Abstract
During invasion, the obligate intracellular pathogen, Toxoplasma gondii, secretes into its host cell a variety of effector molecules, several of which have been implicated in strain-specific variation in disease. The largest family of these effectors, defined by the canonical member ROP2, quickly associates with the nascent parasitophorous vacuole membrane (PVM) after secretion. Here we demonstrate that the NH2-terminal domain of the ROP2-family contains a series of amphipathic helices that are necessary and sufficient for membrane association. While each of the amphipathic helices is individually competent to bind cellular membranes, together they act to bind the PVM preferentially, possibly through sensing its strong negative curvature. This previously uncharacterized helical domain is an evolutionarily robust and energetically efficient design for membrane association.
Keywords: Toxoplasma, amphipathic helix, membrane association
Introduction
Efficient transduction of complex signals across membranes requires exquisite organization of signaling machinery. Receptors, adaptor proteins (such as scaffolds), and effector molecules must associate specifically with one another and their downstream targets. Such organization is often facilitated by direct interaction of the component proteins with the membrane (1, 2). Understanding how these molecules associate with their target membranes is thus critical to elucidating how information is transduced.
While all cells transmit information across membranes, the interaction of an intracellular pathogen with its host cell is particularly complex. In addition to processing information from its own signaling networks, most infectious agents monitor or co-opt host signaling using a variety of factors secreted into the host cell.
One such organism, the protozoan parasite Toxoplasma gondii, survives in its host cell in a specialized membranous organelle known as the parasitophorous vacuole (PV, Figure 1a). The membrane surrounding the vacuole (the PVM) is originally formed from the host plasma membrane as the parasite invades. During invasion, Toxoplasma appears to strip the PVM of most host proteins (3), which are replaced with factors secreted by the parasite from specialized secretory organelles, the rhoptries and dense granules. The rhoptries contain a series of virulence factors (4), while dense granular proteins serve to form a complex network of membrane tubules that vesiculate from the PVM and extend into the vacuolar lumen (5, 6).
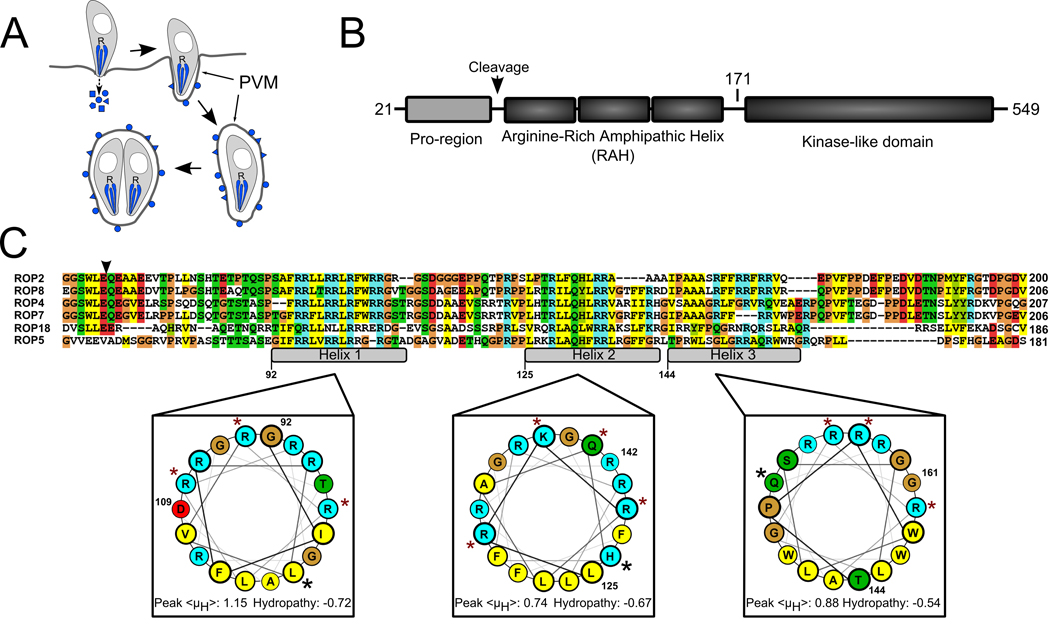
Figure 1. The ROP2-family domain architecture.
A) During Toxoplasma invasion of a host cell, its rhoptries (R) secrete their contents of effector molecules (blue) into the host cytosol. Many of these molecules associate with the PVM, which expands as the parasite replicates inside. B) Members of the ROP2-family have three domains following their signal peptides: a pro-region that is proteolytically processed in all members except for ROP5, a series of three arginine-rich amphipathic helices (RAH domain) and a protein kinase-like domain that appears to be catalytically inactive in all family members except for ROP18. Numbering is according to ROP5. The schematic is not drawn to scale. C) The RAH domain is the region of highest identity among the various family members. The putative processing site that precedes the RAH is marked with an arrowhead. The three amphipathic helices are marked in the alignment and numbered according to the ROP5 sequence. The ROP5 helices are projected on helical wheels and the average hydropathy and peak hydrophobic moment, <μH>, of each is shown, calculated according to the Eisenberg consensus scale (27). Blue shading indicates basic residues, red indicates acidic residues, yellow indicates hydrophobic residues, green indicates polar, and glycines and prolines are shaded tan. Residues that have been mutated to glutamate in the present study are marked with a red star. Residues that have been mutated to proline are marked with a black star.
The largest family of proteins known to be secreted by Toxoplasma into its host cell is the ROP2-superfamily (4, 7). Each superfamily member possesses a related C-terminal kinase-like domain and a divergent N-terminal sequence. Members of the superfamily include two described virulence factors: ROP16, a soluble kinase that translocates to the host nucleus and alters host gene expression (8) and ROP18 which is localized to the PVM, where it affects virulence through an unknown mechanism (9, 10). ROP18 is a member of a closely related clade of the superfamily comprising ROP2, ROP4, ROP5, ROP7, ROP8, and ROP18 (Figure 1), which we refer to as the ROP2-family.
The ROP2-family proteins, as with all known Toxoplasma rhoptry proteins, are secreted into the host cytosol early in invasion (4, 11). The ROP2-family proteins immediately associate with the nascent PVM, where they remain localized until lysis of the host cell. Thus, their functions seem tied to their specific membrane association; for at least one family member, ROP18, such functions include a profound effect on parasite growth and virulence (9, 10). Previous work suggested that the ROP2-family members are integral membrane proteins with a transmembrane helix located toward the C-terminus (12). It was shown recently, however, that the C-terminal region of the ROP2-family contains a domain with a kinase-like fold and the “transmembrane helix” is a portion of the hydrophobic core of the domain (7), making this model of membrane association unlikely. As the family also contains no conserved sites with predicted post-translational modifications that would enable membrane association, we hypothesized that the ROP2-family must have a previously undefined domain with the intrinsic ability to associate with membranes.
Proteins localize and bind to membranes by a variety of mechanisms, including lipid-binding domains (e.g. PH, FYVE, C1 domains) that bind a specific phospholipid (2), covalent addition of a lipid or fatty acid to the protein (13), and the insertion of amphipathic regions of a protein into the membrane (14). As the latter two mechanisms require a much lower amino acid sequence complexity, they can be evolved more easily than a folded protein domain. However, this low sequence complexity makes such motifs difficult to predict. In the present study, we describe such a low-complexity membrane association domain present in the ROP2-family and the determinants of its specificity for the PVM. In addition, we show that possession of a similar domain in another ROP2-superfamily member correctly predicts its PVM localization.
Results
The ROP2-family NH2-terminus contains a conserved membrane association domain
The N-terminal 100 amino acids of mature ROP2-family proteins contains a region that we predict to form three amphipathic helices, each with a polar face substantially enriched in arginine (Figure 1c). This basic, arginine-rich amphipathic helix domain (RAH) is the region with the highest similarity between each of the members of the family, suggesting a common function. Recently, short, basic polypeptide sequences have been shown to be required for membrane localization of the MAP kinase Ste5 (15) and several families of small GTPases (16). While the sequence of the amphipathic helical domain is substantially longer in the ROP2-family than in the preceding cases, we hypothesized that it associates with membranes using mechanisms similar to those involving the short basic sequences of Ste5 and the GTPases.
To test this, we created a series of deletion constructs to map the domains responsible for membrane association (Supplemental Figure S1). Selected ROP2-family members were cloned into vectors for heterologous expression in mammalian cells as fusions to either a monomeric yellow or red fluorescent protein (the variants Venus (17) and mCherry (18), respectively). We expressed the RAH domains of ROP2, ROP5, or ROP18 (representing the most divergent family members) as soluble proteins in human foreskin fibroblasts (HFF) using constructs that yielded proteins with an N-terminus approximating that of the respective mature ROP2 family member (constructs outlined in Supplemental Figure S1). ROP2 and ROP18 are both thought to be processed at their N-termini producing a polypeptide with the RAH domain very close to the N-terminus (Figure 1c, (7)), and were therefore C-terminally tagged. Conversely, ROP5 is not processed and retains its “pro” domain in its mature form (19).
In host cells infected with Toxoplasma, the full-length ROP2, ROP5 and ROP18 proteins, fused to fluorescent protein, localized strongly to the PVM (Figure 2a and data not shown). This is consistent with recent work in which ROP18 was shown to associate with the PVM when similarly expressed in mammalian cell culture (20). Strikingly, proteins lacking the RAH (that is, containing only the kinase-like domain, or, in the case of ROP5, the N-terminal and kinase-like domains) were unable to interact with membrane, even in infected cells (Figure 2b and data not shown). In fact, YFP-tagged ΔRAH proteins displayed diffuse localization throughout the cell, indistinguishable from YFP alone (Figure 2c). Proteins containing only the RAH domain of any of the three family members fused either N- or C-terminally to the fluorescent protein mimicked the localization of the full-length proteins (Figure 2d–f and data not shown). The RAH domain of the ROP2-family, therefore, appears to be both necessary and sufficient for its PVM association.
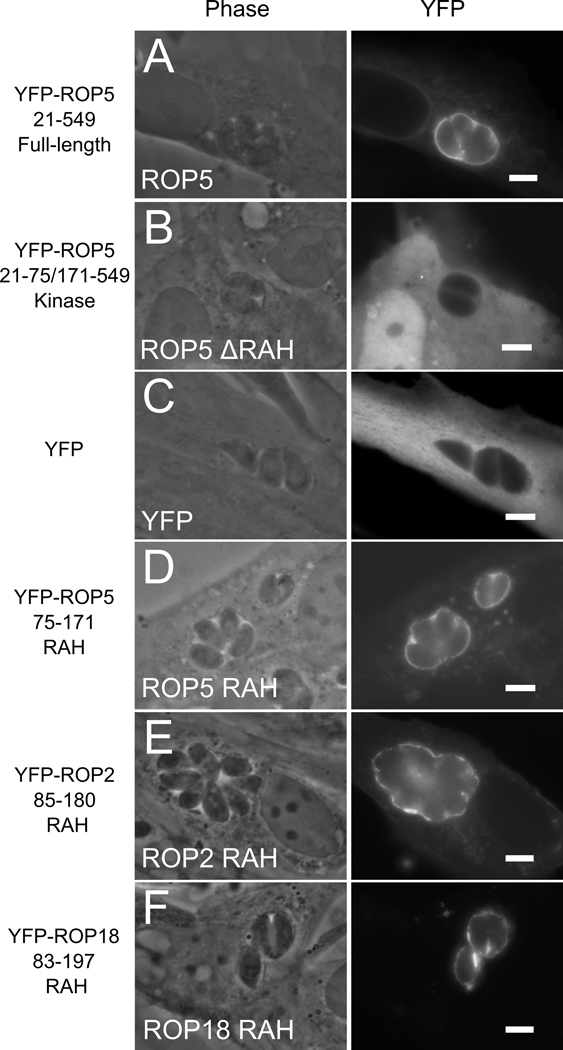
Figure 2. The N-terminal RAH domain of ROP2-family is necessary and sufficient for membrane association.
ROP2, ROP5, and ROP18 were fused to yellow fluorescent protein (YFP) and heterologously expressed in human fibroblasts. The localization of the protein after Toxoplasma infection was visualized by fluorescence. (A) Full-length mature YFP-ROP5 localizes strongly to the PVM. (B) YFP-ROP5 protein lacking the RAH domain fails to associate with host membranes and the PVM; note the diffuse localization identical to (C) YFP alone. (D) The RAH of ROP5 is sufficient to target YFP to the PVM. (E) YFP-ROP2RAH also shows strong and specific PVM association, as does (E) YFP-ROP18RAH.
The ROP2-family RAH domain preferentially associates with the PVM
We next determined how specific the ROP2-family RAH domain was in its tropism for the PVM. While native ROP2-family proteins secreted by Toxoplasma exclusively associate with the PVM , the proteins we have heterologously expressed in the host cells have drastically different localization in uninfected and infected cells (compare Figures 2c–e with Figure 3 and Figure 4). Constructs of ROP2 that contain portions of the RAH domain have been previously reported to co-localize with both host endoplasmic reticulum (ER) and mitochondria when expressed in uninfected host-cells. These observations have been used to suggest that ROP2 mediates the interaction between the host mitochondria and the PVM (21). We were thus interested in the degree to which the RAH domain displays specificity for association with various host membranes and the PVM.
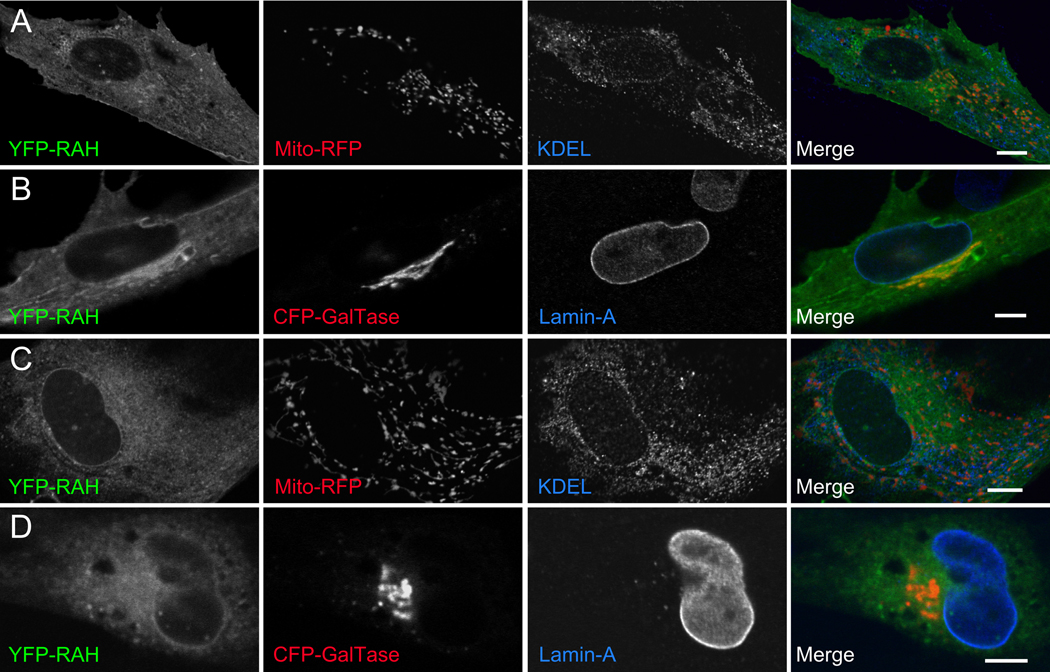
Figure 3. The RAH domain associates weakly with various membranous structures in uninfected cells.
1µm slices of confocal micrographs of uninfected cells expressing either YFP-ROP2RAH (A, B) or YFP-ROP5RAH (C, D) exhibit distributed, punctate fluorescence. Little YFP fluorescence specifically colocalizes with host mitochondrial (A, C; MitoRFP; red), ER (A, C; anti-KDEL; blue), or trans-Golgi (B, D; CFP-GalTase; red) membranes is apparent. However, some YFP-fluorescence colocalizes with host nuclear envelope (B, D; anti-lamin; blue). Scale bar represents 10µm.
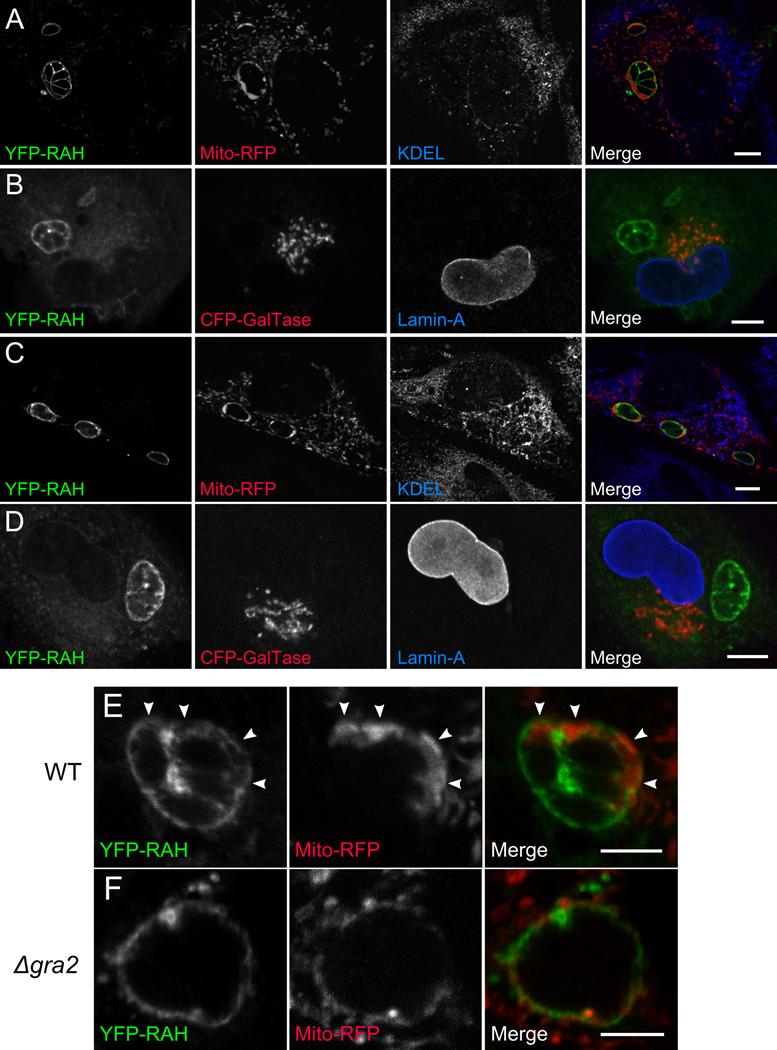
Figure 4. The RAH domain associates specifically with the PVM.
1 µm slices of confocal micrographs of Toxoplasma-infected cells expressing YFP-ROP2RAH. YFP-RAH (green) localizes strongly to the PVM of infected cells, and does not colocalize with (A) the mitochondrial-targeted RFP (red) and the endoplasmic reticular marker KDEL (blue) or (B) the trans-Golgi membrane (CFP-GalTase; red) and the nuclear envelope marker lamin A (blue). (C,D) The same is true for infected cells expressing YFP-ROP5RAH. (E) Even mitochondria associated with the PVM (arrows) do not colocalize with YFP-RAH, nor does YFP-RAH signal intensity correspond to proximity to mitochondria. YFP-RAH localizes not just to the cytosolic-facing PVM, but also to regions reminiscent of the tubular vesicular network of the PV, which is seen as the complex fluorescence “between” parasites. (F) In cells infected with Δgra2 parasites, which have a severely attenuated tubular network, YFP-RAH localizes only to the perimeter of the PV, not to the internal spaces between parasites. Scale bar for (A–D) represents 10 µm. Scale bar for (E–F) represents 5 µm.
To test this idea, we examined the specificity of RAH domain association with various host membranes in uninfected and infected cells by confocal microscopy. We generated constructs to express YFP fusions of the RAH domains from either ROP2 or ROP5 in HFFs , as above . We transfected these constructs into HFFs, which we then examined for the degree of fluorescence colocalization with various host membranous structures labeled by antibodies or targeted fluorescent protein markers. In uninfected cells, the RAH domains gave rise to a punctate, non-diffuse fluorescence as might be expected for proteins associated with membrane; however, they showed limited specificity for the host cellular membranes that we tested (Figure 3). There was minimal specific overlap of RAH signal with host mitochondria, ER, and Golgi, whereas we observed a noticeable increase in YFP-RAH intensity that colocalizes with the nuclear envelope marker lamin A, circumscribing the host nucleus (Figure 3).
We next examined the membrane specificity of YFP-RAH localization in infected cells using the approach just described for uninfected cells. All infected cells we examined had a punctate YFP-RAH fluorescence throughout the cytoplasm, as in uninfected cells. However, the fluorescence around the PVM was much more intense than any other signal in the cell (Figure 4), as we saw in our wide-field imaging (Figure 2). Host ER and mitochondria were found concentrated around the PV (Figure 4a,c), as previously reported (22). In spite of the close proximity of these organelles to the PV, there was surprisingly little colocalization of signal from the RAH domains and host membranes in infected cells. Rather than exhibiting corresponding intensities, we instead observed gaps in the RAH signal around the PVM where mitochondria and ER were clustered, and those regions of PVM with the brightest RAH signal usually appeared largely devoid of associated host organelles (Figure 4a–e).
The PV is distinct from host membranous structures in part due to its extreme negative curvature; a dense network of narrow-bore (60–90nm diameter) membranous tubules protrude into the vacuolar lumen (5, 6). To test whether the RAH domain’s preference for the PVM was due to a specific interaction with this tubular network, we repeated the above colocalization studies using Δgra2 mutant parasites, which have a greatly reduced PVM tubular network (5). The RAH signal on the Δgra2-PVM lacked the bright, complex fluorescence “between parasites” seen in the wild-type PVM (Figure 4e,f), and the association of the RAH with the perimeter of the PVM appeared mildly attenuated. Thus the association of the RAH domain with the regions “between parasites” appears to be dependent on the tubular network, perhaps through a recognition of, and concentration within, the negative curvature of its component tubules.
We then examined the localization of endogenous ROP2-family proteins to verify that the apparent localization of the RAH domains to the tubular network was not an artifact of host cell transfection, of heterologous overexpression, or of YFP-tagging of the protein. We infected host cells with parasites that express ROP5 C-terminally tagged with a 3xFLAG epitope. Previous studies have shown that C-terminal epitope tags do not affect ROP5 localization to the PVM (19). The parasites also express cytosolic RFP (tdTomato, (18)), allowing them to be visualized without disrupting the PVM. We then permeabilized the host cells and stained with anti-FLAG antibody. Figure 5 shows that endogenous ROP5 protein (i.e., expressed and secreted by Toxoplasma) localizes both to the perimeter of the PVM and to regions “between” the parasites. The heterologously expressed YFP-RAH thus faithfully reproduces the localization of the ROP2-family proteins.
Figure 5. Endogenous ROP2-family proteins appear resident in the PVM tubular network.
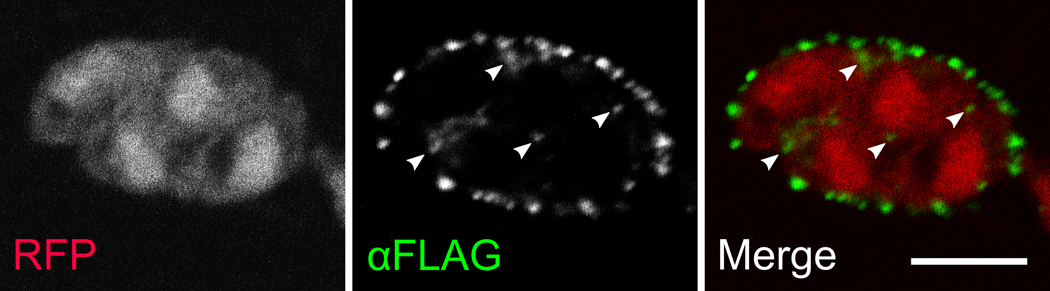
1µm slice through the center of a confocal stack of a Toxoplasma PV. The Toxoplasma strain is an otherwise wild-type strain that transgenically expresses RFP (red) as a cytosolic morphology marker and ROP5 C-terminally tagged with the 3xFLAG epitope (green). Note the typical punctate ROP2-family staining on the PVM, which, like the YFP-RAH, is apparent both on the perimeter of the PVM and in what appears to be the PVM tubular network (arrowheads). Scale bar represents 5µm.
The RAH domain’s ability to associate with membrane is robust to mutation
We next sought to determine the features of the RAH domain that contribute to membrane association and provide specificity to the interaction with the PVM. We created a series of mutants in which individual helices of the ROP5 RAH domain were expressed as N-terminal fusions to mCherry. We then expressed these in HFF, infected with parasites, and tested localization, as above (Figure 6). We also biochemically assayed membrane association of the RAH domain in the absence of other parasite factors. The ROP2-family members are resistant to carbonate extraction from membrane when purified from parasite lysate (23) or when heterologously expressed in host cells (21), which has supported the belief that they are integral membrane proteins. To assay membrane association, each of the mCherry fusion constructs was expressed in uninfected NIH-3T3 cells, the cells were lysed, and soluble and peripheral membrane proteins extracted by treatment with sodium carbonate.
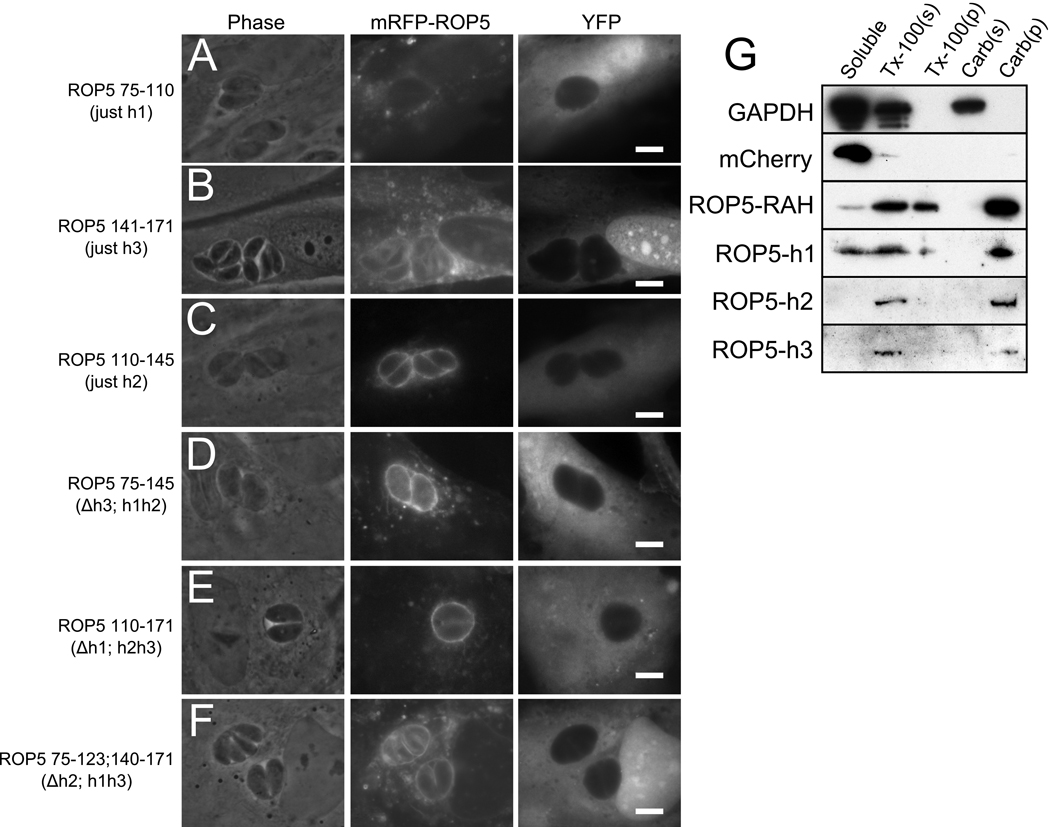
Figure 6. The RAH helices act in concert to bind membrane.
Deletion mutants of mCherry(mRFP)-ROP5RAH were expressed in infected HFF concurrently with soluble YFP as a cytosolic morphology marker. Constructs containing (A) the first helix of ROP5RAH or (B) the third helix are insufficient for strong PVM association; note the high level of cytosolic punctate fluorescence as compared to that localized to the PVM. (C) The second helix of ROP5RAH is sufficient to strongly target the fusion protein to the PVM. Similarly, constructs containing (D) both ROP5RAH helices 1 & 2 or (E) both ROP5RAH helices 2 & 3 show strong localization to the PVM. (F) mRFP fused to ROP5RAH helices 1 & 3 (Δhelix 2) exhibits intermediate specificity for the PVM. (G) Membrane association was assessed biochemically by fractionation. Individual mRFP-ROP5RAH constructs were expressed in uninfected fibroblasts and the soluble fraction was compared to the amount of protein extracted from membrane either with treatment with Triton-x100 or with sodium carbonate. While mCherry (mRFP) is found entirely in the soluble fraction, mRFP-ROP5RAH strongly associates with the membrane. Detergent extracts RAH from membrane, but carbonate does not. Each of the individual RAH helices show similar carbonate-resistant membrane association.
mCherry fusions to either the first or the third helices displayed almost no detectable association with the PVM, but still exhibited punctate fluorescence, suggesting membrane association (Figure 6a,b). Indeed, both the first and third helices of the RAH domain individually caused mCherry to be highly resistant to carbonate extraction suggesting a strong association with membranes (Figure 6g). These constructs were, however, extractable from membrane with the non-ionic detergent Triton-x100, indicating that the proteins were not simply aggregating.
Surprisingly , when a construct containing only the second helix of the ROP5 RAH was expressed in infected HFF, it strongly associated with the PVM (Figure 6c). Like the other two individual helices, the second helix made mCherry resistant to carbonate extraction from the membrane. This indicates that while each of the RAH helices is equally competent to associate with cellular membranes, the specificity for the PVM is largely concentrated in the central region of the RAH. Resistance to carbonate extraction is accepted to indicate an integral rather than peripheral , association with membrane (24, 25), i.e., an association in which the protein is buried, at least in part, into the hydrocarbon core of the bilayer. As the biochemical assays (Figure 6g) were conducted in uninfected cells, the RAH is able to integrate into cellular membrane in the absence of other parasite factors.
We then tested combinations of two of the three helices from the RAH. Not surprisingly, constructs containing both the first and second, or both the second and third helices localized robustly to the PVM, in a pattern that was indistinguishable from the full-length domain (Figure 6d,e). A construct containing only the first and third helices (i.e., with the second helix deleted) displayed weak specificity for the PVM (Figure 6f). Taken together, these data indicate that while the second RAH helix plays the largest role in targeting the PVM, the three RAH helices likely work in concert to achieve the robust membrane association of ROP2-family proteins.
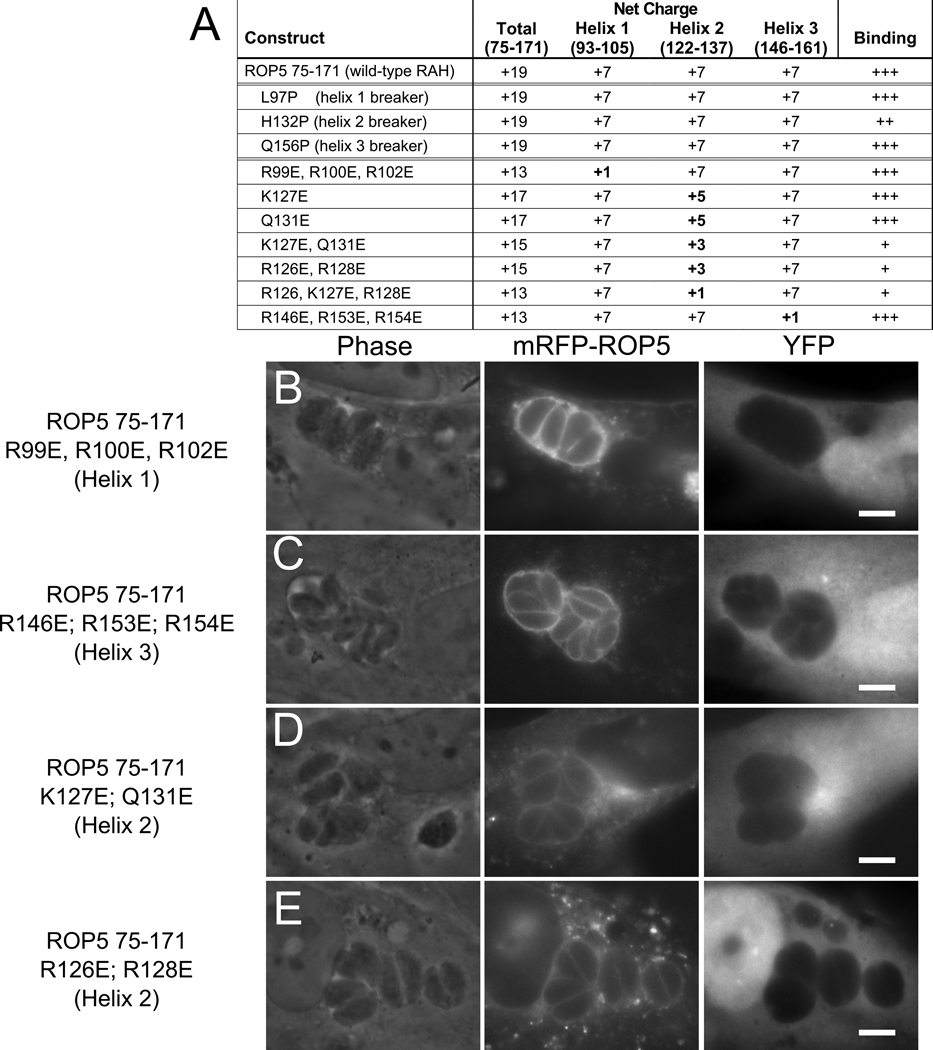
Next, we tested the importance of the charge of the polar face of the predicted helices. The domain is strongly positively charged (ROP5-RAH has a predicted net charge of +19 at neutral pH), and the arginines that provide the majority of this charge are among the most conserved residues in the family (see Figure 1c). This suggests that neutralization of the charge might have drastic effects on the ability of the RAH to associate with membrane. To test this, we created a series of mutations in which basic residues were changed to glutamate (summarized in Figure 7a). Mutations that essentially neutralized the charge of the first or third helices, in the context of a construct containing all three helices, had no effect on the ability of the RAH region to target the fluorescent fusion to the PVM, echoing the effects of deleting either of these two helices (Figure 7b,c). In contrast, mutating combinations of two arginines in the second helix to glutamate, halving its net charge, severely attenuated PVM specificity (Figure 7d,e). While several of the residues mutated are completely conserved in each of the ROP2-family RAH domains, individual mutations had no effect on PVM specificity (data not shown), suggesting that there are cooperative effects and/or that the positions of the mutations are less important than the net charge.
Figure 7. The polar surface of RAH helix 2 encodes PVM-specific binding.
(A) Overview of mutant constructs. The net charge of each construct and a summary of the quality of PVM association are listed. Mutants of mRFP-ROP5RAH were expressed in infected fibroblasts concurrently with soluble YFP as a cellular morphology marker. (B) Protein containing mutations in the first helix (R99E, R100E, R102E) that neutralize the positive charge of the polar face displays wild-type specificity for the PVM. (C) Similar mutations in the third helix (R146E, R153E, R154E) also have no effect on PVM specificity. Mutations in the second helix (D) K127E, Q131E or (E) R126E, R128E, that halve the net charge, attenuate the RAH PVM specificity.
Finally, we tested the degree to which the helicity of the RAH domain contributes to PVM association by creating a “helix-breaker” mutation in each one of the helices through mutating a residue in the middle of the predicted helix to proline (Figure 1a, Figure 7a). When expressed in infected HFF, each of these helix-breaker constructs associated with the PVM similarly to wild-type, though the helix 2 mutant specificity for the PVM was mildly attenuated (data not shown). This further highlights the importance of the second helix in the RAH domain’s targeting of the PVM.
The RAH domain as a predictor of PVM localization
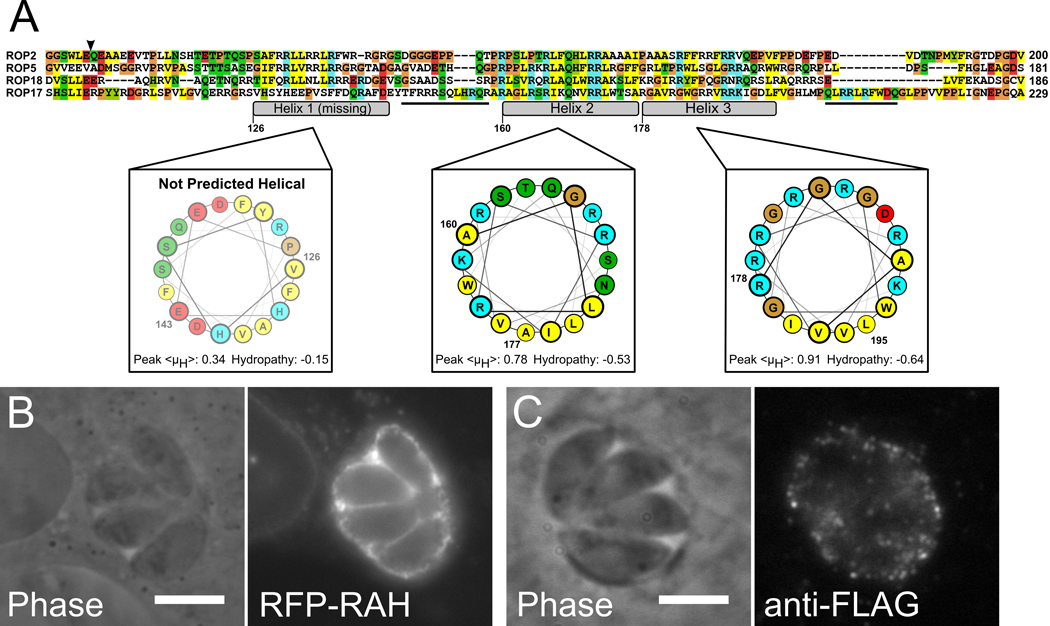
Having determined the characteristics of the ROP2-family RAH that are important for PVM association, we asked whether we could use this information to locate other RAH among Toxoplasma proteins, and thus predict PVM association. Due to the low complexity of the RAH domain sequence, it is difficult to locate significant hits outside of the ROP2-family with either a profiling search (e.g., hidden Markov models) or BLAST (26). We therefore began our search by examining the sequences of Toxoplasma genes that are more distant members of the ROP2-superfamily. While many of the kinase-domain-containing proteins related to the ROP2-family (partially detailed in (7)) have N-termini with low complexity, very few have a substantial enrichment in basic residues (Supplemental Figure S1). In fact, only one protein sequence we examined has a region of sequence that is basic, amphipathic, and predicted to be helical. This gene, ROP17, has a stretch of sequence directly N-terminal to its kinase domain that appears to contain a divergent RAH domain (Figure 8a). The helices of the ROP17-RAH, like the helices of the RAH domains described above, share the physical chemical properties common to membrane-targeting amphipathic helices (as opposed to amphipathic helices that are part of a globular protein fold) (27); they each have a high predicted hydrophobic moment, and a strongly negative mean hydropathy.
Figure 8. An RAH motif correctly predicts PVM association.
A) Alignment of ROP17, a more divergent member of the ROP2-superfamily, with ROP2, ROP5, and ROP18. ROP17 has a divergent RAH that lacks the first amphipathic helix (nor is that region of ROP17 sequence predicted to be helical), but has additional, short arginine-rich segments (underlined sequences) outside of the conserved amphipathic helices. Numbering is according to ROP17 sequence, coloring is as in Figure 1. B) mRFP-ROP17RAH (residues 104–223) expressed in infected host cells strongly localizes to the PVM. C) Cells infected with Toxoplasma that stably express ROP17-3xFLAG show staining for ROP17-3xFLAG on the surface of the PVM.
To test whether ROP17’s N-terminus was, in fact, an RAH domain, we repeated our heterologous expression assay with the ROP17-RAH. When a construct containing the ROP17-RAH (residues 104–223) N-terminally fused to mCherry was expressed in HFF infected with Toxoplasma, the ROP17-RAH caused the mCherry to localize strongly to the PVM (Figure 8b), in a manner indistinguishable from the results with members of the ROP2-family we tested above. We then tested whether native ROP17 secreted by Toxoplasma exhibited similar localization to the PVM. The ROP17 locus was endogenously tagged with Venus-His63xFLAG. The tagged protein was correctly trafficked to the rhoptries, and secreted into the host cell, where it associated with the PVM and stained in a punctate pattern reminiscent of staining with other members of the ROP2 family (Figure 8c). Conversely, the N-termini for two of the members of the ROP2-superfamily that we identified as not containing RAH domains (ROP2L6 and ROP2L5) did not have affinity for membrane when heterologously expressed fused to mCherry in HFF (Figure S2). Similarly, while tagged versions of these latter two proteins expressed in Toxoplasma correctly trafficked to the rhoptries, no PVM staining was observed in infected cells (data not shown). Thus possession of an RAH domain appears to be a good predictor of a Toxoplasma protein’s localization to the PVM.
Discussion
We have delineated a novel membrane interaction domain in the ROP2-family that displays remarkable specificity for the PVM. Through mutagenesis, we have demonstrated that the membrane association is driven by series of arginine-rich amphipathic helices and that the majority of the specificity for the PVM is encoded in a single membrane-associating helix.
Amphipathic proteins generally exist in thermodynamic equilibrium between soluble and membrane-associated states. An amphipathic helix is a common structural motif that often mediates reversible , regulated protein association with cellular membranes (14). ROP2-family proteins are initially secreted into the host cytosol upon Toxoplasma invasion and must then associate with the PVM as it forms (4, 11). For such proteins, a membrane association motif that allows the proteins to exist transiently in a soluble state before binding membrane has clear benefits.
Highly basic amphipathic helices, like those found in the RAH domain, sometimes preferentially interact with negatively charged phospholipids (14, 15). This interaction represents an opportunity for regulation of membrane association. For instance, modulation of membrane charge by second messengers such as phosphatidylinositol-4,5-bisphosphate can direct membrane association of a protein to the site of a signaling event (15, 16). The RAH domain of the ROP2 family is able to interact weakly with a variety of host membranes (Figure 3), suggesting that it lacks a clear preference for a particular phospholipid in uninfected cells. However, the RAH shows a remarkable specificity for the PVM, which, at least initially, is composed largely, if not entirely, from membrane derived from the host plasma membrane.
Another means of regulating the membrane association of amphipathic helices is modulation of bulk electrostatics through such mechanisms as phosphorylation (28). The regions preceding the first and second helices in each of the ROP2-family RAH domains are enriched in serines and threonines, and at least one member of the family, ROP4, has been shown to be extensively phosphorylated (29). Such phosphorylation would be expected to alter the affinity of an RAH-containing protein for membrane and/or the protein’s conformation on the membrane.
Ultimately , the RAH membrane association is resistant to extraction with a basic carbonate solution, which disrupts charge-charge interactions. This observation suggests that the major driving force of its final membrane interaction is hydrophobic, rather than primarily electrostatic. Such an interaction model is consistent with biophysical studies that demonstrate amphipathic helices integrate their hydrophobic faces into the hydrocarbon core of a bilayer on which they “float” (30). Insertion of the RAH helices into the membrane is supported by the previous observation that the RAH domain is resistant to proteolysis when associated with membrane (21). The model also reconciles the observation that an antibody that recognizes an epitope in the RAH domain (21) does not recognize the ROP2-family once it has associated with the PVM (without disruption of the PVM with solvent, e.g., acetone) (12), presumably as it is buried in the membrane. In further support of the importance of hydrophobic forces to the RAH domain’s membrane interaction, our mutagenesis experiments that eliminated the positive charge on either the first or third helix of the RAH had no effect on PVM association. However, mild mutation of the basic residues in the second helix severely attenuated specificity for the PVM, though not membrane association, suggesting that the RAH specificity for the PVM is driven, at least in part, by electrostatic forces.
In comparing RAH-localization at the PVM to other host cell membranes, we observed little specific colocalization of the RAH with markers for host Golgi, ER, or mitochondria. The lack of localization between the RAH and these host organelles suggests that the RAH domains of the ROP2-family are not the primary mediators of the interaction of host organelles with the PVM, in contrast to previous models (21). However, as we have demonstrated that the multiple helices of the RAH are individually capable of associating with membrane, the RAH could stabilize host organellar interactions with the PVM by individually associating with, and thus “bridging,” different membranes.
The mechanism of RAH specificity for the PVM remains unknown. It is possible that specificity for the PVM is due to an interaction with an unidentified parasite-dependent factor, such as another ROP or a specific lipid anchored to, or integral within, the PVM. Our data are also consistent with an alternative model in which the RAH recognizes a specific physical property of the PVM that host membranes lack. We have shown that the RAH has the intrinsic ability to integrate into membrane in the absence of other parasite factors. Further, we found that the RAH localizes to regions of the PV “between” parasites dependent on the presence of the tubular membrane network, and that disrupting this network attenuates the RAH’s localization to the PVM. It is thus possible that the RAH achieves its specificity for the PVM by recognizing the strong negative curvature of the narrow-bore tubular network. Intriguingly, the host membrane with which the RAH had the strongest colocalization, the nuclear envelope, also has membranous tubular invaginations (31), though not as extensive as the PVM.
Amphipathic helix motifs are used by many proteins to recognize and respond to membrane curvature. BAR-domain containing proteins such as endophilin and amphiphysin recognize and stabilize positive curvature during endocytosis (32, 33). The amphipathic helix of CTP:Phosphocholine cytidylyltransferase recognizes negative curvature in binding membrane (34, 35). In addition, Toxoplasma GRA2 has predicted amphipathic helices which have been implicated in formation of the PVM tubular network (36).
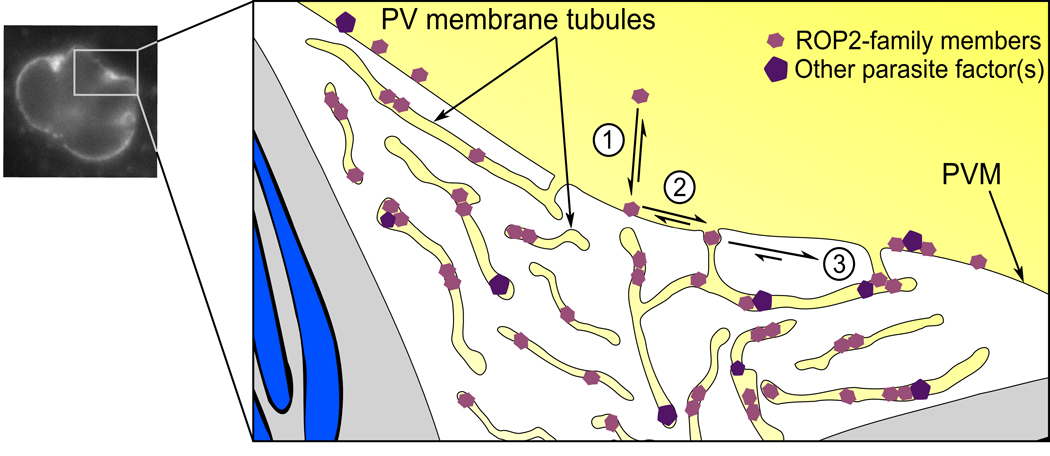
The tubular network is a dynamic structure that forms shortly after invasion of a host cell (5, 6). Interestingly, in our study, both the soluble ΔRAH constructs and YFP were observed to weakly localize “between” parasites in regions that appear as if internal to the PV (Figure 2b,c). We found both endogenous ROP2-family protein and heterologously expressed YFP-RAH localized to these “between parasite” regions (Figure 4f, Figure 5). However, we did not observe this “ between parasite” localization of YFP-RAH or soluble YFP in cells infected with Δgra2 parasites (Figure 4f and data not shown). This suggests that rather than the “between parasite” signal being soluble protein free in the PV space, it is due to protein within the tubular network. That is, this network, which is topologically cytosolic, may preferentially attract molecules from the cytosolic face of the PVM that have an affinity for extreme negative curvature (Figure 9). Molecules that have been recruited to the PVM tubules may be retained there by two cooperative mechanisms. First, interaction with potential binding partners would reduce both the off-rate from the membrane and the rate of diffusion. Simultaneously, the narrow, elongated tubules would constrain the avenues of diffusion, promoting interactions and reducing the likelihood of a given molecule’s escape (Figure 9). Such a model would explain the remarkable specificity for the ROP2-family for the PVM.
Figure 9. Model for ROP2-family interaction with the PVM tubular network.
A schematic of a Toxoplasma PV with the parasites in grey, their unsecreted rhoptries blue, and the host cytosolic space yellow. Note that though the PV tubular network spreads through the vacuole, it is topologically cytosolic. 1. RAH-containing proteins (pink hexagons), due to their amphipathic nature, can transition between soluble and membrane associated states. 2. However, they appear to preferentially associate with negatively curved membranes, such as the PV membrane tubules. 3. Once recruited to the tubular network, they may associate with other proteins (purple pentagons), reducing their off-rate from the membrane. In addition, the narrow tubules may limit the diffusion of their resident proteins, helping retain them.
The members of the ROP2-family have evolved in the context of the interplay between host and parasite and the RAH domain’s interaction with the PVM situates them at the literal interface of this dialog. There appear to be many evolutionary advantages to using a low sequence complexity domain such as the RAH to determine localization. First, there is no requirement for the evolution or maintenance of a complex three-dimensional fold. Also, we have determined that the PVM specificity is encoded in a short sequence of ca. 30 amino acids. Thus, such a domain is both initially simple to evolve and easy to diverge into different functionalities as a family expands. Furthermore, a small, contiguous domain can easily be swapped to other genes. Amphipathic helices have the additional advantage that they require no ATP for integration into the membrane, and are thus energetically efficient. It is interesting to note the enrichment for Arg in the RAH coexists with a relative absence of Lys. This may be due, in part, to a selection against sequences that could be misinterpreted as nuclear localization signals, an additional function that is associated with other basic amphipathic helices (15). Indeed, at least one member of the ROP2-superfamily, ROP16, that is not found associated with the PVM and that does not have a RAH domain, has evolved a lysine-rich nuclear localization signal in its N-terminus (8).
Overall, the results presented here demonstrate that the ROP2-family has evolved a relatively simple but potent mechanism for associating with membranes, especially the PVM. Further understanding of how this association facilitates their role in the parasite’s intracellular lifestyle will require identification of their binding partners and/or the targets of their kinase activity.
Materials and Methods
Plasmid construction and PCR
Primers are listed in Supplemental Figure S3. PCR was carried out using Phusion DNA polymerase (NEB) unless otherwise noted. ROP2-family constructs were amplified from genomic Toxoplasma RH-strain DNA. For N-terminal fusions to fluorescent protein, inserts were cloned into pCDNA3.1(+) (Invitrogen) containing either Venus or mCherry cloned 5’ of the multi-cloning site. For C-terminal fusions, inserts were cloned into pEGFP-N1 (Clonetech), where the eGFP had been replaced with Venus. ROP5 deletion constructs were made by inverse PCR from pcDNA3 . 1-mCherry-ROP5. Mutagenesis of ROP5 was carried out according to the Quikchange protocol, using Pfu-Ultra polymerase (Strategene). To create the construct for the endogenous tagging of ROP17, 5’ and 3’ targeting sequences were amplified from genomic Toxoplasma DNA and cloned into pTVFTAP. pTVFTAP contains an HPRT-cassette flanked by multi-cloning sites (MCS) for 5’ and 3’ targeting sequences. The 5’ MCS is in frame with sequence encoding a Venus-His63xFLAG tag. To create the constructs for the tagging of ROP5, ROP2L5, and ROP2L6, the coding sequence and promoter region (600bp upstream) were cloned into pHPRT (9) in frame with a sequence encoding a 3xFLAG tag.
Parasite and Host Cell Culture
HFF and NIH-3T3 were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Invitrogen) and 2 mM glutamine. Toxoplasma tachyzoites were maintained in confluent monolayers of HFF. Transgenic parasites strains were made by electroporating RH(ΔHPRT) parasites with 15µg of linearized plasmid encoding the construct of interest and selecting for HPRT-positive parasites, as previously described (37). Clonal parasites were grown from populations by limiting dilution.
Host Cell Transfection and Fluorescence Microscopy
HFF were grown to 80–90% confluency on coverslips in 24 well plates. Cells were transfected with lipofectamine-LTX reagent (Invitrogen) according to the manufacturer’s instructions . 500ng of ROP2-family fusion protein, YFP, or CFP-GalTase (38) DNA was used per well. 125ng/well of MitoRFP (39) plasmid was used to reduce fluorescence bleedthrough. Cells were incubated with transfection reagent and DNA for 6–8h, the media changed, and parasites added, as appropriate. Cells were fixed 24–30h post-transfection for 10m in phosphate buffered saline (PBS) with 2.5% formaldehyde. For immunofluorescent analysis, cells were permeabilized for 5m in PBS, 0.1% Triton-x100, 3% BSA. Cells were then blocked for 1h in PBS, 0.01% saponin, 3% BSA. Cells were incubated for 2h at room temperature with mouse monoclonal antibodies against lamin A (Abcam) or KDEL (Stressgen) at 1:100 or 1:500 dilution, respectively, in PBS, 3% BSA. Cells were washed 3 times with PBS and incubated for 1h with Alexa647-conjugated secondary antibody (Molecular Probes). Cells were again washed 3 times with PBS and mounted in VectaShield (Vector). Wide-field images were captured at 100x on an Olympus BX60 and a Hamamatsu Orca100 CCD. Confocal micrographs were taken as stacks of 1µm slices using a 63x objective on a Zeiss LSM 510. All images were analyzed with ImageJ (40).
Carbonate Extraction Assay of Membrane Association
Low passage NIH-3T3 cells were allowed to grow to 80% confluency in 6-well plates. Cells were transfected with lipofectamine LTX, as above, using various mCherry-ROP5 fusions expressed in pCDNA3.1. After 24h, cells were washed twice with ice cold PBS, and 2 wells of cells were scraped into 100µl ice cold lysis buffer (50mM Tris pH 8.0, 150mM NaCl) with complete protease inhibitor cocktail (Roche). All subsequent manipulation was performed at 4°C. Cells were mechanically lysed by repeated passage through a 27 gauge needle. Lysate was sedimented for 10m at maximum speed in a microcentrifuge. The soluble fraction was removed and the pellet resuspended in 100µl lysis buffer and split equally, then sedimented. The soluble fractions were combined with the initial soluble lysate and the two pellets resuspended in 200µl of either lysis buffer with 1% Triton-x100 or 0.1M Na2CO3. The samples were again sedimented and soluble fractions removed. Pellets were resuspended in SDS-PAGE loading buffer. Equal volumes of each sample were loaded on an SDS-PAGE for western blot. After transfer to PVDF membrane (Millipore), the membranes were blocked for 1–2h in TBST + 5% milk and then incubated with mouse monoclonal anti-dsRed (Abcam) in TBST + 3% BSA overnight at 4°C. Membranes were washed with PBS and incubated for 1h with horseradish-peroxidase conjugated secondary antibody in TBST. Membranes were again washed with PBS and signal visualized with ECL reagent (Pierce).
Supplementary Material
Acknowledgements
This work was supported in part by grants from the NIH (AI21423 and AI73756). MLR was supported in part by a fellowship from the Amercian Cancer Society. The mitoRFP, CFP-GalTase, pCDNA(Venus), and pCDNA(mCherry) vectors were kind gifts from S. Finkbeiner. Δgra2 parasites were a kind gift of L.D. Sibley. We thank S. Finkbeiner and E. LaDow for microscopy advice, the Boothroyd lab for helpful discussion, and CEC, SME, AAK, SEK, ESL, and RCY for critical reading of the manuscript.
References
- 1.Baekkeskov S, Kanaani J. Palmitoylation cycles and regulation of protein function (Review) Mol Membr Biol. 2009:1–13. doi: 10.1080/09687680802680108. [DOI] [PubMed] [Google Scholar]
- 2.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9(2):99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 3.Charron AJ, Sibley LD. Molecular partitioning during host cell penetration by Toxoplasma gondii. Traffic. 2004;5(11):855–867. doi: 10.1111/j.1600-0854.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- 4.Boothroyd JC, Dubremetz JF. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol. 2008;6(1):79–88. doi: 10.1038/nrmicro1800. [DOI] [PubMed] [Google Scholar]
- 5.Mercier C, Dubremetz JF, Rauscher B, Lecordier L, Sibley LD, Cesbron-Delauw MF. Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced by parasite proteins. Mol Biol Cell. 2002;13(7):2397–2409. doi: 10.1091/mbc.E02-01-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibley LD, Niesman IR, Parmley SF, Cesbron-Delauw MF. Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host-cell vacuoles occupied by Toxoplasma gondii. J Cell Sci. 1995;108(Pt 4):1669–1677. doi: 10.1242/jcs.108.4.1669. [DOI] [PubMed] [Google Scholar]
- 7.El Hajj H, Demey E, Poncet J, Lebrun M, Wu B, Galeotti N, Fourmaux MN, Mercereau-Puijalon O, Vial H, Labesse G, Dubremetz JF. The ROP2 family of Toxoplasma gondii rhoptry proteins: Proteomic and genomic characterization and molecular modeling. Proteomics. 2006;6(21):5773–5784. doi: 10.1002/pmic.200600187. [DOI] [PubMed] [Google Scholar]
- 8.Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445(7125):324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saeij JP, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, Ajioka JW, Boothroyd JC. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314(5806):1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, Beatty WL, Hajj HE, Jerome M, Behnke MS, White M, Wootton JC, Sibley LD. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314(5806):1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 11.Bradley PJ, Sibley LD. Rhoptries: an arsenal of secreted virulence factors. Curr Opin Microbiol. 2007;10(6):582–587. doi: 10.1016/j.mib.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckers CJ, Dubremetz JF, Mercereau-Puijalon O, Joiner KA. The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J Cell Biol. 1994;127(4):947–961. doi: 10.1083/jcb.127.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8(1):74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 14.Cornell RB, Taneva SG. Amphipathic helices as mediators of the membrane interaction of amphitropic proteins, and as modulators of bilayer physical properties. Curr Protein Pept Sci. 2006;7(6):539–552. doi: 10.2174/138920306779025675. [DOI] [PubMed] [Google Scholar]
- 15.Winters MJ, Lamson RE, Nakanishi H, Neiman AM, Pryciak PM. A membrane binding domain in the ste5 scaffold synergizes with gbetagamma binding to control localization and signaling in pheromone response. Mol Cell. 2005;20(1):21–32. doi: 10.1016/j.molcel.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314(5804):1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20(1):87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- 18.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22(12):1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 19.El Hajj H, Lebrun M, Fourmaux MN, Vial H, Dubremetz JF. Inverted topology of the Toxoplasma gondii ROP5 rhoptry protein provides new insights into the association of the ROP2 protein family with the parasitophorous vacuole membrane. Cell Microbiol. 2006 doi: 10.1111/j.1462-5822.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 20.El Hajj H, Lebrun M, Arold ST, Vial H, Labesse G, Dubremetz JF. ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog. 2007;3(2):e14. doi: 10.1371/journal.ppat.0030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinai AP, Joiner KA. The Toxoplasma gondii protein ROP2 mediates host organelle association with the parasitophorous vacuole membrane. J Cell Biol. 2001;154(1):95–108. doi: 10.1083/jcb.200101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinai AP, Webster P, Joiner KA. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J Cell Sci. 1997;110:2117–2128. doi: 10.1242/jcs.110.17.2117. [DOI] [PubMed] [Google Scholar]
- 23.Bradley PJ, Ward C, Cheng SJ, Alexander DL, Coller S, Coombs GH, Dunn JD, Ferguson DJ, Sanderson SJ, Wastling JM, Boothroyd JC. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J Biol Chem. 2005;280(40):34245–34258. doi: 10.1074/jbc.M504158200. [DOI] [PubMed] [Google Scholar]
- 24.Fujiki Y, Hubbard AL, Fowler S, Lazarow PB. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howell KE, Palade GE. Hepatic Golgi fractions resolved into membrane and content subfractions. J Cell Biol. 1982;92(3):822–832. doi: 10.1083/jcb.92.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- 28.Strickfaden SC, Winters MJ, Ben-Ari G, Lamson RE, Tyers M, Pryciak PM. A mechanism for cell-cycle regulation of MAP kinase signaling in a yeast differentiation pathway. Cell. 2007;128(3):519–531. doi: 10.1016/j.cell.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carey KL, Jongco AM, Kim K, Ward GE. The Toxoplasma gondii rhoptry protein ROP4 is secreted into the parasitophorous vacuole and becomes phosphorylated in infected cells. Eukaryot Cell. 2004;3(5):1320–1330. doi: 10.1128/EC.3.5.1320-1330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Low C, Weininger U, Lee H, Schweimer K, Neundorf I, Beck-Sickinger AG, Pastor RW, Balbach J. Structure and dynamics of helix-0 of the N-BAR domain in lipid micelles and bilayers. Biophys J. 2008;95(9):4315–4323. doi: 10.1529/biophysj.108.134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fricker M, Hollinshead M, White N, Vaux D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J Cell Biol. 1997;136(3):531–544. doi: 10.1083/jcb.136.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farsad K, Ringstad N, Takei K, Floyd SR, Rose K, De Camilli P. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol. 2001;155(2):193–200. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303(5657):495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 34.Attard GS, Templer RH, Smith WS, Hunt AN, Jackowski S. Modulation of CTP:phosphocholine cytidylyltransferase by membrane curvature elastic stress. Proc Natl Acad Sci U S A. 2000;97(16):9032–9036. doi: 10.1073/pnas.160260697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies SM, Epand RM, Kraayenhof R, Cornell RB. Regulation of CTP: phosphocholine cytidylyltransferase activity by the physical properties of lipid membranes: an important role for stored curvature strain energy. Biochemistry. 2001;40(35):10522–10531. doi: 10.1021/bi010904c. [DOI] [PubMed] [Google Scholar]
- 36.Mercier C, Cesbron-Delauw MF, Sibley LD. The amphipathic alpha helices of the toxoplasma protein GRA2 mediate post-secretory membrane association. J Cell Sci. 1998;111(Pt 15):2171–2180. doi: 10.1242/jcs.111.15.2171. [DOI] [PubMed] [Google Scholar]
- 37.Donald RG, Carter D, Ullman B, Roos DS. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J Biol Chem. 1996;271(24):14010–14019. doi: 10.1074/jbc.271.24.14010. [DOI] [PubMed] [Google Scholar]
- 38.Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003;23(15):6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23(15):5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11(7):36–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.