tissue within the trapezoid was examined outside-in from the pial surface, along the tissue-resin interface. The border between layers 1 and 2 was determined by the transition from the cell-sparse structure of layer 1 into cell-rich layer 2. Areas sampled 100–400 μm ventral to the layer 1–2 border were deemed to be located in layer II–III. For layer IV, the tissue was examined inside-out from white matter. Once large pyramidal cells of layer 5 were detected, their apical dendrites were followed into the region where smaller, round cell bodies became prevalent. We are content that this criterion is reliable for identifying layer IV because, when we applied the same criteria in previous studies using tissue stained with a specific marker for layer IV terminals, we have always found labeled thalamocortical terminals (Nahmani and Erisir 2005). This region also typically corresponded to the midpoint between the pial surface and the transition between myelinated axons of white matter and layer 6. In Nissl stained near-adjacent sections, layer IV identified by characteristic cell packing also typically found about the midpoint of the cortical depth. Once the lamina to be sampled was determined, the tissue that was within no more than 100 μm from the tissue-resin border was photographed in marginally overlapping frames with a 16 megapixel CCD camera (SIA; Duluth, GA) at 8000x magnification. The images were then viewed at a final magnification of 60–100k, every synapse in the image was located, and the synapse length along the parallel membranes and the total area of the image excluding cell bodies, capillaries and myelinated axons were measured using Image Pro Plus (MediaCy; Bethesda, MD). Only synapses in which both the synaptic cleft and postsynaptic density were clearly discernable were included in this analysis.
The volumetric densities of labeled and total synapses that appeared within the photographed areas were calculated using previously published quantification approaches (Colonnier and Beaulieu 1985; DeFelipe et al. 1999; Erisir and Harris 2003). Volumetric density was calculated by dividing the number of synapses encountered in a unit area by the average synapse length of those synapses. This calculation yields the density of synapses per unit cube, as synaptic zones are circular by assumption. Thus, the average length of the synapses counted yields the average depth of tissue examined. Then the proportion of NMDAR labeled synapses out of total cortical synapses was computed by dividing the volumetric density of labeled synapses by the volumetric density of all synapses within the same area. This measure is referred as normalized volumetric density of labeled synapses throughout the text. A single volumetric density measurement obtained from photographs representing 250–350μm2 of immunostained neuropil constituted an observation. We aimed to obtain at least 3 observations obtained at equal brain areas (250–350μm2) for each experimental condition. We based the size of the area for observations on recommendations outlined in deFelipe et al, 1999. However, the actual area studied for each case was measured post factum (that is, by measuring the area represented in the photographs and subtracting the area occupied by perikarya, myelinated axons, and capillaries), and if the photographs yielded an area larger than 350μm2, we arbitrarily parsed the data. This resulted in obtaining more than one observation per brain for some cases. Since all analysis of variance analysis comparing within brain and between brain observations revealed no statistical differences (all ANOVA comparisons p>0.5), all observations obtained from the same case, we treated each observation as an independent measure.
The dataset for control conditions of NR2A or NR2B labeled brains at P34–P45, P55–65 and adult (older than P90) age groups are formed from the data collected for a previous study, and from additional brains analyzed for the present study. This enabled us to include an appropriate number of observations in each age group for statistical comparisons. As a consequence, the numerical values in this data set are not identical to those reported in the previous study. However, the new dataset displays the same trends. Specifically, the control dataset for NR2A revealed sequential volumetric density increases among P34–45, P55–65 and adult brains (35.6±2.5, 44.1±4.2 and 56.6±4.1, respectively); each age group was significantly different than the preceding age group (Dunnett T3, p<0.01 for both comparisons). For NR2B staining, normalized volumetric density of labeled synapses at P35–45, P55–65, and in ferrets older than P90 were 53.1±3.4, 28.5±4.6 and 24.5±5.5 (means±SD) respectively. This reduction in density between the critical period and the older age groups were statistically significant (ANOVA, Dunnett T3, p<0.0001 for both comparisons), and agreed with the findings of the previous paper (Erisir and Harris, 2003).
Statistics
The statistic program SPSS (Chicago, Il) was used for all comparisons. Normalized volumetric density of labeled synapses found in experimental brains was compared to that in control animals that are grouped in ages representing the peak and the end of the critical period for layer IV plasticity, and the adult ages. Descriptive statistics, one-way ANOVA, test of homogeneity of variances (Levene statistic) and post hoc pair-wise comparisons were performed for all datasets. Significance values were obtained with Bonferroni and Dunnett t (2-sided) or Dunnett T3 tests. The maximum p value for significance was set as 0.01.
Methodological Concerns
We chose to use a DAB technique in rendering antibodies into electron dense particles because of its high sensitivity (or low threshold) to tag antibody-antigen complexes when compared to other visualization techniques available, such as silver enhancement of ultra-small colloidal gold tagged secondary antibodies or postembedding visualization with colloidal gold (Baschong and Stierhof, 1998). This is particularly important when measuring a ratio of labeled synapses to all synapses, because high sensitivity for antibody-antigen complexes increases the signal to background ratio, and reduces the possibility that labeling intensity falls below detection threshold at experimental conditions at which receptor proteins are downregulated. On the other hand, DAB is a water-soluble chromagen, which can diffuse from its main accumulation site. This renders estimations for number of antigen binding sites represented by individual DAB accumulation sites unfeasible. Therefore we refrain from indicating the amount of receptor proteins at individual synapses or whether the receptor protein is located at the postsynaptic or immediate extrasynaptic zones, However, despite its water solubility, the presence of DAB is unambiguous, and the localization of DAB accumulation sites are very specific. At no instance did we observe a leakage of DAB label across profile membranes, even into the synaptic cleft.
Results
Immunocytochemistry with NR2A and NR2B antibodies yielded staining that was aggregated at postsynaptic densities in all cortical layers (Fig 2A–B). Labeling was also present in cytoplasmic compartments of dendrites, somata and axons. There were no discernible qualitative differences in cellular compartments in which NR2A or NR2B labeling were observed.
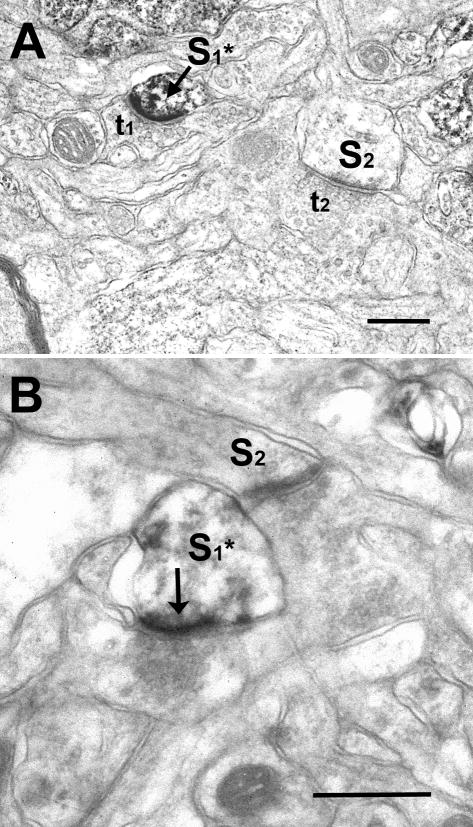
Figure 2.
Examples of cortical synapses that are immuno-stained for NR2A (A) or NR2B (B) subunits. Dense DAB accumulation is often encountered over postsynaptic densities (arrows) of spines (S1* in A and B) that receive synaptic contacts from vesicle filled profiles (t1 in A). Chromagen diffusion into the spine cytoplasm is encountered at varying degrees. DAB label is easily differentiable from electron dense appearance of unlabeled synapses (S2 in A and B). No qualitative differences are noted for NR2A or NR2B staining. Scale bar= 250nm.
In quantifying the amounts of NMDARs, we used a measure of receptor labeled synapse density relative to overall synapse density, rather than simply the density of labeled synapses. This choice is based on the unique properties of synaptogenesis during cortical development. In normally reared animals, synapse density in cortex precipitously increases after eye opening at P32 (Erisir and Harris, 2003), and these newly added glutamatergic synapses comprise additional sites for NMDARs. Furthermore, changes in rates of synaptogenesis may occur following dark-rearing and subsequent light exposure (Albanese et al. 1983; Gabbott and Somogyi 1986). Therefore, a measure of NR2 prevalence must account for variations in the numbers of synapses across developmental ages. Accordingly, normalized receptor density measurements were employed to allow for the interpretation of changes in receptor levels within a framework of concurrent developmental change.
Dark Rearing
In order to reveal the effects of complete light deprivation on NMDA receptor subunit composition, newborn kits were housed in continuous darkness from 10 days before natural eye opening until 20 or 35 days later, at which point animals were perfused without any subsequent light exposure. The ages of perfusion correspond to the peak (P45) and the decline (P55–60) of the critical period for plasticity in layer IV (Issa et al. 1999). Dark rearing affected both NR2A and NR2B prevalence in layer IV of ferret visual cortex. However, the age at which these effects became evident differed for the two subunits.
NR2A
In normally-reared animals, NR2A density precipitously increases after eye opening at P32 (Erisir and Harris 2003). Yet, in ferrets reared in darkness until P45 or P60, NR2A-positive synapse density in layer IV was significantly lower than age-matched controls (Table 1: comparisons a and b1; Figure 3A). In layer II–III, NR2A was modulated similarly to that in layer IV. That is, at P60 with dark-rearing, NR2A levels were significantly lower than age matched controls (Table 1: comparison f1; Figure 3B). This suggests that a lack of normal sensory activity either downregulates synaptic NR2A or hinders its developmental increase at both the peak and the end for critical period plasticity. This effect is not layer specific.
Table 1.
Descriptive statistics (mean ± standard deviation) and pairwise posthoc comparisons (Bonferrani and Dunnett T3; minimum significance level is set to 0.01) of NR2A and NR2B labeled synapse density in layers IV and II–III of control (normal light experience), dark reared (DR-L0), recovery (DR-L2 and longer periods of light exposure), light deprivation (LD) and early eyelid opening (EO) experiments. The conventions for variable names: Pxx or/Pxx=postnatal age at perfusion; Lxx=days of light exposure after sensory deprivation; EO-Pxx= age at eyelid opening surgery. Lowercase letters in the statistical significance column designate the post hoc comparisons to the age-matched control that bears the same uppercase letter; these are followed by the significance levels (p) in parenthesis (ns= not significant). Number of regions examined may include multiple areas from the same brain and are reported in parenthesis next to the values.
| Condition/Age at perfusion | Normalized volumetric density of labeled synapses ± SD (n) | Pairwise comparison: X vs. x (p) | Normalized volumetric density of labeled synapses ± SD (n) | Pairwise comparison X vs. x (p): |
|---|---|---|---|---|
| NR2A | NR2B | |||
| Normal Experience | ||||
| Layer IV | ||||
| Control/P43–P46 | 35.6 ± 2.5 (4) | A | 53.1 ± 3.4 (5) | H |
| Control/P55–P65 | 44.1 ± 4.2 (5) | B | 28.5 ± 4.6 (5) | I; h1(<0.0001) |
| Control/Adult (>P90) | 56.6 ± 4.1 (7) | C | 24.4 ± 5.5 (6) | J; h2 (<0001), i1 (ns) |
| Layer II–III | ||||
| Control/P34 | 20.9 ±6.3 (3) | D | 58.9 ±6.7 (3) | K |
| Control/P43–P46 | 27.4 ±3.9 (3) | E | 57.1±6.4 (4) | L |
| Control/P55–P65 | 35.8 ±4.3 (3) | F | 56.8±8.9 (4) | M |
| Control/Adult | 53.1 ±7.1 (4) | G | 56.1 ±8.5 (5) | N |
| Dark-Rearing | ||||
| Layer IV | ||||
| DR-L0/P45 | 23.5 ± 2.6 (3) | a (<0.01) | 44.0 ± 11 (3) | h3 (ns) |
| DR-L0/P60 | 29.2 ± 4.3 (4) | b1 (<0.0001) | 52.3 ± 7.8 (4) | i2 (<0.0001) |
| DR-L2/P62 | 42.2 ± 1.7 (4) | b2 (ns) | 50.2 ± 0.4 (5) | i3 (<0.0001) |
| DR-L10/P70 | 51.5 ± 1.1 (4) | b3 (ns) | 53.5 ±2.6 (6) | i4 (<0.0001) |
| DR-L20/P80 | - | - | 39.7 ± 8.5 (3) | i5 (ns) |
| DR-L30-40/P90-100 | 51.4 ± 5.0 (4) | c1, b4 (ns) | 29.9 ± 7.3 (4) | i6, j1 (ns) |
| Layer II-III | ||||
| DR-L0/P60 | 23.5 ± 2.8 (3) | f1 (<0.01) | 53.4 ± 5.2 (4) | m1 (ns) |
| DR-L2/P62 | - | - | 50.1±3.5 (3) | m2 (ns) |
| DR-L10/P70 | 47.8 ± 7.0 (3) | f2, g (ns) | - | - |
| DR-L30-40/P90-100 | - | - | 53.3±3.6 (2) | m3, n1 (ns) |
| Light Deprivation | ||||
| Layer IV: | ||||
| LD-L0/Adult | 31.6 ± 6.5 (4) | c2 (<0.005) | 29.9 ± 5.1 (5) | j2 (ns) |
| LD-L10-20/Adult | 50.0 ± 8.2 (3) | c3 (ns) | 32.2 ± 1.2 (3) | j3 (ns) |
| Layer II–III: | ||||
| LD-L0/Adult | 29.7 ± 7.5 (5) | g (<0.005) | 51.1 ± 5.3 (6) | n2 (ns) |
| LD-L12/Adult | 51.4 ± 2.3 (3) | g (ns) | 54.3 ± 7.0 (3) | n3 (ns) |
| Early eyelid opening | ||||
| Layer IV: | ||||
| Littermate control/P30 | 22.0 ± 1.4 (3) | O | 51.20 ± 6.5 (3) | S |
| Littermate control/P46 | 34.7 ± 5.1 (3) | P, a (ns) | 60.0 ± 3.4 (3) | T; h (ns) |
| EEO-L9/P30 | 39.4 ± 2.6 (6) | o1 (<0.0001) | 57.6 ± 5.4 (6) | s1 (ns) |
| EEO-L2/P30 | 38.8 ± 9.3 (6) | o2 (<0.0001) | 59.9 ± 3.0 (3) | s2 (ns) |
| EEO-L25/P46 | 40.8 ± 3.2 (3) | p (ns) | 27.4 ± 6.0 (6) | t (<0.0001) |
| Layer II/III: | ||||
| Littermate control/P30 | 21.7±2.8 (3) | Q; d (ns) | 52.2±6.4 (3) | U; k(ns) |
| Littermate control/p46 | 27.5±3.3 (3) | R; e (ns) | 49.5 ± 4.6 (4) | V; l(ns) |
| EEO-L9/P30 | 38.7±5.5 (6) | q (<0.005) | 49.6±4.1 (4) | u (ns) |
| EEO-L2/P30 | 33.2±8.7 (3) | q (<0.005) | - | |
| EEO-L25/P46 | 31.0±2.2 (3) | r (ns) | 50.8 ± 9.7 (5) | v (ns) |
Figure 3.
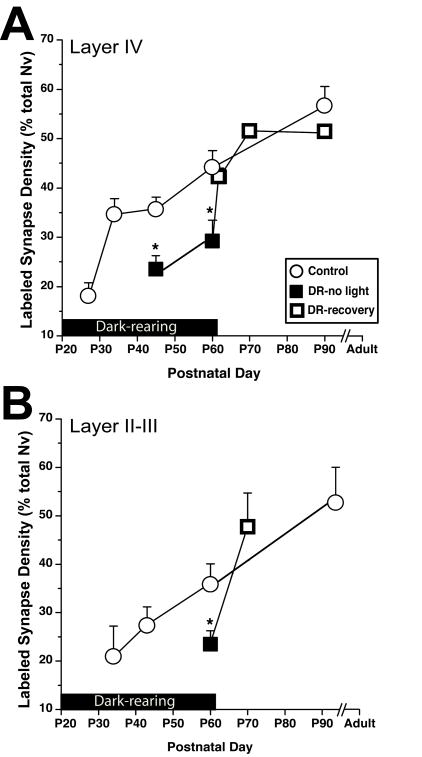
Sensory activity acutely modulates synaptic NR2A subunits during development. Prevalence of labeled synapses (Labeled synapse density as a percent ratio of total synapse volumetric density) is plotted for postnatal ages from before natural eye opening through adult ages. The black bar above x-axis marks the duration that the lights were off. This applies to both dark-reared animals (DR-no light; black squares) and the animals that were exposed to normal light cycle after dark-rearing (DR-recovery; open squares).
A) Compared to age-matched controls (open circles), dark-rearing both until P45 and P60 led to a smaller ratio of NR2A-containing synapses in layer IV. Two days of light exposure after dark-rearing was sufficient to return NR2A levels to normal. **=p<0.001
B) In layer II–III of dark-reared animals, the ratio of NR2A-containing synapses was significantly lower than control animals at P60. **=p<0.001
NR2B
On the other hand, when compared with controls, the pattern of layer IV NR2B subunit development in dark-reared P45 animals did not show any change (Table 1: comparison h3). However, at P60, the age at which layer IV NR2B levels decline in control animals, dark reared animals continued to display significantly higher levels of this subunit (Table 1: comparison i2; Figure 4A). Thus, elimination of sensory input through open eyelids leads to higher than normal levels of synaptic NR2B only at critical period closure. The lack of any change in NR2B at P45 suggests that the effect of dark-rearing on this subunit is not an upregulation, but instead is a blockade of the developmental reduction of NR2B seen in normally reared animals at P60. Dark-rearing until P60 did not have an effect in NR2B containing synapse ratio in supragranular layers (Table 1: comparison m1; Figure 4B). Thus, the effect of dark rearing on NR2B is layer specific.
Figure 4.
Sensory deprivation delays maturation of synaptic NR2B subunits in visual cortex, and this effect is lamina-specific. Conventions of the graph are as in Figure 3.
A) About 50% reduction that is observed in layer IV of control brains (open circles) by P60 was delayed more than 10 days after termination of dark-rearing. *= p<0.0001.
B) In layer II–III, where the ratio of NR2B-containing synapses sustains a uniform level through all ages, deprivation did not lead to any change.
Recovery From Dark-Rearing
As well as presumably affecting a plethora of activity dependent cortical processes (Fox et al. 1992; Chapman and Stryker 1993; Fagiolini et al. 1994; White et al. 2001; Li et al. 2006), dark-rearing prolongs cortical sensitivity to monocular deprivation, thus delaying the end of the critical period for developmental plasticity (Cynader and Mitchell 1980; Timney et al. 1980; Mower and Christen 1985; Mower 1991). Based on the assumption that factors related to the duration and/or end of the critical period were delayed by dark rearing, the regulation of NR2 subunits after normal sensory activity following dark rearing was examined next.
NR2A
In layer IV, two days of light exposure following dark rearing until P60, increased synaptic NR2A labeling to control levels (Figure 3A; Table 1: comparison b2). Moreover, two days of recovery appeared sufficient because, compared to control animals longer light exposures did not result in a significant increase NR2A labeling beyond that of 2 days (Table 1: comparisons b3, b4 and c1). Similar effects were observed in layer II–III. In P70 animals that were exposed to ten days of normal light after dark-rearing, NR2A levels were restored to levels observed in P60 and P90 normal controls (Figure 3B; Table 1, comparisons f2 and g). This demonstrates that, similar to in rodents (Quinlan et al. 1999a), the suppression of developmental upregulation (or downregulation, in absolute terms) of synaptic NR2A due to lack of activity during dark-rearing rapidly recovers after restoration of sensory activity.
NR2B
In contrast to NR2A, NR2B levels remained higher than age-matched controls despite 2 or 10 days of restored sensory activity following dark-rearing until P60 (Table 1: comparisons i3 and i4; Figure 4A). Yet, 20 days or more light exposure following dark-rearing was sufficient to return NR2B expression to age-appropriate control levels (Table 1: comparisons i5, i6 and j1). Therefore, dark-rearing delays the developmental reduction of layer IV synaptic NR2B that is observed in normal P60 brains.
In contrast to layer IV, layer II–III of control animals does not display any significant NR2B changes at the postnatal ages examined (Erisir and Harris, 2003). Accordingly, if the effect of light recovery after dark rearing were to reinstitute an age-appropriate expression pattern, rather than to cause a global, late-occurring downregulation, no changes in layer II–III NR2B would be expected. Indeed, dark rearing and subsequent recovery did not lead to any changes in layer II–III NR2B levels. Animals that had no light experience, or those that had 2 or 30 days of light following dark rearing, were not different than age-matched controls (Figure 4B; Table 1: comparisons m2, m3, and n1).
Light-Deprivation in Adults
In order to test whether the activity-dependence of synaptic NR2 changes observed in neonatal animals represents the disruption of a developmental event or a life-long sensitivity to the absence of sensory activity, we examined the effects of light-deprivation on cortical NR2A and NR2B levels in adult animals. In animals housed in total darkness for 25 days as adults, synaptic NR2A in layer IV and II–III was reduced, suggesting that activity regulates the NR2A subunit both in neonatal and adult ages (Table 1: comparisons c2 and g2, respectively; Figure 5A). Moreover, similar to younger animals, 10–12 days of light-exposure following light-deprivation recovered NR2A expression to normal levels (Table 1: comparison c3 and g3). In contrast, synaptic NR2B levels in both layers IV and II–III in adult animals were unaffected by any light perturbation (Figure 5B; Table 1: comparisons j2, j3, n2 and n3).
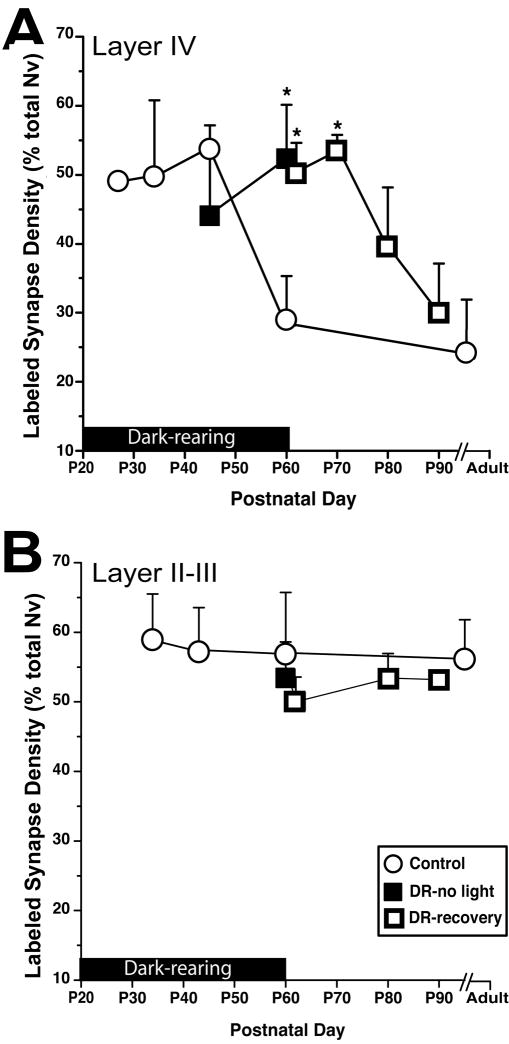
Figure 5.
Light deprivation at adult ages leads to acute modulation of NR2A subunits, and no discernable change in the prevalence of NR2B-containing synapses. A) Light deprivation for 10 days in adult animals led to a reduction in the ratio of NR2A-containing synapses both in layer II–III and in layer IV. NR2A density was restored to normal by subsequent normal stimulation for 10 days. *= p<0.005
B) Adult light deprivation or subsequent normal light cycle did not lead to any changes in NR2B ratios in layer II–III or in layer IV.
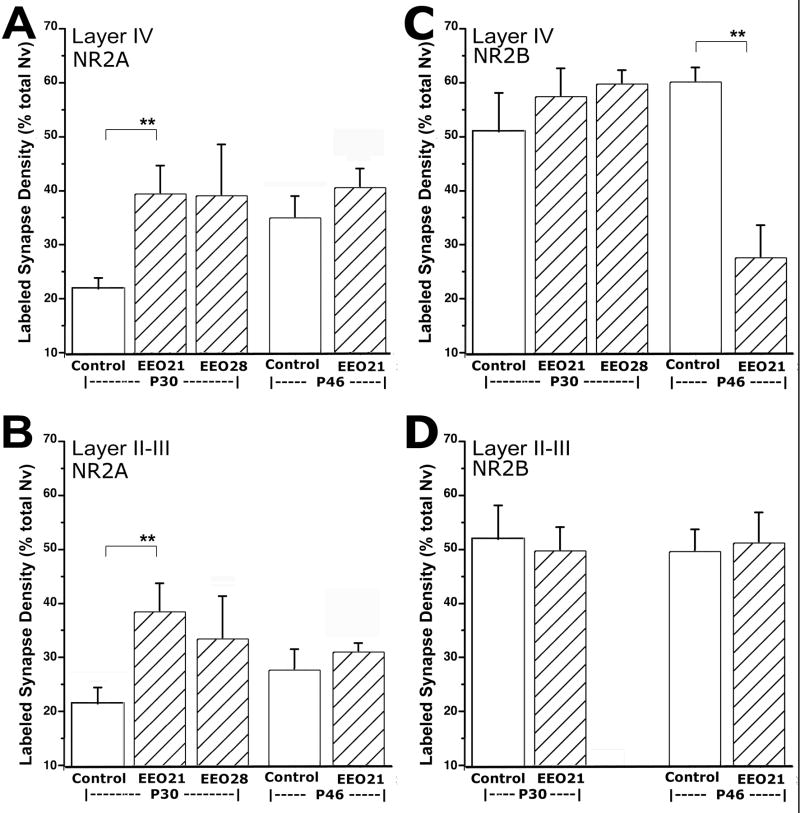
Early Eyelid Opening
Dark-rearing and light-deprivation paradigms remove the sensory stimulus, thus revealing the changes in NMDAR subunits due to activity suppression, rather than the actual effects of activity. In contrast, surgical opening of ferret eyelids prior to natural eye opening prematurely initiates sensory stimulation. In comparison to cats, primates, and rodents, the natural eye-opening in ferret occurs only days before the onset of critical period plasticity, making this species advantageous for testing the direct effects of sensory activity on the course of the critical period, as well as regulation of NMDAR subunits by sensory activity rather than sensory deprivation.
Four ferret littermates underwent early eyelid-opening (EEO) surgery at P21 (EEO21) or P28 (EEO28) (Fig 1). These animals were perfused at P30 (n=3) or P46 (n=1). Two littermates, one at each matching age were also perfused. The eyelids of the P46 control littermate opened naturally at P32. The eyelids of the P30 control littermate were still fused. Although no behavioral tests were performed, observations of animals that underwent early eyelid opening surgery indicated that they were using vision for simple orienting behaviors. Similar to the deprivation paradigms, synaptic NR2A and NR2B prevalence in EEO animals revealed that these subunits were differentially regulated by visual activity.
NR2A
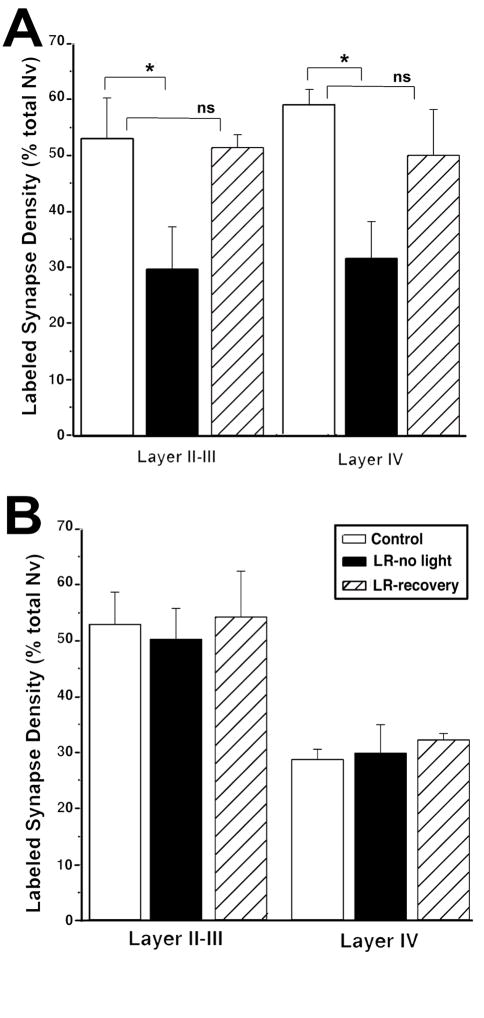
In layer IV of P30 animals that had experienced sensory activity through open eyelids for 2 days, there was a significant increase in the ratio of NR2A-containing synapses over age-matched littermates (Table 1: comparison o2; Figure 6A). Nine days of early sensory activity led to a similar statistically significant increase of NR2A-containing synapses in P30 animals compared to controls (Table 1: comparison o1). NR2A levels found at P30 after 9 or 2 days of premature vision were not statistically different (39.4 ± 2 vs. 38.9 ± 3, respectively). Furthermore, in one animal that received eyelid opening surgery at P21, about 10 days prior to normal eyelid opening, and perfused at P46, NR2A levels were different neither from a P46 littermate control (Table 1: comparison p), nor from a littermate that received eyelid opening surgery at P28 (Fig 6A). A similar effect was observed for layer II–III, in that two days of early visual activity was sufficient to cause an early upregulation of NR2A (Table 1: comparisons q1, q2, and r; Figure 6B). Together with the 9-day results, these results suggest that sensory activity induced an acute, and possibly saturating upregulation of NR2A that rapidly reached a plateau after initial light exposure. It should be noted that a plateau in our measure (normalized volumetric density) indicates successive increases in receptor containing synapses at a rate that is equal to the rate of increase in total synapse density in cortex. During the early developmental ages, glutamatergic synapse density continues to increase. Thus, our results indicate that the initial sensory activity coming through open eyelids serves as an inductive factor for NR2A. As the favorable conditions, (i.e., sensory activity) are maintained, newly formed synapses may maintain the same probability to utilize NR2A as the already existing ones.
Figure 6.
Premature sensory activity upregulates NR2A prevalence, and leads to a precocious downregulation of NR2B in layer IV.
A–B) In both layers II–III and IV, animals that received eyelid opening surgery at P21 (EEO21) or P28 (EEO28) displayed significantly higher NR2A-containing synapse ratios at P30, about two days before natural eyelid opening in littermates (Control). In another ferret that received eyelid opening surgery at P28, the NR2A-containing synapse ratio at P46 was not different than same age control littermate. *= p<0.0001; *= p<0.005.
C–D) Premature light exposure led to reduction of NR2B-containing synapse ratio in layer IV of animals that received eyelid opening surgery at P28 (EO P28) and survived until P46, but not in EO P21 or EO P28 animals that survived until P30. In layer II–III, no NR2B changes were detected at P30 or P46 between EEO and control littermates. **= p<0.0001
NR2B
In contrast to NR2A, premature visual activity differentially regulated the pattern of synaptic NR2B. After 2 or 9 days of premature vision, there were no differences in synaptic NR2B levels at P30 (Table 1: comparisons s1 and s2; Fig 6B). However by P46, the animal that had eyelid surgery at P21 (i.e. activity started 10 days prematurely and lasted 25 days) had significantly lower synaptic NR2B in layer IV (Table 1: comparison t; Figure 6C). Levels of NR2B under these circumstances were not different than those found in layer IV of control animals at P55–60 (28.7 ± 5%). A similarly precocious development of NR2B was not observed in layer II–III (Table 1: comparisons u and v; Figure 6D), where this subunit does not normally display a temporal downregulation. While the findings at P46 should be regarded tentative, these results suggest that unlike NR2A expression, premature sensory activation does not acutely regulate synaptic NR2B, but may rather lead to a precocious development of this subunit.
Discussion
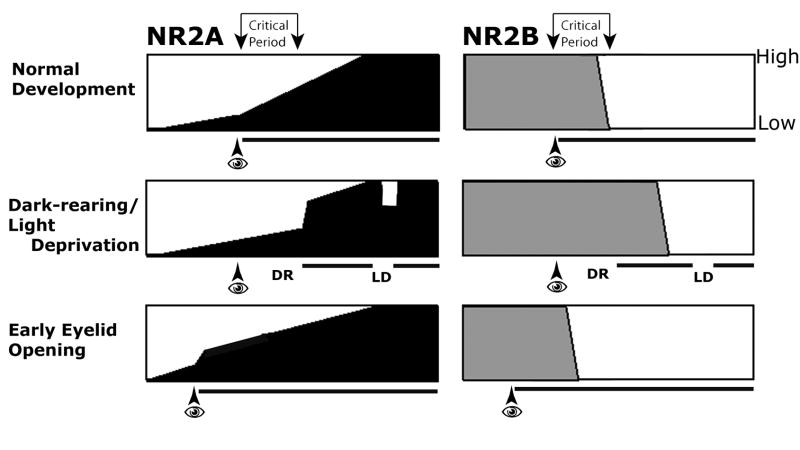
Using quantitative electron microscopy, we examined the effects of four complementary sensory activity manipulation paradigms on synaptic NR2A and NR2B localization in layer IV and layer II–III of the primary visual cortex (Figure 7). NR2A subunits displayed acute and bidirectional modulation: Dark-rearing in early developmental ages, and light deprivation in adulthood downregulated the synaptic expression of NR2A in both layers II–III and IV. In contrast, both restoration of normal light conditions after dark-rearing and premature eyelid opening rapidly upregulated NR2A subunits. The age-independence of NR2A response to sensory activity suggests that once the cortical circuitry containing this subunit is established, NR2A containing NMDAR may mediate a plastic process that takes place both during developmental ages and adulthood, and involves more than one hierarchical step of cortical glutamatergic circuitry.
Figure 7.
The normal development and the activity-dependent modulation of NR2A and NR2B subunits in layer IV of developing ferrets.
Row 1: In layer IV of ferret visual cortex, natural eye opening (eye symbol), which initiates patterned visual activity (black horizontal line), coincides with a surge of increase in prevalence of NR2A-containing synapses (darkly shaded areas in left panels) from low to high levels. In contrast, the prevalence NR2B-containing synapses (right panels) remains constant for the early post-eye opening ages, declining to low levels by P60.
Row 2: Dark-rearing (DR) until ages corresponding to the end of the critical period leads to a reduction in the prevalence of NR2A-containing synapses, which rapidly recovers to control levels by subsequent restoration of normal activity. A similar reduction occurs if the visual stimulation is removed in adulthood via light deprivation (LD). In contrast, the effect of visual deprivation and subsequent restoration of activity is a delay of NR2B downregulation that normally occurs concurrently with the decline of plasticity in layer IV.
Row 3. Early eyelid opening prematurely exposes animals to visual activity, and leads to an early upregulation of NR2A-containing synapses. This upregulation is a probably an early induction of NR2A in existing synapses, and does not push NR2A levels beyond normal in subsequent ages. Prematurely initiated vision may lead to an early downregulation of the ratio of NR2B containing synapses in layer IV. This effect is unique to layer IV, suggesting that early vision prematurely initiated a cascade of events that eventually leads to loss of NR2B from a unique population of synapses in this layer.
In contrast, manipulation of sensory experience modulates NR2B only during developmental ages, and only in layer IV. Specifically, dark-rearing until the end of the critical period led to higher amounts of synaptic NR2B compared to controls in layer IV. This change is not an upregulation, but it is a suppression of the normal developmental downregulation that occurs at the end of the critical period. Similarly, early sensory activity led to a premature downregulation of synaptic NR2B. Yet, at ages or cortical layers that do not display a normal developmental drop in this subunit, the manipulations of sensory activity did not affect NR2B levels. These results suggest that NR2A or NR2B subunit-containing NMDA-type glutamate receptors may have unique and independent roles in developmental and life-long cortical plasticity.
In this study, quantification with electron microscopy was optimized for sampling the ocurrence of NR2A and NR2B proteins that are located at the synapses, while excluding cytoplasmically located proteins that are in storage or in transport as well as presynaptic or extrasynaptic sites. As such, while this technique is effective in quantifying the prevalence of receptor containing synapses, it does not detect any protein synthesis induction, which can more appropriately be measured using brain homogenates. As such, we cannot directly compare our results to past studies that quantified NR2A or NR2B amounts in developing and deprived animals using western blotting to quantify total protein levels. However, several parallels can be drawn. The effects of manipulating sensory activity via dark-rearing, dark-exposure, monocular deprivation or light deprivation on NMDAR subunit composition have been previously studied using visual cortex homogenates, synaptosome or cell surface fractions from rat and mouse (Quinlan et al. 1999a; Philpot et al. 2001; He et al. 2006; Chen and Bear, 2007). Both dark-rearing in juvenile rats and light deprivation in adult rats decrease the NR2A/NR2B ratio in total cortex synaptosome preparations (Quinlan et al. 1999a; He et al. 2006). In these studies, the change in 2A/2B ratio is attributed to a decrease in NR2A in juvenile, but an increase in NR2B in adult deprivation. In mice, a significant increase in NR2B protein expression in cortex homogenates and surface fractions has also been demonstrated (Chen and Bear 2007). Our findings with NR2A did not reveal any laminar differences, and thus they are relatively more suitable for comparisons with synaptoneurosome measurements. Using this latter approach, Quinlan and colleagues demonstrated a decrease of NR2A via dark-rearing (Quinlan et al., 1999a, He et al., 2006), which is in agreement with our NR2A results. We also expand upon those findings by demonstrating laminar differences in the susceptibility of synaptic NR2B that is located in layer II–III or IV circuits in ferrets, and that layer-specific effects of altered sensory experience are age dependent for NR2B, but not for NR2A.
Early eyelid opening is a novel paradigm, which provided de novo patterned visual activity, to study activity dependence profiles of cortical NMDA receptors in the ferret. The ferret is an opportune species in that, unlike in the cat, the monkey and the rodent, natural eyelid opening is delayed until the end of first postnatal month, and is immediately followed by the critical period for thalamocortical plasticity (Hubel and Wiesel 1970; Hubel et al. 1977; Issa et al. 2000). Thus, it is tempting to hypothesize that the molecular machinery needed for visual activity-induced plasticity is in place well before natural eyelid opening, and that premature eye opening may lead to a precocious critical period. While this possibility awaits confirmation by independent means of measuring critical period duration and synaptic NR2B in the same animals, our observation of precocious NR2B subunit development in layer IV in early eye opening animals implies that retraction of NR2B from synapses may be a latent consequence of the first visual experience.
An important corollary to the postsynaptic localization of NMDA receptors quantified herein, is the heterogeneity of glutamatergic inputs to layers II–III and IV, which arise from different sources (Gilbert 1983; Wiesel and Gilbert 1983). As detailed in cat visual cortex (Binzegger et al. 2004; Douglas and Martin 2004), glutamatergic synapses in layer IV come from thalamocortical relay cells, spiny stellate cells of layer IV and pyramidal cells of layer VI. In layer II–III, glutamatergic synapses originate from pyramidal cells of layer VI, spiny stellate and pyramidal cells of layer IV, pyramidal cells of layer V, and intracortical long-range projections. While some unique features have been described for individual glutamatergic inputs, morphological criteria to identify the origins of different glutamatergic synapses within each layer are not available. Thus, we did not attempt to dissociate glutamatergic inputs that may have been differentially affected by sensory deprivation. Regardless, the specificity of NR2B modulation to layer IV, but not layer II–III neurons is a strong indicator that NMDA receptors with similar NR2 subunit compositions yet located at different glutamatergic input synapses may respond differentially to sensory deprivation.
Understanding the roles of different NR2 subunits distinct cortical circuits is also complicated by the fact that different glutamatergic inputs mature at different rates. Patterned visual activity that starts at birth in primates and at eyelid opening in carnivores coincide with a massive wave of synaptogenesis in cortex (Cragg 1975; Bourgeois and Rakic 1993; Erisir and Harris 2003; White and Fitzpatrick 2007). This wave of synapse formation that lasts about 2–3 months, depending on the species, constitutes a period during which long-range horizontal connections of layer II–III cells and axons of layer IV spiny stellate cells are elaborated (Callaway and Katz 1990; Luhmann et al. 1990; Callaway and Katz 1992; Burkhalter et al. 1993; Durack and Katz 1996), and orientation and direction selectivity maps are established (Chapman et al. 1996; White et al. 2001; Li et al. 2006). Presence of distinct glutamatergic circuits serving these different functions, and the temporal overlap of their development present challenges in understanding the contribution of NMDAR with distinct NR2 subunit compositions to cortical function.
Based on the assumption that sensory conditions that delay the end of the critical period also delay the development of factors associated with it (Daw 1994), we tested the hypothesis that the observed reduction in NR2B subunits from layer IV (Erisir and Harris 2003) is one such factor. Indeed, dark-rearing delayed, and premature light exposure hastened the reduction of NR2B containing synapse prevalence from layer IV, respectively. These findings suggest that NR2B plays a crucial role in the plasticity observed in layer IV during the critical period. It should be noted that not all NR2B is eliminated from layer IV, bearing the question that whether loss of this subunit occurs at a selective input. Thalamocortical synapses may be a possible site of this loss because, while many new intracortical glutamatergic synapses that conceivably contain NR2A and/or NR2B are added into cortical circuits throughout the weeks of critical period plasticity, dynamic changes occur in synapse frequency of thalamocortical axons, and their selectivity for postsynaptic cell types (Erisir and Dreusicke, 2005). Furthermore, the maturation of thalamocortical axons is marked by a weakening in their NMDAR-mediated responses (Hagihara et al. 1988; Fox et al. 1989). Thus, we propose the possibility that removal of NR2B containing NMDAR from thalamocortical synapses, and consequent anatomical stability at this input may contribute to the resistance to reorganization observed at the critical period end.
Accumulating evidence now suggests that visual cortex is a site of several distinct activity-dependent plastic processes during early developmental ages (Hensch et al. 1998; Rittenhouse et al. 1999; White et al. 2001; Desai 2003; Fagiolini et al. 2003; Frenkel and Bear 2004; Maffei et al. 2006; Li et al. 2006; Crozier et al. 2007; Mrsic-Flogel et al. 2007; Liu et al. 2004, and NR2A or NR2B containing NMDA-type receptors may selectively contribute to these processes. For example, while the development of orientation selectivity is dependent on the maturation and normal function of NR2A, but not NR2B containing circuitry (Fagiolini et al. 2003), plastic processes that are deemed to contribute to effects of monocular deprivation seem to require both NR2A and NR2B function (Heynen 2003; Bear 2003; Iny et al. 2006; Cao et al. 2007; Philpot et al. 2007). Similarly, NR2A and NR2B subunits of layer II–III act in concert to contribute to persistence of LTP/LTD induction and to mediation of metaplasticity in adulthood (Philpot et al. 2003; Jiang et al. 2007; Yashiro & Philpot, 2008). The fundamental differences in patterns of sensory activity dependence of NR2 subunits demonstrated in this study emphasize the diversity of NMDAR- mediated visual functions.
The results from this study indicate that NR2A and NR2B are independent and distinct in their susceptibility to sensory deprivation. The changes in NR2A are acute, bidirectional, and lifelong, while the changes in NR2B are restricted to a subpopulation of layer IV synapses, delayed, and they can only be induced during a critical period in development. Thus, changes in the NR2A subunit may underlie a lifelong, experience-dependent plasticity of cortical synapses, while changes in the NR2B subunit underlie a developmental, experience-dependent plasticity of only a specific population of layer IV synapses. This conclusion is consistent with findings that suggest a dissociation in mechanisms that underlie developmental and adult synaptic plasticity (Goodman and Shatz 1993; Rampon and Tsien 2000; Yasuda et al. 2003; Dumas 2005). It is also consistent with duality of distinct plasticities that are studied in visual cortex. In early development, the most striking feature of critical period plasticity, thalamocortical reorganization, seems to coexist with physiological, molecular and synaptic plasticity of various glutamatergic circuits in visual cortex. However, axonal reorganization is very limited in adulthood. It is possible that during the critical period for thalamocortical reorganization, the activity of NR2A containing synapses in layers II–III or IV serve for acute detection of perturbations in sensory activity, whereas the presence of NR2B at layer IV synapses may serve to render their presynaptic axons amenable to retracting or to sprouting. In adult ages, NR2A may continue to detect sensory perturbations in visual environment, eliciting physiological measures of synaptic plasticity, while circuit specific lack of NR2B-containing NMDAR prevents anatomical plasticity of thalamocortical axons.
Acknowledgments
This work was supported by Jeffress Memorial Funds #J-779, NIH EY12138, F31 NS059189 and Predoctoral Training in Neuroscience 2T32GM008328-16. We thank Bonnie Sheppard for excellent technical support. We thank Jason Coleman for critical reading of the manuscript.
Comprehensive Abbreviation List
- NR2A
NMDA receptor subunit 2A
- NR2B
NMDA receptor subunit 2B
- NMDA
N-methyl-D-aspartate
- NMDAR
N-methyl-D-aspartate receptor
- DAB
Diaminobeinzidine
- EEO
Early eyelid opening
- DR
Dark rearing
- LD
Light deprivation
- LTP
Long Term Potentiation
- LTD
Long Term Depression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19(4):126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Albanese A, Albanese E, Brusco A, Saavedra JP. A quantitative study of visual cortex synapses during the postnatal development of dark-reared rats. J Neurobiol. 1983;14(1):1–8. doi: 10.1002/neu.480140102. [DOI] [PubMed] [Google Scholar]
- Baschong W, Stierhof YD. Perparation, use, and enlargement of ultrasmall gold particles in immunoelectron microscopy. Microsc Res Tech. 1998;42(1):66–79. doi: 10.1002/(SICI)1097-0029(19980701)42:1<66::AID-JEMT8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358(1432):649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Kleinschmidt A, Gu QA, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci. 1990;10(3):909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Rittenhouse CD. Molecular basis for induction of ocular dominance plasticity. J Neurobiol. 1999;41(1):83–91. doi: 10.1002/(sici)1097-4695(199910)41:1<83::aid-neu11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55(5):779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2(1):32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzegger T, Douglas RJ, Martin KA. A quantitative map of the circuit of cat primary visual cortex. J Neurosci. 2004;24(39):8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13(7):2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter A, Bernardo KL, Charles V. Development of local circuits in human visual cortex. J Neurosci. 1993;13(5):1916–1931. doi: 10.1523/JNEUROSCI.13-05-01916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. Emergence and refinement of clustered horizontal connections in cat striate cortex. J Neurosci. 1990;10(4):1134–1153. doi: 10.1523/JNEUROSCI.10-04-01134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. Development of axonal arbors of layer 4 spiny neurons in cat striate cortex. J Neurosci. 1992;12(2):570–582. doi: 10.1523/JNEUROSCI.12-02-00570.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Liu L, Lickey M, Graves A, Pham T, Gordon B. Virally mediated knock-down of NR2 subunits ipsilateral to the deprived eye blocks ocular dominance plasticity. Exp Brain Res. 2007;177(1):64–77. doi: 10.1007/s00221-006-0647-8. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258(5084):1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990;33(2–3):113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Stryker MP. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J Neurosci. 1993;13(12):5251–5262. doi: 10.1523/JNEUROSCI.13-12-05251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Stryker MP, Bonhoeffer T. Development of orientation preference maps in ferret primary visual cortex. J Neurosci. 1996;16(20):6443–6453. doi: 10.1523/JNEUROSCI.16-20-06443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WS, Bear MF. Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology. 2007;52(1):200–214. doi: 10.1016/j.neuropharm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Colonnier M, Beaulieu C. An empirical assessment of stereological formulae applied to the counting of synaptic disks in the cerebral cortex. J Comp Neurol. 1985;231(2):175–179. doi: 10.1002/cne.902310205. [DOI] [PubMed] [Google Scholar]
- Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci. 2007;27(37):9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg BG. The development of synapses in kitten visual cortex during visual deprivation. Exp Neurol. 1975;46(3):445–451. doi: 10.1016/0014-4886(75)90118-1. [DOI] [PubMed] [Google Scholar]
- Crozier RA, Wang Y, Liu CH, Bear MF. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc Natl Acad Sci U S A. 2007;104(4):1383–1388. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M, Mitchell DE. Prolonged sensitivity to monocular deprivation in dark-reared cats. J Neurophysiol. 1980;43(4):1026–1040. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- Daw N, Rao Y, Wang XF, Fischer Q, Yang Y. LTP and LTD vary with layer in rodent visual cortex. Vision Res. 2004;44(28):3377–3380. doi: 10.1016/j.visres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Daw NW. Mechanisms of plasticity in the visual cortex. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1994;35(13):4168–4179. [PubMed] [Google Scholar]
- Daw NW, Fox K, Sato H, Czepita D. Critical period for monocular deprivation in the cat visual cortex. J Neurophysiol. 1992;67(1):197–202. doi: 10.1152/jn.1992.67.1.197. [DOI] [PubMed] [Google Scholar]
- Daw NW, Reid SN, Wang XF, Flavin HJ. Factors that are critical for plasticity in the visual cortex. Ciba Found Symp. 1995;193:258–276. doi: 10.1002/9780470514795.ch13. discussion: 258–276;discussion 322–324. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Marco P, Busturia I, Merchan-Perez A. Estimation of the number of synapses in the cerebral cortex: methodological considerations. Cereb Cortex. 1999;9(7):722–732. doi: 10.1093/cercor/9.7.722. [DOI] [PubMed] [Google Scholar]
- Desai NS. Homeostatic plasticity in the CNS: synaptic and intrinsic forms. J Physiol Paris. 2003;97(4–6):391–402. doi: 10.1016/j.jphysparis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Dumas TC. Developmental regulation of cognitive abilities: Modified composition of a molecular switch turns on associative learning. Prog Neurobiol. 2005;76(3):189–211. doi: 10.1016/j.pneurobio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Durack JC, Katz LC. Development of horizontal projections in layer 2/3 of ferret visual cortex. Cereb Cortex. 1996;6(2):178–183. doi: 10.1093/cercor/6.2.178. [DOI] [PubMed] [Google Scholar]
- Erisir A, Dreusicke M. Quantitative morphology and postsynaptic targets of thalamocortical axons in criticl period and adult ferret visual cortex. J Comp Neurol. 2005;485(1):11–31. doi: 10.1002/cne.20507. [DOI] [PubMed] [Google Scholar]
- Erisir A, Harris JL. Decline of the critical period of visual plasticity is concurrent with the reduction of NR2B subunit of the synaptic NMDA receptor in layer 4. J Neurosci. 2003;23(12):5208–5218. doi: 10.1523/JNEUROSCI.23-12-05208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, Hensch TK. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc Natl Acad Sci U S A. 2003;100(5):2854–2859. doi: 10.1073/pnas.0536089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34(6):709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Fox K, Daw N, Sato H, Czepita D. The effect of visual experience on development of NMDA receptor synaptic transmission in kitten visual cortex. J Neurosci. 1992;12(7):2672–2684. doi: 10.1523/JNEUROSCI.12-07-02672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Sato H, Daw N. The location and function of NMDA receptors in cat and kitten visual cortex. J Neurosci. 1989;9(7):2443–2454. doi: 10.1523/JNEUROSCI.09-07-02443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44(6):917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Somogyi P. Quantitative distribution of GABA-immunoreactive neurons in the visual cortex (area 17) of the cat. Exp Brain Res. 1986;61(2):323–331. doi: 10.1007/BF00239522. [DOI] [PubMed] [Google Scholar]
- Gilbert CD. Microcircuitry of the visual cortex. Annu Rev Neurosci. 1983;6:217–247. doi: 10.1146/annurev.ne.06.030183.001245. [DOI] [PubMed] [Google Scholar]
- Goodwin CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Hagihara K, Tsumoto T, Sato H, Hata Y. Actions of excitatory amino acid antagonists on geniculo- cortical transmission in the cat’s visual cortex. Exp Brain Res. 1988;69(2):407–416. doi: 10.1007/BF00247586. [DOI] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006;26(11):2951–2955. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282(5393):1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynen AJ, Yoon BJ, Liu CH, Chung HJ, Huganir RL, Bear MF. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat Neurosci. 2003;6(8):854–862. doi: 10.1038/nn1100. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol (Lond) 1970;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Iny K, Heynen AJ, Sklar E, Bear MF. Bidirectional modifications of visual acuity induced by monocular deprivation in juvenile and adult rats. J Neurosci. 2006;26(28):7368–7374. doi: 10.1523/JNEUROSCI.0124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The Critical Period for Ocular Dominance Plasticity in the Ferret’s Visual Cortex. J Neurosci. 1999;19(16):6965–6978. doi: 10.1523/JNEUROSCI.19-16-06965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa NP, Trepel C, Stryker MP. Spatial frequency maps in cat visual cortex. J Neurosci. 2000;20(22):8504–8514. doi: 10.1523/JNEUROSCI.20-22-08504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Trevino M, Kirkwood A. Sequential development of long-term potentiation and depression in different layers of the mouse visual cortex. J Neurosci. 2007;27(36):9648–52. doi: 10.1523/JNEUROSCI.2655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A, Bear MF, Singer W. Blockade of “NMDA” receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987;238(4825):355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- Li Y, Fitzpatrick D, White LE. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat Neurosci. 2006;9(5):676–681. doi: 10.1038/nn1684. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci. 2004;24(40):8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann HJ, Singer W, Martinez-Millan L. Horizontal Interactions in Cat Striate Cortex: I. Anatomical Substrate and Postnatal Development. Eur J Neurosci. 1990;2(4):344–357. doi: 10.1111/j.1460-9568.1990.tb00426.x. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443(7107):81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Brain Res Dev Brain Res. 1991;58(2):151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Mower GD, Christen WG. Role of visual experience in activating critical period in cat visual cortex. J Neurophysiol. 1985;53(2):572–589. doi: 10.1152/jn.1985.53.2.572. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54(6):961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Nahmani M, Erisir A. VGluT2 immunocytochemistry identifies thalamocortical terminals in layer 4 of adult and developing visual cortex. J Comp Neurol. 2005;484(4):458–473. doi: 10.1002/cne.20505. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci. 2003;23(13):5583–8. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Cho KK, Bear MF. Obligatory role of NR2A for metaplasticity in visual cortex. Neuron. 2007;53(4):495–502. doi: 10.1016/j.neuron.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29(1):157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D- aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci USA. 1999a;96(22):12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999b;2(4):352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Rampon C, Tsien JZ. Genetic analysis of learning and behavior-induced structural plasticity. Hippocampus. 2000;10(5):605–609. doi: 10.1002/1098-1063(2000)10:5<605::AID-HIPO11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ramoa AS, Mower AF, Liao D, Jafri SI. Suppression of cortical NMDA receptor function prevents development of orientation selectivity in the primary visual cortex. J Neurosci. 2001;21(12):4299–4309. doi: 10.1523/JNEUROSCI.21-12-04299.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y, Daw NW. Layer variations of long-term depression in rat visual cortex. J Neurophysiol. 2004;92(5):2652–2658. doi: 10.1152/jn.00298.2004. [DOI] [PubMed] [Google Scholar]
- Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 1999;397(6717):347–350. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- Rittenhouse CD, Siegler BA, Voelker CC, Shouval HZ, Paradiso MA, Bear MF. Stimulus for rapid ocular dominance plasticity in visual cortex. J Neurophysiol. 2006;95(5):2947–2950. doi: 10.1152/jn.01328.2005. [DOI] [PubMed] [Google Scholar]
- Rivadulla C, Sharma J, Sur M. Specific roles of NMDA and AMPA receptors in direction-selective and spatial phase-selective responses in visual cortex. J Neurosci. 2001;21(5):1710–1719. doi: 10.1523/JNEUROSCI.21-05-01710.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EB, Meredith MA, Ramoa AS. Suppression of NMDA receptor function using antisense DNA block ocular dominance plasticity while preserving visual responses. J Neurophysiol. 1998;80(3):1021–1032. doi: 10.1152/jn.1998.80.3.1021. [DOI] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38(6):977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Scheetz AJ, Contstantine-Paton M. Modulation of NMDA receptor function: Implications for vertebrate neural development. FASEB J. 1994;8(10):745–752. doi: 10.1096/fasebj.8.10.8050674. [DOI] [PubMed] [Google Scholar]
- Smith GB, Heynen AJ, Bear MF. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philos Trans R Soc Long B Biol Sci. 2009;364(1515):357–364. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timney B, Mitchell DE, Cynader M. Behavioral evidence for prolonged sensitivity to effects of monocular deprivation in dark-reared cats. J Neurophysiol. 1980;43(4):1041–1054. doi: 10.1152/jn.1980.43.4.1041. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Trepel C, Stryker MP. Rapid extragranular plasticity in the absence of thalamocortical plasticity in the developing primary visual cortex. Science. 2000;287(5460):2029–2032. doi: 10.1126/science.287.5460.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert B, Yoshii A, Constantine-Paton M. Receptor compartmentalization and trafficking at glutamate synapses: a developmental proposal. Trends Neurosci. 2004;27(7):428–437. doi: 10.1016/j.tins.2004.05.010. [DOI] [PubMed] [Google Scholar]
- White LE, Coppola DM, Fitzpatrick D. The contribution of sensory experience to the maturation of orientation selectivity in ferret visual cortex. Nature. 2001;411(6841):1049–1052. doi: 10.1038/35082568. [DOI] [PubMed] [Google Scholar]
- White LE, Fitzpatrick D. Vision and cortical map development. Neuron. 2007;56(2):327–338. doi: 10.1016/j.neuron.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Gilbert CD. The Sharpey-Schafer lecture. Morphological basis of visual cortical function. Q J Exp Physiol. 1983;68(4):525–543. doi: 10.1113/expphysiol.1983.sp002747. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Higashi H, Kudo Y, Inoue T, Hata Y, Mikoshiba K, Tsumoto T. Imaging of calcineurin activated by long-term depression-inducing synaptic inputs in living neurons of rat visual cortex. Eur J Neurosci. 2003;17(2):287–297. doi: 10.1046/j.1460-9568.2003.02449.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Ohmura T, Komatsu Y. Two forms of synaptic plasticity with distinct dependence on age, experience, and NMDA receptor subtype in rat visual cortex. J Neurosci. 2003;23(16):6557–6566. doi: 10.1523/JNEUROSCI.23-16-06557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]