Abstract
Pulmonary arterial hypertension (PAH) is a significant disease process characterized by elevated pulmonary vascular resistance leading to increased right ventricular afterload and ultimately progressing to right ventricular dysfunction and often death. Irreversible remodeling of the pulmonary vasculature is the hallmark of pulmonary hypertension and frequently leads to progressive functional decline in patients with PAH despite treatment with currently available therapies. Metabolites of the arachidonic acid cascade play an important homeostatic role in the pulmonary vasculature and dysregulation of pathways downstream of arachidonic acid play a central role in the pathobiology of PAH. Cyclooxygenase-2 (COX-2) is upregulated in pulmonary artery smooth muscle cells (PASMC) and inflammatory cells during hypoxia and plays a protective role in the lung’s response to hypoxia. We recently demonstrated that absence of COX-2 was detrimental in a mouse model of hypoxia-induced pulmonary hypertension. Exposure of COX-2 null mice to hypoxia resulted in severe pulmonary hypertension characterized by enhanced pulmonary vascular remodeling and significant upregulation of the ET-1 receptor (ETAR) in the lung following hypoxia. Absence of COX-2 in vitro led to enhanced contractility of PASMC following exposure to hypoxia that could be attenuated by iloprost, a prostaglandin I2 analog. These findings suggest that selective inhibition of COX-2 may have detrimental pulmonary vascular consequences in patients with pre-existing pulmonary hypertension or underlying hypoxemic lung diseases. Here we discuss our recent data demonstrating the adverse consequences of COX-2 inhibition on pulmonary vascular remodeling and PASMC contractility.
Introduction
Pulmonary hypertension (PH) is a severe and frequently fatal disease characterized by an elevation in mean pulmonary arterial (PA) pressure greater than 25 mm Hg at rest or greater than 30 mm Hg with exercise (Farber and Loscalzo 2004). The etiology of pulmonary hypertension based on the most recent World Health Organization (WHO) classification (Simonneau et al. 2004) includes 5 categories: 1) pulmonary arterial hypertension (PAH), 2) PH associated with left heart disease, 3) PH associated with lung disease and/or hypoxemia, 4) PH associated with chronic thromboembolic disease and 5) PH associated with other disorders. Category 1, PAH, includes idiopathic PAH, familial PAH, PAH associated with collagen vascular disease, congenital systemic-to-pulmonary shunts, portal hypertension, HIV, drugs/toxins and other conditions such as hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome), glycogen storage diseases, and hemoglobinopathies. Familial forms of idiopathic pulmonary hypertension have recently been identified and are associated with genetic mutations in members of the TGF-β receptor family [bone morphogenetic protein receptor 2 (BMPR2) and activin-receptor-like kinase (ALK1)] and allelic variants of the serotonin transporter 5-HTT (Said 2006).
The hallmark of PH is the development of increased pulmonary vascular resistance leading to increased right ventricular afterload and ultimately progression to right heart failure and death if untreated. The mechanisms by which low resistance arterioles in the pulmonary circulation narrow include pulmonary vascoconstriction, in situ thrombosis, and pulmonary vascular remodeling (Farber and Loscalzo 2004). Vascular remodeling involves pathological changes in all three layers of the pulmonary arteries (Stenmark and Mecham 1997). These changes include endothelial dysfunction with release of vasoactive mediators, growth factors, and cytokines; smooth muscle cell hyperplasia and hypertrophy with resultant medial wall thickening; and adventitial fibroblast proliferation, extracellular matrix deposition, and myofibroblast differentiation (Stenmark and Mecham 1997).
Mechanisms of PAH
Recent genetic studies of patients with familial PAH strongly support a genetic association in the pathogenesis of PAH. Most notably, BMPR2 mutations account for 70% of patients with familial PAH and 10–30% of patients with idiopathic PAH (Aldred et al. 2006). As the penetrance of BMPR2 mutations is only 20% in familial PAH, it is likely that, in addition to genetic predisposition additional physiologic or acquired factors (e.g. chronic hypoxia, female gender, anorexigens) are necessary for the development of PAH. Downstream of these “multiple hits”, endothelial dysfunction results in an imbalance of multiple vascular effectors (Said 2006) leading to dysregulation of smooth muscle cell growth and proliferation, vasoconstriction, and intravascular thrombosis. Endothelial injury leads to release of potent vasoconstrictors, including thromboxane A2 (TXA2) and endothelin-1 (ET-1), which can overwhelm the effects of endothelial-derived vasodilators such as prostacyclin (PGI2) and nitric oxide (NO), thereby promoting remodeling of the arteriolar wall (Farber and Loscalzo 2004; Stenmark and Mecham 1997). Current therapy for PAH targets three of these dysregulated pathways, including the PGI2, ET-1, and NO pathways (Figure 1) (Humbert et al. 2004). However, imbalance of other mediators, including serotinin (Eddahibi et al. 2000), 5-lipoxygenase (5-LO) (Jones et al. 2004), vasoactive intestinal peptide (VIP) (Said et al. 2007), vascular endothelial growth factor (VEGF) (Voelkel et al. 2006), platelet-derived growth factor (PDGF) (Voelkel et al. 2006), adrenomedullin (Matsui et al. 2004), and angiopoietin-1 (Sullivan et al. 2003) have also been observed in patients and implicated in animal models of PAH. More recently, emerging therapeutic targets for treatment of PAH include Rho kinase inhibition (Oka et al. 2008), voltage gated K+ (Kv) channel openers (Michelakis et al. 2002), elastase inhibition (Cowan et al. 2000), anti-inflammatory therapy/NFAT (nuclear factor of activated T cells) inhibition (Bonnet et al. 2007), survivin inhibition (McMurtry et al. 2005), hypercapnia (Kantores et al. 2006), and progenitor cell regenerative therapy (Zhao et al. 2005).
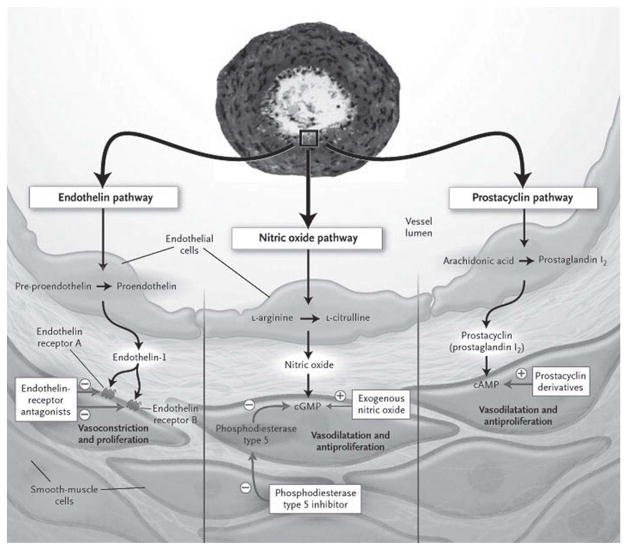
Figure 1.
Current therapeutic targets in pulmonary arterial hypertension. Current therapy for PAH targets imbalance of three mediators including ET-1, NO, and PGI2. These pathways become dysregulated in patients with PAH, leading to enhanced vasoconstriction, in situ thrombosis, and pulmonary vascular remodeling. A transverse section of a remodeled pulmonary artery in a patient with PAH is shown, demonstrating intimal proliferation and medial thickening. Patients with PAH have increased levels of the potent vasoconstrictor ET-1 and decreased levels of endothelial vasodilators NO and PGI2 leading to aberrant PASMC proliferation and hypertrophy. Current therapy with endothelin-receptor antagonists, phosphodiesterase inhibitors, and prostacylin analogues aims to reestablish the balance of these vascular effectors. Plus signs (+) indicate an increase in the concentration; minus signs (−) denote a decrease in the concentration, inhibition of an enzyme, or blockage of a receptor. cGMP, cyclic guanosine monophospate; cAMP, cyclic adenosine monophospate. Reproduced from (Humbert et al. 2004) by permission of the Massachusetts Medical Society. Copyright © [2004] Massachusetts Medical Society. All rights reserved.
The arachidonic acid cascade and PH
The arachidonic acid cascade plays a central role in homeostasis of the endothelium and vascular smooth muscle cells and dysregulation of pathways downstream of arachidonic acid have been observed in patients with PAH and are associated with development of PAH in animal models. The cyclooxygenase enzymes (COX-1 and COX-2) catalyze the conversion of arachidonic acid to the intermediate prostaglandin H2, which is then converted to a series of prostanoids by cell-specific synthases (FitzGerald and Loll 2001; Funk 2001) (Figure 2). Whereas COX-1 is constitutively expressed, COX-2 is inducible and is upregulated by many pro-inflammatory stimuli (Murakami and Kudo 2004) and by hypoxia (Chida and Voelkel 1996; Pidgeon et al. 2004). Cyclooxygenase-derived prostanoids play a key role in the pathophysiology of pulmonary vascular disease. PGI2 is the major arachidonic acid metabolite of the vascular endothelium, and an imbalance between PGI2 and thromboxane A2 (TXA2) has been demonstrated in patients with both idiopathic and secondary forms of pulmonary hypertension (Christman et al. 1992). Overexpression of PGI2 synthase in the lung protects against the development of hypoxia-induced pulmonary hypertension in mice (Geraci et al. 1999) and continuous administration of prostacyclin to patients with PAH extends survival and improves quality of life (Rubin et al. 1990). Furthermore, deletion of the PGI2 receptor exacerbates vascular remodeling in a mouse model of hypobaric hypoxia-induced pulmonary hypertension (Hoshikawa et al. 2001).
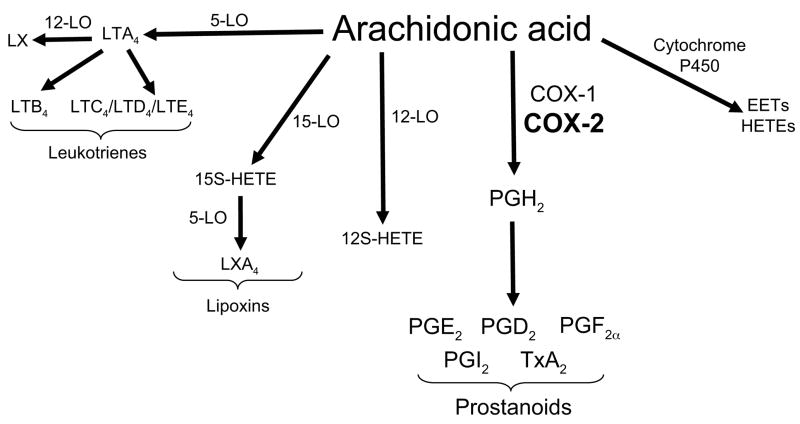
Figure 2.
Metabolic pathways of arachidonic acid metabolism. Arachidonic acid is metabolized via three major enzymatic pathways: cyclooxygenases, lipoxygenases, and cytochrome P450 to generate prostanoids, leukotrienes, and hydroxyeicosatetraenoic acids (HETEs) and epoxyeicosatrienoic acids (EETs), respectively. The COX-2, 5-LO, and 12-LO pathways have been demonstrated to play an important role in pulmonary hypertension. LT, leukotriene; PG, prostaglandin; TxA2, thromboxane A2; LO, lipoxygenase; COX, cyclooxygenase; LX, lipoxin; HETE, hydroxyeicosatetraenoic acid; EETs, epoxyeicosatrienoic acids.
In addition to the COX pathway, emerging data suggest that alternative arachidonic acid metabolism via the 5-LO, 12-LO, and 15-LO pathways (Jones et al. 2004; Preston et al. 2006) (Figure 2) may play pivotal roles in the vascular remodeling process as well. 5-LO catalyzes conversion of arachidonic acid to leukotriene A4 (LTA4), which is subsequently converted to the potent chemoattractant LTB4 and the cysteinyl leukotrienes (cysLT; LTC4, LTD4, and LTE4), which may increase vascular permeability and accelerate pulmonary artery endothelial cell (PAEC) proliferation in the setting of endothelial cell injury (Walker et al. 2002). 5-LO expression is increased in PAEC in patients with PAH (Wright et al. 1998) and in a rat model of hypoxia-induced PH (Voelkel et al. 1996). Pharmacologic inhibition of 5-LO (Morganroth et al. 1984) or 5-LO activating protein (FLAP) (Voelkel et al. 1996), as well as antagonism of the cysLT receptor (Morganroth et al. 1985), attenuates hypoxia-induced PH in animal models. Similarly, deficiency of 5-LO (Voelkel et al. 1996) reduced hypoxia-induced RVH in mice, while overexpression of 5-LO accelerated monocrotaline-induced PH in rats (Jones et al. 2004). More recently, it has been shown that adenoviral overexpression of 5-LO in heterozygous BMPR2 mice exposed to monocrotaline resulted in increased inflammation and exacerbation of PH compared with wild-type mice (Song et al. 2008).
The 12-LO pathway has also recently been identified as contributing to PASMC proliferation and thus may exacerbate pulmonary vascular remodeling. 12-LO is upregulated in lungs of hypoxic rats and in PASMC exposed to hypoxia (Preston et al. 2006). Administration of 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE) to PASMC increased proliferation in vitro, while 12-LO inhibition attenuated PASMC proliferation (Preston et al. 2006), suggesting a potential role for 12-LO and its metabolite 12(S)-HETE in hypoxia-induced pulmonary vascular remodeling.
COX-2 and vascular remodeling
Selective inhibition of COX-2 is associated with an increased incidence of myocardial infarction and stroke in patients with cardiovascular risk factors. (Grosser et al. 2006; Kearney et al. 2006). In addition, selective COX-2 inhibition in a rat model of hypobaric hypoxia-induced pulmonary hypertension (Pidgeon et al. 2004) led to enhanced platelet deposition and intravascular thrombosis. Recent studies have also demonstrated accelerated atherosclerosis (Cheng et al. 2002) and vascular remodeling in mice lacking the PGI2 receptor (Rudic et al. 2005). Deletion of the PGI2 receptor or selective COX-2 inhibition enhances vascular hyperplasia and remodeling of the systemic vasculature in murine models of transplant arteriosclerosis and flow-dependent vascular remodeling (Rudic et al. 2005). These potential vascular sequelae associated with pharmacologic COX-2 inhibition appear to arise from alterations in multiple vascular effectors, including PGI2 and PGE2, which may directly or indirectly modulate platelet function, vascular tone, and remodeling (Grosser et al. 2006). Selective COX-2 inhibition may thus perturb the complex balance of vascular mediators and promote vascular remodeling and/or a pro-thrombotic state in susceptible patients (Grosser et al. 2006).
COX-2 and hypoxia-induced pulmonary hypertension and vascular remodeling
Given the potential consequences of COX-2 inhibition on the systemic vasculature, we examined the effect of COX-2 deficiency on the development of pulmonary hypertension and vascular remodeling in a mouse model of chronic hypoxia. We exposed COX-2 deficient (COX-2−/−) and wild-type (COX-2+/+) mice to 2 weeks of normobaric hypoxia or normoxia. Absence of COX-2 led to exaggerated elevation of right ventricular systolic pressure (RVSP) (Figure 3A) and severe right ventricular hypertrophy (RVH) following chronic hypoxia (Fredenburgh et al. 2008). Similarly, pharmacologic inhibition of COX-2 with nimesulide led to an exaggerated response to hypoxia with elevated RVSP and profound RVH in wild-type mice following hypoxia. In addition, deficiency (Figure 3B–C) or pharmacologic inhibition of COX-2 (Fredenburgh et al. 2008) was associated with accelerated vascular remodeling characterized by PASMC hypertrophy as well as significant upregulation of the endothelin A receptor (ETA) during exposure to hypoxia.
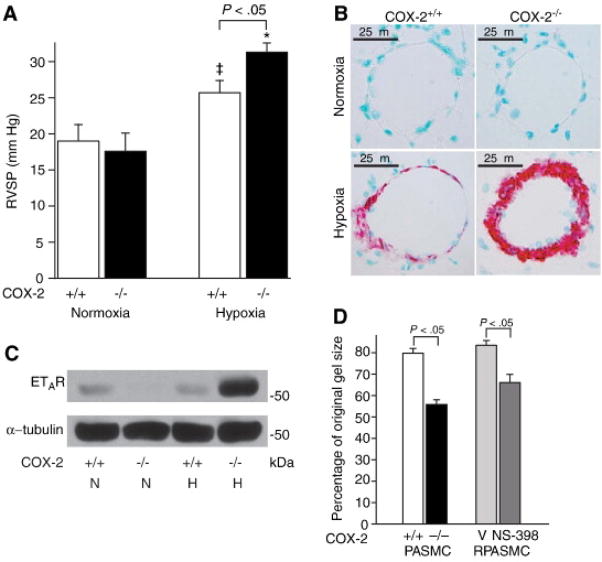
Figure 3.
Absence of COX-2 exacerbates hypoxia-induced pulmonary hypertension and enhances contractility of PASMC. A. RVSP in COX-2+/+ (□) and COX-2−/− (■) mice following hypoxia (n=18 per group) and normoxia (n=8 per group). Error bars represent SE (p<0.05 for hypoxic COX-2−/− mice vs. hypoxic COX-2+/+ mice, *p<0.05 for hypoxic COX-2−/− mice vs. normoxic controls, and ‡p<0.05 for hypoxic COX-2+/+ mice vs. normoxic controls). B. Immunostaining of lungs from COX-2+/+ (left) and COX-2−/− (right) mice after normoxia (top) and hypoxia (bottom) for α-SMA. C. Total protein was isolated from lungs of COX-2+/+ and COX-2−/− mice following hypoxia and normoxia and Western blot analysis performed for the ETA receptor. Loading was quantified with an anti-tubulin antibody. A representative of three experiments is shown. D. Cells were plated on collagen gels and exposed to hypoxia for 24 h. Gel contraction was measured 4 h following matrix release. Data are presented as the percentage of the original collagen gel size for mouse PASMC (COX-2+/+ □ and COX-2−/− ■) and RPASMC (vehicle  and NS-398
and NS-398  ) exposed to hypoxia. Data are expressed as mean ± SE (p<0.05 for COX-2−/− PASMC vs. COX-2+/+ PASMC; p<0.05 for NS-398-treated vs. vehicle-treated RPASMC).
) exposed to hypoxia. Data are expressed as mean ± SE (p<0.05 for COX-2−/− PASMC vs. COX-2+/+ PASMC; p<0.05 for NS-398-treated vs. vehicle-treated RPASMC).
Our findings also revealed that absence of COX-2 in PASMC led to enhanced contractility of a 3-dimensional collagen matrix following hypoxia. COX-2−/− PASMC demonstrated exaggerated contraction of collagen matrices following hypoxia compared with COX-2+/+ PASMC (Figure 3D). In addition, pharmacologic inhibition of COX-2 in a rat pulmonary artery smooth muscle (RPASMC) cell line resulted in increased contraction during hypoxia compared with vehicle control (Figure 3D). To determine the COX-2-derived mediator(s) responsible for these results, we measured concentrations of PGE2 and 6-keto-PGF1α, a stable PGI2 metabolite, in COX-2+/+ and COX-2−/− PASMC exposed to hypoxia. Wild type PASMC had significantly higher levels of both PGE2 and 6-keto-PGF1α following hypoxia compared with COX-2−/− PASMC. However, as expected, levels of 6-keto-PGF1α were almost 8-fold higher than PGE2 levels in wild type PASMC exposed to hypoxia (Fredenburgh et al. 2008). Given these findings, we examined whether repletion of these COX-2-derived prostanoids would alter the contractile phenotype of COX-2−/− PASMC during hypoxia. Indeed, we found that the enhanced contractility of COX-2−/− PASMC could be significantly attenuated by either exogenous iloprost, a PGI2 analog, PGE2, or forskolin, an adenylate cyclase activator (Fredenburgh et al. 2008).
Furthermore, as alternative enzymatic pathways of arachidonic acid metabolism may be upregulated in the absence of COX-2, additional eicosanoid mediators (e.g. cysLT, HETEs, EETs) may also play a role in exacerbating pulmonary hypertension and vascular remodeling under hypoxic conditions. Although alteration of multiple eicosanoid and non-eicosanoid vascular mediators may occur in the absence of COX-2, our findings suggest that COX-2-derived PGI2 and PGE2 play a critical protective role in the pulmonary vasculature under hypoxic conditions and that selective COX-2 inhibition may be hazardous to patients with pulmonary hypertension, particularly under conditions of hypoxemia.
In addition to enhanced pulmonary vascular remodeling, inhibition of COX-2 has been associated with increased platelet deposition and intravascular thrombosis in a rat model of hypobaric hypoxia-induced pulmonary hypertension (Pidgeon et al. 2004). Administration of ifetroban, a TXA2 receptor antagonist, attenuated deposition of platelets in the lung and decreased platelet aggregation in COX-2 inhibitor-treated rats during hypoxia. Furthermore, administration of ifetroban to COX-2 deficient mice similarly decreased intravascular thrombosis and attenuated the rise in RVSP during hypoxia (Cathcart et al. 2008). Taken together, these studies demonstrate that absence of COX-2 or pharmacologic inhibition of COX-2 during chronic hypoxia is associated with both exaggerated pulmonary vascular remodeling and enhanced intravascular thrombosis, both of which exacerbate the rise in pulmonary vascular resistance in response to hypoxia.
Interestingly, treatment of rats with the COX-2 inhibitor celecoxib in a monocrotaline model of PH was beneficial, with attenuation of pulmonary hypertension, pulmonary vascular remodeling, and RVH (Rakotoniaina et al. 2008). While these appear to be contrasting results, the monocrotaline model differs significantly from the hypoxia model of PH in that it is characterized by endothelial cell injury and thus inhibition of COX-2 may have different effects in this model. COX-2 is upregulated in several malignancies, including colorectal cancer (Sheehan et al. 1999) and non-small cell lung cancer (NSCLC) (Wolff et al. 1998), and increasing evidence suggests that COX-2 plays a critical role in tumorigenesis (Greenhough et al. 2009). Intringuingly, the plexiform lesions observed in patients with PAH share many neoplastic features, with excessive proliferation and suppressed apoptosis in the arterial wall, leading investigators to propose a cancer paradigm for treating PAH (Rai et al. 2008). Selective inhibition of COX-2 induces apoptosis in several malignant cell lines (Chang and Weng 2001), and the selective COX-2 inhibitor celecoxib is currently being evaluated as a chemotherapeutic agent in several malignancies, including NSCLC (Mascaux et al. 2006). As the authors reported no difference in apoptosis in celecoxib-treated rats exposed to monocrotaline (Rakotoniaina et al. 2008), the beneficial effects of celecoxib in their study were likely secondary to anti-angiogenic effects (Tsujii et al. 1998), or inhibition of other known targets such as carbonic anhydrase, 3-phosphoinositide-dependent protein kinase 1 (PDK1) and sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) (Schonthal 2007), which may be responsible for non-COX-2 dependent anti-neoplastic properties of celecoxib. Taken together, while there may be a role for antiproliferative therapy in the treatment of PAH, this potential benefit of COX-2 inhibitors will quite likely be offset by the adverse consequences of reduced PGI2 expression in the endothelium.
Conclusions and future directions
Pulmonary arterial hypertension is a complex disorder with significant morbidity and mortality. Despite recent advances in our understanding of the mechanisms and genetic determinants of disease, novel strategies that target reversal of pulmonary vascular remodeling are necessary to improve outcomes from this disease process. While prostacyclin is a known cornerstone of therapy for patients with PAH, the significance of COX-2 in the pathophysiology of hypoxia-induced pulmonary vascular remodeling is less well understood. We have shown that absence or inhibition of COX-2 is detrimental during exposure to hypoxia leading to exacerbation of pulmonary hypertension, accelerated vascular remodeling characterized by PASMC hypertrophy, and significant upregulation of the ETA receptor (Fredenburgh et al. 2008).
Our findings suggest that hypoxic induction of COX-2 and subsequent enhanced synthesis of PGI2 attenuate expression of the ETA receptor via a cAMP-dependent signaling pathway, thereby modulating the contractile and growth-promoting effects of ET-1 (Figure 4). While PGI2 has well recognized inhibitory effects on platelet aggregation in addition to anti-proliferative and vasodilatory properties, this study demonstrates an additional novel mechanism whereby PGI2 may modulate pulmonary vascular remodeling via attenuation of ETAR expression. These findings suggest that combination therapy with a PGI2 analog and an endothelin receptor antagonist may be complementary and strategies to downregulate expression of ETAR may be an important therapeutic target to reverse pulmonary vascular remodeling in PAH. Potential downstream signaling mechanisms by which PGI2 may attenuate ETAR expression and mediate protection against hypoxia-induced pulmonary vascular remodeling include the protein kinase A (Taurin et al. 2007) and Epac (exchange protein directly activated by cAMP) (Bos 2006) signaling pathways, which are currently under investigation. While our study specifically examined the effects of COX-2 derived prostanoids on pulmonary vascular remodeling, alternative eicosanoid mediators may also be dysregulated in the absence of COX-2 and may have additional consequences on pulmonary artery smooth muscle proliferation and contractility.
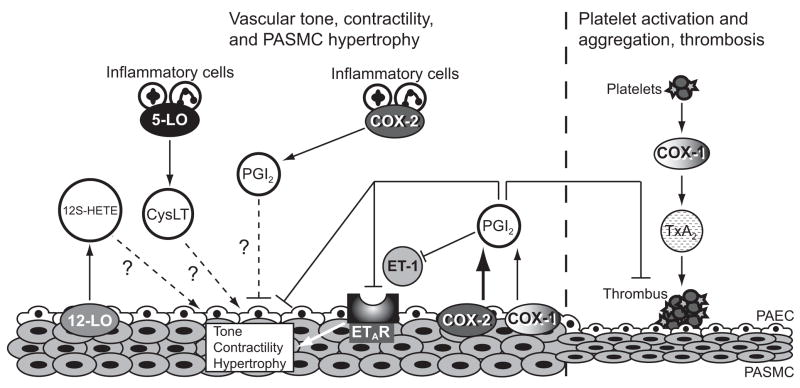
Figure 4.
Role of COX-2 in hypoxia-induced pulmonary hypertension and pulmonary vascular remodeling. COX-2 is upregulated in PASMC following hypoxia leading to enhanced synthesis of PGI2 in the endothelium of pulmonary arteries. COX-2-derived PGI2 inhibits platelet aggregation and subsequent thrombus formation and promotes vasodilation. Inhibition of COX-2 during hypoxia leads to increased thrombosis that is TXA2 dependent. In addition, our data suggest that COX-2-derived PGI2 decreases ETAR expression leading to attenuation of ET-1-induced PASMC contractility and hypertrophy. COX-2 is also highly induced in inflammatory cells following hypoxia and may play a role in modulating PASMC remodeling in a paracrine fashion. Alternative metabolites of arachidonic acid, including cysLT and 12S-HETE, may exacerbate pulmonary vascular remodeling in the absence of COX-2 via modulation of PASMC proliferation and vascular tone. Arrows (→) denote an increase in the indicated downstream mediator; flat arrowheads (⊥) indicate an inhibition or a decrease in concentration; dashed lines (···) indicate a proposed mechanism. PGI2, prostaglandin I2; ET-1 endothelin-1; TXA2, thromboxane A2; 12S-HETE, 12S-hydroxyeicosatetraenoic acid; cysLT, cysteinyl leukotrienes; ETAR, endothelin A receptor; PASMC, pulmonary artery smooth muscle cells; PAEC, pulmonary artery endothelial cells.
Finally, while our study specifically examined the consequences of COX-2 deficiency in PASMC, it is clear that COX-2 is also upregulated in inflammatory cells following hypoxia (Figure 4), and increasing evidence suggests that early inflammation may contribute to the development of pulmonary hypertension and pulmonary vascular remodeling (Stenmark et al. 2005). Future work will examine the effect of COX-2-derived eicosanoids and the consequences of COX-2 deficiency in inflammatory cells on the development of hypoxia-induced pulmonary hypertension and pulmonary vascular remodeling. Regardless of the cell type responsible for this phenotype however, it is clear that in addition to well-recognized pro-thrombotic cardiovascular risks, selective COX-2 inhibition may have detrimental pulmonary vascular consequences in patients with hypoxemic lung diseases or pre-existing pulmonary hypertension.
Acknowledgments
We apologize to the many authors whose significant works were not cited owing to space constraints. The authors thank Dr. Stephen R. Walsh for careful review of the manuscript.
This work was supported by a Parker B. Francis Fellowship (LEF), an American Lung Association Biomedical Research Grant (LEF), as well as grants from the National Institute of General Medical Sciences, including K08 GM083207 (LEF) and R01 GM053249 (MAP), and grants from the National Heart, Lung, and Blood Institute, including T32 HL0007633 (LEF) and R01 HL60788 (MAP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldred MA, Vijayakrishnan J, James V, Soubrier F, Gomez-Sanchez MA, Martensson G, et al. BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. Hum Mutat. 2006;27:212–213. doi: 10.1002/humu.9398. [DOI] [PubMed] [Google Scholar]
- Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, et al. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci U S A. 2007;104:11418–11423. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Cathcart MC, Tamosiuniene R, Chen G, Neilan TG, Bradford A, O’Byrne KJ, et al. Cyclooxygenase-2-linked attenuation of hypoxia-induced pulmonary hypertension and intravascular thrombosis. J Pharmacol Exp Ther. 2008;326:51–58. doi: 10.1124/jpet.107.134221. [DOI] [PubMed] [Google Scholar]
- Chang HC, Weng CF. Cyclooxygenase-2 level and culture conditions influence NS398-induced apoptosis and caspase activation in lung cancer cells. Oncol Rep. 2001;8:1321–1325. doi: 10.3892/or.8.6.1321. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296:539–541. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- Chida M, Voelkel NF. Effects of acute and chronic hypoxia on rat lung cyclooxygenase. Am J Physiol. 1996;270:L872–878. doi: 10.1152/ajplung.1996.270.5.L872. [DOI] [PubMed] [Google Scholar]
- Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327:70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M. Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med. 2000;6:698–702. doi: 10.1038/76282. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, Hamon M, Adnot S. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest. 2000;105:1555–1562. doi: 10.1172/JCI8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- FitzGerald GA, Loll P. COX in a crystal ball: current status and future promise of prostaglandin research. J Clin Invest. 2001;107:1335–1337. doi: 10.1172/JCI13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredenburgh LE, Liang OD, Macias AA, Polte TR, Liu X, Riascos DF, et al. Absence of cyclooxygenase-2 exacerbates hypoxia-induced pulmonary hypertension and enhances contractility of vascular smooth muscle cells. Circulation. 2008;117:2114–2122. doi: 10.1161/CIRCULATIONAHA.107.716241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CD. Prostaglandin and leukotrienes: Advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Geraci MW, Gao B, Shepherd DC, Moore MD, Westcott JY, Fagan KA, et al. Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J Clin Invest. 1999;103:1509–1515. doi: 10.1172/JCI5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshikawa Y, Voelkel NF, Gesell TL, Moore MD, Morris KG, Alger LA, et al. Prostacyclin receptor-dependent modulation of pulmonary vascular remodeling. Am J Respir Crit Care Med. 2001;164:314–318. doi: 10.1164/ajrccm.164.2.2010150. [DOI] [PubMed] [Google Scholar]
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- Jones JE, Walker JL, Song Y, Weiss N, Cardoso WV, Tuder RM, et al. Effect of 5-lipoxygenase on the development of pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol. 2004;286:H1775–1784. doi: 10.1152/ajpheart.00281.2003. [DOI] [PubMed] [Google Scholar]
- Kantores C, McNamara PJ, Teixeira L, Engelberts D, Murthy P, Kavanagh BP, Jankov RP. Therapeutic hypercapnia prevents chronic hypoxia-induced pulmonary hypertension in the newborn rat. Am J Physiol Lung Cell Mol Physiol. 2006;291:L912–922. doi: 10.1152/ajplung.00480.2005. [DOI] [PubMed] [Google Scholar]
- Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. Br Med J. 2006;332:1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascaux C, Martin B, Paesmans M, Berghmans T, Dusart M, Haller A, et al. Has Cox-2 a prognostic role in non-small-cell lung cancer? A systematic review of the literature with meta-analysis of the survival results. Br J Cancer. 2006;95:139–145. doi: 10.1038/sj.bjc.6603226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Shimosawa T, Itakura K, Guanqun X, Ando K, Fujita T. Adrenomedullin can protect against pulmonary vascular remodeling induced by hypoxia. Circulation. 2004;109:2246–2251. doi: 10.1161/01.CIR.0000127950.13380.FD. [DOI] [PubMed] [Google Scholar]
- McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, et al. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, et al. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- Morganroth ML, Stenmark KR, Morris KG, Murphy RC, Mathias M, Reeves JT, Voelkel NF. Diethylcarbamazine inhibits acute and chronic hypoxic pulmonary hypertension in awake rats. Am Rev Respir Dis. 1985;131:488–492. doi: 10.1164/arrd.1985.131.4.488. [DOI] [PubMed] [Google Scholar]
- Morganroth ML, Stenmark KR, Zirrolli JA, Mauldin R, Mathias M, Reeves JT, et al. Leukotriene C4 production during hypoxic pulmonary vasoconstriction in isolated rat lungs. Prostaglandins. 1984;28:867–875. doi: 10.1016/0090-6980(84)90040-6. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res. 2004;43:3–35. doi: 10.1016/s0163-7827(03)00037-7. [DOI] [PubMed] [Google Scholar]
- Oka M, Fagan KA, Jones PL, McMurtry IF. Therapeutic potential of RhoA/Rho kinase inhibitors in pulmonary hypertension. Br J Pharmacol. 2008;155:444–454. doi: 10.1038/bjp.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidgeon GP, Tamosiuniene R, Chen G, Leonard I, Belton O, Bradford A, Fitzgerald DJ. Intravascular thrombosis after hypoxia-induced pulmonary hypertension: regulation by cyclooxygenase-2. Circulation. 2004;110:2701–2707. doi: 10.1161/01.CIR.0000145613.01188.0B. [DOI] [PubMed] [Google Scholar]
- Preston IR, Hill NS, Warburton RR, Fanburg BL. Role of 12-lipoxygenase in hypoxia-induced rat pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L367–374. doi: 10.1152/ajplung.00114.2005. [DOI] [PubMed] [Google Scholar]
- Rai PR, Cool CD, King JA, Stevens T, Burns N, Winn RA, et al. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:558–564. doi: 10.1164/rccm.200709-1369PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakotoniaina Z, Guerard P, Lirussi F, Rochette L, Dumas M, Goirand F, Bardou M. Celecoxib but not the combination of celecoxib+atorvastatin prevents the development of monocrotaline-induced pulmonary hypertension in the rat. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:241–251. doi: 10.1007/s00210-008-0298-3. [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Mendoza J, Hood M, McGoon M, Barst R, Williams WB, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 1990;112:485–491. doi: 10.7326/0003-4819-112-7-485. [DOI] [PubMed] [Google Scholar]
- Rudic RD, Brinster D, Cheng Y, Fries S, Song WL, Austin S, et al. COX-2-derived prostacyclin modulates vascular remodeling. Circ Res. 2005;96:1240–1247. doi: 10.1161/01.RES.0000170888.11669.28. [DOI] [PubMed] [Google Scholar]
- Said SI. Mediators and modulators of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;291:L547–558. doi: 10.1152/ajplung.00546.2005. [DOI] [PubMed] [Google Scholar]
- Said SI, Hamidi SA, Dickman KG, Szema AM, Lyubsky S, Lin RZ, et al. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation. 2007;115:1260–1268. doi: 10.1161/CIRCULATIONAHA.106.681718. [DOI] [PubMed] [Google Scholar]
- Schonthal AH. Direct non-cyclooxygenase-2 targets of celecoxib and their potential relevance for cancer therapy. Br J Cancer. 2007;97:1465–1468. doi: 10.1038/sj.bjc.6604049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan KM, Sheahan K, O’Donoghue DP, MacSweeney F, Conroy RM, Fitzgerald DJ, Murray FE. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA. 1999;282:1254–1257. doi: 10.1001/jama.282.13.1254. [DOI] [PubMed] [Google Scholar]
- Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Song Y, Coleman L, Shi J, Beppu H, Sato K, Walsh K, et al. Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice. Am J Physiol Heart Circ Physiol. 2008;295:H677–690. doi: 10.1152/ajpheart.91519.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark KR, Davie NJ, Reeves JT, Frid MG. Hypoxia, leukocytes, and the pulmonary circulation. J Appl Physiol. 2005;98:715–721. doi: 10.1152/japplphysiol.00840.2004. [DOI] [PubMed] [Google Scholar]
- Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol. 1997;59:89–144. doi: 10.1146/annurev.physiol.59.1.89. [DOI] [PubMed] [Google Scholar]
- Sullivan CC, Du L, Chu D, Cho AJ, Kido M, Wolf PL, et al. Induction of pulmonary hypertension by an angiopoietin 1/TIE2/serotonin pathway. Proc Natl Acad Sci U S A. 2003;100:12331–12336. doi: 10.1073/pnas.1933740100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurin S, Hogarth K, Sandbo N, Yau DM, Dulin NO. Gbetagamma-mediated prostacyclin production and cAMP-dependent protein kinase activation by endothelin-1 promotes vascular smooth muscle cell hypertrophy through inhibition of glycogen synthase kinase-3. J Biol Chem. 2007;282:19518–19525. doi: 10.1074/jbc.M702655200. [DOI] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- Voelkel NF, Tuder RM, Wade K, Hoper M, Lepley RA, Goulet JL, et al. Inhibition of 5-lipoxygenase-activating protein (FLAP) reduces pulmonary vascular reactivity and pulmonary hypertension in hypoxic rats. J Clin Invest. 1996;97:2491–2498. doi: 10.1172/JCI118696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290:L209–221. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- Walker JL, Loscalzo J, Zhang YY. 5-Lipoxygenase and human pulmonary artery endothelial cell proliferation. Am J Physiol Heart Circ Physiol. 2002;282:H585–593. doi: 10.1152/ajpheart.00003.2001. [DOI] [PubMed] [Google Scholar]
- Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58:4997–5001. [PubMed] [Google Scholar]
- Wright L, Tuder RM, Wang J, Cool CD, Lepley RA, Voelkel NF. 5-Lipoxygenase and 5-lipoxygenase activating protein (FLAP) immunoreactivity in lungs from patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 1998;157:219–229. doi: 10.1164/ajrccm.157.1.9704003. [DOI] [PubMed] [Google Scholar]
- Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]