Abstract
Schizophrenia is a common psychotic mental disorder that is believed to result from the effects of multiple genetic and environmental factors. In this study, we explored gene-gene interactions and main effects in both case-control (657 cases and 411 controls) and family-based (273 families, 1350 subjects) datasets of English or Irish ancestry. Fifty three markers in 8 genes were genotyped in the family sample and 44 markers in 7 genes were genotyped in the case-control sample. The Multifactor Dimensionality Reduction Pedigree Disequilibrium Test (MDR-PDT) was used to examine epistasis in the family dataset and a 3-locus model was identified (permuted p = 0.003). The 3-locus model involved the IL3 (rs2069803), RGS4 (rs2661319), and DTNBP1 (rs2619539) genes. We used MDR to analyze the case-control dataset containing the same markers typed in the RGS4, IL3 and DTNBP1 genes and found evidence of a joint effect between IL3 (rs31400) and DTNBP1 (rs760761) (Cross-validation consistency 4/5, balanced prediction accuracy = 56.84%, p = 0.019). While this is not a direct replication, the results obtained from both the family and case-control samples collectively suggest that IL3 and DTNBP1 are likely to interact and jointly contribute to increase risk for schizophrenia. We also observed a significant main effect in DTNBP1, which survived correction for multiple comparisons, and numerous nominally significant effects in several genes.
Keywords: Epistasis, Association, Multifactor Dimensionality Reduction algorithm, Pedigree Disequilibrium Test
2 Introduction
Schizophrenia is a complex disorder in which both genetic and environmental factors are likely involved. Heritability estimates are as high as 80% (Sullivan et al. 2003), and several chromosomal regions have been linked to the trait (Owen et al. 2004b). Due to this observed heterogeneity, some of the etiology of schizophrenia is likely the result of both biological and statistical epistasis (O'Donovan et al. 2003). Biological epistasis (Bateson 1909) is the result of physical interactions of biomolecules in individuals (Moore and Williams 2002), while statistical epistasis, or gene-gene interaction, initially described by R.A. Fisher, is a deviation from additivity in a mathematical model (Fisher 1918). Statistical epistasis is thereby a population-level event measured on average outcomes among samples, whereas biological epistasis is only observable in individuals. It has been suggested that variation among individuals in biologically epistatic processes is likely to yield statistically epistatic effects (Moore and Williams 2005).
Interest in statistical epistasis among Schizophrenia investigators has recently increased. Schizophrenia samples have been examined for statistical epistasis using several approaches (Macgregor and Khan 2006;Zammit et al. 2004;Qin et al. 2005b;Ott et al. 2005;Sullivan et al. 2001). Some studies of epistasis rely exclusively on regression and relatively restricted hypotheses (Norton et al. 2006;Norton et al. 2002;De, V et al. 2006;Corvin et al. 2007;Nicodemus et al. 2007), and sometimes couple statistical results with functional studies (Talkowski et al. 2007). Other investigations of statistical epistasis in schizophrenia use non-parametric methods that do not rely on the strict modeling assumptions of regression to detect and test for interaction. One such method which has been shown to have good power in studies with binary categorical outcomes is the multifactor dimensionality reduction method (MDR) (Ritchie et al. 2001;Hahn et al. 2003;Hahn and Moore 2004;Moore 2004;Moore 2007;Ritchie et al. 2003). MDR is a nonparametric method that performs an exhaustive search of all possible interactions and maps the data into a single dimension relevant to association. Critical values for significance are obtained via permutation testing, which inherently adjusts for the multiple comparisons from the search performed. Several recently published studies have applied MDR to the statistical epistasis problem in schizophrenia (Qin et al. 2005a;Yasuno et al. 2007;Vilella et al. 2007;Zhao et al. 2007).
In this study, we employ the recently developed multifactor dimensionality reduction pedigree disequilibrium test (MDR-PDT) (Martin et al. 2006) method to screen for potential interactions in our Irish Study of High Density Schizophrenia Families (ISHDSF) sample and use an independent case-control sample, the Irish Case Control Study of Schizophrenia (ICCSS) to verify the finding using MDR. MDR-PDT functions very similarly to MDR, searching through all possible interactions for association with the genotype pedigree disequilibrium test (Martin et al. 2003a). This method is especially useful for family-based samples that have been collected for the purposes of linkage analysis. Some statistical epistasis investigation is possible with linkage analysis, as in (Schulze et al. 2004). However, association analysis is required to map susceptibility to combinations of specific genes rather than chromosomal regions (Risch and Merikangas 1996). Our results suggest that this approach may be effective to identify real interactions for biological testing.
In the last several years, promising candidate genes (Stefansson et al. 2002;Brzustowicz et al. 2004;Pimm et al. 2005;Chowdari et al. 2002;Hodgkinson et al. 2004;Duan et al. 2004) have been identified, including dystrobrevin-binding protein 1 (DTNBP1) (Straub et al. 2002a), the SPEC2/PDZ-GEF2/ACSL6 locus (Chen et al. 2006) and interleukin 3 (IL3) (Chen et al. 2007b) which were reported from observations made in the ISHDSF.
Our group has been working with the ISHDSF for many years now and found suggestive linkages at several chromosome regions (Straub et al. 2002b). Following up these linkages, several candidate genes, i.e. DTNBP1(Straub et al. 2002a), the SPEC2/PDZ-GEF2/ACSL6 locus (Chen et al. 2006) and IL3 (Chen et al. 2007b) have been identified. We have also studied the regulator of G-protein signaling 4 (RGS4) (Chen et al. 2004a) and catechol-o-methyltransferase (COMT)(Chen et al. 2004b) genes in this sample, and modest associations were also observed. These studies, in detecting multiple associations from the same sample, provided an opportunity to examine whether these genes work in concert and collectively contribute to increase the risk of schizophrenia. Combined with the ICCSS samples, this study has the capability to replicate multilocus associations in two independent study designs and significantly advance the understanding of the genetic etiology for these functional and positional candidates in schizophrenia.
3 Methods and Materials
3.1 Subjects
3.1.1 The ISHDSF sample
The ISHDSF was collected in Northern Ireland, United Kingdom and the Republic of Ireland. Phenotypes were assessed using DSM-III-R. Individuals in the study were determined to be cases if they were diagnosed as having schizophrenia, poor-outcome schizoaffective disorder, simple schizophrenia, schizotypal personality disorder, schizophreniform disorder, delusional disorder, atypical psychosis and good-outcome schizoaffective disorder. The final inclusion criteria for pedigrees in the ISHDSF sample required two or more first, second or third degree relatives with a diagnosis above, one or more of whom had a schizophrenia, poor-outcome schizoaffective disorder or simple schizophrenia diagnosis. The sample contained 273 pedigrees and about 1350 subjects had a DNA sample for genotyping. Of these, 654 were diagnosed as cases (427 males and 227 females). Detailed descriptions of the sample were published previously (Kendler et al. 2000).
3.1.2 The ICCSS sample
The ICCSS sample was collected in the same geographic regions as that of the ISHDSF sample. In this study, we used 657 affected subjects (436 males and 221 females) and 411 controls (233 males and 178 females). The affected subjects were selected from in-patient and outpatient psychiatric facilities in the Republic of Ireland and Northern Ireland. Subjects were eligible for inclusion if they had a diagnosis of schizophrenia or poor-outcome schizoaffective disorder by DSM-III-R criteria. Controls, selected from several sources, including blood donation centers, were included if they denied a lifetime history of schizophrenia. Both cases and controls were included only if they reported all four grandparents as being born in Ireland or the United Kingdom. The broader trait definition for the ISHDSF was used to provide more cases and offset the lesser sensitivity per sample for family-based tests of association.
3.2 Marker selection and genotyping
In this study, we selected to use a total of 53 markers from several genes that had shown significant associations in the ISHDSF sample. These markers covered the CSF2RB, DTNBP1, RGS4, SPEC2, PDZ-GEF2, FNIP1, ACSL6 and IL3 genes. For the ICCSS sample, we typed the same markers in the DTNBP1, RGS4, SPEC2, PDZ-GEF2, FNIP1, ACSL6 and IL3 genes. The details for the markers typed in the ISHDSF and ICCSS are listed in Supplemental Table I.
We used two techniques for SNP genotyping. A majority of SNPs were typed by the TaqMan method (Livak 1999). SNPs typed by this method were either validated assays or custom designed assays developed by Applied BioSystems Corporation (Foster city, CA). The remaining SNPs were typed using the FP-TDI protocol (Chen et al. 1999;van den Oord et al. 2003a). For the FP-TDI procedures, DNA sequences of SNPs obtained from dbSNP [http://www.ncbi.nlm.nih.gov/SNP/index.html] were masked by the RepeatMasker program (Bedell et al. 2000) and PCR and FP-TDI extension primers were designed by the PRIMER 3 program (Rozen and Skaletsky 2000). We used the FP-TDI genotyping products from the Perkin Elmer Corporation (Boston, MA) and followed the recommended procedures for PCR and single base extension. All markers typed were checked for deviation from the Hardy-Weinberg Equilibrium (HWE) and Mendelian errors by the PEDSTATS program (Wigginton et al. 2005).
3.3 Statistical analyses
3.3.1 ISHDSF sample
We used the allele and genotype pedigree disequilibrium sum tests (PDT, Geno-PDT) (Martin et al. 2000;Martin et al. 2003a) to analyze single marker association in the entire ISHDSF sample. Analyses of these markers using the PDT averaged statistics were reported elsewhere (Chen et al. 2004a;Chen et al. 2006;Straub et al. 2002a;Chen et al. 2007b). These analyses were performed in this manner due to the use of the PDT-sum statistics in the current implementation of MDR-PDT, and the desire for consistency among signals detected by multilocus and single-locus methods. Additionally, the PDT averaged statistics can be biased to the alternate hypothesis in some pedigrees and the sum statistics are generally more powerful (Martin et al. 2001). PDT methods and MDR-PDT do not support covariate analysis of non-SNP variables, and although sex may be associated with schizophrenia, these data contain extended pedigrees that cannot be partitioned by sex. To address this issue the whole data was analyzed to assess for the presence of signals that could affect PDT and MDR-PDT results and inferences.
Haplotype analysis was performed using the Association in the Presence of Linkage (APL) (Martin et al. 2003b;Chung et al. 2006) statistic and HBAT (Horvath et al. 2004) with the –e option for regions of known linkage for the ISHDSF samples. These analyses were conducted with a cutoff frequency of 1% for rare haplotypes.
The MDR-PDT (Martin et al. 2006) was used to explore multi-locus associations in the ISHDSF sample. The MDR-PDT is a within-family measure of indirect or direct association between genotype and disease. As described previously (Martin et al. 2006), the PDT statistic (Martin et al. 2003a) functions within the framework of the MDR algorithm. Genotypes are classified as high and low-risk by comparing the genoPDT statistic to a threshold of 0, where positive statistics indicate evidence for association at that genotype. The MDR-PDT statistic is then calculated for the pooled high-risk genotypes for each set of loci. The models are ordered and evaluated by MDR-PDT statistics. A permutation test is applied to estimate the significance of the result, which inherently adjusts for the size of the search performed. The MDR-PDT procedure is illustrated in Figure 1 and includes the following steps: 1. All possible discordant sib pairs (DSPs) are generated within each sibship and pooled; 2. Each genotype is determined to be high or low risk by comparing the geno-PDT statistic for each multilocus genotype to a threshold τ (τ = 0); where whether above or below the threshold indicates positive or negative association with affected status; 3. Statistics for the pooled high-risk genotypes are calculated using the PDT. This is the MDR-PDT statistic for this model. A classification error (CE), calculated as the number of low-risk affecteds plus high-risk unaffecteds divided by the total sample, is also calculated for the model; 4. Steps 2–3 are repeated for all possible combinations of loci, calculating an MDR-PDT statistic and CE for each, choosing the largest MDR-PDT statistic as the final result; And 5. A permutation test is performed to determine the distribution of the statistic under the null hypothesis, to which the result from step 4 is compared for significance assessment.
Figure 1.
Diagram illustrating the multifactor dimensionality reduction pedigree disequilibrium test (MDR-PDT) algorithm. Details of the procedure are in the text.
To examine whether the best model of a given order that is observed by MDR-PDT is a real signal or is likely to be the result of sampling error, a permutation test is conducted. The permutation test consists of randomizing status for offspring, holding the proportion of affected individuals constant across permutations, calculating the statistic, and repeating many times to estimate the distribution of the null hypothesis. The test based on the permutation procedure should have the correct type I error, even for sparse data. This validity is due to all contingency table cells from each permutation containing the same number of observations as those from the unpermuted data. In this study, we limit our search to include only 2- and 3-locus interactions.
3.3.2 ICCSS sample
Hardy-Weinberg equilibrium (HWE) and single marker allele and genotype frequency association analyses were performed using chi-squared statistics from Powermarker v3.25 statistical software (Liu and Muse 2005). A Fisher’s exact test was used when cell counts in the table relating genotypes to status for a marker were less than 5. Where we observed Hardy-Weinberg disequilibrium, we calculated inbreeding coefficients separately in cases and controls to determine whether the disequilibrium was evidence of association (Wittke-Thompson et al. 2005). We used the MDR method with 5-fold cross-validation to screen for potential interactive effects. As in the family sample, we limited our search to 2- and 3-locus interactions. Permutation testing is used to estimate the statistical significance of an MDR result. Haplotype trend regression from Powermarker with permutation testing (Liu and Muse 2005) and the Haplo.Stats (Schaid 2004) module from the R project for the ICCSS. Again these analyses were conducted with a cutoff frequency of 1% for rare haplotypes.
3.3.3 Multiple Testing Corrections
Multiple testing was accounted for depending on the type of analysis. MDR and MDR-PDT both inherently correct for the search conducted with permutation testing. Haplotype analysis significance in the ICCSS and ISHDSF was also adjusted with permutation testing with either the HBAT –p option or in haplotype trend regression. Multiple tests of main effects were corrected for using Nyholt’s SNPSpD method (Nyholt 2004) with the modification of (Li and Ji 2005). The effective number of tests for the 44 markers which were in both datasets was 17.133 for the family data and 17.277 for the controls from the case-control data, showing the similarity of correlation among SNPs for these independent samples. For the full 53 markers from the family data, there were 24.148 effectively independent tests. For significance assessment for tests of main effects where any null may be rejected from either sample to observe association, there are 17.277 + 24.148 = 41.425 independent hypothesis tests, for an alpha threshold of 0.0012. This threshold is determined by the Sidak correction for multiple tests (Sidak 1967) which is slightly more liberal than the Bonferroni correction but provides the exact correction necessary to return the experiment-wise error rate to the desired level.
However, we also would like to assess significance where two tests have been performed for the same null hypothesis in the independent samples. For this analysis, requiring that both tests reject at the level established for any individual test is conservative. To investigate the combined evidence for association across all the data, we used Fisher’s method (Fisher 1950) to merge p-values from each SNP in different samples. We then compared the merged p-value to the threshold for significance for the effective number of independent tests established by SNPSpD for the ICCSS controls, alpha = 0.0021, since this was the higher of the two estimated effective independent tests for the two samples. We note here that these tests are supplemental to the single hypothesis tests described above, using the same data, and thus are not independent tests requiring further corrections.
We also report all nominally significant findings here for the use of other investigators looking at these genes.
4 Results
4.1 Single locus results
4.1.1 ISHDSF sample
Based on our previous studies, we selected a total of 53 SNPs to study in the ISHDSF. These SNPs cover the DTNBP1, RGS4, SPEC2, PDZ-GEF2, FNIP1, ACSL6, IL3 and CSF2RB genes. These genes were reported to be associated with schizophrenia in our ISHDSF sample (Straub et al. 2002a;Chen et al. 2004a;Chen et al. 2006;Chen et al. 2007b;Chen et al. 2007a). Of these markers, many are nominally associated with schizophrenia at the allele and/or genotype level in the ISHDSF (Table 1). The most significant results were observed in the SPEC2 and DTNBP1 genes. In SPEC2, both allele and genotype tests exceeded the threshold for multiple tests at rs3756295 and in DTNBP1 rs760761 exceeded the threshold for genotype test.
Table 1.
Single-locus association results from the ISHDSF grouped by gene.
| P-Value
|
|||||||
|---|---|---|---|---|---|---|---|
| Marker | Position | Alleles (minor) | Gene | Role | Risk allele | Allele- PDT | Geno- PDT |
| rs26195391 | chr6:15728834 | C/G (G) | DTNBP1 | Intron | - | 0.415 | 0.282 |
| rs168767381 | chr6:15735532 | A/G (A) | DTNBP1 | Intron | - | 0.317 | 0.053 |
| rs10113131 | chr6:15741411 | A/G (G) | DTNBP1 | Intron | G | 0.861 | 0.049 |
| rs20059761 | chr6:15758781 | A/G (G) | DTNBP1 | Intron | G | 0.006 | 0.008 |
| rs7607611 | chr6:15759111 | C/T (T) | DTNBP1 | Intron | T | 0.006 | 0.001a |
| rs26195221 | chr6:15761628 | C/A (A) | DTNBP1 | Intron | T | 0.021 | 0.026 |
| rs10183811 | chr6:15765049 | C/T (T) | DTNBP1 | Intron | - | 0.116 | 0.155 |
| rs2072707 | chr22:35643581 | G/T (T) | CSF2RB | Intron | - | 0.217 | 0.496 |
| rs2284031 | chr22:35645580 | C/T (T) | CSF2RB | Intron | - | 0.063 | 0.200 |
| rs17811365 | chr22:35647320 | G/T (G) | CSF2RB | Intron | - | 0.389 | 0.555 |
| Rs909486 | chr22:35648488 | C/T (T) | CSF2RB | Intron | - | 0.059 | 0.096 |
| rs2075936 | chr22:35653251 | A/G (G) | CSF2RB | Intron | - | 0.730 | 0.787 |
| rs11705394 | chr22:35654176 | C/T (C) | CSF2RB | Intron | - | 0.387 | 0.133 |
| rs7285064 | chr22:35654499 | C/T (T) | CSF2RB | S/S | - | 0.277 | 0.031 |
| Rs131840 | chr22:35658294 | C/T (T) | CSF2RB | P/P | - | 0.310 | 0.583 |
| Rs131842 | chr22:35660273 | C/T (C) | CSF2RB | 3' UTR | - | 0.285 | 0.507 |
| rs10302711 | chr5:130660554 | C/G (C) | SPEC2 | Intron | C | 0.024 | 0.095 |
| rs25490121 | chr5:130687657 | A/C (C) | SPEC2 | Intron | C | 0.005 | 0.004 |
| rs47060201 | chr5:130701975 | A/G (A) | SPEC2 | Intron | A | 0.022 | 0.087 |
| rs37562951 | chr5:130720739 | C/G (G) | SPEC2 | Intron | G | 0.001a | 0.001a |
| Rs403961 | chr5:130735943 | C/G (G) | SPEC2 | Intron | G | 0.009 | 0.037 |
| Rs277081 | chr5:130771440 | A/G (G) | G | 0.019 | 0.065 | ||
| rs49965221 | chr5:130789369 | A/G (A) | PDZ-GEF2 | 3' UTR | G | 0.004 | 0.023 |
| rs12916021 | chr5:130794561 | C/T (T) | PDZ-GEF2 | Q/R | - | 0.148 | 0.321 |
| rs7399521 | chr5:130824467 | A/T (T) | PDZ-GEF2 | Intron | T | 0.009 | 0.036 |
| rs2447391 | chr5:130834773 | A/C (C) | PDZ-GEF2 | Intron | C | 0.005 | 0.021 |
| Rs312511 | chr5:130861845 | A/G (G) | PDZ-GEF2 | Intron | G | 0.045 | 0.009 |
| rs68735821 | chr5:130920640 | A/G (A) | PDZ-GEF2 | Intron | G | 0.018 | 0.074 |
| rs14228711 | chr5:130970380 | C/T (T) | PDZ-GEF2 | Intron | T | 0.011 | 0.043 |
| rs1528151 | chr5:131054117 | A/G (A) | KIAA1961 | Intron | A | 0.005 | 0.028 |
| Rs131642361 | chr5:131107707 | C/T (C) | KIAA1961 | Intron | T | 0.019 | 0.072 |
| rs15664271 | chr5:131219571 | A/G (G) | ACSL6 | Intron | G | 0.011 | 0.027 |
| rs13550951 | chr5:131276668 | A/G (G) | ACSL6 | Intron | - | 0.478 | 0.650 |
| rs6674371 | chr5:131303408 | A/G (A) | ACSL6 | Intron | - | 0.065 | 0.242 |
| rs4770861 | chr5:131312509 | C/T (T) | ACSL6 | Intron | - | 0.074 | 0.264 |
| rs6153051 | chr5:131319755 | A/G (G) | ACSL6 | Intron | G | 0.013 | 0.040 |
| rs22405251 | chr5:131343783 | A/G (G) | ACSL6 | Intron | G | 0.009 | 0.003 |
| rs3997141 | chr5:131346687 | C/T (T) | ACSL6 | Intron | T | 0.019 | 0.095 |
| Rs779381 | chr5:131350393 | C/T (C) | ACSL6 | Intron | - | 0.161 | 0.349 |
| rs4409701 | chr5:131364186 | G/T (G) | ACSL6 | Intron | - | 0.263 | 0.626 |
| rs2469991 | chr5:131376070 | A/G (G) | ACSL6 | Intron | G | 0.029 | 0.076 |
| rs39140251 | chr5:131381184 | C/T (T) | ACSL6 | Intron | - | 0.344 | 0.635 |
| rs38467261 | chr5:131386898 | A/G (T) | ACSL6 | Promoter | - | 0.289 | 0.790 |
| rs39164411 | chr5:131397140 | A/G (A) | - | 0.412 | 0.564 | ||
| Rs314001 | chr5:131417406 | C/T (T) | IL3 | Promoter | - | 0.454 | 0.779 |
| Rs314801 | chr5:131424231 | C/T (T) | IL3 | Promoter | T | 0.029 | 0.109 |
| Rs404011 | chr5:131424377 | C/T (T) | IL3 | P/S | T | 0.017 | 0.082 |
| Rs314811 | chr5:131425101 | A/G (A) | IL3 | Intron | - | 0.074 | 0.179 |
| rs20698031 | chr5:131428332 | C/T (C) | IL3 | 3' UTR | T | 0.005 | 0.026 |
| Rs109176701 | chr1:159764500 | C/T (T) | RGS4 | Promoter | - | 0.569 | 0.442 |
| rs9514361 | chr1:159765000 | A/T (T) | RGS4 | Promoter | - | 0.906 | 0.459 |
| rs9514391 | chr1:159765349 | C/T (T) | RGS4 | Promoter | - | 0.871 | 0.315 |
| rs26613191 | chr1:159771435 | A/G (G) | RGS4 | Intron | - | 0.359 | 0.704 |
Markers also genotyped in the ICCSS
Exceeds the threshold for significance for any single-locus test from either sample
4.1.2 ICCSS sample
In the ICCSS, 44 markers in RGS4, DTNBP1, and the chromosome 5 linkage region including, SPEC2, PDZ-GEF2, FNIP1, ACSL6, and IL-3 were genotyped. Four markers were nominally significantly associated with disease at either alleles or genotypes, and 3 additional markers showed a trend (Table 2). Some of these markers deviated slightly from Hardy-Weinberg equilibrium; however, this can be considered evidence of association if the disequilibrium of heterozygotes is in opposite directions in cases and controls (rs2549012, genotype p = 0.02, control F = 0.05, case F = −0.13; rs3756295, genotype p = 0.08, control F = 0.02, case F = −0.11; rs31251, genotype p = 0.027, control F = 0.06, case F = −0.10; and rs2240525 in genotype p = 0.06, control F = 0.05, case F = −0.09). However, only one marker, rs760761, in DTNBP1 exceeded the threshold for multiple testing.
Table 2.
Association detected with χ2 tests on alleles and genotypes from the ICCSS
| Gene (Role) | Marker | Minor Allele Freq.
|
HWE P-Value
|
χ2 P-Value
|
|||
|---|---|---|---|---|---|---|---|
| Controls | Cases | Controls | Cases | Allele | Genotype | ||
| No gene | rs2549012 | 0.386 | 0.389 | 0.008 | 0.23 | 0.877 | 0.017 |
| SPEC2 (prom) | rs3756295 | 0.373 | 0.36 | 0.023 | 0.548 | 0.538 | 0.078 |
| PDZ-GEF2 (intron) | rs31251 | 0.395 | 0.381 | 0.039 | 0.113 | 0.514 | 0.027 |
| ACSL6 (intron) | rs2240525 | 0.389 | 0.377 | 0.054 | 0.207 | 0.555 | 0.06 |
| DTNBP1(intron) | rs16876738 | 0.12 | 0.094 | 0.32 | 0.817 | 0.062 | 0.172 |
| DTNBP1(intron) | rs2005976 | 0.199 | 0.162 | 0.409 | 0.055 | 0.039 | 0.071 |
| DTNBP1(intron) | rs760761 | 0.247 | 0.181 | 0.495 | 0.987 | 0.001a | 0.002b |
Exceeds the threshold for significance for any single-locus test for either sample
Exceeds the threshold for significance for tests within the ICCSS
The chromosome 5 markers rs2549012, rs3756295, rs31251 and rs2240525 and the chromosome 6 markers rs2005976 and rs760761 all showed evidence of association from both the ISHDSF and the ICCSS. The association at DTNBP1 marker rs760761 exceeded the threshold for multiple tests for all tests from either sample.
4.1.3 Merged results
Fisher’s method was employed to obtain combined estimates of significance for all tests which examined a single null hypothesis in both independent samples (Supplementary Table 2). The threshold for significance for these tests is the more stringent of the thresholds established by SNPSpD for either sample as described in section 2.5. In these results there were many nominally significant findings, several exceeded the threshold for significance taking into account multiple testing. Two tests of genotypes in SPEC2 at rs2549012 (p = 0.0007) and rs3756295 (p = 0.0004) were strongly associated with the disease. Also the genotype test for ACSL6 SNP rs2240525 (p = 0.0015) was significantly associated. Two markers in DTNBP1 were significantly associated, rs2005976 at alleles (p = 0.0021) and rs760761 at alleles and genotypes (allele p = 0.00005, genotype p = 0.00003).
4.2 Haplotype results
Association signals were strong and distributed throughout the chromosome 5 region among these samples in the ISHDSF (Table 3). Previously unreported associated haplotypes were observed using a 3-locus scan with APL and testing the full haplotype with HBAT using the –e option which adjusts the null hypothesis for association in regions of known linkage, and the HBAT –p option which permutation tests the results for multiple testing adjustment. A 4-locus haplotype was found overlapping the intergenic space and 5’ ends of ACSL6 and IL3 including rs3914025, rs3846726, rs3916441, and rs31400. APL and HBAT observed nominal signals for the minor allele haplotype. An adjacent region contained a 5-marker haplotype overlapping the intergenic haplotype at rs31400 and also included rs31480, rs40401, rs31481, and rs2069803. The overlap of associated haplotypes at rs31400 is relevant for the subsequent epistasis analyses, and in previous analyses this marker was found to associate with disease in females in the ISHDSF and ICCSS (Chen et al. 2007b). If the haplotypes underlying the interaction is dependent on family history and sex, then the ICCSS would be less powerful for detecting the haplotype associations because less than one third of the affected subjects had positive family history of schizophrenia. The tests for these chromosome 5 haplotypes in the ICCSS in the full sample and in females were not significant.
Table 3.
APL and HBAT-e haplotype analysis for associated blocks in ISHDSF
| Analysis | Gene | Markers | Haplotype | Frequency | P-Value |
|---|---|---|---|---|---|
| APL | - | rs3914025-rs3846726-rs3916441 | T-A-A | 0.392 | 0.001 |
| APL | - | rs3846726-rs3916441-rs31400 | A-A-T | 0.356 | 0.025 |
| HBAT -e | - | rs3914025-rs3846726-rs3916441-rs31400 | T-A-A-T | 0.361 | 0.046 |
| HBAT -p | - | rs3914025-rs3846726-rs3916441-rs31400 | T-A-A-T | 0.361 | 0.038 |
|
| |||||
| APL | IL3 | rs31400-rs31480-rs40401 | T-C-C | 0.392 | 0.043 |
| APL | IL3 | rs40401-rs31481-rs2069803 | C-G-T | 0.341 | 0.011 |
| HBAT -e | IL3 | rs31400-rs31480-rs40401-rs31481-rs2069803 | T-C-C-G-T | 0.344 | 0.037 |
| HBAT -p | IL3 | rs31400-rs31480-rs40401-rs31481-rs2069803 | T-C-C-G-T | 0.344 | 0.032 |
|
| |||||
| APL | DTNBP1 | rs2005976-rs760761-rs2619522 | G-T-A | 0.158 | 0.045 |
| APL | DTNBP1 | rs760761-rs2619522-rs1018381 | T-A-C | 0.072 | 0.067 |
| HBAT -e | DTNBP1 | rs2005976-rs760761-rs2619522-rs1018381 | G-T-A-C | 0.091 | 0.057 |
| HBAT -p | DTNBP1 | rs2005976-rs760761-rs2619522-rs1018381 | G-T-A-C | 0.091 | 0.078 |
Additionally, a haplotype was observed in DTNBP1 in the ISHDSF and the ICCSS spanning 4 markers including rs2005976, rs760761, rs2619522, and rs1018381 (Tables 3 and 4). This haplotype was highly significantly associated in both the ICCSS and ISHDSF, reflecting both the strength and diffuse nature of the association in this gene.
Table 4.
Haplo.stats results for haplotype association from the ICCSS
| Gene | Markers | Haplotype | Frequency | OR | 95% CI | P-value | Global P | |
|---|---|---|---|---|---|---|---|---|
| Controls | Cases | |||||||
| - | rs3914025-rs3846726-rs3916441-rs31400 | T-A-A-T | 0.401 | 0.384 | 0.935 | 0.78–1.12 | 0.430 | 0.533 |
| IL3 | rs31400-rs31480-rs40401-rs31481-rs2069803 | T-C-C-G-T | 0.342 | 0.336 | 0.942 | 0.75–1.13 | 0.280 | 0.702 |
| DTNBP1 | rs2005976-rs760761-rs2619522-rs1018381 | G-T-A-C | 0.089 | 0.12 | 1.49 | 1.14–1.73 | 0.001 | 0.0001 |
4.3 Epistasis results
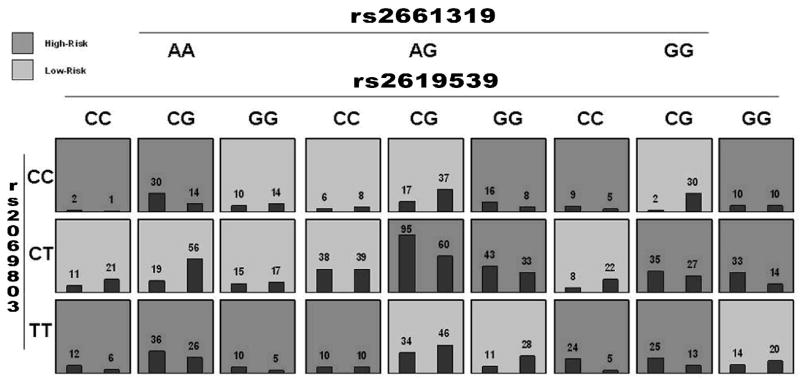
Epistasis was explored in ISHDSF samples using the MDR-PDT (Figure 1). These analyses revealed a significant 3-locus model (p = 0.003). This model includes IL3 SNP rs2069803, DTNBP1 SNP rs2619539, and RGS4 SNP rs2661319 (Figure 2).
Figure 2.
MDR-PDT three-locus model summary of interaction between RGS4 SNP rs2661319, IL3 SNP rs2069803, and DTBPN1 SNP rs2619539. Each multifactorial cell is labeled as “high risk” (T = 3) or “low risk” (T < 3). For each multilocus stratification, empirical distributions of affected (left bar in cell) and unaffected (right bar in cell) are shown. The classification accuracy for the 3-locus model is 63.31% (permuted p-value = 0.008) with a MDR-PDT statistic of 5.41 (permuted p-value = 0.003).
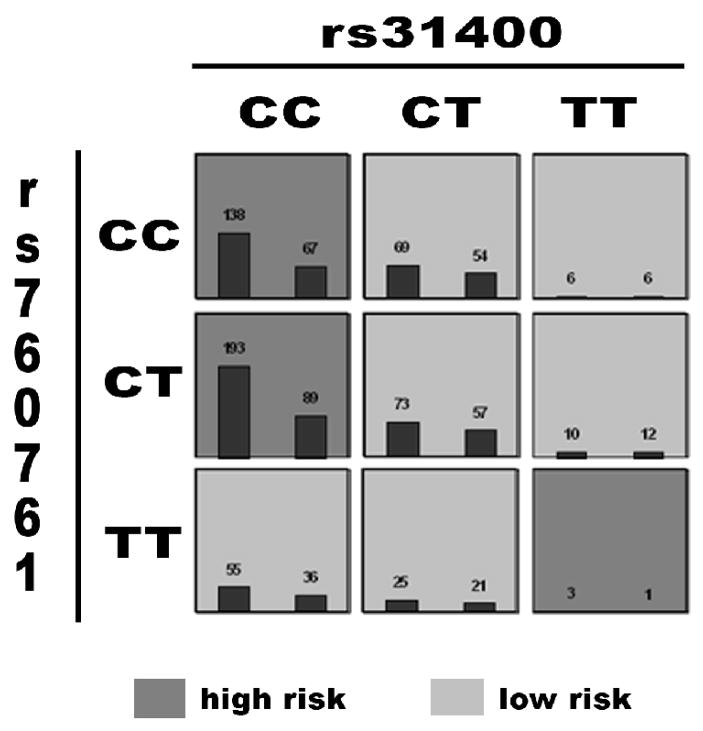
We also used MDR with 5-fold cross-validation to evaluate 2- and 3-locus interactions in the ICCSS sample. A significant 2-locus interaction was observed by MDR between IL3 SNP rs31400 and DTNBP1 SNP rs760761, with a cross-validation consistency of 4/5 and average balanced prediction accuracy = 56.84%, p = 0.019 (Figure 3).
Figure 3.
MDR two-locus model of IL3 SNP rs31400 and DTNBP1 SNP rs760761. Distribution of cases (left bar in cell) and controls (right bar in cell) stratified by multilocus genotype. Each multifactorial cell is labeled as “high risk” or “low risk”. Average balanced prediction accuracy for the model was 56.84%, permuted p = 0.019.
To test whether the signals from the interacting markers found in the ISHDSF and ICCSS came from the same genetic background, we carried out 2-locus haplotype analyses in both IL3 and DTNBP1 using the two loci implicated in the models. If significant, these results would indicate that both SNPs from each model were in linkage disequilibrium with the causal variant functioning in the interaction. For the ISHDSF, we used the APL statistic and observed nominally significant associations in both DTNBP1 (global p = 0.04) and IL-3 (global p = 0.01). For the ICCSS sample, haplotype trend regression with permutation testing was used. There was no significant risk haplotype for the IL3 gene while a nominally significant haplotype was identified for the DTNBP1 gene (global p = 0.01). These negative results in IL3 were not unexpected, because we knew that the associations in the ICCSS for IL3 are sex-specific and family-history dependent (Chen et al. 2007b).
5 Discussion
Schizophrenia is believed to have complex etiology and multiple risk factors may be required for the manifestation of the disease. In recent years, several candidate genes have been reported in the ISHDSF samples. Taking advantage of these data, we use the MDR-PDT method to explore potential epistatic interactions in this sample. We tested 2- and 3-locus interactions for 53 SNPs covering the DTNBP1, RGS4, CSF2RB, IL3, SPEC2, PDZ-GEF2, FNIP1 and ACSL6 genes. We found a 3-locus model involving RGS4, IL3 and DTNPB1. We attempted but failed to replicate these results with an independent case-control sample (ICCSS). However, a 2-locus interaction involving both IL3 and DTBNP1 genes with different markers was identified. To understand whether the markers implicated in the ISDHSF and ICCSS came from the same genetic background, we conducted 2-locus haplotype analyses for rs31400-2069803 and rs2619539-rs760761 for the IL3 and DTNBP1 genes respectively. We found that the haplotypes in the ISHDSF sample were indeed derived from previously identified risk haplotypes in the IL3 (Chen et al. 2007b) and DTNBP1 genes (van den Oord et al. 2003b). For the ICCSS sample, a major haplotype (1-1) in the DTNBP1 gene was marginally significant. This haplotype was the risk haplotype reported in a German sample (Schwab et al. 2003) and it differed from the one identified in ISHDSF. No significant haplotype was observed in the IL3 gene when the entire ICCSS sample was used. Two-locus haplotype analyses using only those subjects with positive family history identify the same risk haplotype (p = 0.0762) as that observed in the ISHDSF sample (data not shown).
This study found 2 multilocus models featuring different markers in IL3 and DTNBP1. In the ISHDSF sample, MDR-PDT reported a 3-locus interaction while the ICCSS reported a 2-locus interaction with MDR. The difference between the two models was the presence of an RGS4 SNP in the MDR-PDT model. This may be due to the generally weaker associations observed in the ICCSS. RGS4 is a strong functional candidate for schizophrenia susceptibility, since RGS4 transcripts are significantly less abundant in postmortem samples of dorsolateral prefrontal cortex in schizophrenics than in normal controls (Mirnics et al. 2001). Significant correlation has been observed between RGS4 genetic variation and dorsolateral prefrontal cortex morphometry in schizophrenic patients relative to controls (Prasad et al. 2005). It was also observed that when sex was used as the outcome instead of disease status, the geno-PDT found a trend toward transmission disequilibrium among genotypes of the MDR-PDT model RGS4 SNP rs2661319 to either sex (global p = 0.09). This sampling-error effect might also explain the presence of the RGS4 SNP in the MDR-PDT model, as it may be functioning as a surrogate for the sex variable, allowing the true interaction between IL3 and DTNBP1 to be detected.
A similar 2-locus interaction between IL3 and DTNBP1 was identified by MDR; however, the markers involved were different. As reported previously, we observed associations in multiple markers in the chromosome 5 region covering a large genomic distance and many markers in this region share high LD. Variations in signal strength were observed for individual markers between different samples. This variation may contribute to the difference of 2-locus interaction between ISHDSF and ICCSS. Another possible reason to explain the difference between the ISHDSF and ICCSS is that the ISHDSF consists of extended families with multiple affected individuals in each family, while the ICCSS consists of largely sporadic cases. If family history is important for this interaction, then the marginal interaction observed in the ICCSS might be due to the lack of power since only about 30% of the cases in the ICCSS have positive family history. This rationale is based on our previous report that the association observed in IL3 in ICCSS is family history dependent. It may be possible that the markers associated in both the MDR and MDR-PDT models are in tight LD with the same functional variant in both IL3 and DTNBP1 that is contributing to increased susceptibility for Schizophrenia. Although we cannot exclude the possibility that this finding is false, since it does not meet the stringent criteria of replication (Sullivan 2007). But the interactions in the ISHDSF and ICCSS involve the same 2 genes, this does meet part of the criteria (replication at the level of gene) proposed by Neale and Sham (Neale and Sham 2004). Taken together, while the markers in the interaction observed in the ICCSS are not the same as that in the ISHDSF sample, we believe that it does support the interaction observed in the ISHDSF sample.
The biological connection between IL3 and DTNBP1, however, is not obvious. This may be the case for many genetic interactions that are observed statistically. Many such interactions are observed at the population level in S. cerevisiae which include genes with no known physical interaction (Schuldiner et al. 2005;Tong et al. 2001). These investigations have uncovered novel biology in pathways which were previously extensively studied (Tong et al. 2004). These studies demonstrated that variation in genes which function together in and across biochemical pathways can have unforeseen effects on phenotypes, and that exhaustive searches through these spaces can reveal models which predict gene functions and phenotype outcomes (Segre et al. 2005).
IL3 is a cytokine secreted by Th0 immune cells in the immune system. Th2 activity has been shown to be increased in schizophrenic patients (Avgustin et al. 2005). Other Th2 cytokines have also been associated with schizophrenia, such as IL2 and IL4 (Schwarz et al. 2006). As a pleiotropic growth factor, IL3 is essential for the development and differentiation of all hematopoietic cell types. Withdrawal of IL3 triggers apoptosis, a process which has been implicated in schizophrenia pathophysiology (Jarskog et al. 2005;Margolis et al. 1994). While both IL3 and IL3 receptors are expressed in the brain (Konishi et al. 1995), little is known about their functions. Transgenic mice with over- or suppressed-expression of IL3 all have neurological dysfunctions (Chavany et al. 1998;Chiang et al. 1996;Cockayne et al. 1994). In animal models, IL3 is capable of activating microglia and astrocytes in the brain (Chiang et al. 1996;Powell et al. 1999). When activated, both microglia and astrocytes participate in and modulate synaptogenesis via production of cytokines, chemokines and other permeable molecules (Bessis et al. 2007;Natarajan et al. 2004).
On the other hand, DTNBP1 is a structural protein, and it has been shown to be expressed in the brain and associated with the synaptic complex (Weickert et al. 2004;Talbot et al. 2006). DTNBP1 appears to play a role in cognitive function and memory (Owen et al. 2004a). Dysbindin has been observed to be present in significantly reduced concentration in schizophrenic brain tissue relative to normal controls (Weickert et al. 2004). Because of these relevant functions and impact on multiple traits, DTNBP1 has been postulated to mediate the course of schizophrenia (McClellan et al. 2007). But its role in synapsis is not clear. Based on our current knowledge of both IL3 and DTNBP1, we can only speculate that DTNBP1 is a downstream regulatory target of IL3. To understand the nature and mechanism of this regulation, further studies are necessary. These results may assist investigators who study molecular mechanisms and pathophysiology to design experiments which elucidate the process by which these genes interact to produce schizophrenia.
Supplementary Material
Acknowledgments
This study is supported by a research grant (RO1MH41953) to KSK from the National Institute of Mental Health and by a young investigator award to XC from the National Alliance for Research on Schizophrenia and Depression. We thank the patients and their families for participating in this study. The Northern Ireland Blood Transfusion Service assisted with collection of control sample.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avgustin B, Wraber B, Tavcar R. Increased Th1 and Th2 immune reactivity with relative Th2 dominance in patients with acute exacerbation of schizophrenia. Croat Med J. 2005;46:268–274. [PubMed] [Google Scholar]
- Bateson W. Mendel's Principles of Heredity. Cambridge University Press; Cambridge: 1909. [Google Scholar]
- Bedell JA, Korf I, Gish W. MaskerAid: a performance enhancement to RepeatMasker. Bioinformatics. 2000;16:1040–1041. doi: 10.1093/bioinformatics/16.11.1040. [DOI] [PubMed] [Google Scholar]
- Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- Brzustowicz LM, Simone J, Mohseni P, Hayter JE, Hodgkinson KA, Chow EW, Bassett AS. Linkage disequilibrium mapping of schizophrenia susceptibility to the CAPON region of chromosome 1q22. Am J Hum Genet. 2004;74:1057–1063. doi: 10.1086/420774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavany C, Vicario-Abejon C, Miller G, Jendoubi M. Transgenic mice for interleukin 3 develop motor neuron degeneration associated with autoimmune reaction against spinal cord motor neurons. Proc Natl Acad Sci U S A. 1998;95:11354–11359. doi: 10.1073/pnas.95.19.11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Wang X, O'Neill FA, Walsh D, Fanous A, Kendler KS, Chen X. Association study of CSF2RB with schizophrenia in Irish family and case - control samples. Mol Psychiatry. 2007a doi: 10.1038/sj.mp.4002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Dunham C, Kendler S, Wang X, Oneill F, Walsh D, Kendler KS. Regulator of G-protein signaling 4 (RGS4) gene is associated with schizophrenia in Irish high density families. Am J Med Genet. 2004a;129B:23–26. doi: 10.1002/ajmg.b.30078. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang X, Hossain S, O'Neill AF, Walsh D, van den Oord EJ, Fanous A, Kendler KS. Interleukin 3 and schizophrenia: The impact of sex and family history. Mol Psychiatry. 2007b;12:273–282. doi: 10.1038/sj.mp.4001932. [DOI] [PubMed] [Google Scholar]
- Chen X, Levine L, Kwok PY. Fluorescence polarization in homogeneous nucleic acid analysis. Genome Res. 1999;9:492–498. [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang X, Hossain S, O'Neill FA, Walsh D, Pless L, Chowdari KV, Nimgaonkar VL, Schwab SG, Wildenauer DB, Sullivan PF, van den OE, Kendler KS. Haplotypes spanning SPEC2, PDZ-G EF2 and ACSL6 genes are associated with schizophrenia. Hum Mol Genet. 2006;15:3329–3342. doi: 10.1093/hmg/ddl409. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang X, O'Neill AF, Walsh D, Kendler KS. Variants in the catechol-o-methyltransferase (COMT) gene are associated with schizophrenia in Irish high-density families. Mol Psychiatry. 2004b;9:962–967. doi: 10.1038/sj.mp.4001519. [DOI] [PubMed] [Google Scholar]
- Chiang CS, Powell HC, Gold LH, Samimi A, Campbell IL. Macrophage/microglial-mediated primary demyelination and motor disease induced by the central nervous system production of interleukin-3 in transgenic mice. J Clin Invest. 1996;97:1512–1524. doi: 10.1172/JCI118574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdari KV, Mirnics K, Semwal P, Wood J, Lawrence E, Bhatia T, Deshpande SN, BKT, Ferrell RE, Middleton FA, Devlin B, Levitt P, Lewis DA, Nimgaonkar VL. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum Mol Genet. 2002;11:1373–1380. doi: 10.1093/hmg/11.12.1373. [DOI] [PubMed] [Google Scholar]
- Chung RH, Hauser ER, Martin ER. The APL test: extension to general nuclear families and haplotypes and examination of its robustness. Hum Hered. 2006;61:189–199. doi: 10.1159/000094774. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Bodine DM, Cline A, Nienhuis AW, Dunbar CE. Transgenic mice expressing antisense interleukin-3 RNA develop a B-cell lymphoproliferative syndrome or neurologic dysfunction. Blood. 1994;84:2699–2710. [PubMed] [Google Scholar]
- Corvin A, McGhee KA, Murphy K, Donohoe G, Nangle JM, Schwaiger S, Kenny N, Clarke S, Meagher D, Quinn J, Scully P, Baldwin P, Browne D, Walsh C, Waddington JL, Morris DW, Gill M. Evidence for association and epistasis at the DAOA/G30 and D-amino acid oxidase loci in an Irish schizophrenia sample. Am J Med Genet B Neuropsychiatr Genet. 2007;144:949–953. doi: 10.1002/ajmg.b.30452. [DOI] [PubMed] [Google Scholar]
- De LV, Tharmalingam S, Muller DJ, Wong G, de Bartolomeis A, Kennedy JL. Gene-gene interaction between MAOA and COMT in suicidal behavior: analysis in schizophrenia. Brain Res. 2006;1097:26–30. doi: 10.1016/j.brainres.2006.04.053. [DOI] [PubMed] [Google Scholar]
- Duan J, Martinez M, Sanders AR, Hou C, Saitou N, Kitano T, Mowry BJ, Crowe RR, Silverman JM, Levinson DF, Gejman PV. Polymorphisms in the trace amine receptor 4 (TRAR4) gene on chromosome 6q23.2 are associated with susceptibility to schizophrenia. Am J Hum Genet. 2004;75:624–638. doi: 10.1086/424887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. The correlation between relatives on the supposition of Mendelian inheritance. Trans R Soc Edin. 1918;52:399–433. [Google Scholar]
- Fisher RA. Statistical methods for research workers. Vol. 11. Hafner; New York: 1950. p. xv–354. [Google Scholar]
- Hahn LW, Moore JH. Ideal discrimination of discrete clinical endpoints using multilocus genotypes. In Silico Biol. 2004;4:183–194. [PubMed] [Google Scholar]
- Hahn LW, Ritchie MD, Moore JH. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics. 2003;19:376–382. doi: 10.1093/bioinformatics/btf869. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Goldman D, Jaeger J, Persaud S, Kane JM, Lipsky RH, Malhotra AK. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet. 2004;75:862–872. doi: 10.1086/425586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Xu X, Lake SL, Silverman EK, Weiss ST, Laird NM. Family-based tests for associating haplotypes with general phenotype data: application to asthma genetics. Genet Epidemiol. 2004;26:61–69. doi: 10.1002/gepi.10295. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Glantz LA, Gilmore JH, Lieberman JA. Apoptotic mechanisms in the pathophysiology of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:846–858. doi: 10.1016/j.pnpbp.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers JM, O'Neill FA, Martin R, Murphy B, MacLean CJ, Walsh D, Straub RE. Clinical features of schizophrenia and linkage to chromosomes 5q, 6p, 8p, and 10p in the Irish Study of High-Density Schizophrenia Families. Am J Psychiatry. 2000;157:402–408. doi: 10.1176/appi.ajp.157.3.402. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Chui DH, Kunishita T, Yamamura T, Higashi Y, Tabira T. Demonstration of interleukin-3 receptor-associated antigen in the central nervous system. J Neurosci Res. 1995;41:572–582. doi: 10.1002/jnr.490410503. [DOI] [PubMed] [Google Scholar]
- Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95:221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- Liu K, Muse SV. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21:2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- Livak KJ. Allelic discrimination using fluorogenic probes and the 5' nuclease assay. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Macgregor S, Khan IA. GAIA: an easy-to-use web-based application for interaction analysis of case-control data. BMC Med Genet. 2006;7:34. doi: 10.1186/1471-2350-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RL, Chuang DM, Post RM. Programmed cell death: implications for neuropsychiatric disorders. Biol Psychiatry. 1994;35:946–956. doi: 10.1016/0006-3223(94)91241-6. [DOI] [PubMed] [Google Scholar]
- Martin ER, Bass MP, Gilbert JR, Pericak-Vance MA, Hauser ER. Genotype-based association test for general pedigrees: the genotype-PDT. Genet Epidemiol. 2003a;25:203–213. doi: 10.1002/gepi.10258. [DOI] [PubMed] [Google Scholar]
- Martin ER, Bass MP, Hauser ER, Kaplan NL. Accounting for linkage in family-based tests of association with missing parental genotypes. Am J Hum Genet. 2003b;73:1016–1026. doi: 10.1086/378779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ER, Bass MP, Kaplan NL. Correcting for a potential bias in the pedigree disequilibrium test. Am J Hum Genet. 2001;68:1065–1067. doi: 10.1086/319525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ER, Monks SA, Warren LL, Kaplan NL. A test for linkage and association in general pedigrees: the pedigree disequilibrium test 4. Am J Hum Genet. 2000;67:146–154. doi: 10.1086/302957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ER, Ritchie MD, Hahn L, Kang S, Moore JH. A novel method to identify gene-gene effects in nuclear families: the MDR-PDT. Genet Epidemiol. 2006;30:111–123. doi: 10.1002/gepi.20128. [DOI] [PubMed] [Google Scholar]
- McClellan JM, Susser E, King MC. Schizophrenia: a common disease caused by multiple rare alleles. Br J Psychiatry. 2007;190:194–199. doi: 10.1192/bjp.bp.106.025585. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry. 2001;6:293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- Moore JH. Computational analysis of gene-gene interactions using multifactor dimensionality reduction. Expert Rev Mol Diagn. 2004;4:795–803. doi: 10.1586/14737159.4.6.795. [DOI] [PubMed] [Google Scholar]
- Moore JH. Genome-wide analysis of epistasis using mutifactor dimensionality reduction: feature selection and construction in the domain of human genetics. In: Zu X, Davidson I, editors. Knowledge Discovery and Data Mining: Challenges and Realities with Real World Data. IGI Press; Hershey: 2007. [Google Scholar]
- Moore JH, Williams SM. New strategies for identifying gene-gene interactions in hypertension 5. Ann Med. 2002;34:88–95. doi: 10.1080/07853890252953473. [DOI] [PubMed] [Google Scholar]
- Moore JH, Williams SM. Traversing the conceptual divide between biological and statistical epistasis: systems biology and a more modern synthesis. Bioessays. 2005;27:637–646. doi: 10.1002/bies.20236. [DOI] [PubMed] [Google Scholar]
- Natarajan C, Sriram S, Muthian G, Bright JJ. Signaling through JAK2-STAT5 pathway is essential for IL-3-induced activation of microglia. Glia. 2004;45:188–196. doi: 10.1002/glia.10316. [DOI] [PubMed] [Google Scholar]
- Neale BM, Sham PC. The future of association studies: gene-based analysis and replication. Am J Hum Genet. 2004;75:353–362. doi: 10.1086/423901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus KK, Kolachana BS, Vakkalanka R, Straub RE, Giegling I, Egan MF, Rujescu D, Weinberger DR. Evidence for statistical epistasis between catechol-O-methyltransferase (COMT) and polymorphisms in RGS4, G72 (DAOA), GRM3, and DISC1: influence on risk of schizophrenia. Hum Genet. 2007;120:889–906. doi: 10.1007/s00439-006-0257-3. [DOI] [PubMed] [Google Scholar]
- Norton N, Kirov G, Zammit S, Jones G, Jones S, Owen R, Krawczak M, Williams NM, O'Donovan MC, Owen MJ. Schizophrenia and functional polymorphisms in the MAOA and COMT genes: no evidence for association or epistasis. Am J Med Genet. 2002;114:491–496. doi: 10.1002/ajmg.10517. [DOI] [PubMed] [Google Scholar]
- Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, Spurlock G, Kirov G, Buckland P, Waddington JL, Gill M, Corvin AP, Owen MJ, O'Donovan MC. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan MC, Williams NM, Owen MJ. Recent advances in the genetics of schizophrenia. Hum Mol Genet. 2003;12 Spec No 2:R125–R133. doi: 10.1093/hmg/ddg302. [DOI] [PubMed] [Google Scholar]
- Ott U, Reuter M, Hennig J, Vaitl D. Evidence for a common biological basis of the Absorption trait, hallucinogen effects, and positive symptoms: epistasis between 5-HT2a and COMT polymorphisms. Am J Med Genet B Neuropsychiatr Genet. 2005;137:29–32. doi: 10.1002/ajmg.b.30197. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Williams NM, O'Donovan MC. The molecular genetics of schizophrenia: new findings promise new insights. Mol Psychiatry. 2004b;9:14–27. doi: 10.1038/sj.mp.4001444. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Williams NM, O'Donovan MC. The molecular genetics of schizophrenia: new findings promise new insights. Mol Psychiatry. 2004a;9:14–27. doi: 10.1038/sj.mp.4001444. [DOI] [PubMed] [Google Scholar]
- Pimm J, McQuillin A, Thirumalai S, Lawrence J, Quested D, Bass N, Lamb G, Moorey H, Datta SR, Kalsi G, Badacsonyi A, Kelly K, Morgan J, Punukollu B, Curtis D, Gurling H. The epsin 4 gene on chromosome 5q, which encodes the clathrin-associated protein enthoprotin, is involved in the genetic susceptibility to schizophrenia. Am J Hum Genet. 2005;76:902–907. doi: 10.1086/430095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell HC, Garrett RS, Brett FM, Chiang CS, Chen E, Masliah E, Campbell IL. Response of glia, mast cells and the blood brain barrier, in transgenic mice expressing interleukin-3 in astrocytes, an experimental model for CNS demyelination. Brain Pathol. 1999;9:219–235. doi: 10.1111/j.1750-3639.1999.tb00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Chowdari KV, Nimgaonkar VL, Talkowski ME, Lewis DA, Keshavan MS. Genetic polymorphisms of the RGS4 and dorsolateral prefrontal cortex morphometry among first episode schizophrenia patients. Mol Psychiatry. 2005;10:213–219. doi: 10.1038/sj.mp.4001562. [DOI] [PubMed] [Google Scholar]
- Qin S, Zhao X, Pan Y, Liu J, Feng G, Fu J, Bao J, Zhang Z, He L. An association study of the N-methyl-D-aspartate receptor NR1 subunit gene (GRIN1) and NR2B subunit gene (GRIN2B) in schizophrenia with universal DNA microarray. Eur J Hum Genet. 2005b;13:807–814. doi: 10.1038/sj.ejhg.5201418. [DOI] [PubMed] [Google Scholar]
- Qin S, Zhao X, Pan Y, Liu J, Feng G, Fu J, Bao J, Zhang Z, He L. An association study of the N-methyl-D-aspartate receptor NR1 subunit gene (GRIN1) and NR2B subunit gene (GRIN2B) in schizophrenia with universal DNA microarray. Eur J Hum Genet. 2005a;13:807–814. doi: 10.1038/sj.ejhg.5201418. [DOI] [PubMed] [Google Scholar]
- Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273:1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- Ritchie MD, Hahn LW, Moore JH. Power of multifactor dimensionality reduction for detecting gene-gene interactions in the presence of genotyping error, missing data, phenocopy, and genetic heterogeneity. Genet Epidemiol. 2003;24:150–157. doi: 10.1002/gepi.10218. [DOI] [PubMed] [Google Scholar]
- Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF, Moore JH. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001;69:138–147. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Schaid DJ. Evaluating associations of haplotypes with traits 2. Genet Epidemiol. 2004;27:348–364. doi: 10.1002/gepi.20037. [DOI] [PubMed] [Google Scholar]
- Schuldiner M, Collins SR, Thompson NJ, Denic V, Bhamidipati A, Punna T, Ihmels J, Andrews B, Boone C, Greenblatt JF, Weissman JS, Krogan NJ. Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell. 2005;123:507–519. doi: 10.1016/j.cell.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Buervenich S, Badner JA, Steele CJ, tera-Wadleigh SD, Dick D, Foroud T, Cox NJ, MacKinnon DF, Potash JB, Berrettini WH, Byerley W, Coryell W, DePaulo JR, Jr, Gershon ES, Kelsoe JR, McInnis MG, Murphy DL, Reich T, Scheftner W, Nurnberger JI, Jr, McMahon FJ. Loci on chromosomes 6q and 6p interact to increase susceptibility to bipolar affective disorder in the national institute of mental health genetics initiative pedigrees. Biol Psychiatry. 2004;56:18–23. doi: 10.1016/j.biopsych.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Schwab SG, Knapp M, Mondabon S, Hallmayer J, Borrmann-Hassenbach M, Albus M, Lerer B, Rietschel M, Trixler M, Maier W, Wildenauer DB. Support for association of schizophrenia with genetic variation in the 6p22.3 gene, dysbindin, in sib-pair families with linkage and in an additional sample of triad families. Am J Hum Genet. 2003;72:185–190. doi: 10.1086/345463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz MJ, Kronig H, Riedel M, Dehning S, Douhet A, Spellmann I, Ackenheil M, Moller HJ, Muller N. IL-2 and IL-4 polymorphisms as candidate genes in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2006;256:72–76. doi: 10.1007/s00406-005-0603-9. [DOI] [PubMed] [Google Scholar]
- Segre D, Deluna A, Church GM, Kishony R. Modular epistasis in yeast metabolism. Nat Genet. 2005;37:77–83. doi: 10.1038/ng1489. [DOI] [PubMed] [Google Scholar]
- Sidak Z. Rectangular Confidence Regions for Means of Multivariate Normal Distributions. Journal of the American Statistical Association. 1967;62:626. [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, Wormley B, Sadek H, Kadambi B, Cesare AJ, Gibberman A, Wang X, O'Neill FA, Walsh D, Kendler KS. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002a;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, Wormley B, Sadek H, Kadambi B, O'Neill FA, Walsh D, Kendler KS. Genome-wide scans of three independent sets of 90 Irish multiplex schizophrenia families and follow-up of selected regions in all families provides evidence for multiple susceptibility genes. Mol Psychiatry. 2002b;7:542–559. doi: 10.1038/sj.mp.4001051. [DOI] [PubMed] [Google Scholar]
- Sullivan PF. Spurious genetic associations. Biol Psychiatry. 2007;61:1121–1126. doi: 10.1016/j.biopsych.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, O'Neill FA, Walsh D, Ma Y, Kendler KS, Straub RE. Analysis of epistasis in linked regions in the Irish study of high-density schizophrenia families. Am J Med Genet. 2001;105:266–270. doi: 10.1002/ajmg.1324. [DOI] [PubMed] [Google Scholar]
- Talbot K, Cho DS, Ong WY, Benson MA, Han LY, Kazi HA, Kamins J, Hahn CG, Blake DJ, Arnold SE. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Hum Mol Genet. 2006;15:3041–3054. doi: 10.1093/hmg/ddl246. [DOI] [PubMed] [Google Scholar]
- Talkowski ME, Kirov G, Bamne M, Georgieva L, Torres G, Mansour H, Chowdari KV, Milanova V, Wood J, McClain L, Prasad K, Shirts B, Zhang J, O'Donovan MC, Owen MJ, Devlin B, Nimgaonkar VL. A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum Mol Genet. 2007 doi: 10.1093/hmg/ddm347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AH, Evangelista M, Parsons AB, Xu H, Bader GD, Page N, Robinson M, Raghibizadeh S, Hogue CW, Bussey H, Andrews B, Tyers M, Boone C. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science. 2001;294:2364–2368. doi: 10.1126/science.1065810. [DOI] [PubMed] [Google Scholar]
- Tong AH, Lesage G, Bader GD, Ding H, Xu H, Xin X, Young J, Berriz GF, Brost RL, Chang M, Chen Y, Cheng X, Chua G, Friesen H, Goldberg DS, Haynes J, Humphries C, He G, Hussein S, Ke L, Krogan N, Li Z, Levinson JN, Lu H, Menard P, Munyana C, Parsons AB, Ryan O, Tonikian R, Roberts T, Sdicu AM, Shapiro J, Sheikh B, Suter B, Wong SL, Zhang LV, Zhu H, Burd CG, Munro S, Sander C, Rine J, Greenblatt J, Peter M, Bretscher A, Bell G, Roth FP, Brown GW, Andrews B, Bussey H, Boone C. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–813. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- van den Oord EJ, Jiang Y, Riley BP, Kendler KS, Chen X. FP-TDI SNP scoring by manual and statistical procedures: a study of error rates and types. Biotechniques. 2003a;34:610–20. 622. doi: 10.2144/03343dd04. [DOI] [PubMed] [Google Scholar]
- van den Oord EJ, Sullivan PF, Jiang Y, Walsh D, Neill FA, Kendler KS, Riley BP. Identification of a high-risk haplotype for the dystrobrevin binding protein 1 (DTNBP1) gene in the Irish study of high-density schizophrenia families. Mol Psychiatry. 2003b;8:499–510. doi: 10.1038/sj.mp.4001263. [DOI] [PubMed] [Google Scholar]
- Vilella E, Costas J, Sanjuan J, Guitart M, De Diego Y, Carracedo A, Martorell L, Valero J, Labad A, De Frutos R, Najera C, Molto MD, Toirac I, Guillamat R, Brunet A, Valles V, Perez L, Leon M, de Fonseca FR, Phillips C, Torres M. Association of schizophrenia with DTNBP1 but not with DAO, DAOA, NRG1 and RGS4 nor their genetic interaction. J Psychiatr Res. 2007;42:278–288. doi: 10.1016/j.jpsychires.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Human Dysbindin (DTNBP1) Gene Expression in Normal Brain and in Schizophrenic Prefrontal Cortex and Midbrain. Arch Gen Psychiatry. 2004;61:544–555. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76:887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittke-Thompson JK, Pluzhnikov A, Cox NJ. Rational inferences about departures from Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76:967–986. doi: 10.1086/430507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno K, Ando S, Misumi S, Makino S, Kulski JK, Muratake T, Kaneko N, Amagane H, Someya T, Inoko H, Suga H, Kanemoto K, Tamiya G. Synergistic association of mitochondrial uncoupling protein (UCP) genes with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2007;144:250–253. doi: 10.1002/ajmg.b.30443. [DOI] [PubMed] [Google Scholar]
- Zammit S, Jones G, Jones SJ, Norton N, Sanders RD, Milham C, McCarthy GM, Jones LA, Cardno AG, Gray M, Murphy KC, O'Donovan MC, Owen MJ. Polymorphisms in the MAOA, MAOB, and COMT genes and aggressive behavior in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2004;128:19–20. doi: 10.1002/ajmg.b.30021. [DOI] [PubMed] [Google Scholar]
- Zhao X, Qin S, Shi Y, Zhang A, Zhang J, Bian L, Wan C, Feng G, Gu N, Zhang G, He G, He L. Systematic study of association of four GABAergic genes: glutamic acid decarboxylase 1 gene, glutamic acid decarboxylase 2 gene, GABA(B) receptor 1 gene and GABA(A) receptor subunit beta2 gene, with schizophrenia using a universal DNA microarray. Schizophr Res. 2007;93:374–384. doi: 10.1016/j.schres.2007.02.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.