Abstract
Circadian rhythms in mammals are coordinated by the suprachiasmatic nuclei (SCN) of the hypothalamus, which are most potently synchronized to environmental light-dark cycles. Large advances in the light-dark cycle typically yield gradual advances in activity rhythms on the order of 1–2 hours per day until re-entrainment is complete due to limitations on the circadian system which are not yet understood. In humans, this delay until re-entrainment is accomplished is experienced as jetlag, with accompanying symptoms of malaise, decreased cognitive performance, sleep problems and gastrointestinal distress. In these experiments, locomotor rhythms of BALB/cJ mice monitored by running wheels were shown to re-entrain to large 6 or 8-hour shifts of the light-dark cycle within 1–2 days, as opposed to the 5–7 days required for C57BL/6J mice. A single-day 6-hr advance of the LD cycle followed by release to constant darkness yielded similar phase shifts, demonstrating that exaggerated re-entrainment is not explained by masking of activity by the light-dark cycle. Responses in BALB/cJ mice were similar when monitored instead by motion detectors, indicating that wheel-running exercise does not influence the magnitude of responses. Neither brief (15 min) light exposure late during subjective nighttime nor 6-hr delays of the light-dark cycle produced exaggerated locomotor phase shifts, indicating that BALB/cJ mice do not merely experience enhanced sensitivity to light. Fos protein was expressed in cells of the SCN following acute light exposure at ZT10 of their previous light-dark cycle, a normally non-responsive time in the circadian cycle, but only in BALB/cJ (and not C57BL/6J) mice that had been subjected two days earlier to a single-day 6-hr advance of the light-dark cycle, indicating that their SCN had been advanced by that treatment. BALB/cJ mice may thus serve as a useful comparative model for studying molecular and physiological processes that limit responsiveness of circadian clocks to photic input.
Keywords: circadian, jetlag, suprachiasmatic, re-entrainment
INTRODUCTION
The central circadian pacemaker in mammals is located in the suprachiasmatic nuclei (SCN) of the hypothalamus. It is entrained most potently by photic environmental input via direct and indirect retinofugal projections, which ultimately yield alterations in multiple feedback loops of clock-related gene products within SCN cells to effect shifts in their rhythmic output that allow synchronization with environmental light-dark cycles (reviewed in [1]). Photic input also induces expression of the immediate early gene, c-fos, in retinorecipient SCN cells. Induction of phase shifts and Fos mRNA and protein expression by light are both restricted roughly to the dark phase of the light-dark cycle or to the subjective night-time of a subject held under constant lighting conditions in most species [2–5]. Light early in the responsive period typically yields delays in the onset of activity whereas light late in the responsive period yields advances in onset of activity [6–9]. Differences in neurochemical and molecular signal transduction processes during phase advances and delays have been documented (reviewed in [10]) but the exact mechanisms dictating the direction and relative magnitude of light-induced phase shifts are not yet understood.
Re-entrainment of circadian activity rhythms in mammals after large shifts of the light-dark (LD) cycle is usually gradual and requires multiple circadian cycles for completion due to limiting factors not yet identified. Here, we show evidence that locomotor rhythms of Balb/cJ mice do not demonstrate this characteristic 5–7 day transient lag until re-entrainment observed in C57BL/6J mice following a large (6–8 hour) phase advance of the light-dark cycle. Moreover, examination of circadian activity rhythms and Fos expression in the SCN following a variety of manipulations of the light-dark cycle in BALB/cJ and C57BL/6J mice suggests that the enhanced phase advance response of BALB/cJ mice does not involve masking or depend on wheel-running exercise, and may not extend to phase delays of the light-dark cycle.
METHODS
All experimental and animal care procedures were performed with the approval of the Rider University Institutional Animal Care and Use Committee and in accordance with the NIH Guidelines for the Care and Use of Animals in Research.
Circadian Activity Measurement
Male and female BALB/cJ and C57BL/6J mice (approximately 6 weeks old) were placed individually in standard laboratory cages with access to food and water ad libitum. Mice were obtained directly from Jackson Laboratories (Bar Harbor, ME) or from a short-term colony inbred over several generations in our animal facility from Jackson Laboratories stock. For most experiments, each mouse had access to a running wheel connected to a computer which collected wheel rotation data using the Clocklab circadian collection and analysis software (Actimetrics, Inc., Evanston, IL). In one experiment designed to determine whether running behavior influenced rate of re-entrainment, activity was monitored instead by infrared motion detectors (Slimline 5V, SmarthomePro.com). Mice were maintained under white light illumination provided by Daylight™ (General Electric) fluorescent bulbs, approximately 300–400 lux at cage level.
Mice entrained to a 12:12 light-dark cycle were subjected sequentially to 6- or 8-hour advances of the light-dark cycle, single day 6- or 8-hour advances of the light-dark cycle followed by release to constant darkness, or simply released to constant darkness or constant light. A separate group of mice was subjected to 6-hour delays of the light-dark cycle. Phase was determined by regression line fit through five consecutive daily onsets of activity (2 hours of inactivity followed by 1 hour of activity), using Clocklab software. Re-entrainment was defined as re-establishment of phase to within 30 minutes of the phase relationship demonstrated prior to the shift of the light cycle, maintained for at least 3 consecutive days. Phase angle was determined by measuring the difference between the onset of darkness on the last day of LD and the predicted onset of activity derived from a regression line fit to the five days following release to constant conditions and extrapolated back to the last day of the LD cycle.
During the course of a 6-hr advance of the light-dark cycle, mice are exposed to 6 hours of light during the end of subjective nighttime and 6 hours of darkness during late subjective daytime. To assess the effects of these components separately, mice were subjected to 6 hrs of dark during the latter half of the 12-hr day followed by release to DD for 10 days, then after 2 weeks of re-entrainment, were subjected to 6 hours of light during the latter half of night then released to DD for 10 days.
Given the possibility that ocular sensitivity to light is altered in Balb/cJ mice, which are albino, mice free-running under constant darkness were subjected to acute light exposure (15 mins, 200 lux) at CT21, the peak phase advance time for BALB/c according to Schwartz & Zimmerman [11], then returned to constant darkness for 10 days and assessed for shifts in phase of circadian locomotor activity rhythms. Phase shifts were measured as the difference between regression lines fitted to onsets of activity for 5 days before and after treatments, extrapolated to the day following treatment.
Photic Fos production
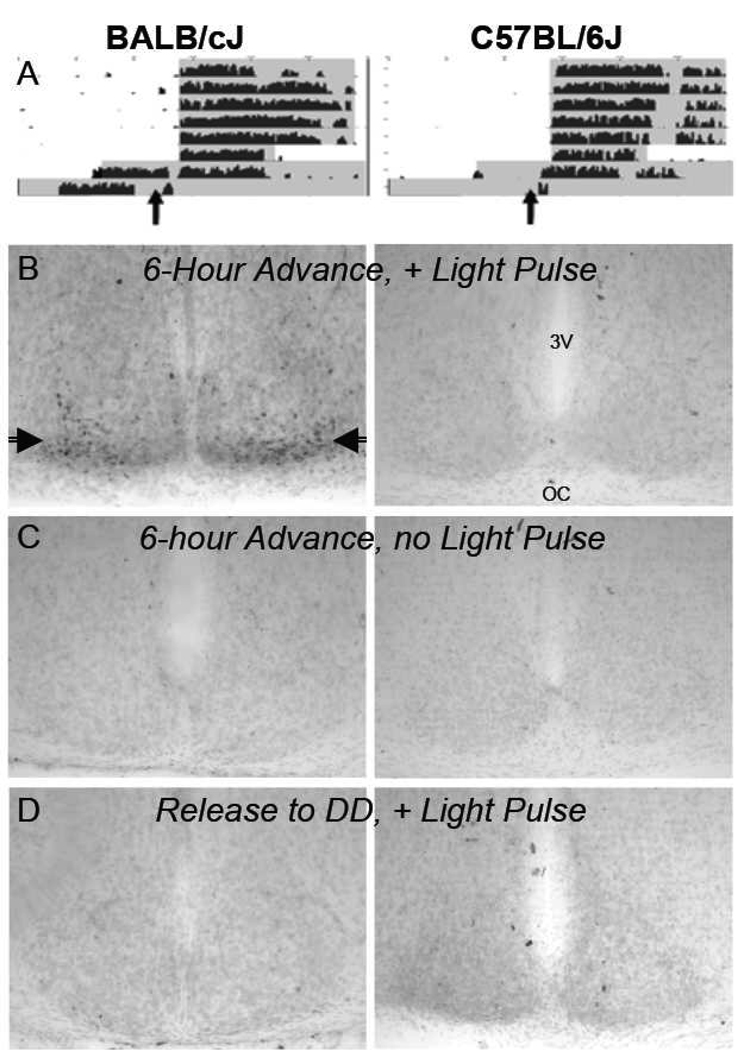
Three groups of BALB/cJ and C57BL/6J mice (n=4 each/group) were entrained for a minimum of 2 weeks to a 12:12 LD cycle. Since the onset of activity following manipulation of the LD cycle as an accurate measure of phase of SCN-generated rhythms relative to the light-dark cycle for these two strains of mice was in question and thus being tested in these experiments, timing of the light pulse is expressed relative to the prior LD cycle rather than by subsequent onsets of activity in DD. The first group was presented with a single day 6-hour advance of the light dark cycle and subsequently released to DD followed two days later by a 15-minute light pulse (200 lux) at zeitgeber time 10 (ZT 10) of their previous light dark cycle. (Zeitgeber time: time expressed relative to the light-dark cycle, where ZT12 corresponds to dark onset.) The second group was also subjected to a single day 6-hour advance of the light dark cycle and release to DD but received no light pulse when placed in the light stimulator two days later. The third group was simply released to DD and presented with a light pulse two days later. Light pulses were administered 2 days after phase shifts so that sufficient onsets of activity would permit determination about whether wheel-running rhythms had also been advanced by that treatment. Light pulses were administered by moving the mouse to a transparent 500 ml cup in a ventilated chamber illuminated by a fiber optic lamp. Handling of the mice in the dark was performed with the aid of night-vision goggles.
Ninety minutes subsequent to the light pulse presentation the mice were deeply anesthetized with sodium pentobarbital (30 mg/kg i.p.) then perfused transcardially with 35 ml of heparin-saline (1000U/ml heparin in 0.9% NaCl, delivered 7.5ml/min) followed by 35 ml of 4% paraformaldehyde (PFA) in 0.1M phosphate buffer at the same rate. Brains were removed and stored in 4% PFA overnight at 4–8°C then transferred to phosphate buffer. Brains were sectioned (40 µm) through the rostrocaudal extent of the SCN using a Vibroslice and stained immunohistochemically for Fos. Sections were incubated overnight in 400 µL of primary antibody (Calbiochem Ab-2, 1:2,000 dilution in 0.1M phosphate buffer with 0.5% BSA and 3% Triton X-100), then visualized with a Vectastain horseradish peroxidase kit (Vector Labs, Burlingame, CA) using DAB (SigmaFast tablets, Sigma Chemical Co., St. Louis, MO) as a chromagen. Slices were mounted on microscope slides and lightly counterstained with methyl green before dehydration and coverslipping. Fos-immunoreactive cells were counted throughout the rostro-caudal extent of the SCN by an observer blind to the treatment of each animal.
Statistical Analysis
Data from 6-hour advances, release to DD, and single day 6-hour delays were analyzed using t-tests. Mann Whitney U tests were applied to (non-parametric) data from single day 6-hour advances and single day 8-hour advances. Responses to acute light exposure or “control” placement in the apparatus were subjected to ANOVA followed by Tukey analysis. Phase shifts are expressed as group averages ± standard error of the mean (SEM). Fos-immunoreactive cell counts, expressed in the Results as group averages ± SEM, were subjected to two-way ANOVA, with strain and treatment as factors, followed by Tukey analysis.
RESULTS
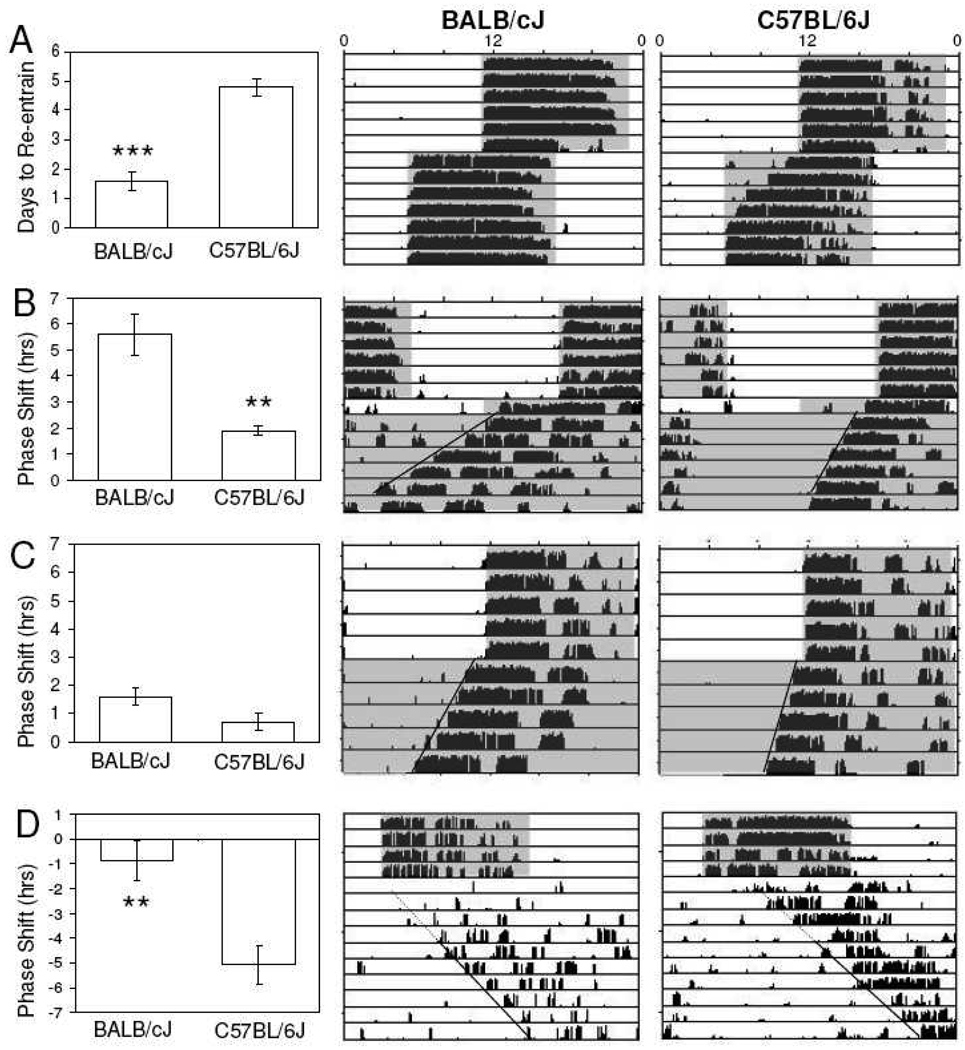
In response to a 6-hour advance of their light-dark cycle, locomotor rhythms of BALB/c mice were found to re-entrain more rapidly (1.6 ± 0.3 days; n=15) than C57BL/6J mice (4.8 ± 0.3 days, n=15; p < 0.001)(Fig. 1A). Re-entrainment was also significantly more rapid in BALB/cJ than C57BL/6J mice following 8 hour advances of the light-dark cycle (1.8 ± 0.3 days (n=10) vs 5.7 ± 0.7 days (n=7); data not shown graphically; p<0.05).
Fig. 1.
Representative wheel-running actograms from mice following various light-dark manipulations. (A) BALB/cJ mice re-entrain to a 6-hr advance of the light-dark cycle within 1–2 circadian cycles, whereas C57BL/6J mice require 4–5 days. (B) A single-day 6-hr advance of the light-dark cycle followed by release to constant darkness induces large phase advances in BALB/cJ, but not C57BL/6J mice. (C) Release to constant darkness reveals similar phase angles of entrainment for both strains. (D) Release to constant light has no effect on phase of BALB/cJ rhythms, but yields phase delays in C57BL/6J mice. Shaded areas correspond to lights off. (**, p<.01; ***, p<.001)
When mice were exposed to a 6-hour advance of the LD cycle for one day then released into constant darkness, locomotor rhythms in BALB/cJ mice advanced significantly greater (5.6 ± 0.8 hrs, n=11) than in C57BL/6J mice (1.9 ± 0.2 hrs, n= 10; p< 0.001)(Fig. 1B). One-day 8-hour advances of the light-dark cycle yielded 7.2 ± 1.1 hr advances in Balb/cJ mice (n=13), compared to 0.4 ± 0.4 hr advances in C57BL6J mice (n=13; data not shown graphically; p<0.001).
Upon release to constant darkness (DD), no significant difference in the phase of locomotor rhythms with respect to the previous light-dark cycle was observed for BALB/cJ mice (1.6 ± 0.3 hrs, n=11) versus C57BL/6J mice (0.7 ± 0.3 hrs, n=7; p= 0.06)(Fig. 1C). Upon release to constant darkness, free-running periods of BALB/cJ mice were measurably shorter than those of C57BL/6J mice (22.5 ±0.1 versus 23.3 ±0.1 hrs, respectively; p<.05, Mann-Whitney rank sum test). Upon being released instead to constant light (LL), significantly larger delays in onset of activity were observed in C57BL/6J mice (−5.1 ± 0.8 hrs, n=7) than in Balb/cJ mice (−0.9 ± 0.8 hrs, n=5; p<0.01)(Fig. 1D). Upon release to constant light, free-running periods were not significantly different for BALB/cJ (25.3 ±0.2 hrs) and C57BL/6J mice (25.2 ±0.1 hrs)(p>.05, Student’s t-test).
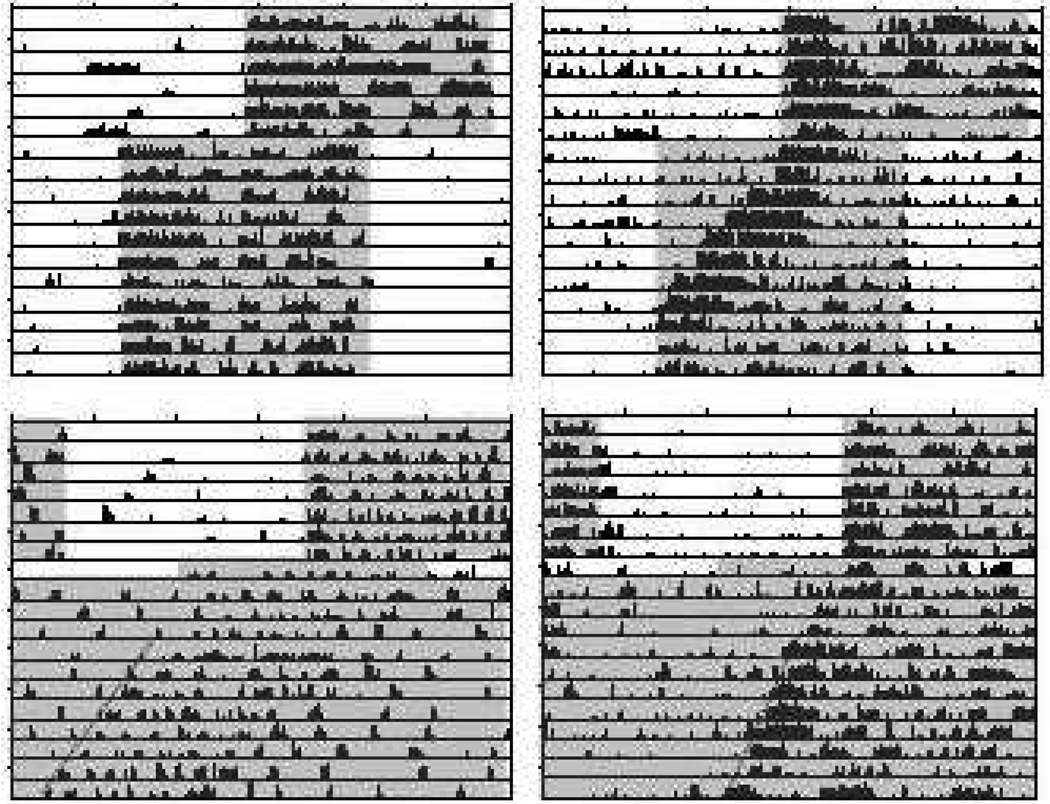
Strain differences in rate of re-entrainment were similarly apparent following 6-hour phase shifts when mice did not have access to running wheels and were instead monitored with motion detectors (Balb/cJ, 1.6 ± 0.4 days (n=7) versus C57BL/6J, 7.6 ± 0.3 days (n=8); p<0.001)(Fig. 2A). BALB/cJ mice without running wheels also showed exaggerated phase shifts following single-day 6-hr advances of the light-dark cycle followed by release to constant darkness (4.9 ± 0.5 hrs, n=4) compared to C57BL/6J mice (0.8 ± 0.4 hrs, n=5)(p<.01)(Fig. 2B). Three mice of each strain for which activity was absent due to detector malfunction or where the detector indicated activity consistently across the entire circadian cycle were removed from the latter analysis due to an inability to accurately calculate phase from their activity records.
Fig. 2.
Representative actograms constructed from motion detector data on mice without running wheels. (A) BALB/cJ mice re-entrain to 6-hr advances of the light-dark cycle within 1–2 circadian cycles in the absence of running wheels, whereas C57BL/6J mice require 7–8 cycles. (B) Large phase advances in activity rhythms are expressed by BALB/cJ mice but not C57BL/6J mice after single-day 6-hr advances of the light-dark cycle followed by release to constant darkness. Shaded areas correspond to lights off. (**, p<.01; ***, p<.001)
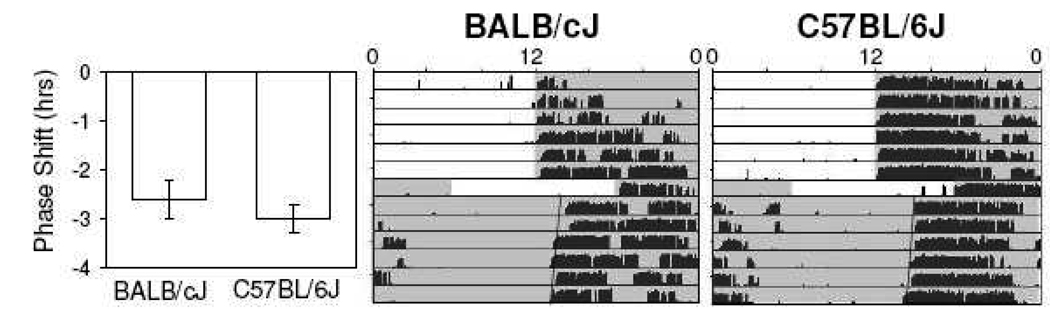
No strain difference in magnitude of phase shifts was observed following one-day 6-hour delays of the light-dark cycle followed by release to constant darkness for BALB/cJ mice (−2.6 ± 0.4 hrs, n=7) and C57BL/6J mice (−3.0 ± 0.3 hrs, n=5; p>0.05)(Fig. 3).
Fig. 3.
Representative wheel-running actograms following single-day 6-hr delays of the light-dark cycle followed by release to constant darkness. Magnitude of responses for BALB/cJ and C57BL/6J mice are similar (p>.05). Shaded areas correspond to lights off.
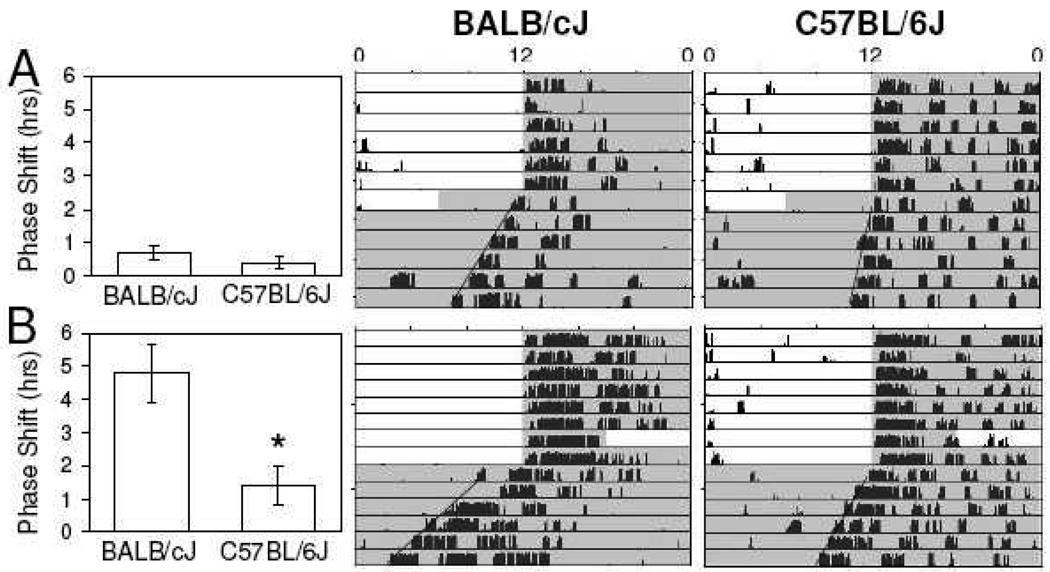
Following an advance of the dark onset by 6 hours while keeping the subsequent light onset the same (i.e. dark pulse during late day), Balb/cJ and C57BL6J mice displayed similarly small mean shifts of 0.7 ± 0.2 hrs (n=5) and 0.4 ± 0.2 hrs (n=5), respectively (p>0.05)(Fig. 4A). On the other hand, exposure to a 6-hour advance of the onset of light while maintaining the original subsequent onset of darkness (i.e. 6-hour light pulse in late night) yielded large phase advances in Balb/cJ (4.8 ± .9 hrs, n=5) compared to C57BL/6J mice (1.4 ± 0.6 hrs, n=5; p<0.05)(Fig. 4B).
Fig. 4.
Representative actograms following single-day 6-hr dark pulses late during the day or single-day 6-hr light pulses late at night. Mice subjected to single-day 6-hr advances of the light-dark cycle experience 6 hrs of darkness late in their day and 6 hrs of light late at night. (A) Exposure to only the 6-hr dark pulse in the latter half of the light period yields no noteworthy change in phase of activity rhythms. (B) Exposure to only the 6-hr light pulse at night yields large phase advances in BALB/cJ but not C57BL/6J mice. Shaded areas correspond to lights off. (*, p<.05)
Phase shifts in response to an acute light pulse at CT21 were not significantly different for Balb/cJ mice (0.7 ± 0.2 hrs, n=5) and C57BL/6J mice (0.9 ± 0.1 hrs, n=7), but were significantly greater than shifts produced by placement in the light stimulation apparatus without light exposure (0.3 ± 0.1 hr, n=6 Balb/cJ; 0.3 ± 0.2 hr, n=6 C57BL/6J)[F=24.37, df=3,20, p<.001].
Photic Fos Induction
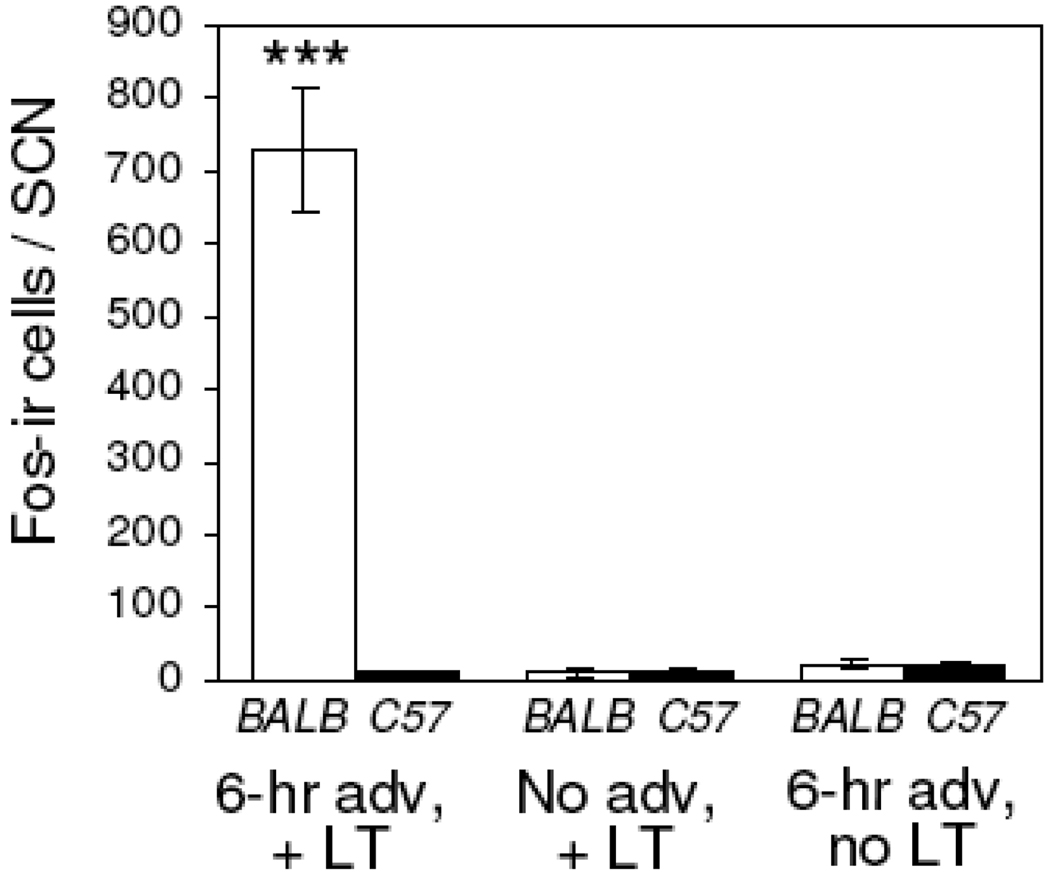
Two-way ANOVA indicated a significant interaction of mouse strain and treatment on light-induced Fos expression in the SCN [F(2,23)=92.80, p<.001]. A light pulse administered at ZT10 following a single-day 6-hr phase advance of the LD cycle yielded significantly greater Fos expression in the SCN of BALB/cJ (728 ±85 cells, n=4) but not C57BL/6J (8 ±2, n=4) mice (Fig. 5, Fig. 6). Few Fos-immunoreactive cells were observed in the SCN of mice given a light pulse at ZT10 following release to constant darkness (BALB/cJ, 12 ±5 cells, n=4; C57BL/6J, 8 ±5 cells, n=4), or when no light pulse was administered after a single-day 6-hr advance of the light-dark cycle (BALB/cJ, 22 ±8 cells, n=4; C57BL/6J, 18 ±7 cells, n=4).
Fig. 5.
Light-induced Fos production in the suprachiasmatic nuclei of BALB/cJ and C57BL/6J mice. (A) Mice were subjected to acute exposure to light (~200 lux for 15 mins) at ZT10 of their original light-dark cycle (indicated by vertical arrows), a normally unresponsive time of the circadian cycle, following either a single-day 6-hr phase advance of the light-dark cycle or release to constant darkness. Activity rhythms were advanced by the phase shifts in the BALB/cJ mice (left panel), so that the acute light exposure occurred several hours after their apparent onset of activity. Shaded areas correspond to lights off. (B) Acute light exposure two days following a single 6-hr advance of the light cycle stimulated prominent stereotypical Fos production in cells of the ventrolateral suprachiasmatic nucleus (indicated by horizontal arrows) in all of the BALB/cJ but none of the C57BL/6J mice. (C) No Fos was detected in the suprachiasmatic nuclei of any of the mice when no acute light pulse was administered following a 6-hr phase advance. (D) Acute light exposure did not yield Fos production in any of the mice if not preceded by a single-day 6-hr phase shift. (3V= third ventricle; OC= optic chiasm)
Fig. 6.
Quantification of Fos-positive cells in the paired SCN of BALB/cJ (open bars) and C57BL/6J mice (filled bars). Acute light exposure at ZT10 of the prior light-dark cycle yielded a significant increase in Fos-positive cells in BALB/cJ mice following a single-day 6-hr advance of the light-dark cycle, compared to similarly treated C57BL/6J mice. Few Fos-immunoreactive cells were observed in the SCN of either strain following a phase shift but no light pulse, or a light pulse without the single-day phase shift. (* p<.001, two-way ANOVA followed by Tukey analysis)
DISCUSSION
From the results of these experiments, it is evident that the circadian system of BALB/cJ mice can achieve re-entrainment to shifted light-dark cycles at a rate significantly greater than in C57BL/6J mice. It has been documented previously that the circadian rhythms of BALB/cJ mice sometimes, albeit inconsistently, display advanced phase angles relative to the light-dark cycle [11, 12]. Our experiments showed little or no difference in phase angles of entrainment between BALB/cJ mice and C57BL/6J mice upon release to constant darkness, dispelling any suggestion that both rapid re-entrainment to 6- or 8-hour advances in the light cycle and large magnitude phase shifts following single-day phase advances of the light cycle in BALB/cJ mice merely reflect unmasking of activity rhythms with large advanced phase angles. This is supported also by the typical pattern of light-induced Fos expression in the SCN that could be generated only in BALB/cJ mice whose circadian system had obviously been advanced to a photically responsive time point as a result a 6-hr phase shift two days earlier.
Free-running period in constant darkness was nearly 1 hour shorter for BALB/cJ than for C57BL/6J mice. This may have contributed to, but could not have been responsible for, the rapidity of re-entrainment to large advances of the light-dark cycle. The approximate 1-hour daily “gain” associated with the shorter period in BALB/cJ mice contributed to a phase advance that was almost complete on the first day after both 6-hour and 8-hour shifts of the light-dark cycle, as well as after single-day 6-hr and 8-hr shifts of the light-dark cycle. The observation that relatively brief discrete light pulses yield phase shifts of similar magnitude in these two strains of mice, yet shifts of the complete light-dark cycle yield distinctly different patterns of resetting, is suggestive of a continuous mechanism of entrainment at work in the circadian system in BALB/cJ mice. In the context of limit cycle organization of the clock mechanism (for review, see Johnson et al., 2003), this could reflect strain-specific differences in kinetics of one or more “state variables” (i.e., the level or function of molecular clock components) in response to light exposure across the circadian cycle, to be revealed by future experiments.
Locomotor activity has been shown to influence various aspects of circadian rhythmicity (reviewed in [13]). A comparison of responses of mice with and without running wheels in our experiments demonstrate that the rapid re-entrainment of BALB/cJ mice following large phase shifts of the light-dark cycle is not influenced by wheel-running exercise. It should be noted however that C57BL/6J mice running on wheels required less time to re-entrain to 6-hr phase advances, suggesting that exaggeration of BALB/cJ responses may entail diminished sensitivity to inhibitory locomotor or other feedback that may be more influential in other species or strains of mice. Alterations in locomotor feedback or other putative feedback pathways could be acting to directly potentiate photic input or indirectly diminish feedback inhibitory to photic input in BALB/cJ mice.
Presumably, some combination of altered inputs to the SCN, response of the SCN itself, and/or coupling of SCN output to effector systems differs sufficiently to produce this enhanced response in BALB/cJ mice. The Fos results here demonstrate that the light-responsive portion of the circadian cycle in the SCN of the BALB/cJ group is rapidly advanced by one-time shifts of the light-dark cycle, suggesting that the differential responses to shifted light-dark cycles occurs at the level of the SCN and not to altered coupling to downstream outputs such as locomotor responses. The notably large phase shifts elicited in BALB/cJ mice by long light pulses but not by long dark pulses also point toward an altered response of the SCN to light in this strain of mouse. It is unlikely that photic input is generally more potent in BALB/cJ mice, given that exaggerated phase shifts are not observed after single-day 6-hr delays of the light-dark cycle, and acute light pulses at ZT21 yield phase shifts similar in magnitude to those in C57BL/6J mice, consistent with previously published findings [11]. In that study, Schwartz and Zimmerman propose a correlation between endogenous period of a free-running rhythm and the ratio of light-induced delays to advances which would be necessary to maintain entrainment to a light-dark cycle. In general, proportionately larger daily phase advances of activity would be required of mice with endogenously shorter endogenous rhythms. While this assertion is supported by their study of acute light exposures across the circadian cycle (and by our own unpublished observations), it is not supported by the results of this study on re-entrainment to larger shifts of the light-dark cycle. While light-induced phase shifts following acute light exposure in both strains of mice were significantly larger (statistically speaking) than the “control” mice in our experiment, these light-induced phase shifts were the same or smaller in magnitude than daily advances in onset of activity observed in mice in the first experiment which were merely released to DD. This suggests that the suprachiasmatic nuclei in BALB/cJ but not C57BL/6J mice are capable of integrating extended nighttime light exposures into proportionately larger phase shifts, as reflected by even greater responses to single-day 8-hr shifts than to single-day 6-hr shifts seen in BALB/cJ but not C57BL/6J mice in our study. Future experiments will examine the effects of intensity, duration and total irradiance of light exposures relative to circadian phase in order to determine whether altered responses to longer and/or brighter light exposure underlie the comparatively rapid rate of re-entrainment in BALB/cJ mice.
In conclusion, our results suggest that factors restricting re-entrainment rates in C57BL/6J mice (and many other mammals studied to date) are absent or ineffective in BALB/cJ mice. Future comparisons of BALB/cJ to C57BL/6J or other strains at a pharmacological or molecular level may permit identification of those regulatory factors, which could ultimately yield targets in the treatment of maladies associated with the circadian system such as seasonal affective disorders, altered sleep phase syndromes, shift-work fatigue and jetlag.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reppert SM, Weaver DR. Coordination of Circadian Timing in Mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 2.Earnest D, Iaradora M, Yeh HH, Olschowka JA. Photic regulation of c-fos expression in neural components governing the entrainment of circadian rhythms. Exp. Neurol. 1990;109:353–361. doi: 10.1016/s0014-4886(05)80027-5. [DOI] [PubMed] [Google Scholar]
- 3.Kornhauser JM, Nelson DE, Mayo DE, Takahashi JS. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron. 1990;5:127–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- 4.Colwell CS, Foster RG. Photic regulation of Fos-like immunoreactivity in the suprachiasmatic nucleus of the mouse. J. Comp. Neurol. 1992;324:135–142. doi: 10.1002/cne.903240202. [DOI] [PubMed] [Google Scholar]
- 5.Rusak B, Robertson HA, Wisden W, Hunt SP. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;248:1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- 6.DeCoursey PJ. Daily light sensitivity rhythm in a rodent. Science. 1960;131:33–35. doi: 10.1126/science.131.3392.33. [DOI] [PubMed] [Google Scholar]
- 7.Hoban TM, Sulzman FM. Light effects on circadian timing system of a diurnal primate, the squirrel monkey. Am. J. Physiol. 1985;249:R274–R280. doi: 10.1152/ajpregu.1985.249.2.R274. [DOI] [PubMed] [Google Scholar]
- 8.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: pacemaker as clock. J. Comp. Physiol.[A] 1976;106:291–331. [Google Scholar]
- 9.Rea MA. Neurochemistry of Photic Entrainment. Chronobiol. Int. 1998;15:395–423. doi: 10.3109/07420529808998699. [DOI] [PubMed] [Google Scholar]
- 10.Meijer JH, Schwartz WJ. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J. Biol. Rhythms. 2003;18:235–249. doi: 10.1177/0748730403018003006. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz WJ, Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J. Neurosci. 1990;10:3685–3694. doi: 10.1523/JNEUROSCI.10-11-03685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimomura K, Low-Zeddies SS, King DP, Steeves TD, Whiteley A, Kushla J, Zemenides PD, Lin A, Vitaterna MH, Churchill GA, Takahashi JS. Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res. 2001;11:959–980. doi: 10.1101/gr.171601. [DOI] [PubMed] [Google Scholar]
- 13.Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol. Rev. Camb. Philos. Soc. 1996;71:343–372. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]