Abstract
We have considered a novel “rational” gene targeting approach for treating pathologies whose genetic bases are defined using select phytochemicals. We reason that one such potential application of this approach would be conditions requiring immunosuppression such as autoimmune disease and transplantation, where the genetic target is clearly defined; i.e., interleukin-2 and associated T-cell activation. Therefore, we hypothesized that select phytochemicals can suppress T-lymphocyte proliferation both in vitro and in vivo. The immunosuppressive effects of berry extract, curcumin, quercetin, sulforaphane, epigallocatechin gallate (EGCG), resveratrol, α-tocopherol, vitamin C and sucrose were tested on anti-CD3 plus anti-CD28-activated primary human T-lymphocytes in culture. Curcumin, sulforaphane, quercetin, berry extract and EGCG all significantly inhibited T-cell proliferation, and this effect was not due to toxicity. IL-2 production was also reduced by these agents, implicating this important T-cell cytokine in proliferation suppression. Except for berry extract, these same agents also inhibited mouse splenic T-cell proliferation and IL-2 production. Subsequent in vivo studies revealed that quercetin (but not sulforaphane) modestly suppressed mouse splenocyte proliferation following supplementation of BALB/c mice diets. This effect was especially prominent if corrected for the loss of supplement “recall” as observed in cultured T-cells. These results suggest the potential use of these select phytochemicals for treating autoimmune and transplant patients, and support our strategy of using select phytochemicals to treat genetically-defined pathologies, an approach that we believe is simple, healthy, and cost-effective.
Keywords: immunosuppression, healthy dietary nutrients, T-cells, IL-2, splenocyte, lymphocyte, mouse, autoimmune, transplant
1. INTRODUCTION
Deliberately-induced clinical immunosuppression is used during bone marrow transplantation, organ transplantation, and in the treatment of auto-immune disease [1-3]. Inhibition of intracellular calcineurin, a phosphatase that stimulates interleukin-2 expression during the normal T-lymphocyte immune response, has been a favorite target of immunosuppressive approaches. Cyclosporine and FK506 are the most notable of these calcineurin inhibitors, and have been used for many years as highly effective immunosuppressants [3-5]. More recent approaches use these agents in combination with others that do not target calcineurin. Thus, current immunosuppression regimens often include a combination of agents including tacrolimus (Prograf, FK506), mycophenolate mofetil (CellCept, MMF), sirolimus (Rapamune, Rapamycin), cyclosporine (Gengraf, Neoral, Sandimmune), azathioprine (Imuran) and others.
Despite their effectiveness, clinical immunosuppressants have historically produced numerous side-effects [1-5]. These include risk of infection, nephrotoxicity, nausea, stomach upset, vomiting, diarrhea, high blood pressure, neurotoxicity, headaches, tremors, diabetogenicity, altered salt levels, numbness, cholesterol elevation, triglyceride elevation, anemia, reduced platelet count, rash or mouth sores, delayed wound healing, joint pain and others. In addition, patients on these immunosuppression regimens have a significantly higher risk of cancer than the general population, particularly lymphomas and skin cancer [6-9]. Thus, there is significant interest in developing immunosuppressive approaches with less toxicity while maintaining sufficient immunosuppression and acceptable acute rejection rates.
Our laboratory became interested in immunosuppression research due to our studies on the gene RCAN1/ADAPT78 [10-12], whose protein product (RCAN1) has been found to inhibit calcineurin [13-17]. Calcineurin is a major mediator of calcium signaling and, as mentioned above, a target for effective immunosuppressants such as cyclosporine and FK506. In evaluating immunosuppression, we considered a novel alternative approach based on the knowledge that phytochemicals can modulate gene expression [18-21]. Specifically, we hypothesize that select phytochemicals can potentially be used to purposely modulate gene expression to gain clinical benefit. Even though it is now known that phytochemicals can modulate gene expression, their use in treating genetically-defined clinically-related conditions has not been a focus. In order to use this approach, the genes that need to be modulated have to be clearly defined. In the case of clinical immunosuppression, it is IL-2 (and accompanying T-cell proliferation). That is, the activation of T-lymphocytes and their induction of IL-2 production are what sustain their proliferation during the immune response, and therefore underlie transplant allograft rejection and autoimmunity.
Since our approach focuses on select phytochemicals, it has the major advantages of using phytochemicals with known health benefits. This is in contrast to existing toxic immunosuppressants, or future experimental ones with unknown long-term effects and usually high cost. By “healthy”, we mean that reports of a dietary agent's benefits to health have been published in respected peer-reviewed journals, and that the agent has been used for health benefit for a long period of time. Many studies have demonstrated the beneficial effects of phytochemicals from fresh fruits and vegetables including their prevention or delay of chronic degenerative diseases [18-20]. These plants contain numerous and diverse nutrients including polyphenols, antioxidants, vitamins, phase II inducers (such as sulforaphane), and potent chemoprotectants that are thought to be major contributors to their healthy effects, and their individual nutrients are also known to modulate gene expression [25-43]. Health benefits have been reported for all of the plant-based compounds that we have tested for their effects on immunosuppression [22-25, 29,31-35,42].
To date, only a modest number of studies have investigated the effects of select phytochemicals on T-cell suppression [26,27,30,43-48]. Most notably, those that have have focused on curcumin, an anti-inflammatory and antioxidant component of turmeric [26,27,30,44,45]. Interestingly, two curcumin studies report a strong suppression of Tlymphocyte proliferation with near-identical inhibitory IC50 values of 3 uM [30,44]. Based on these and other studies in our laboratory, we hypothesized that select phytochemicals can suppress T-lymphocyte proliferation both in vitro and in vivo. Our objectives were to test selected phytochemicals, including those previously untested, for their in vitro suppression of human T-cells and mouse splenocytes; to include selected phytochemicals for in vivo analyses; and to assess the effect of cell culture on the recall of in vivo phytochemical effects. Additionally, we included curcumin as a positive control to compare our results with studies from other laboratories. Combined, we believe that our approach of using select phytochemicals as clinical immunosuppressants is simple, healthy, and cost-effective.
2. METHODS AND MATERIALS
2.1. Human T-lymphocyte purification
T-lymphocytes were purified from peripheral blood mononuclear cells (PBMCs) obtained from healthy human volunteers according to the Albany Medical Center Institutional Review Board Protocol. The PBMCs were first applied to a Ficoll-Hypaque density gradient, and white blood cells collected. After chilling, the cells were further processed using anti-CD19 Dynabeads (Invitrogen, Carlsbad, CA) to remove B-lymphocytes and the remaining purified T-lymphocytes were collected. These cells were centrifuged and resuspended in RPMI 1640 whole media (RPMI 1640 solution + 10% heat-inactivated fetal bovine serum + 2 mM glutamine + 50 units/ml penicillin + 50 μg/ml streptomycin), then added to 96-well round bottom flasks at a density of 150,000 cell/ml and cultures maintained in a humidified incubator atmosphere of 95% air and 5% CO2 at 37°C.
2.2. Human T-lymphocyte activation
Following preincubation with select phytochemicals as described below, the purified T-lymphocytes were activated with soluble anti-human CD3 plus soluble anti-human CD28 (49) obtained from eBioscience (San Diego, CA), both at 5 μg/ml. After 48 hours, the cells were pulsed with tritiated thymidine at 1 μCi/well (Perkin Elmer Health Sciences, Boston, Mass). After 14 hours, the cells were lysed and genomic DNA collected using a Skatron Cell Harvester. Tritiated thymidine radioactivity was then counted by Liquid Scintillation spectrometer as a measure of cell proliferation.
2.3. Effects of select phytochemicals on T-cell activation
Cells were pretreated in culture for 30 minutes with various phytochemicals at multiple concentrations or solvent control prior to activation to assess their effects on proliferation and IL-2 production. These select nutrients and phytochemicals included curcumin, sulforaphane, OptiBerry (a mixture of six berry extracts; 50-53), (−)-epigallocatechin gallate (EGCG, from green tea), the flanonoid quercetin, the Vitamin E component alpha tocopherol, ascorbic acid (Vitamin C), resveratrol, and sucrose (as a non-phytochemical negative control), all from Sigma Chemical Co. (St. Louis, MO) except OptiBerry (InterHealth, CA) and sulforaphane (LKT Labs, St. Paul, Minn.). All agents were dissolved in DMSO or ethanol except ascorbic acid (PBS), and 0 μM concentration controls contained these solvents only. In the case of OptiBerry, the dried extract was first dissolved in DMSO for 5 minutes at room temperature, then centrifuged 2 minutes at 12,000 × g and the soluble fraction used for our studies.
2.4. Effects of select phytochemicals on IL-2 levels
Cells were treated with and without select phytochemicals as described above, and culture media aliquots removed 24 hours after stimulation with anti-CD3 and anti-CD28. Interleukin-2 (IL-2) levels in the media were then analyzed using the BD Biosciences (San Jose, CA) Cytometric Bead Array Flex Set to human IL-2 according to the manufacturer. These analyses were carried out at the Center for Immunology Core Center. IL-2 levels were quantified by interpolation from a standard curve and expressed as pg per ml.
2.5. Cell viability
Twenty-four hours after stimulation with anti-CD3 and anti-CD28, cells treated as described above were centrifuged, washed once with PBS, resuspended in PBS and cell death assessed by flow cytometry after propidium iodide addition (12).
2.6. Mice
Six week old BALB/c female mice (obtained from Jackson Labs, Bar Harbor, ME) were used for the in vivo study and as a source of splenocytes. They were maintained under 12 hour light/dark cycles with food and water provided ad libitum, and housed at 4 mice per cage. All animal studies were approved by the Albany Medical College Institutional Animal Care and Use Committee, and animals handled and treated according to the regulations set forth in the approved Albany Medical College Institutional Animal Care and Use Protocol
2.7. Mouse dietary treatments
Mice were randomly assigned into three groups of four mice each. Control and supplemented diets were then provided to each group for 10 days. The control group was fed unaltered powdered AIN-93M mouse chow (LabDiet, Richmond, IND). Animals in the two experimental groups were fed analytical grade quercetin (0.25% w/w; Sigma) and sulforaphane (0.05% w/w; LKT Labs), respectively, incorporated into the powdered AIN-93M mouse chow. Thus, there were three groups for this study: control mice fed AIN-93M only; mice fed AIN-93M supplemented with quercetin; and mice fed AIN-93M supplemented with sulforaphane. The powders were placed in dry-diet feeder jars to avoid significant food loss that often accompanies pellet feeding, and the jars were weighed daily to determine the amount of food consumed. General animal health and water intake were also monitored during the 10 day test period.
2.8. Splenocyte harvesting and culture
At the conclusion of the 10-day feeding regimen, the animals were euthanized by carbon dioxide inhalation followed by decapitation. The spleens were removed, teased in cold PBS, forced through nylon mesh, centrifuged, resuspended and applied to Lympholyte-M lymphocyte separation medium (Accurate Chemical and Scientific, Westbury, NY). The white blood cell-positive band was then removed, centrifuged, and the splenocyte pellet resuspended in RPMI 1640 whole media. These cells were then counted, added to 96 round bottom well culture dishes at 200,000 cell per well, stimulated with 5 μg/ml each of soluble anti-mouse CD3 plus anti-mouse CD28, and proliferation analysis carried out as described above for human T-lymphocytes.
2.9. Select phytochemical effects on in vitro recall
Cultured mouse splenocytes and human T-lymphocytes were treated with 40 μM quercetin, 9 μM curcumin or 6 μM sulforaphane for 30 minutes. The cultured cells were then centrifuged, washed once with media, and split prior to plating. Half of these culture wells were then re-supplemented with the same concentration of phytochemical supplement (40 μM quercetin, 9 μM curcumin or 6 μM sulforaphane), and the other half with solvent (DMSO) only. A Control (unsupplemented) culture was also included. Proliferation was then assessed as above.
2.10. Statistical Analyses
Proliferation and IL-2 levels are expressed as the means of duplicate analyses, or when N≥3, the mean +/− SEM (standard error of the mean). In comparing experiments, each result was normalized to controls, which were set at 100%. Statistically significant differences between control and treated samples were assessed by the Kruskal-Wallis test using the SPSS Statistics (release 8.0.0) program (SPSS Inc., Chicago, IL). P < .05 was considered significant.
3. RESULTS
3.1. Curcumin, sulforaphane, quercetin, berry extract and EGCG all significantly inhibit human T-cell proliferation in vitro
The potential use of a number of select phytochemicals as clinical immunosuppressants was first assessed using isolated T-lymphocytes from human volunteers. Following purification, flow cytometry was carried out to assess purity, and revealed that 91% of the isolated cells were CD3+ T-cells, confirming the strong enrichment of this purification protocol. These T-cells were then used to assess the effects of various select phytochemicals on proliferation. Initially, we compared the relative stimulatory effects of plate bound anti-CD3 plus soluble anti-CD28; soluble anti-CD3 plus soluble anti-CD28; plate bound anti-CD3 by itself; and soluble anti-CD3 by itself. The best stimulatory effect was observed using a combination of the two soluble antibodies, and this was used for all future studies (data not shown).
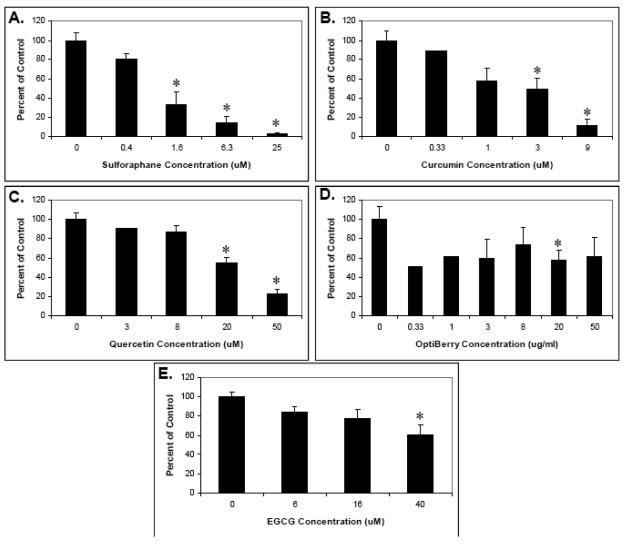
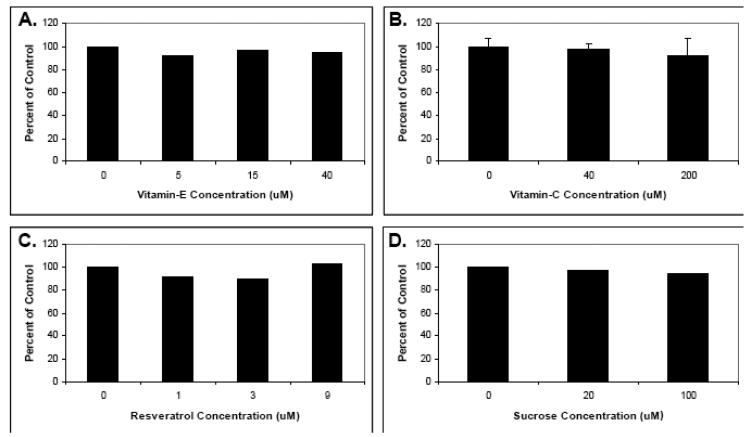
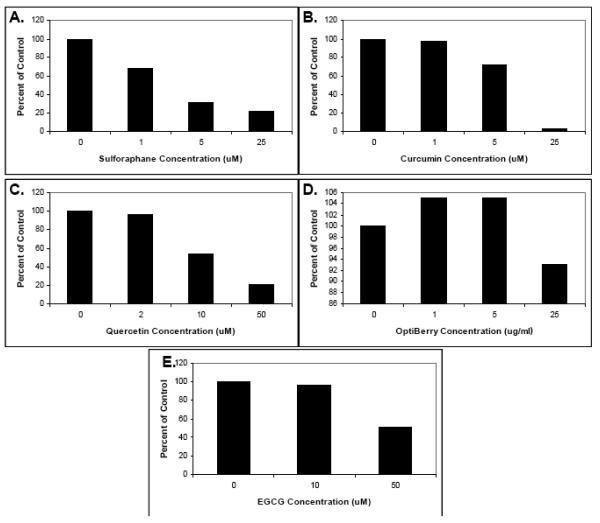
The isolated T-cells were then preincubated with various concentrations of the selected phytochemicals as well as purified α-tocopherol, vitamin C, and sucrose (as a control) and their effect on cell proliferation assessed. As shown in Figs. 1 and 2, a range of effects was observed depending upon the dietary phytochemical supplement used. Specifically, curcumin, sulforaphane, quercetin, berry extract and EGCG all significantly inhibited T-cell proliferation and, with the exception of Optiberry, in a dose-dependent manner (Fig. 1). All other agents showed little or no effect (Fig. 2). The strongest suppression was observed for sulforaphane (IC50 approximately 1μM) and curcumin (IC50 approximately 3 μM). Consistent with reports from other labs, the 3 μM IC50 for curcumin was essentially identical to that reported for this agent in two other similar T-lymphocyte proliferation studies [30,44]. Thus, select phytochemicals are able to inhibit human T-cell proliferation in vitro.
Fig. 1. Human T-lymphocyte suppression by select phytochemicals.
Human T-lymphocytes were isolated, pretreated with the indicated phytochemical supplements at various concentrations for 30 minutes, and stimulated with 5 ug/ml each of soluble anti-human CD3 plus anti-human CD28. Proliferation was then measured by tritiated thymidine incorporation. (A) Sulforaphane. (B) Curcumin. (C). Quercetin. (D) Berry extracts (E) EGCG. Data is expressed as means +/− SEM (N=3-5). For data points without error bars, N=2. Statistically significant differences between control and treated samples were assessed by the Kruskal-Wallis test. *P < .05.
Fig. 2. The effects of resveratrol, Vitamin C, alpha tocophereol, and sucrose on human T-lymphocyte proliferation.
Human T-lymphocytes were isolated, pretreated with indicated phytochemical supplements at various concentrations for 30 minutes, and stimulated with anti-CD3 plus anti-CD28. Proliferation was then measured by tritiated thymidine incorporation. (A) Resveratrol. (B) Vitamin C. (C) alpha tocopherol. (D) Sucrose. Data is expressed as the means of two separate experiments. Statistically significant differences between control and treated samples for Vitamin C (N=3) were assessed by the Kruskal-Wallis test.
3.2. Curcumin, quercetin, berry extract and EGCG all significantly inhibit human IL-2 production in vitro
Based on the strong proliferative inhibition observed for curcumin, quercetin, berry extract and EGCG, we also evaluated their effect on IL-2 levels under the same conditions 24 hours after stimulation (note: sulforaphane IL-2 levels were not tested here, but were in the below mouse splenocyte studies). As shown in Fig. 3, these phytochemical supplements also suppressed IL-2 levels, and actually did so to an even greater extent than proliferation. Thus, select phytochemicals are also able to inhibit human T-cell IL-2 production in vitro. Since T-cell activation involves IL-2 induction and IL-2 autocrine stimulation, these results strongly suggest that the observed proliferative inhibitions are due, at least in part, to diminished IL-2 levels.
Fig. 3. The effects of select phytochemicals on human T-lymphocyte IL-2 production.
Human T-lymphocytes were isolated, pretreated with the indicated phytochemical supplements at various concentrations for 30 minutes, and stimulated with anti-CD3 plus anti-CD28. Twenty four hours later, media was removed and assayed for IL-2 using the Cytometric Bead Array assay. (A) Sulforaphane. (B) Curcumin. (C). Quercetin. (D) Berry extracts (E) EGCG. Data is expressed as the means of two separate experiments.
3.3. Select phytochemical suppression of human T-cell proliferation and IL-2 production is not due to toxicity
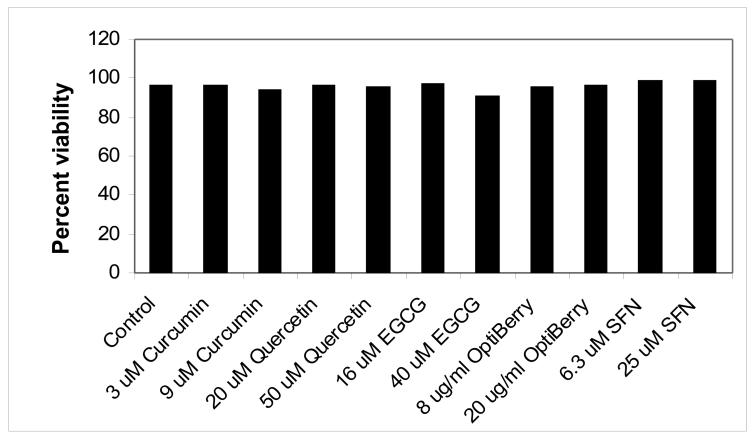
To rule out toxicity as the reason for the above suppressions, propidium iodide studies were carried out. As shown in Fig. 4, no significant cell toxicity was observed with any of the select phytochemicals used after 24 hours. Thus, curcumin, sulforaphane, quercetin, berry extract and EGCG inhibitions of T-cell proliferation and IL-2 production are not due to toxicity.
Fig. 4. The effects of select phytochemicals on human T-lymphocyte viability.
Human T-lymphocytes were treated with phytochemical supplements, stimulated, and 24 hours later, propidium iodide uptake assessed. Data is expressed as the means of two separate experiments.
3.4. Curcumin, sulforaphane, quercetin, and EGCG all significantly inhibit mouse T-cell proliferation in a mixed splenocyte population in vitro
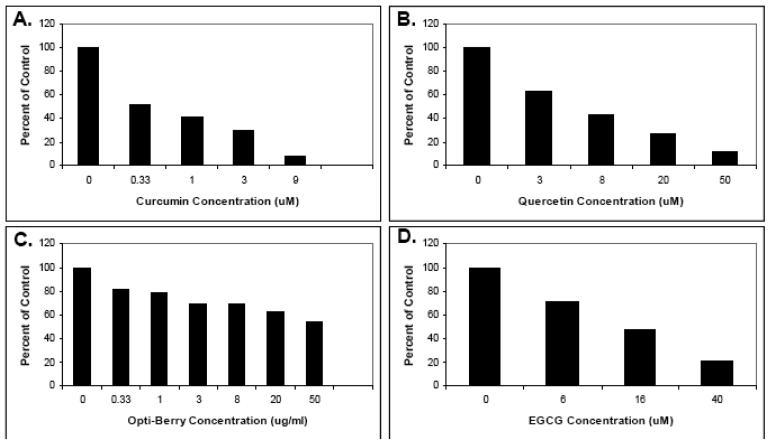
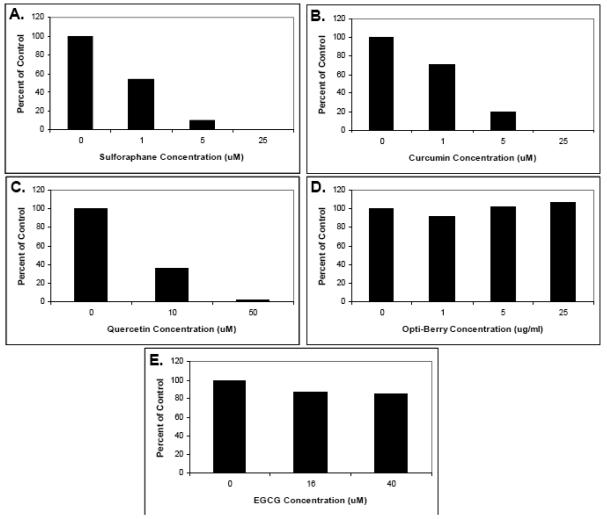
Since we were interested in other T-cell sources as well as pursuing in vivo feeding studies in mice, we first assessed the ability of the above selected phytochemicals to suppress mouse T-cell activation. Here, we used whole splenocyte populations, which are a mixture of T-cells with other immune cell types, to get a more realistic appraisal of the effect of these phytochemicals on cells as they exist in an organism; i.e., as part of mixed cell populations. With the possible exception of berry extracts, all select phytochemicals that inhibited human purified T-cells (curcumin, sulforaphane, quercetin, EGCG) also inhibited mouse splenocyte T-cell proliferation (Fig. 5). For Berry extract, stimulation was observed a lower concentrations followed by inhibition at the highest concentration. This variability may be due, in part, to the lesser number of samples analyzed here (N=2) as compared with human.
Fig. 5. The effects of select phytochemicals on mouse splenocyte proliferation.
Mouse splenocytes were isolated from BALB/c mouse spleens, pretreated with the indicated phytochemical supplements at various concentrations for 30 minutes, and stimulated with 5 ug/ml each of anti-mouse CD3 plus anti-mouse CD28. Proliferation was then measured by tritiated thymidine incorporation. (A) Sulforaphane. (B) Curcumin. (C). Quercetin. (D) Berry extracts (E) EGCG. Data is expressed as the means of two separate experiments.
3.5. Curcumin, sulforaphane, quercetin, and (weakly) EGCG inhibit mouse IL-2 production in a mixed splenocyte population in vitro
The above splenocyte cultures were also analyzed 24 hours after stimulation for IL-2 levels in the culture media. Consistent with the proliferation results, sulforaphane, curcumin and quercetin strongly suppressed IL-2 production, and EGCG exhibited a relatively weak effect (Figs. 6A-C and 6E). No suppression of IL-2 levels was observed with Berry extract (Fig. 6D).
Fig. 6. The effects of select phytochemicals on mouse splenocyte IL-2 production.
Mouse splenocytes were isolated from BALB/c mouse spleens, pretreated with the indicated phytochemical supplements at various concentrations for 30 minutes, and stimulated with anti-CD3 plus anti-CD28. Twenty four hours later, media was removed and assayed for IL-2 using the Cytometric Bead Array assay. (A) Sulforaphane. (B) Curcumin. (C). Quercetin. (D) Berry extracts (E) EGCG. Data is expressed as the means of two separate experiments.
3.6. Quercetin but not sulforaphane inhibits mouse splenocyte proliferation after in vivo feeding
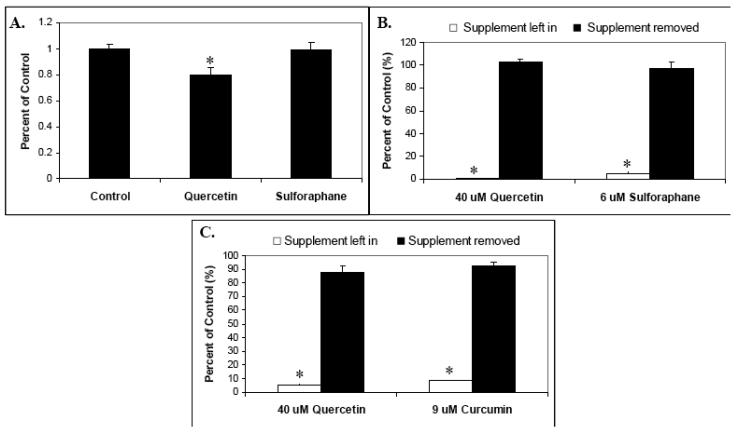
To assess the effect of these select phytochemicals on T-cell activation in vivo, we selected two of these agents, quercetin and sulforaphane, for further study. BALB/c mice were fed a powdered diet of AIN-93M with or without quercetin (0.25 w/w) or sulforaphane (0.05 w/w) for 10 days. No significant difference in food consumed per day was observed, and there was a modest loss of weight in the sulforaphane-fed mice (6.7%) as compared with Control and quercetin fed. A 20% statistically significant reduction in proliferation was observed in the quercetin-fed mice as compared with Control (Kruskal-Wallis test; P < .05). However, sulforaphane-fed mice splenocytes exhibited proliferation near equal to Controls (Fig. 7A).
Fig. 7.
The effects of in vivo phytochemical supplementation on mouse splenocyte proliferation, and the retention of phytochemical supplement effect “recall”. (A) BALB/c mice were fed a powdered diet of AIN-93M with or without quercetin (0.25 w/w) or sulforaphane (0.05 w/w) for 10 days. After sacrifice, splenocytes were isolated from spleens, pretreated with the indicated phytochemical supplements at various concentrations for 30 minutes, stimulated with anti-CD3 plus anti-CD28, and proliferation measured by tritiated thymidine incorporation. Data is expressed as means +/− SEM (N=4). (B) and (C) Retention of phytochemical supplement recall. (B) Mouse splenocytes were supplemented with 40 uM quercetin or 6 uM sulforaphane for 30 minutes, centrifuged, washed, and split prior to plating. Half of these culture wells were then resupplemented with 40 uM quercetin and 6 uM sulforaphane, and the other half with solvent (DMSO) only. Control (unsupplemented) cultures were also included. Cells were then stimulated with anti-CD3 plus anti-CD28 and proliferation measured by tritiated thymidine incorporation. (C) Human T-lymphocytes were supplemented with 40 uM quercetin or 9 uM curcumin for 30 minutes, centrifuged, washed, and split prior to plating. Half of these culture wells were then resupplemented with 40 uM quercetin and 9 uM curcumin, and the other half with solvent (DMSO) only. Control (unsupplemented) cultures were also included. Cells were then stimulated with anti-CD3 plus anti-CD28 and proliferation measured by tritiated thymidine incorporation. Statistically significant differences between control and treated samples (A) and between supplement left in versus supplement removed (B and C) were assessed by the Kruskal-Wallis test. *P < .05.
3.7. Select phytochemical effects ('recall”) are significantly lost in vitro, suggesting an even greater in vivo suppression by quercetin
For the above in vivo study, splenocytes from the supplement-fed mice were harvested and then analyzed for in vitro proliferation in the absence of additional quercetin or sulforaphane. We thus considered the likelihood that at least some of the effects (“recall”) of the in vivo phytochemical supplement feeding may be lost during this cell culturing. To assess this, we treated both mouse splenocytes and human T-lymphocytes with 40 μM quercetin, 9 μM curcumin or 6 μM sulforaphane, all known suppressive concentrations for proliferation (Figs. 1 and 5), then removed the selected phytochemicals for half the wells as described in Methods and Materials prior to stimulation. The results in Fig 7B for splenocytes and Fig. 7C for human T-lymphocytes indicate that removal of supplement in cell culture leads to a dramatic and statistically significant loss of suppression as compared with supplemented (Kruskal-Wallis test; P < .05). Thus, the 20% inhibition of splenocyte proliferation observed for quercetin-fed mice (Fig. 5) is probably a significant underestimation when considering the loss of actual suppressive effects that occurs during subsequent cell culture. These results also underscore the major importance of considering the loss of recall when carrying out supplement-based in vivo studies followed by in vitro analysis.
4. DISCUSSION
In this study, we identify select phytochemicals that are able to significantly suppress T-cell activation in both human T-lymphocytes and mouse splenocytes. These results raise the possibility of using these phytochemical supplements as an alternative or complementary approach to treating autoimmune and transplant patients. Most likely, their use would be as adjuvants, leading to a reduction (rather than elimination) in the concentration of present clinical immunosuppressants that exhibit toxic side effects. Since many of these select phytochemicals have anti-carcinogenic activity, they may also reduce the increased risk of cancer associated with present clinical immunosuppression. The healthy nature of the agents we have tested may also confer reduced infection susceptibility to patients.
A critical part to our strategic approach is our use of select phytochemicals with known health benefits. Sulforaphane is a component of broccoli and other Cruciferous vegetables that is reportedly cytoprotective and anticarcinogenic primarily due to its ability to induce phase II enzymes [32,33]. OptiBerry fruit extract, the purified flanonoid quercetin, and tea epigallocatechin-3-gallate (EGCG) were chosen because these polyphenol and other nutrient-containing extracts and purified compounds have also been shown to improve health and modulate gene expression [18,28,33,34,50-53]. Curcumin is considered a healthy phytochemical with documented antioxidant, anti-inflammatory, and anti-carcinogenesis activities, and it has also been shown to modulate gene expression [25,29-31,42]. We added purified antioxidant Vitamin C and tocopherol to our studies because all of our tested phytochemicals have antioxidant (or antioxidant-inducing) activities, and thus, any observed suppression may be due to this activity. However, our lack of a suppressive effect with either Vitamin C or tocopherol argue against antixoxidant activity as being a key mechanism by which the tested phytochemicals suppress T-cell activation.
Our parallel IL-2 studies provide major mechanistic insight into how these phytochemical supplements are able to immunosuppress. T-cell activation involves IL-2 induction and IL-2 autocrine stimulation to sustain proliferation of this cell-type. The coordinate decrease in IL-2 levels and cell proliferation strongly suggest that the suppression of cell division by the phytochemical supplements tested is due, at least in part, to diminished IL-2 levels. While beyond the scope of these studies, other mechanistic factors may also contribute to the observed suppression of proliferation including changes in IL-2 receptor and interferon gamma levels, and NFkB and NF-AT signaling. The latter would implicate calcineurin, a known mediator of IL-2 production and the target of present immunosuppressants cyclosporine and tacrolimus. It is also possible that our select phytochemicals induce T-cell cycle arrest, although preliminary studies suggest that this is not the case. Interestingly, there is evidence for the suppression of NF-AT and/or NFkB pathways, both of which are known mediators of IL-2 transcriptional induction, by our select phytochemicals (31).
While these present studies are promising, additional ones are needed before the use of such phytochemical supplements can be recommended for human autoimmune and transplant patients. For one, their suppressive effects in humans would have to be demonstrated. If successful, their proper dosage would also have to be then titrated. Additionally, while we are advocating these select phytochemicals as future immunosuppressant treatments (or more likely, co-treatments), we believe that their chronic use over a long period of time should be investigated more thoroughly. That is, they must be amenable to ingestion at the doses and concentrations needed over prolonged periods without toxicity. It will also be important to assess the effect of these phytochemical supplements on other arms of the immune system such as overall white blood cell distribution to see if this balance is altered by select phytochemical intervention.
In addition, the issue of bioavailability is often a concern when carrying out in vivo phytochemical studies, including the fact that this varies from species to species [54,55]. This is a potential limitation of our in vivo studies since here, we did not include an analysis of blood quercetin and sulforaphane to confirm that sufficient uptake occurred. However, it has been demonstrated that in vivo effects can occur in mice fed quercetin or sulforaphane, and at similar levels to those that we administered [56-58]. Furthermore, we do detect in vivo effects with quercetin as presented in Fig. 7. Thus, even with expected bioavailability constraints, we believe that our Fig. 7 studies are still able to assess the in vivo effects of quercetin and sulforaphane. It would also be important to ultimately test the effect of these select phytochemicals in antigen-challenged animals, since this is what occurs and causes autoimmunity and transplantation.
Relative to this, one curious and unresolved question is why we see suppression of T-cells using our selected phytochemicals, since they are considered healthy [22-25, 29,31-35,42] and a healthy diet is usually associated with improved immune system function. Most likely, such suppression is most observed during activation of T-cells such as occurs in transplantation procedures or with certain autoimmune diseases. Otherwise, these select phytochemicals may actually enhance other arms of the immune system (e.g., macrophages, NK cells, dendritic cells, B-cells, etc.). If so, this could represent yet another potential valuable use of select phytochemicals for intentional immunosuppression: that of reducing infection susceptibility and cancer that is associated with current immunosuppression regimens.
Our approach would not only reduce treatment toxicity, but also significantly reduce the high drug cost associated with clinical immunosuppression and improve compliance. The typical cost of immunosuppressants is tens of thousands of dollars per year. A recent estimated U.S. average cost summary for the first year of a kidney transplant is $27,000 (compiled by Milliman USA, Inc. for 2006). If successful, our strategy could dramatically cut these costs since much less expensive phytochemical supplements could be obtained at a small fraction of the cost. It is also likely to improve compliance, as some patients do not keep to their medication schedule due to toxicity and cost.
In summary, our data provide evidence that select phytochemicals can act as T-lymphocyte immunosuppressants. These results suggest the potential use of these dietary supplements for treating autoimmune and transplant patients, and support our strategy of using select phytochemicals to treat genetically-defined pathologies including those requiring clinical immunosuppression. Combined, we believe that our approach of using select phytochemicals is simple, healthy, and cost-effective.
Acknowledgment
The authors would like to thank Stan Mudzinski, David Conti, Ed Gosselin, Robert Jordan, Julie Hasselbarth, Devender Kumar, Girish Kirimanjeswara, Betsy Bashaw and Eileen Graffunder for assistance, suggestions, and reagents. This work was supported by grants from the NIH STIRT grant program and The Community Foundation for the Capital Region's Bender Scientific Fund.
List of Abbreviations
- T-cell
T-lymphocyte
- IL-2
interleukin-2
- EGCG
epigallocatechin gallate
- PBMC
peripheral blood mononuclear cells
- SEM
standard error of the mean
- NF-κB
nuclear factor κB
- NF-AT
nuclear factor of activated T cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Knoll G. Trends in kidney transplantation over the past decade. Drugs. 2008;68(Suppl 1):3–10. doi: 10.2165/00003495-200868001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Tang IY, Meier-Kriesche HU, Kaplan B. Immunosuppressive strategies to improve outcomes of kidney transplantation. Semin Nephrol. 2007;27:377–92. doi: 10.1016/j.semnephrol.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Eisen HJ. Immunosuppression on the horizon. Heart Fail Clin. 2007;3:43–9. doi: 10.1016/j.hfc.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol. 2007;2:374–84. doi: 10.2215/CJN.03791106. [DOI] [PubMed] [Google Scholar]
- 5.Guerra G, Srinivas TR, Meier-Kriesche HU. Calcineurin inhibitor-free immunosuppression in kidney transplantation. Transpl Int. 2007;20:813–27. doi: 10.1111/j.1432-2277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 6.Wong G, Chapman JR. Cancers after renal transplantation. Transplant Rev. 2008;22:141–9. doi: 10.1016/j.trre.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor A. Malignancy in kidney transplant recipients. Drugs. 2008;68(Suppl 1):11–9. doi: 10.2165/00003495-200868001-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman DB, Shapiro R, Lucey MR, et al. Immunosuppression: practice and trends. Am J Transplant. 2004;4(Suppl 9):38–53. doi: 10.1111/j.1600-6135.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- 9.Ponticelli C, Tarantino A, Campise M, et al. From cyclosporine to the future. Transplant Proc. 2004;36:557S–560S. doi: 10.1016/j.transproceed.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 10.Crawford DR, Leahy KP, Abramova N, et al. Hamster adapt78 mRNA is a Down syndrome critical region homologue that is inducible by oxidative stress. Arch Biochem Biophys. 1997;342:6–12. doi: 10.1006/abbi.1997.0109. [DOI] [PubMed] [Google Scholar]
- 11.Leahy KP, Davies KJ, Dull M, et al. adapt78, a stress-inducible mRNA, is related to the glucose-regulated protein family of genes. Arch Biochem Biophys. 1999;368:67–74. doi: 10.1006/abbi.1998.1059. [DOI] [PubMed] [Google Scholar]
- 12.Leahy KP, Crawford DR. adapt78 protects cells against stress damage and suppresses cell growth. Arch Biochem Biophys. 2000;379:221–228. doi: 10.1006/abbi.2000.1897. [DOI] [PubMed] [Google Scholar]
- 13.Fuentes JJ, Genesca L, Kingsbury TJ, et al. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet. 2000;9:1681–1690. doi: 10.1093/hmg/9.11.1681. [DOI] [PubMed] [Google Scholar]
- 14.Rothermel B, Vega RB, Yang J, et al. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem. 2000;275:8719–8725. doi: 10.1074/jbc.275.12.8719. [DOI] [PubMed] [Google Scholar]
- 15.Kingsbury TJ, Cunningham KW. A conserved family of calcineurin regulators. Genes Dev. 2000;14:1595–1604. [PMC free article] [PubMed] [Google Scholar]
- 16.Lin HY, Michtalik HJ, Zhang S, et al. Oxidative and calcium stress regulate DSCR1 (Adapt78/MCIP1) protein. Free Rad Biol Med. 2003;35:528–539. doi: 10.1016/s0891-5849(03)00358-7. [DOI] [PubMed] [Google Scholar]
- 17.Michtalik MJ, Narayan AV, Bhatt N, et al. Multiple oxidative stress-response members of the Adapt78 family. Free Rad Biol Med. 2004;37:454–462. doi: 10.1016/j.freeradbiomed.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Weinreb O, Amit T, Youdim MB. A novel approach of proteomics and transcriptomics to study the mechanism of action of the antioxidant-iron chelator green tea polyphenol (−)-epigallocatechin-3-gallate. Free Rad Biol Med. 2007;43:546–56. doi: 10.1016/j.freeradbiomed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Delabar JM, Park MY, Lee KS, Sung MK. Effects of dietary mulberry, Korean red ginseng, and banaba on glucose homeostasis in relation to PPAR-alpha, PPAR-gamma, and LPL mRNA expressions. Life Sci. 2005;77:3344–54. doi: 10.1016/j.lfs.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Pung D, Leong V, et al. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Rad Biol Med. 2004;37:1578–1590. doi: 10.1016/j.freeradbiomed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson SK, Tucker GA, John M. Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc Nutr Soc. 2008;67:42–7. doi: 10.1017/S0029665108006009. [DOI] [PubMed] [Google Scholar]
- 22.Everitt AV, Hilmer SN, Brand-Miller JC, et al. Dietary approaches that delay age-related diseases. Clin Interv Aging. 2006;1:11–31. doi: 10.2147/ciia.2006.1.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stan SD, Kar S, Stoner GD, Singh SV. Bioactive food components and cancer risk reduction. J Cell Biochem. 2008;104:339–56. doi: 10.1002/jcb.21623. [DOI] [PubMed] [Google Scholar]
- 24.Lila MA. From beans to berries and beyond: teamwork between plant chemicals for protection of optimal human health. Ann N Y Acad Sci. 2007;1114:372–80. doi: 10.1196/annals.1396.047. [DOI] [PubMed] [Google Scholar]
- 25.Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006;8:E443–E449. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma S, Chopra K, Kulkarni SK, Agrewala JN. Resveratrol and curcumin suppress immune response through CD28/CTLA-4 and CD80 co-stimulatory pathway. Clin Exp Immunol. 2007;147:155–163. doi: 10.1111/j.1365-2249.2006.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav VS, Mishra KP, Singh DP, et al. Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol. 2005;27:485–97. doi: 10.1080/08923970500242244. [DOI] [PubMed] [Google Scholar]
- 28.Yasmin T, Sen CK, Hazra S, Bagchi M. Antioxidant capacity and safety of various anthocyanin berry extract formulations. Res Commun Pharmacol Toxicol. 2003;8:IV-25–IV-35. [Google Scholar]
- 29.Hatcher H, Planalp R, Cho J, et al. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deters M, Knochenwefel H, Lindhorst, et al. Different Curcuminoids Inhibit T-Lymphocyte Proliferation Independently of Their Radical Scavenging Activities. Pharmacol Res. 2008;25:1822–7. doi: 10.1007/s11095-008-9579-2. [DOI] [PubMed] [Google Scholar]
- 31.Jeong W-S, Kim I-W, Hu R, Kong A-NT. Modulatory Properties of Various Natural Chemopreventive Agents on the Activation of NF-kB Signaling Pathway. Pharmaceutical Res. 2004;21:661–670. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 32.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol. Life Sci. 2007;64:1105–27. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res. 2008;52(Suppl 1):S128–38. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- 34.Chen D, Milacic V, Chen MS, et al. Tea polyphenols, their biological effects and potential molecular targets. Histol Histopathol. 2008;23:487–96. doi: 10.14670/hh-23.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Q:Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–37. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Yu ES, Min HJ, An SY, et al. Regulatory mechanisms of IL-2 and IFNgamma suppression by quercetin in T helper cells. Biochem Pharmacol. 2008;76:70–8. doi: 10.1016/j.bcp.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Singh B, Kaur P, Gopichand, et al. Biology and chemistry of Ginkgo biloba. Fitoterapia. 2008;79:401–418. doi: 10.1016/j.fitote.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Dias MC, Rodrigues MAM, Reimberg MCH, Barbisan LF. Protective effects of Ginkgo biloba against rat liver carcinogenesis. Chemico-Biological Interactions. 2008;173:32–42. doi: 10.1016/j.cbi.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Masteikova R, Muselik J, Bernatonienė J, Bernatonienė R. Antioxidative activity of Ginkgo, Echinacea, and Ginseng tinctures. Medicina. 2007;43:306–9. [PubMed] [Google Scholar]
- 40.Gum SIl, Jo SJ, Ahn SH, et al. The potent protective effect of wild ginseng (Panax ginseng C.A. Meyer) against benzo[α]pyrene-induced toxicity through metabolic regulation of CYP1A1 and GSTs. J. Ethnopharmacology. 2007;112:568–576. doi: 10.1016/j.jep.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Belloir C, Singh V, Daurat C, et al. Links Protective effects of garlic sulfur compounds against DNA damage induced by direct- and indirect-acting genotoxic agents in HepG2 cells. Food Chem Toxicol. 2006;44:827–34. doi: 10.1016/j.fct.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Dairam A, Fogel R, Daya S, Limson JL. Antioxidant and Iron-Binding Properties of Curcumin, Capsaicin, and S-Allylcysteine Reduce Oxidative Stress in Rat Brain Homogenate. J Agri Food Chem. 2007;56:3350–3356. doi: 10.1021/jf0734931. [DOI] [PubMed] [Google Scholar]
- 43.Wilasrusmee C, Siddiqui J, Bruch D, et al. Immunosuppression by green tea mediated by IL-2 decrease, but this not observed for ginseng In vitro immunomodulatory effects of herbal products. Am Surgery. 2002;68:860–4. [PubMed] [Google Scholar]
- 44.Ranjan D, Johnston TD, Wu G, et al. Curcumin Blocks Cyclosporine A-Resistant CD28 Costimulatory Pathway of Human T-Cell Proliferation. J Surgical Res. 1998;77:174–178. doi: 10.1006/jsre.1998.5374. [DOI] [PubMed] [Google Scholar]
- 45.Gao X, Kuo J, Jiang H, et al. Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem Pharmacol. 2004;68:51–61. doi: 10.1016/j.bcp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Thejass P, Kuttan G. Immunomodulatory activity of Sulforaphane, a naturally occurring isothiocyanate from broccoli (Brassica oleracea) Phytomedicine. 2007;14:538–545. doi: 10.1016/j.phymed.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Lugli E, Ferraresi R, Roat E, et al. Quercetin inhibits lymphocyte activation and proliferation without inducing apoptosis in peripheral mononuclear cells. Leuk Res. 2009;33:140–50. doi: 10.1016/j.leukres.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 48.Lee SI, Kim BS, Kim KS, et al. Immune suppressive activity of punicalagin via inhibition of NFAT activation. Biochem Biophys Res Commun. 2008;371:799–803. doi: 10.1016/j.bbrc.2008.04.150. [DOI] [PubMed] [Google Scholar]
- 49.Catalano A, Caprari P, Moretti S, Faronato M, Tamagnone L, Procopio A. Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood. 2006;107:3321–3329. doi: 10.1182/blood-2005-06-2445. [DOI] [PubMed] [Google Scholar]
- 50.Zafra-Stone S, Yasmin T, Bagchi M, et al. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51:675–683. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]
- 51.Myhrstad MCW, Carlsen H, Dahl LI, et al. Bilberry Extracts Induce Gene Expression Through the Electrophile Response Element. Nutrition and Cancer. 2006;54:94–101. doi: 10.1207/s15327914nc5401_11. [DOI] [PubMed] [Google Scholar]
- 52.Bagchi D, Sen CK, Bagchi M, Atalay M. Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry. 2004;69:75–80. doi: 10.1023/b:biry.0000016355.19999.93. [DOI] [PubMed] [Google Scholar]
- 53.Bagchi D, Roy S, Patel V, et al. Safety and whole-body antioxidant potential of a novel anthocyanin-rich formulation of edible berries. Mol Cell Biochem. 2006;281:197–209. doi: 10.1007/s11010-006-1030-6. [DOI] [PubMed] [Google Scholar]
- 54.Meng X, Maliakal P, Lu H, et al. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J Agric Food Chem. 2004;52:935–942. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 55.Scholz S, Williamson G. Interactions affecting the bioavailability of dietary polyphenols in vivo. Int J Vitam Nutr Res. 2007;77:224–35. doi: 10.1024/0300-9831.77.3.224. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Li H, Zhao Y, Gao Z. Dietary supplementation of baicalin and quercetin attenuates iron overload induced mouse liver injury. Eur J Pharmacology. 2006;535:263–9. doi: 10.1016/j.ejphar.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 57.Myzak MC, Dashwood WM, Orner GA, et al. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apcmin mice. FASEB J. 2006;20:506–8. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Izawa H, Kohara M, Aizawa K, et al. Alleviative effects of quercetin and onion on male reproductive toxicity induced by diesel exhaust particles. Biosci. Biotechnol. Biochem. 2008;72:1235–1241. doi: 10.1271/bbb.70705. [DOI] [PubMed] [Google Scholar]