Abstract
Sphingosine-1-phosphate (S1P), which mediates pleiotropic actions within the vascular system, is a prominent regulator of microvascular tone. By virtue of its S1P-degrading function, we hypothesized that S1P-phosphohydrolase 1 (SPP1) is an important regulator of tone in resistance arteries. Hamster gracilis muscle resistance arteries express mRNA encoding SPP1. Overexpression of SPP1 (via transfection of a SPP1wt) reduced resting tone, Ca2+ sensitivity, and myogenic vasoconstriction, whereas reduced SPP1 expression (antisense oligonucleotides) yielded the opposite effects. Expression of a phosphatase-dead mutant of SPP1 (SPP1H208A) had no effect on any parameter tested, suggesting that catalytic activity of SPP1 is critical. The enhanced myogenic tone that follows overexpression of S1P-generating enzyme sphingosine kinase 1 (Sk1wt) was functionally antagonized by coexpression with SPP1wt but not SPP1H208A. SPP1 modulated vasoconstriction in response to 1 to 100 nmol/L exogenous S1P, a concentration range that was characterized as S1P2-dependent, based on the effect of S1P2 inhibition by antisense oligonucleotides and 1 μmol/L JTE013. Inhibition of the cystic fibrosis transmembrane regulator (CFTR) (1) restored S1P responses that were attenuated by SPP1wt overexpression; (2) enhanced myogenic vasoconstriction; but (3) had no effect on noradrenaline responses. We conclude that SPP1 is an endogenous regulator of resistance artery tone that functionally antagonizes the vascular effects of both Sk1wt and S1P2 receptor activation. SPP1 accesses extracellular S1P pools in a manner dependent on a functional CFTR transport protein. Our study assigns important roles to both SPP1 and CFTR in the physiological regulation of vascular tone, which influences both tissue perfusion and systemic blood pressure.
Keywords: Ca2+ sensitization, signal transduction, transfection, vascular smooth muscle, myogenic response, cystic fibrosis transmembrane regulator
The bioactive sphingolipid metabolite sphingosine-1-phosphate (S1P) is a potent regulator of diverse biological processes, acting both as an intracellular second messenger and as an extracellular receptor ligand. Intracellular S1P (ie, second messenger) has been shown to mediate mitogenic/antiapoptotic1 and Ca2+-mobilizing responses,2 although the mechanisms controlling the latter remain undefined. As an extracellular receptor ligand, S1P can activate small GTPases (eg, RhoA and Rac), phospholipase C and adenylate cyclase via five distinct, G protein–coupled S1P receptors (S1P1–5) (formerly known as EDG family receptors3). Growing evidence supports an important role for S1P in the development and homeostasis of the vascular system,4–6 in the pathogenesis of vascular diseases,7 and in the regulation of acute vascular responses.8 Our laboratory has recently identified the S1P-generating enzyme sphingosine kinase 1 (Sk1) as a vascular smooth muscle cell (SMC) signaling component necessary for myogenic vasoconstriction in resistance arteries. Alterations in endogenous S1P formation, through transfection of resistance arteries with sphingosine kinase (Sk1wt) or its dominant-negative mutant Sk1G82D, substantially affected resting tone, Ca2+ sensitivity, and Ca2+ handling.8 This suggests that S1P may be an important regulator of blood pressure and tissue perfusion, because these arteries originate from the primary site of pressure regulation.
Because of its pleiotropic effects,9 S1P bioavailability must be tightly regulated in a spatiotemporal manner. The formation and degradation of S1P comprises a dynamic equilibrium. Although the importance of S1P synthesis is evident, little is known about the biological significance of S1P degradation. Because perturbations in S1P metabolism result in prominent effects on microvascular tone and Ca2+ sensitivity,8 from a functional standpoint, S1P degradation may be as important as S1P synthesis.
We hypothesized that in isolated resistance vessels, the S1P-degrading enzyme SPP1 acts as a functional antagonist of Sk1. We focused on SPP1 for several reasons: (1) to be a “functional antagonist,” the S1P-degrading enzyme should yield only the original substrate of Sk1 (ie, sphingosine) and not other metabolites that could have bioactive effects (such as those produced by S1P lyase10); (2) the enzyme should not have broad substrate specificity, like lipid phosphohydrolases (LPPs),11 so that the effects are relatively limited to S1P metabolism; and (3) although both SPP1 and SPP2 fit these 2 metabolic criteria, only SPP1 has been localized to the plasma membrane,12 which would be potentially significant with respect to regulating the availability of S1P for ligation to its cell surface receptors.
Our primary objectives were as follow: to (1) confirm mRNA expression of SPP1 in isolated resistance arteries; (2) assign a tone-regulating role to SPP1; (3) validate SPP1 as a functional antagonist of Sk1; and (4) explore the mechanism by which SPP1 accesses the separate intra- and extracellular S1P pools.
Materials and Methods
Isolation and Transfection of Resistance Arteries
Animal care and experimental protocols were conducted in accordance with German and Canadian federal animal protection laws. We have previously described the procedure for resistance artery isolation and culture.13 Briefly, hamster gracilis muscle resistance artery segments (181±7 μm in diameter, ≈1 mm in length, n=60) were carefully dissected and cannulated under sterile conditions, adjusted to their in vivo lengths, set to a transmural pressure of 45 mm Hg and transfected under culture conditions (using Effectene, Qiagen, Hilden, Germany) for 20 hours.14 To monitor vessel transfection efficacy, routine spot tests using a green fluorescent protein (GFP) construct, RT-PCR analysis, and immunolabeling of the c-myc tag were conducted.
All experiments were conducted at a transmural pressure of 45 mm Hg; pressure-induced responses were induced by a step change to 100 mm Hg. Before experimentation, resistance artery SMCs were loaded with Fura-2/acetoxymethyl ester (2 μmol/L, 2 hours, Invitrogen; Karlsruhe, Germany). Vessel viability at the start and completion of experiments was assessed by initiating vasomotor responses with 0.3 μmol/L noradrenaline followed by 1 μmol/L acetylcholine. Vessels that did not robustly constrict to noradrenaline (>30%) or fully dilate to acetylcholine were deemed to be damaged and excluded. Maximum vessel diameter was determined under depolarizing conditions (125 mmol/L K+) in Ca2+-free buffer solution. As previously detailed,15 the SMC intracellular Ca2+ concentration (Delta Scan, Photomed, Seefeld, Germany) and outside diameter were determined simultaneously.
Isolation of mRNA From Resistance Vessels, RT-PCR, and SPP1 Sequencing
The procedures for mRNA isolation, RT-PCR, and sequencing can be found in the online data supplement at http://circres.ahajournals.org. A list of primers used in the present study is also included in this supplement.
SPP1H208A Plasmid Construction
The pcDNA3(c-myc)-SPP1wt vector has been previously described.16 The QuickChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, Calif) was used to prepare a SPP1 construct with a histidine (H208) to alanine mutation (SPP1H208A). Mutagenesis was verified by DNA sequencing. Catalytic inactivity of SPP1H208A was confirmed by measuring S1P phosphohydrolase activity in membrane fractions isolated from transfected HEK293 cells as previously described.17
Calculation of Tone and Reversal of Initial Distension
All values of tone represent acute diameter measurements that have been normalized; the values represent the magnitude of vessel constriction relative to maximal diameter. The computation of tone is as follows: tone (% of diamax)=(diamax −diameasured)/diamax×100.
Myogenic vasoconstriction was initiated by a stepwise change in transmural pressure from 45 to 110 mm Hg. The immediate passive distension of the vessel from its resting diameter is reversed by a continuous, active vasoconstriction. The “reversal of initial distension” (ie, the magnitude of the myogenic response) is calculated as the percentage of constriction compared to the initial distension and is computed as follows: reversal of initial distension (%)=(diadist−diat=7)/(diadist−diat=0) ×100, where diat=0 is the diameter immediately preceding the pressure step, diadist is the distended diameter measured immediately following the pressure step, and diat=7 is the diameter measured 7 minutes following the pressure step, an arbitrary time point where the constriction is normally stable.
Statistics
Functional experiments were conducted under blinded conditions (ie, the transfected construct was not known to the experimenter until after the measurement of functional parameters). Data represent means±SEM for n experiments. Experimental groups were compared using an unpaired Student t test; when multiple groups were analyzed, an ANOVA followed by a t test with Bonferroni correction was used. Experiments involving JTE013 and CFTR(inh)-172 were analyzed as paired or repeated measures datasets. Differences were considered significant at an error probability of P<0.05.
Results
SPP1 Is Expressed in Resistance Arteries of the Hamster
The RT-PCR primers for SPP1 amplified a sequence from hamster gracilis muscle resistance artery homogenates with the expected size of 574 bp (online data supplement) and 92% homology with the comparable region of mouse SPP1 mRNA. Based on this amplified sequence, which is predicted to be 50 bp downstream of the translation initiation site, an antisense oligonucleotide was generated (5′-TGTGTCTC-CTCGGGATGTG-3′). A Cy5-conjugated version of the oligonucleotide was observed to be effectively delivered into the SMCs of resistance arteries (data not shown).
SPP1wt Modulates Resting Tone and Intracellular Ca2+ in Resistance Arteries
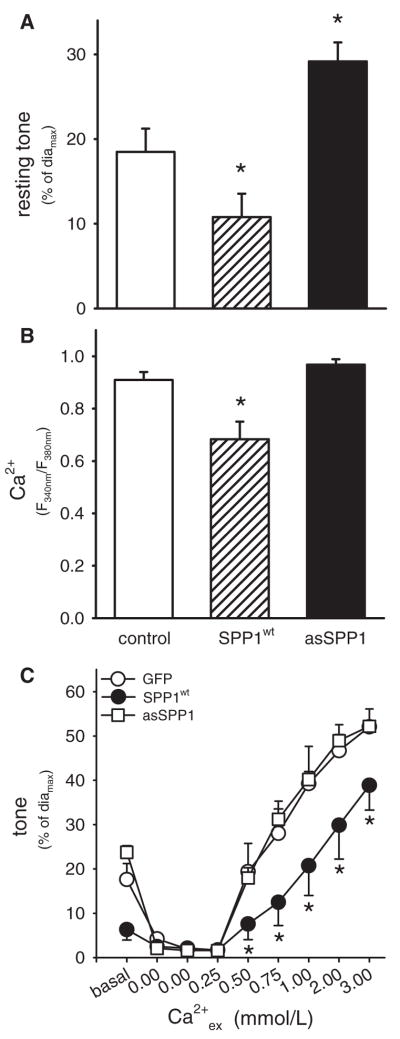
Transfection of the SMCs of resistance arteries with SPP1wt significantly reduced resting tone by 57%, compared to GFP-transfected arteries (n=6; Figure 1A). This decrease in tone was associated with a 25% decrease in resting Ca2+i (n=6; Figure 1B). In contrast, knockdown of endogenous SPP1 expression, via transfection of antisense oligonucleotides, enhanced resting tone by 34% compared to GFP-transfected vessel (n=6; Figure 1A); the resting Ca2+i concentration, however, was not significantly altered compared to GFP-expressing arteries (Figure 1B). Maximal vessel diameter (diamax) was not different in any of the treatment groups (diamaxGFP=191±15 μm, n=6; diamaxSPP1wt=196±6 μm, n=6; diamaxasSPP1=190±8 μm, n=6; P>0.05). Modulation of SPP1 activity did not significantly affect vasomotor responses to 0.3 μmol/L noradrenaline (GFP:52±3%; SPP1wt: 47±5%, asSPP1: 49±6%) or subsequent complete dilation in all three groups following 1 μmol/L acetylcholine.
Figure 1.
A, Enhanced expression of SPP1 in SMCs of resistance arteries (via transfection of SPP1wt; n=6) significantly reduced resting tone. Accordingly, reduction of SPP1 expression (via antisense oligonucleotides; n=6) significantly increased resting tone. Maximal vessel diameters were not significantly different between any of the treatment groups. B, Transfection of resistance arteries with SPP1wt significantly reduced basal SMC Ca2+ (Fura-2 method, ratio F340 nm/F380 nm). Treatment with antisense oligonucleotides directed against SPP1 had no effect on resting Ca2+. C, Constriction of depolarized resistance arteries (120 mmol/L K+) in response to increasing extracellular Ca2+ (Ca2+ex from 0 to 3 mmol/L) was significantly attenuated following transfection of SPP1wt (n=6). Transfection of antisense oligonucleotides against SPP1 had no significant effect on the diameter/Ca2+ex relationship (asSPP1) (n=6). *P<0.05 compared to arteries transfected with GFP.
Forced expression of SPP1wt could have effects independent of its S1P-degrading actions. To examine this possibility, resistance arteries were transfected with a mutant form of SPP1 (SPP1H208A), which is catalytically inactive (see measurements of phosphohydrolase activity in transfected HEK293 cells in Figure 4A). Expression of SPP1H208A had no significant effect on resting tone (Figure 4C; Con diamax=239±8 μm, SPP1H208A diamax=253±14 μm; P>0.05), Ca2+i (control: 1.04±0.04; SPP1H208A: 0.96±0.09; P>0.05), vasomotor responses to noradrenaline (control: 48±4; SPP1H208A: 51±3% P>0.05), or subsequent complete dilation to acetylcholine.
Figure 4.
A, The SPP1H208A mutant did not possess S1P phosphohydrolase activity, as measured in membrane fractions derived from transiently transfected HEK293 cells. Cells transfected with wild-type SPP1 or an empty pcDNA3 vector acted as positive and negative controls, respectively. For all groups, n=3. *P<0.05 compared to the negative control. Expression of SPP1H208A in resistance arteries had no effect on myogenic vasoconstriction (B), diameter/Ca2+ relationship (C), or the dose–response curve (D) to exogenous S1P (for graphs B through D, n=5 for control and n=8 for SPP1H208A groups).
SPP1wt Modulates Ca2+ Sensitivity and Myogenic Vasoconstriction in Resistance Arteries
Exogenous and endogenous S1P has been reported to enhance both VSMC contractile apparatus Ca2+ sensitivity and myogenic vasoconstriction.8,18 Consistent with these reports, a reduced diameter/Ca2+ relationship (constriction in response to increased extracellular Ca2+ [Ca2+ex] under depolarizing conditions18) was observed in resistance vessels transfected with S1P-degrading enzyme SPP1wt (n=6; Figure 1C). For each Ca2+ex concentration, the measured Fura-2 fluorescence ratio (ie, correlate of Ca2+i) was not significantly different between GFP- and SPP1wt-transfected arteries (data not shown). Transfection of resistance vessels with antisense oligonucleotides against SPP1 did not affect the diameter/Ca2+ relationship (n=6; Figure 1C).
Myogenic vasoconstriction in response to increased transmural pressure (from 45 to 110 mm Hg) was significantly reduced (by 69%) in resistance arteries transfected with SPP1wt (n=6; Figure 2). Accordingly, myogenic vasoconstriction was enhanced (by 46%) following transfection of antisense oligonucleotides against SPP1 (n=6; Figure 2). Neither the amplitude nor the kinetics of the pressure-induced increase in intracellular Ca2+ associated with myogenic vasoconstriction was affected by either overexpression or downregulation of SPP1 (data not shown). Expression of the catalytically inactive mutant SPP1H208A did not significantly affect myogenic responses (Figure 4B) or the diameter/Ca2+ relationship (apparent Ca2+ sensitivity; Figure 4C).
Figure 2.
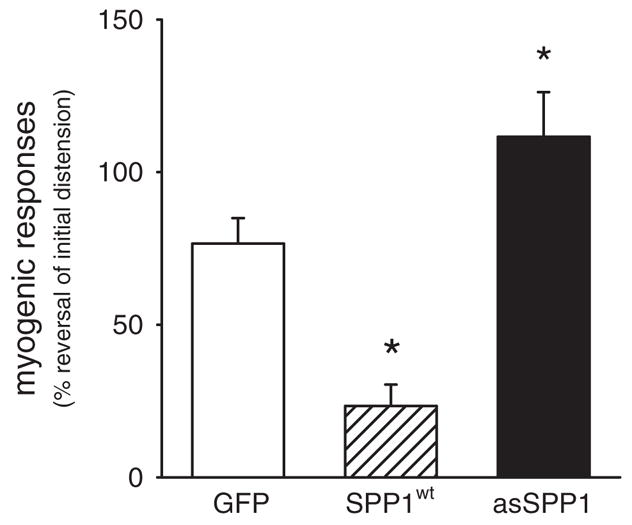
Pressure-induced (from 45 to 110 mm Hg) myogenic vasoconstriction was reduced in resistance arteries transfected with SPP1wt (n=6) and was enhanced following treatment with antisense oligonucleotides (n=6). *P<0.05 compared to arteries transfected with GFP and asSPP1.
S1P2 Is the Predominant Receptor Mediating the Effects of Exogenous S1P
We have previously shown that exogenous S1P induces vasoconstriction that is primarily mediated through activation of the RhoA/Rho kinase pathway.14 RhoA is activated by at least 2 of the 5 identified S1P receptors, namely S1P2 and S1P3 (formerly known as Edg5 and Edg3).19 Hamster resistance artery homogenates possessed mRNA encoding S1P2 but not S1P3 (data not shown; primer efficacy was verified in hamster lung homogenates). Based on this observation, sense and antisense oligonucleotides against the S1P2 receptor were generated (antisense: 5′-CTCTCTACGCCAAGCATTAT-GT-3′; sense: 5′-ACATAATGCTTGGCGTAGAGAG-3′). Transfection of resistance arteries with S1P2 antisense oligonucleotides had no effect on basal tone (diamax: sS1P2=205±8 μm, asS1P2=201±8 μm, n=6, P>0.05; resting diameter: sS1P2=190±9 μm, asS1P2=196±9 μm, n=6, P>0.05) or resting Ca2+i (R340 nm/380 nm: sS1P2=0.80±0.04, asS1P2=0.73±0.03, n=6, P>0.05). However, vasoconstriction to exogenous S1P (1 nmol/L to 10 μmol/L) was significantly reduced compared to the control group transfected with sense oligonucleotides (n=6; Figure 3A). Oligonucleotide treatment did not significantly affect vasomotor responses to noradrenaline or subsequent complete dilation to acetylcholine, compared to GFP controls (data not shown).
Figure 3.
A, Sphingosine-1-phosphate–induced constriction was significantly attenuated in resistance arteries treated with antisense oligonucleotides against S1P2 (asS1P2, n=6) compared to arteries treated with sense oligonucleotides (sS1P2, n=6). *P<0.05. B, Dose-dependent responses to exogenous S1P were reduced following treatment with the S1P2 blocker JTE013 (1 μmol/L, n=6). *P<0.05. C, Constriction induced by exogenous S1P was reduced in resistance arteries transfected with SPP1wt (n=6). Accordingly, treatment of resistance arteries with antisense oligonucleotides against SPP1 (asSPP1) (n=6) enhanced constriction to exogenous S1P. *P<0.05 compared to arteries transfected with GFP.
By using the chemical S1P2 inhibitor JTE013 (1 μmol/L; 30 minutes), we also used a second, mechanistically distinct inhibitory strategy to substantiate our antisense oligonucleotide results. The inhibitory profile for JTE013 was similar to that observed for the S1P2 antisense oligonucleotide treatment: attenuated vasoconstrictor responses to exogenous S1P, with no effect on resting tone (Figure 3B; diamax=241±16 μm). Taken together, the results suggest that S1P indeed acts as an extracellular ligand (S1P2-dependent) within the concentration range of 1 to 100 nmol/L.
SPP1wt Modulates the Vasoconstricting Effects of Exogenously Applied S1P
To assess whether SPP1 accesses extracellular S1P pools, vasoconstriction in response to exogenous S1P (0.1 nmol/L to 10 μmol/L) was monitored following modulation of SPP1 activity. Transfection of resistance arteries with SPP1wt significantly attenuated vasoconstriction in response to exogenous S1P; of note, complete abolition of vasoconstriction was observed at the lower, physiological concentration range of exogenous S1P (≤100 nmol/L; Figure 3C). Conversely, significantly enhanced S1P-mediated vasoconstriction was observed in resistance arteries transfected with SPP1 antisense oligonucleotides (Figure 3C). Responses to exogenously applied S1P were not affected by forced expression of the catalytically inactive mutant SPP1H208A (Figure 4D).
SPP1wt Antagonizes Enhanced Vascular Responsiveness in Sk1wt-Overexpressing Resistance Arteries
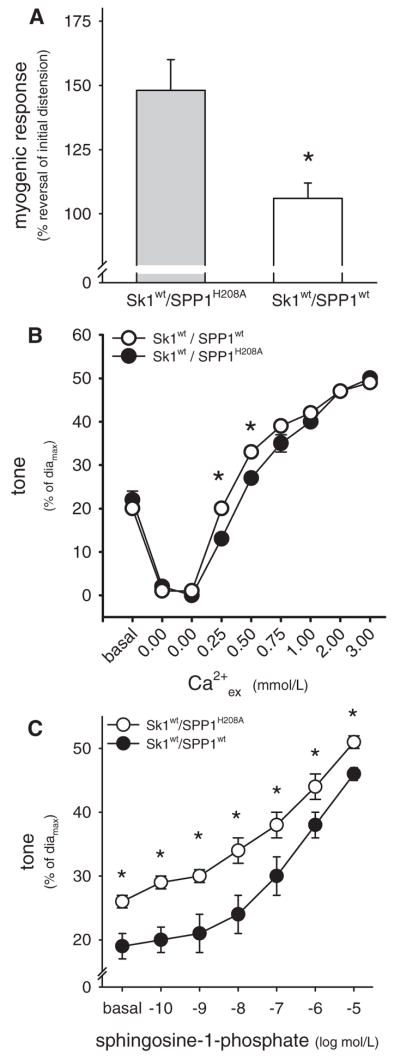
The vascular effects of SPP1wt in resistance arteries are opposite to those of Sk1wt,8 suggesting the existence of functional antagonism between these 2 enzymes that could tightly control S1P bioavailability within the microvascular wall. To prove that SPP1wt can effectively antagonize the tone-enhancing effects of Sk1, both enzymes were coexpressed in the SMCs of intact resistance arteries. Arteries expressing Sk1wt together with the phosphatase-dead SPP1 mutant (SPP1H208A) served as controls. Functional tests showed that the myogenic response in Sk1wt/SPP1wt-coexpressing arteries was (1) significantly weaker compared to arteries coexpressing Sk1wt and SPP1H208A; and (2) not statistically different compared to control arteries expressing GFP (Figure 5). Vasoconstriction in response to 0.3 μmol/L noradrenaline (Sk1wt/SPP1wt=49±1%, diamax=187±6 μm, n=5; Sk1wt/SPP1H208A=53±1%, diamax=184±4 μm, n=7; all values P>0.05 compared to GFP) or exogenous S1P (0.3 nmol/L to 30 μmol/L; Figure 5) was significantly reduced in Sk1wt/SPP1wt-coexpressing arteries, compared to Sk1wt/SPP1H208A expressing arteries. The diameter/Ca2+ relationship (apparent Ca2+ sensitivity) was largely the same in Sk1wt/SPP1wt- and Sk1wt/SPP1H208A-expressing arteries, except for 2 of the lower Ca2+ concentrations tested (Figure 5). Initially, resting tone did not differ between the 2 groups (Figure 5B). Following depolarization, which activates Sk1,20 the new steady-state resting tone was significantly weaker in arteries coexpressing Sk1wt/SPP1wt compared to those coexpressing Sk1wt/SPP1H208A (Figure 5C).
Figure 5.
A, Coexpression of Sk1wt with the phosphatase-dead SPP1H208A resulted in enhanced myogenic vasoconstriction (n=5), compared to the control responses presented in B. Vessels coexpressing Sk1wt/SPP1wt displayed significantly weaker myogenic responses (n=7). B, Shown is the constriction of depolarized resistance arteries (120 mmol/L K+) in response to increasing extracellular Ca2+ (Ca2+ex from 0 to 3 mmol/L). Vasoconstriction in vessels coexpressing Sk1wt/SPP1wt (n=7) was significantly greater at 0.25 and 0.50 mmol/L Ca2+ex, compared to vessels coexpressing Sk1wt/SPP1H208A (n=5). C, Sphingosine-1-phosphate–induced constriction was significantly attenuated in resistance arteries coexpressing Sk1wt/SPP1wt (n=5), compared to vessels coexpressing Sk1wt/SPP1H208A (n=7). *P<0.05 compared to vessels coexpressing Sk1wt/SPP1H208A.
CFTR(inh)-172 Restores S1P Responses in SPP1wr-Transfected Vessels
Our data strongly suggest that SPP1 degrades extracellular S1P. This would require either localization at the plasma membrane or a transport mechanism that shuttles S1P to the intracellular location of SPP1 (smooth endoplasmic reticulum17). Our attempts to localize SPP1 to the plasma membrane, via confocal imaging and fractionation of cellular lysates, were inconclusive (data not shown). The alternate scenario (transport) is supported by recent reports that identify the cystic fibrosis transmembrane conductance regulator (CFTR) (also known as ATP-binding cassette subfamily C member 7 [ABCC7]) as a transporter of S1P.21
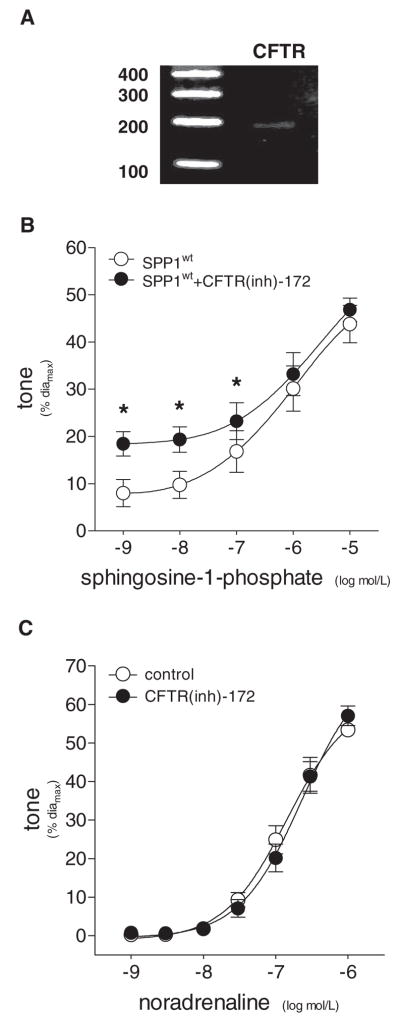
Using primers targeting conserved regions in rat, mouse and human CFTR, we detected mRNA transcripts in hamster gracilis muscle resistance arteries (Figure 6A). To establish a link between CFTR and S1P transport in resistance arteries, we used the reversible and highly specific inhibitor, CFTR(inh)-172, at a concentration comparable to the reported Ki for chloride transport (100 nmol/L).22 Resting tone was unaffected by CFTR inhibition (con=12±2%, CFTR(inh)-172=11±2%; n=16, P>0.05, diamax=293±13 μm). As previously shown in Figure 3C, vessels transfected with SPP1wt displayed reduced vasoconstriction at lower doses of exogenous S1P. Inhibition of CFTR in SPP1wt-transfected vessels restored S1P responses within the physiological concentration range (≤100 nmol/L; Figure 6B). Importantly, CFTR inhibition had no effect on vasomotor responses to noradrenaline (Figure 6C). CFTR(inh)-172 also reversibly enhanced myogenic vasoconstriction and the pressure-induced elevation of intracellular Ca2+ levels (Figure 7).
Figure 6.
A, RT-PCR using homogenates of resistance arteries isolated from the gracilis muscle revealed vascular expression of CFTR mRNA. GAPDH acted as a control for PCR conditions. B, In SPP1wt-transfected vessels, dose-dependent responses to exogenous S1P were enhanced following treatment with the specific CFTR inhibitor CFTR(inh)-172 [100 nmol/L, 30 minutes; n=6]. C, In noncultured vessels, dose-dependent responses to noradrenaline were not affected CFTR inhibition [100 nmol/L CFTR(inh)-172, 30 minutes; n=4]. *P<0.05 comparing pre- and post-CFTR inhibition.
Figure 7.
A, Representative tracings of the myogenic response (following elevation of transmural pressure from 45 to 110 mm Hg) in a single vessel under control conditions, following CFTR(inh)-172 treatment (100 nmol/L; 30 minutes) and washout of the inhibitor (30 minutes). B, Pressure-induced myogenic vasoconstriction (following a pressure step from 45 to 110 mm Hg) was reversibly enhanced following treatment with CFTR(inh)-172 (n=6). C, The amplitude of the pressure-induced Ca2+ elevation (following a pressure step from 45 to 110 mm Hg) was reversibly enhanced following treatment with CFTR(inh)-172 (n=5). *P<0.05 compared to arteries transfected with GFP.
Discussion
There is accumulating evidence that the S1P signaling pathway is a prominent determinant of resting and pressure-induced myogenic microvascular tone8 and, hence, involved in several vascular pathologies.7 Although a link between Sk1-mediated synthesis of S1P in SMCs and the control of vascular tone has been established,8 potential antagonistic pathways that degrade S1P have yet to be investigated. In this regard, we are the first to characterize SPP1 as a negative regulator of resting and myogenic tone in resistance arteries.
We were able to confirm mRNA expression of SPP1 in hamster resistance arteries using RT-PCR and subsequent sequencing. The amplified transcript possessed a high degree of similarity to the published rat, human and mouse SPP1 coding sequences. We attempted to confirm SPP1 protein expression by using both commercial and custom-made antibodies against SPP1; however, both antibodies failed to detect the hamster epitope in protein lysates from arteries, lung, liver and heart (data not shown). We found that the same was true for a commercial antibody against the S1P2 receptor.
The inability to detect hamster SPP1/S1P2 prevents the direct assessment of (1) endogenous protein expression; (2) knockdown of the native protein by antisense oligonucleotides; and (3) in the case of SPP1, elevated protein expression following transfection with plasmids. Because the SPP1 constructs include a c-myc label, we confirmed their expression via immunohistochemistry (online data supplement). We also confirmed that the antisense oligonucleotides were effectively delivered into the intracellular compartment by our transfection method (via fluorescent labels on the nucleotides). At this time, however, protein expression reductions following antisense oligonucleotide treatment can only be inferred by the functional effects (Figures 1 through 3). The fact that the antisense oligonucleotides displayed prominent functional effects, whereas 5 other oligonucleotides (S1P1 and S1P3 antisense/sense and a S1P2 sense) had no effect, minimizes the concern that our results are attributable to nonspecific effects and supports the conclusion that the respective protein levels were indeed reduced.
Consistent with its proposed role in regulating intramural S1P levels, elevated expression of SPP1wt abolished vasoconstriction to exogenous S1P at concentrations of 10−9 and 10−8 mol/L and significantly attenuated vasoconstriction to 10−7 mol/L S1P. Because S1P-mediated vasoconstriction is dependent on RhoA/Rho kinase in this type of artery,14 we propose that a cell surface receptor primarily mediates the response. In this regard, suppression of S1P2 expression (but not S1P1 and S1P3; data not shown) and blockade of the S1P2 receptor with the specific inhibitor JTE013 abolished vasoconstriction within the physiological concentration range of exogenously applied S1P (from 10−9 to 10−7 mol/L; Figure 3).
Based on its ability to regulate extracellular S1P levels, we propose that SPP1 possesses functional effects on vascular tone that are counteractive to those previously observed for Sk1wt.8,18 Consistent with this hypothesis, elevated expression of SPP1 (via transfection of the SPP1wt construct) induced similar functional effects to those previously associated with inhibition of Sk1, including reduced resting tone and basal Ca2+, attenuated myogenic vasoconstriction and decreased apparent Ca2+ sensitivity.8 Expression of a phosphatase-dead mutant of SPP1 (SPP1H208A) in resistance arteries did not affect any of these parameters, excluding proteomic effects of SPP1wt protein overexpression unrelated to its S1P-degrading function.
Although the effects of SPP1wt expression support the notion that SPP1 antagonizes Sk1 function, these results do not address whether endogenous SPP1 levels regulate S1P bioavailability under normal physiological conditions. We investigated this question by studying the effect of inhibited SPP1 protein expression/function. Notably, inhibition of SPP1 function via antisense oligonucleotides produced the opposite effect compared to SPP1wt expression: elevated resting tone, enhanced myogenic vasoconstriction, and increased constriction to exogenous S1P. Together, these results strongly support the conclusion that SPP1 is a potent and physiologically relevant regulator of S1P bioavailability in hamster resistance arteries.
Decreased SPP1 expression would have been expected to increase Ca2+ sensitivity attributable to increased levels of extracellular S1P. Indeed, Figure 1A shows that basal tone is increased, despite unaltered resting Ca2+, however, no leftward shift in the diameter/Ca2+ relationship was observed (Figure 1C). This may represent a methodological limitation whereby measurement of S1P-induced increases in Ca2+ sensitivity are compromised by the intrinsic sensitivity of Sk1 to depolarizing stimuli. The depolarizing conditions required to clamp intracellular Ca2+ (via extracellular Ca2+) may activate Sk120 and stimulate the release of S1P (or activate other Ca2+-sensitizing mechanisms) such that measurement occurs under conditions of near-maximal Ca2+ sensitivity. This is in accordance with one of our previous observations, where exogenously applied S1P also failed to left shift the Ca2+-sensitivity curve under these specific experimental conditions.18
Although our data strongly suggest that SPP1 is a prominent “negative” regulator of S1P bioavailability within the microvascular wall, these data alone do not establish SPP1 as a functional antagonist of Sk1, because resolving the spatiotemporal characteristics of both enzyme activities (a prerequisite for functional antagonism) is required. We performed SPP1wt/Sk1wt cotransfection experiments to provide indirect functional evidence for spatiotemporal overlap. We expected that the coexpression of SPP1wt would attenuate the documented proconstrictive effects of Sk1wt overexpression.23 Indeed, SPP1wt coexpression “normalized” myogenic vasoconstriction (ie, not different from vessels expressing GFP) in vessels where Sk1wt overexpression alone would have augmented this physiological response.23
Independent parameters rule out insufficient expression of one or both enzymes in this experimental series as a cause for observed “control-like” myogenic responses: (1) consistent with arteries expressing SPP1wt alone (Figure 3C), arteries coexpressing SPP1wt and Sk1wt also displayed attenuated responsiveness to exogenous S1P; and (2) arteries coexpressing Sk1wt and SPP1H208A display an augmented myogenic response, similar to that observed in vessels expressing Sk1wt alone.23 A possible inhibition of Sk1wt activity or expression resulting from SPP1 coexpression is unlikely to cause the normalized myogenic response in Sk1wt/SPP1wt coexpressing arteries, because such a situation would resemble the single SPP1wt expression (ie, reduced myogenic response).
Taken together, our functional data suggest that SPP1 effectively degrades newly synthesized S1P before it binds to cell surface receptors and promotes vasoconstriction. To do so, SPP1 would need to be either positioned close to the S1P receptors (ie, outer leaflet of plasma membrane), or in close proximity to the plasma membrane where transmembrane S1P transport occurs. With regard to our results (Figure 3C), plasma membrane localization is the most simplistic explanation for how SPP1 could regulate extracellular S1P. However, our attempts to localize transfected SPP1 in resistance artery vascular SMCs via immunolocalization (staining for the c-myc motif of transfected SPP1wt) and fluorescence protein tagging did not provide convincing evidence for or against plasma membrane localization (data not shown).
In fact, the majority of reports in the literature strongly support an endoplasmic reticulum localization of SPP1, and only one study, where SPP1 was overexpressed in HEK293 cells, has reported a small proportion of SPP1 (less than 10%) localized to the plasma membrane.17 It is the close proximity of the smooth endoplasmic reticulum and plasma membrane in SMCs (eg, the endoplasmic reticulum and plasma membrane can be separated by as little at 20 nm24) that makes the discrete subcellular localization of SPP1 in intact arteries technically challenging for optically based methods.
The close proximity of the smooth endoplasmic reticulum to the plasma membrane24 could serve as the structural basis for a putative S1P transport-degradation mechanism. The proteins that transport S1P across the plasma membrane are largely unknown. To date, only 2 transporters, which belong to the highly conserved ATP-binding cassette (ABC) family, have been shown to translocate S1P: ABCC125 and ABCC7 (CFTR).21 Because ABCC1 unidirectionally exports intracellular S1P,25 CFTR is the only known candidate that could shuttle extracellular S1P to intracellularly localized SPP1. Indeed, Boujaoude et al have demonstrated that CFTR transports extracellular S1P across the plasma membrane. Furthermore, in their model system, CFTR-mediated transport of S1P diverted it from interacting with cell surface receptors and consequently modulated S1P receptor-dependent effects.21
We therefore hypothesized that CFTR shuttles extracellular S1P to intracellularly localized SPP1 in our experimental model, thereby sequestering S1P from the S1P2 receptor and ultimately attenuating the proconstrictive effects of S1P. In accordance with this hypothesis, CFTR inhibition restored S1P responses in arteries expressing SPP1wt (SPP1 overexpression). This effect appeared confined to the physiologically relevant S1P concentration range (Figure 6B) and was specific to S1P, because vasomotor responses to noradrenaline were unaffected (Figure 6C). Consistent with its tone-enhancing effect on exogenous S1P responses, CFTR inhibition enhanced myogenic vasoconstriction and the pressure-induced Ca2+ elevation (Figure 7). Intriguingly, these effects are remarkably similar to those observed for resistance arteries overexpressing Sk1,8 suggestive of increased S1P availability.
Taken together, our data support the hypothesis that CFTR transports S1P across the plasma membrane of resistance artery SMCs for degradation by SPP1. This represents the first proposed mechanistic explanation for recent observations that CFTR is involved in the regulation of vascular tone.26 It should be noted that CFTR is traditionally regarded as a chloride transporter (specifically, chloride secretion). CFTR inhibition, therefore, may have several effects, including a possible impact on membrane potential (a determinant of vascular tone). Because the inhibition of chloride export would result in hyperpolarization and consequently vasodilation, if there are membrane potential-related effects resulting from CFTR inhibition, this should cause us to underestimate the effects of CFTR inhibition with regard to S1P-stimulated and myogenic vasoconstriction.
In summary, we provide the first evidence that SPP1 contributes to the regulation of vascular tone in resistance arteries. Our data indicate that SPP1 can regulate extracellular S1P pools and therefore modulate extracellular S1P-mediated alterations in vascular tone. As an underlying mechanism, our data support a CFTR-dependent transport mechanism that shuttles extracellular S1P to the intracellular compartment, where it is degraded by SPP1. By acting as a functional antagonist to sphingosine kinase, SPP1 possesses a physiologically relevant role in the control of vascular and myogenic tone. We propose that, under normal physiological conditions, SPP1 contributes to the regulation of tissue perfusion, which also feeds back to systemic blood pressure.
Supplementary Material
Acknowledgments
We thank Sabine D’Avis, Marolt Buchner, and Franz Singer for technical assistance and Dr Richard Proia (NIH, Bethesda, Md) for constructive comments and discussion.
Sources of Funding
This work was supported by the Friedrich Baur Stiftung, Munich (to S.-S.B.), the Deutsche Forschungsgemeinschaft-funded Graduate Program 438 (to L.V.), a Canadian Institutes of Health Research fellowship FRN 63761 (to D.L.), start-up funding from the University of Toronto (to S.-S.B.), a joint infrastructure grant from the Canadian Foundation for Innovation and Ontario Research Fund (11810 to S.-S.B.), and research grants from the Heart and Stroke Foundation of Ontario (NA6198 to S.-S.B.), Canadian Institutes of Health Research (MOP-79466 to S.H.), European Union (Exgenesis LSHM-CT2004-005272 to U.P.), and NIH (R37 GM043880 to S.S.).
Footnotes
Disclosures
None.
References
- 1.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 2.Mattie M, Brooker G, Spiegel S. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J Biol Chem. 1994;269:3181–3188. [PubMed] [Google Scholar]
- 3.Van Brocklyn J, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJ, Thangada S, Liu CH, Hla T, Spiegel S. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582:222–227. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waeber C, Blondeau N, Salomone S. Vascular sphingosine-1-phosphate S1P(1) and S1P(3) receptors. Drug News Perspect. 2004;17:365–382. doi: 10.1358/dnp.2004.17.6.829028. [DOI] [PubMed] [Google Scholar]
- 7.Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, Young TR, Gleason LA, Nakajima N, Sabbadini RA. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146:62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 8.Bolz SS, Vogel L, Sollinger D, Derwand R, Boer C, Pitson SM, Spiegel S, Pohl U. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation. 2003;108:342–347. doi: 10.1161/01.CIR.0000080324.12530.0D. [DOI] [PubMed] [Google Scholar]
- 9.Watterson K, Sankala H, Milstien S, Spiegel S. Pleiotropic actions of sphingosine-1-phosphate. Prog Lipid Res. 2003;42:344–357. doi: 10.1016/s0163-7827(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda M, Kihara A, Igarashi Y. Sphingosine-1-phosphate lyase SPL is an endoplasmic reticulum-resident, integral membrane protein with the pyridoxal 5′-phosphate binding domain exposed to the cytosol. Biochem Biophys Res Commun. 2004;325:338–343. doi: 10.1016/j.bbrc.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 11.Jasinska R, Zhang QX, Pilquil C, Singh I, Xu J, Dewald J, Dillon DA, Berthiaume LG, Carman GM, Waggoner DW, Brindley DN. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochem J. 1999;340:677–686. [PMC free article] [PubMed] [Google Scholar]
- 12.Le Stunff H, Galve-Roperh I, Peterson C, Milstien S, Spiegel S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J Cell Biol. 2002;158:1039–1049. doi: 10.1083/jcb.200203123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolz SS, Pieperhoff S, de Wit C, Pohl U. Intact endothelial and smooth muscle function in small resistance arteries after 48 h in vessel culture. Am J Physiol Heart Circ Physiol. 2000;279:H1434–H1439. doi: 10.1152/ajpheart.2000.279.3.H1434. [DOI] [PubMed] [Google Scholar]
- 14.Bolz SS, Pohl U. Highly effective non-viral gene transfer into vascular smooth muscle cells of cultured resistance arteries demonstrated by genetic inhibition of sphingosine-1-phosphate-induced vasoconstriction. J Vasc Res. 2003;40:399–405. doi: 10.1159/000072830. [DOI] [PubMed] [Google Scholar]
- 15.Bolz SS, de Wit C, Pohl U. Endothelium-derived hyperpolarizing factor but not NO reduces smooth muscle Ca2+ during acetylcholine-induced dilation of microvessels. Br J Pharmacol. 1999;128:124–134. doi: 10.1038/sj.bjp.0702775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson KR, Johnson KY, Becker KP, Bielawski J, Mao C, Obeid LM. Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. J Biol Chem. 2003;278:34541–34547. doi: 10.1074/jbc.M301741200. [DOI] [PubMed] [Google Scholar]
- 17.Le Stunff H, Peterson C, Thornton R, Milstien S, Mandala SM, Spiegel S. Characterization of murine sphingosine-1-phosphate phosphohydrolase. J Biol Chem. 2002;277:8920–8927. doi: 10.1074/jbc.M109968200. [DOI] [PubMed] [Google Scholar]
- 18.Bolz SS, Vogel L, Sollinger D, Derwand R, de Wit C, Loirand G, Pohl U. Nitric oxide-induced decrease in calcium sensitivity of resistance arteries is attributable to activation of the myosin light chain phosphatase and antagonized by the RhoA/Rho kinase pathway. Circulation. 2003;107:3081–3087. doi: 10.1161/01.CIR.0000074202.19612.8C. [DOI] [PubMed] [Google Scholar]
- 19.Takuwa Y, Okamoto H, Takuwa N, Gonda K, Sugimoto N, Sakurada S. Subtype-specific, differential activities of the EDG family receptors for sphingosine-1-phosphate, a novel lysophospholipid mediator. Mol Cell Endocrinol. 2001;177:3–11. doi: 10.1016/s0303-7207(01)00441-5. [DOI] [PubMed] [Google Scholar]
- 20.Alemany R, Kleuser B, Ruwisch L, Danneberg K, Lass H, Hashemi R, Spiegel S, Jakobs KH, Meyer zu Heringdorf D. Depolarisation induces rapid and transient formation of intracellular sphingosine-1-phosphate. FEBS Lett. 2001;509:239–244. doi: 10.1016/s0014-5793(01)03168-4. [DOI] [PubMed] [Google Scholar]
- 21.Boujaoude LC, Bradshaw-Wilder C, Mao C, Cohn J, Ogretmen B, Hannun YA, Obeid LM. Cystic fibrosis transmembrane regulator regulates uptake of sphingoid base phosphates and lysophosphatidic acid: modulation of cellular activity of sphingosine 1-phosphate. J Biol Chem. 2001;276:35258–35264. doi: 10.1074/jbc.M105442200. [DOI] [PubMed] [Google Scholar]
- 22.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolz SS, Fisslthaler B, Pieperhoff S, de Wit C, Fleming I, Busse R, Pohl U. Antisense oligonucleotides against cytochrome P450 2C8 attenuate EDHF-mediated Ca(2+) changes and dilation in isolated resistance arteries. FASEB J. 2000;14:255–260. doi: 10.1096/fasebj.14.2.255. [DOI] [PubMed] [Google Scholar]
- 24.Fameli N, van BC, Kuo KH. A quantitative model for linking Na(+)/Ca(2+) exchanger to SERCA during refilling of the sarcoplasmic reticulum to sustain [Ca(2+)] oscillations in vascular smooth muscle. Cell Calcium. 2007;42:565–575. doi: 10.1016/j.ceca.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valero M, Pereboom D, Garay RP, Alda JO. Role of chloride transport proteins in the vasorelaxant action of nitroprusside in isolated rat aorta. Eur J Pharmacol. 2006;553:205–208. doi: 10.1016/j.ejphar.2006.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.