Abstract
Objective
Histone acetylation/deacetylation plays an important role in the control of gene expression, tissue growth and development. In particular, histone deacetylases 7 (HDAC7), a member of class IIa HDACs, is crucial in maintaining vascular integrity. However, whether HDAC7 is involved in the processes of vascular endothelial signaling and angiogenesis remains unclear. Here, we investigated the role of HDAC7 in vascular endothelial growth factor (VEGF) signaling and angiogenesis.
Methods and Results
We show for the first time that VEGF stimulated phosphorylation of HDAC7 at the sites of Ser178, Ser344, and Ser479 in a dose- and time-dependent manner, which leads to the cytoplasmic accumulation of HDAC7. Using pharmacological inhibitors, siRNA and adenoviruses carrying dominant-negative mutants, we found that phospholipase Cγ/protein kinase C/protein kinase D1 (PKD1)-dependent signal pathway mediated HDAC7 phosphorylation and cytoplasmic accumulation by VEGF. Infection of ECs with adenoviruses encoding a mutant of HDAC7 specifically deficient in PKD1-dependent phosphorylation, inhibited VEGF-induced angiogenic gene expression, including matrix metalloproteinases MT1-MMP and MMP10. Moreover, HDAC7 and its targeting genes were involved in VEGF-stimulated endothelial cell migration, tube formation and microvessel sprouting.
Conclusions
Our results demonstrate that VEGF stimulates PKD1-dependent HDAC7 phosphorylation and cytoplasmic accumulation in endothelial cells modulating gene expression and angiogenesis.
Keywords: VEGF, histone deacetylase 7, protein kinase D, gene expression, endothelial cells, angiogenesis
Introduction
Angiogenesis is essential for normal physiological processes such as organ development and wound healing. It is also critical to pathological processes such as tumor growth, atherosclerosis, rheumatoid arthritis, and diabetic retinopathy 1-3. Vascular endothelial growth factor (VEGF)1 is crucial for many angiogenic processes both in normal and pathological conditions 2, 3. The binding of VEGF to its cognate receptors triggers intracellular signal cascades to mediate endothelial gene expression, cell migration and angiogenesis 4-7. In particular, VEGF receptor 2 (VEGFR2)-mediated phospholipase Cγ (PLCγ)/protein kinase C (PKC) pathway regulates the activation of extracellular-regulated kinases and angiogenesis 4, 7, 8. Recently, we have further showed protein kinase D1 (PKD1), a novel serine/thronine protein kinase 9-12, is phosphorylated in endothelial cells (ECs) in response to VEGF, which mediates VEGF-induced activation of extracellular-regulated kinases and endothelial cell proliferation 13. However, the direct downstream targets of PKD1 in VEGF signaling are not fully understood.
Histone acetylation/deacetylation plays an important role in the control of gene expression 14, 15. Histone acetyltransferases stimulate transcription through acetylation of histones, resulting in relaxation of nucleosomes; and histone deacetylases (HDACs) deacetylates histone and repress transcription by condenses the chromatin. Recently we have shown that HDAC5, a member in class IIa HDAC family, is involved in VEGF-induced gene expression and angiogenesis 16. Interestingly, HDAC7, also belongs to class IIa HDAC family, has been shown to maintain vascular integrity by repressing matrix metalloproteinase 10 (MMP10) 17. Specifically, both global deletion and vascular EC-specific deletion of HDAC7 gene in mice resulted in the defects of endothelial cells-cells contacts and consequent dilation and rupture of blood vessels 17. Silencing HDAC7 in ECs impairs cell migration and tube formation 18. HDAC7 also functions as a signal-dependent repressor of gene transcription during T-cell development 19, 20. However, the role of HDAC7 in VEGF signaling and function remains largely unclear.
Here, we demonstrate for the first time that VEGF stimulates phosphorylation of HDAC7 on Ser178/344/479 residues in ECs via PKD1-dependent pathway, and subsequent nuclear exclusion of HDAC7 and myocyte enhance factors 2 (MEF2) transcriptional activation. Importantly, PKD1-HDAC7 pathway modules VEGF-induced gene expression of matrix metalloproteinases and angiogenesis.
Methods
A detailed methods description is included in the supplemental materials. Experiments were performed in primary bovine aortic ECs (BAECs) and human umbilical vein ECs (HUVCEs) 16, 21. HUVCEs were used for the experiments with small interfering RNA (siRNA) that specifically target on genes in human cells. HUVECs were transfected with 21-mer small interfering RNA (siRNA) complexed to Lipofectamine 2000 (Invitrogen) 21, 22. BAECs and HUVECs were infected with adenoviruses encoding HA-HDAC7 wild type (WT) and S178/344/479A mutant (S/A), and YFP-tagged HDAC7-WT 23. The immunofluorescence studies for HDAC7 subcellular localization were performed in fixed cells 16, 21. mRNA was measured by reverse-transcription polymerase chain reaction (RT-PCR) using TaqMan (Applied Biosystems) 16. Cell migration was analyzed under wound closure. Capillary-like tube formation in Matrigel (BD Biosciences) for in vitro angiogenesis was performed 16, and microvessel sprouting was analyzed by aortic ring assay for ex vivo angiogenesis 24, 25.
Results
VEGF stimulates HDAC7 phosphorylation in endothelial cells
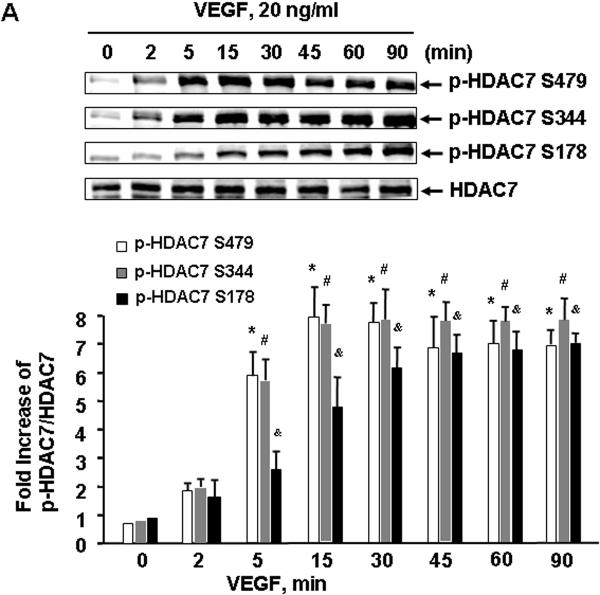
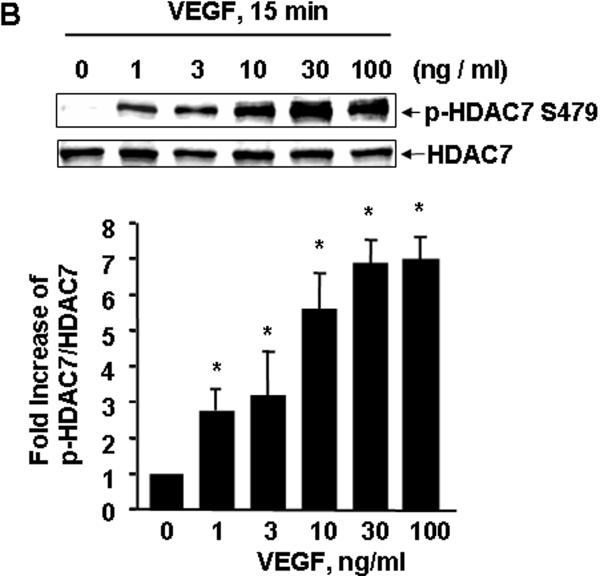
To examine the potential role of HDAC7 in VEGF signaling, we first studied HDAC7 phosphorylation in ECs in response to VEGF. BAECs were stimulated with VEGF (20 ng/ml) at indicated time, and the phosphorylation of HDAC7 in cell lysates was determined by Western blots using phospho-specific HDAC7 antibodies, which recognizes the HDAC7 phosphorylated at Ser178, Ser344, or Ser479, respectively 23. We observed that VEGF rapidly induced HDAC7 phosphorylation within 2 min, and the activation reached a maximum after 15 min and still high at 90 min (Figure 1A). Because of similar pattern among three sites for HDAC7 phosphorylation in response to VEGF, hereafter we only show the results for HDAC7 phosphorylation at Ser479. We also observed that VEGF induced HDAC7 phosphorylation in a dose-dependent manner. VEGF induced HDAC7 phosphorylation at a concentration as low as 1 ng/ml and achieved maximal activation at 10-100 ng/ml (Figure 1B). The induced phosphorylation of HDAC7 by VEGF was not limited on BAECs because VEGF also stimulated HDAC7 phosphorylation in a similar time- and dose-dependent manner in HUVECs (data not shown).
Figure 1. VEGF stimulates HDAC7 phosphorylation in endothelial cells.
(A) BAECs were exposed to VEGF (20 ng/ml) for various times as indicated. (B) BAECs were exposed to VEGF for 15 min with the indicated concentrations. HDAC7 phosphorylation and expression in cell lysates were determined as described in Materials and Methods. Representative immunoblots and quantitative data of HAC7 phosphorylation normalized with the level of HDAC7 were shown (n=4). *, # and &, p < 0.05 in comparison to value from control group without VEGF treatment (0 min).
PKD1 mediates HDAC7 phosphorylation by VEGF
Next we studied the signal pathway(s) for VEGF-induced HDAC7 phosphorylation. Using selective neutralizing antibodies, we found that VEGFR2 but not VEGFR1 mediated VEGF-stimulated HDAC7 phosphorylation in ECs (supplemental data S1). Using specific pharmacological inhibitors, we further observed that PLCγ/PKC-dependent pathway, but not the phosphoinositide 3-kinase (PI3K)-dependent pathway, was required for VEGF-stimulated HDAC7 phosphorylation (supplemental data S2).
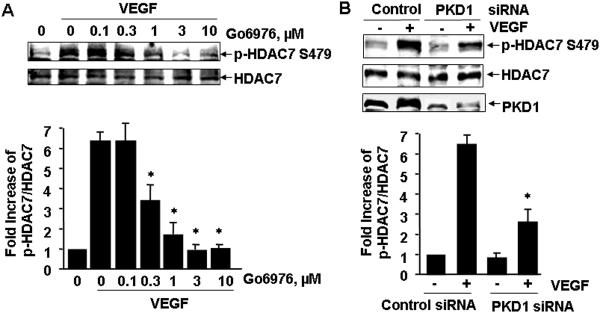
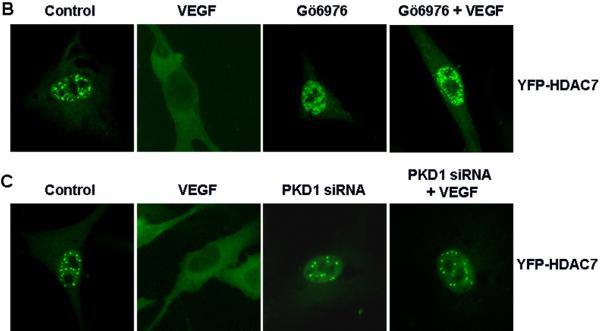
Recently we have demonstrated that PKC activation by VEGF leads to PKD1 phosphorylation and activation in ECs 13. Thus we decided to examine the potential role of PKD1 in VEGF-induced HDAC7 phosphorylation. A PKD inhibitor Go6976 significantly inhibited VEGF-induced HDAC7 phosphorylation in a dose-dependent manner (Figure 2A), suggesting the potential involvement of PKD in phosphorylation of HDAC7 by VEGF. To specifically define the role of PKD1 in VEGF-induced HDAC7 phosphorylation, we knocked down endogenous PKD1 in HUVECs using small interference RNA (siRNA) specifically targeting on human PKD1 as previously described 13. Silencing PKD1 by siRNA significantly inhibited VEGF-induced HDAC7 phosphorylation (Figure 2B), indicating that PKD1 is required for HDAC7 phosphorylation by VEGF in ECs.
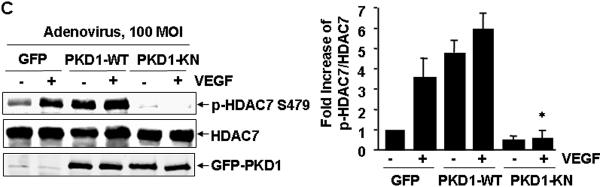
Figure 2. PKD1 mediates HDAC7 phosphorylation by VEGF.
(A) BAECs were pretreated with the potent PKD inhibitor Go6976 at indicated concentration for 30 min, and then exposed to VEGF (20 ng/ml) for 15 min. (B) HUVECs were transfected with scrambled siRNA (100 nM, control) or PKD1 siRNA (100 nM) for 48 h, and then exposed to VEGF for 15 min. (C) BAECs were infected with adenoviruses encoding GFP (Ad-GFP, control), or Ad-GFP-PKD1-KN at 100 MOI for 24 h, and then exposed to VEGF for 15 min. HDAC7 phosphorylation and expression in cell lysates were determined. PKD1 expression levels were also analyzed to confirm the knockdown effect of PKD1 siRNA (B) and overexpression of GFP-PKD1-KN in ECs (C). Representative immunoblots and quantitative data were shown (n=3).
To further determine whether PKD1 activation mediates HDAC7 phosphorylation by VEGF, we generated and tested adenoviruses expressing GFP-tagged PKD1 wild type (Ad-GFP-PKD1-WT) and PKD1 kinase-negative (K612W) mutant (Ad-GFP-PKD1-KN). The dominant negative nature of this ATP-binding site mutant PKD1-KN has been previously characterized 26, 27. Infection of BAECs with Ad-GFP-PKD1-WT and Ad-GFP-PKD1-KN resulted in robust expression of these PKD1 (Figure 2C). In contrast to that Ad-GFP-PKD1-WT enhanced basal and VEGF-induced HDAC7 phosphorylation, Ad-GFP-PKD1-KN significantly reduced VEGF-induced HDAC7 phosphorylation, compared to that in the control infected with adenoviruses encoding GFP alone. Together, these data demonstrate an essential role of PKD1 for VEGF-induced HDAC7 phosphorylation in ECs.
VEGF stimulates HDAC7 cytoplasmic accumulation in endothelial cells through PKD1-dependent phosphorylation
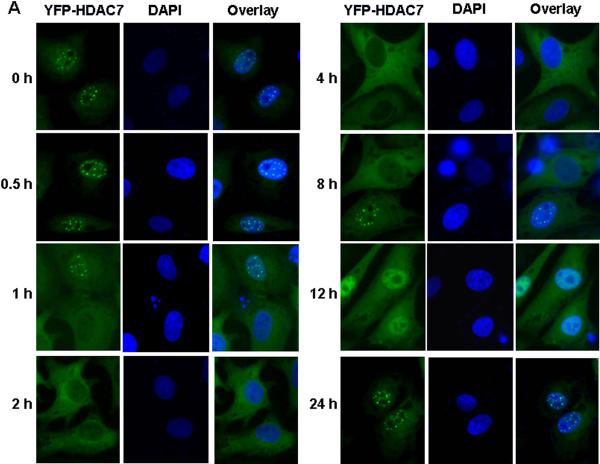
To gain insight into the functional significance of HDAC7 phosphorylation in VEGF-mediated signaling events, we studied the effect of VEGF on HDAC7 subcellular localization in ECs with HDAC7 fused with YFP. BAECs were infected with adenovirus expressing YFP-tagged HDAC7-WT, and then exposed to 20 ng/ml VEGF for indicated times. YFP-HDAC7 localization in cells was analyzed by fluorescence microscope. Without the treatment of VEGF, YFP-HDAC7 was located primarily in the nuclei of ECs (Figure 3A). In response to VEGF stimulation, YFP-HDAC7 in the cells was undergoing time-dependent nucleocytoplasmic shuttling. Cytoplasmic accumulation of HDAC7 was observed at 30 min after the application of VEGF, and reached maximum at 2 h, in which almost all YFP-HDAC7 was accumulated in the cytoplasm from nucleus. Exported YFP-HDAC7 was sustained in cytoplasm for several hours, and then gradually accumulated in the nucleus after 12 h VEGF stimulation. At 24 h after VEGF treatment, almost all of YFP-HDAC7 accumulated in the nuclei from cytoplasm. These results clearly show that VEGF stimulates dynamic nucleocytoplasmic shuttling of HDAC7 in ECs.
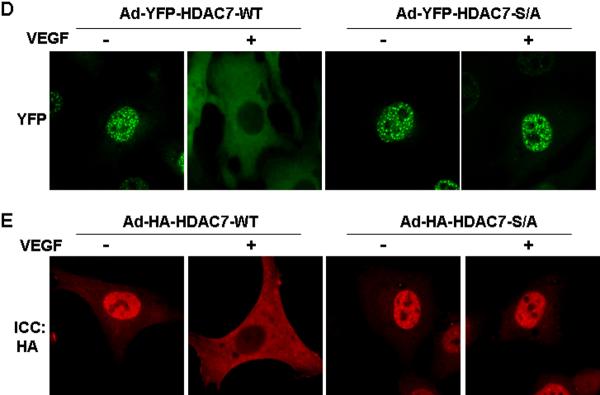
Figure 3. VEGF promotes cytoplasmic accumulation of HDAC7 through PKD1-dependent phosphorylation.
(A-B) BAECs were infected with Ad-YFP-HDAC7 at 50 MOI for 24 h, and then exposed to VEGF (20 ng/ml) for various times as indicated (A); or then pretreated with PKD inhibitor Go6976 (1 μM) for 30 min followed by the exposure of VEGF for 3 h (B). (C) HUVECs were transfected with scrambled siRNA (100 nM, control) or PKD1 siRNA (100 nM) for 24 h, and then infected with Ad-YFP-HDAC7 at 50 MOI for 24 h, followed by the exposure of VEGF (20 ng/ml) for 3 h. (D) BAECs were infected with Ad-YFP-HDAC7-WT or Ad- YFP-HDAC7-S/A at 50 MOI for 24 h, and then stimulated with VEGF for 3 h. (E) BAECs were infected with Ad-HA-HDAC7-WT or Ad- HA-HDAC7-S/A at 50 MOI for 24 h, and then stimulated with VEGF (20 ng/ml) for 3 h. The cells were fixed and YFP-HDAC7 or HA-HDAC7 distribution in the cell was analyzed by fluorescence microscopy. Representative images (magnification, x40 for (A) or x60 for (B-E)) were shown (n=3). Nuclei stained with DAPI (A) were also analyzed by fluorescence microscopy.
In consistent with a role of PLCγ/PKC pathway in regulation of HDAC7 phosphorylation in response to VEGF, we found that the inhibition of PLCγ and PKCs also abolished VEGF-induced HDAC7 cytoplasmic accumulation (supplemental data S3). However, PI3K inhibitor LY294002, calcium chelator BAPTA/AM or calmudulin-dependent kinase inhibitor KN93 had no effect on HDAC7 cytoplasmic accumulation in response to VEGF (supplemental data S3). In agreement with the critical role of PKD1 in VEGF-induced HDAC7 phosphorylation, PKD inhibitor Go6976 and siRNA PKD1 blocked VEGF-induced HDAC7 cytoplasmic accumulation (Figure 3B and 3C).
To determine whether PKD1-dependent phosphorylation of HDAC7 at Ser178/344/479 is required for HDAC7 cytoplasmic accumulation, we studied subcellular localization of YFP-HDAC7-S/A mutant, in which serine178/344/479 residues were replaced with alanine. ECs were infected with Ad-YFP-HDAC7-WT or Ad-YFP-HDAC7-S/A. In basal condition without VEGF stimulation, both YFP-HDAC7-WT and YFP-HDAC7-S/A were localized in the nuclei of ECs (Figure 3D). After VEGF stimulation for 2 h, YFP-HDAC7 was accumulated in the cytoplasm. In contrast, YFP-HDAC7-S/A remained in the nuclei after VEGF stimulation, suggesting that theses three serines are essential for VEGF-induced cytoplamisc accumulation of HDAC7. In addition, YFP-HDAC7-S/A was also resistant to shuttling in response to PKC activator PMA stimulation in ECs (data not shown). Similar results were observed when ECs were infected with Ad-HA-HDAC7-WT and Ad-HA-HDAC7-S/A followed with immunofluorescence staining with anti-HA antibodies (Figure 3E). Collectively, these results demonstrate that PKD1-dependent phsophorylation of HDAC7 mediates its cytoplamisc accumulation in ECs in response to VEGF.
PKD1-HDAC7 pathway modulates VEGF-induced MEF2 transcriptional activation and expression of MMPs
HDAC7 have been shown to interact with MEF2 transcription factors resulting inhibition of MEF2-dependent gene expression as well as apoptosis in T cells 28. To examine whether HDAC7 modulates VEGF-induced MEF2 activation, we examined the effect of HA-HDAC7-S/A on VEGF-induced MEF2 transcriptional activation in ECs. Co-transfection of HA-HADC7-S/A with 3xMEF2 luciferase plasmids abolished MEF2 transcriptional activation in response to VEGF (supplemental data S4).
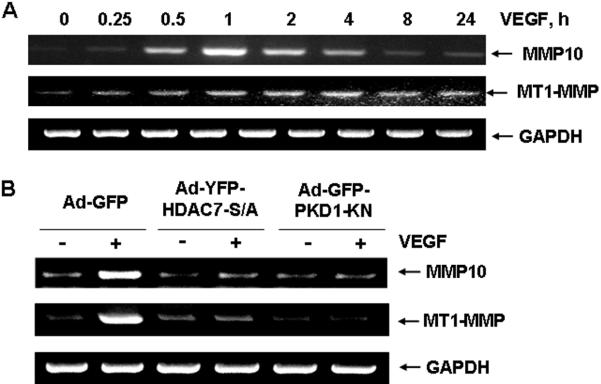
It has been reported that MEF2-dependent gene MMP10 is induced in HDAC7 knockdown HUVECs 17. However, whether VEGF stimulates MMP10 via PKD1-HDAC7 pathway is unknown. Thus, we examined the expression of MMP10 with RT-PCR. We found that VEGF strongly stimulated MMP10 mRNA expression in a time-dependent manner, peaked at 1 hour (Figure 4A). Furthermore, we also found that VEGF stimulated expression of membrane type matrix metalloproteinase 1 (MT1-MMP), which plays a crucial role in regulation of angiogenesis 29. Interestingly, both Ad-HA-HDAC7-S/A and Ad-GFP-PKD1-KN significantly inhibited such an induction of MMP10 and MT1-MMP by VEGF (Figure 4B), indicating a critical role of PKD1-dependent HDAC7 phosphorylation for VEGF induction of MMP10 and MT1-MMP expression in ECs.
Figure 4. PKD1-dependent HDAC7 phosphorylation modulates VEGF-induced MMP10 and MT1-MMP expression in endothelial cells.
(A) HUVECs were stimulated with VEGF (20 ng/ml) for various times as indicated. (B) HUVECs were infected with Ad-GFP (control), Ad-YFP-HDAC7-S/A or GFP-PKD1-KN (100 MOI) for 24 h, and then stimulated with VEGF (20 ng/ml) for 1 h. The mRNA was extracted from the cell lysates, and RT-PCR with the primers of MMP1, MT1-MMP, and GADPH (internal control) were performed as described in Materials and Methods. The representative images and quantitative data were shown (n=4).
HDAC7 and MT1-MMP are involved in VEGF-induced EC migration and tube formation
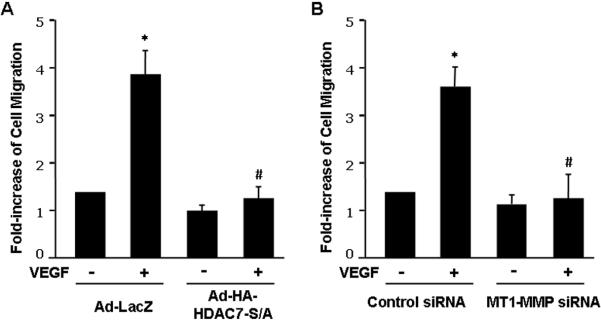
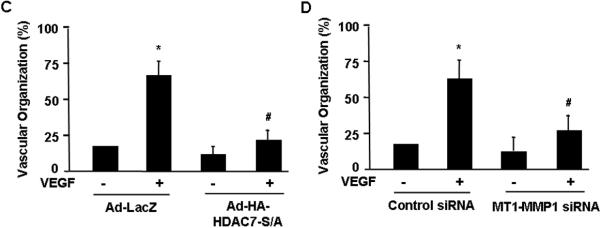
Next we asked whether HDAC7-dependent regulation of MT1-MMP is implicated in the processes of angiogenesis. Using the assay of cell migration during wound closure process, we found that VEGF-induced EC migration was substantially inhibited by Ad-HA-HDAC7-S/A (Figure 5A and supplemental data S5). Knockdown of MT1-MMP by siRNA in HUVECs also significantly inhibited VEGF-induced EC migration during wound closure (Figure 5B and supplemental data S6). In the assay of in vitro angiogenesis, the capability of primary ECs to form capillary-like tube structures was investigated upon cultivation on Matrigel and quantified by measuring the length of capillary-like tube structure 30. VEGF-induced capillary-like tube formation in Matrigel was significantly attenuated by Ad-HA-HDAC7-S/A and MT1-MMP siRNA (Figure 5C and 5D, and supplemental data S7 and S8).
Figure 5. HDAC7 and MT1-MMP are involved in VEGF-induced endothelial cell migration and tube formation.
(A) BAECs were infected with Ad-LacZ (control), or Ad-HA-HDAC7-S/A and then measured for cell migration in response to VEGF in wound closure assay (n=4). (B) HUVECs were transfected with control siRNA or human MT1-MMP1 siRNA and then measured for cell migration in response to VEGF in wound closure assay. The representative images and quantitative data were shown (n=4). (C) HUVECs infected with Ad-LacZ (control), or Ad-HA-HDAC7-S/A were cultured in Matrigel and measured for in vitro angiogenesis in response to VEGF with tube formation assay (n=4). (D) HUVECs transfected with control siRNA or human MT1-MMP1 siRNA were cultured in Matrigel and measured for in vitro angiogenesis in response to VEGF with tube formation assay. The length of tube-like structure were quantified and shown. *, p < 0.05 in comparison to value from control; #, p < 0.05 vs. that from the group treated with VEGF along with Ad-lacZ or control siRNA.
PKD1-HDAC7 pathway is critical for VEGF-induced aorta ring angiogenesis
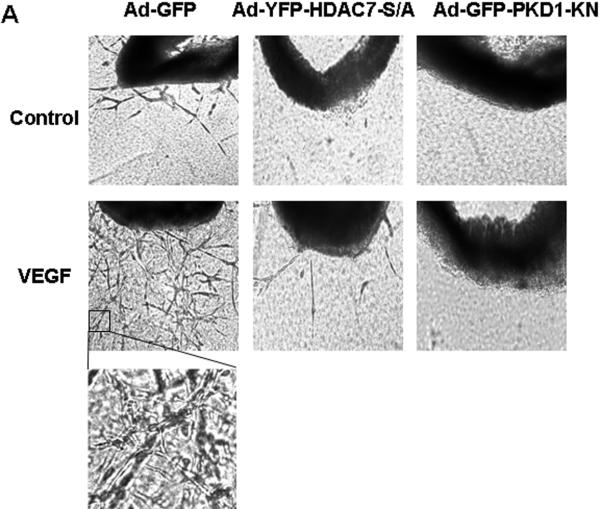
To define the role of PKD1-HDAC7 pathway in angiogenesis in intact vessels, we used aorta ring assay for ex vivo angiogenesis 24, 25. VEGF increased the number of microvessels sprouting from aortic rings isolated from mice (Figure 6), and infection of Ad-PKD1-KN and Ad-HDAC7-S/A markedly inhibited VGEF-induced microvessel sprouting (Figure 6A).
Figure 6. PKD1-dependent HDAC7 phosphorylation is required for VEGF-induced microvessel sprouting in mouse aorta ring assay.
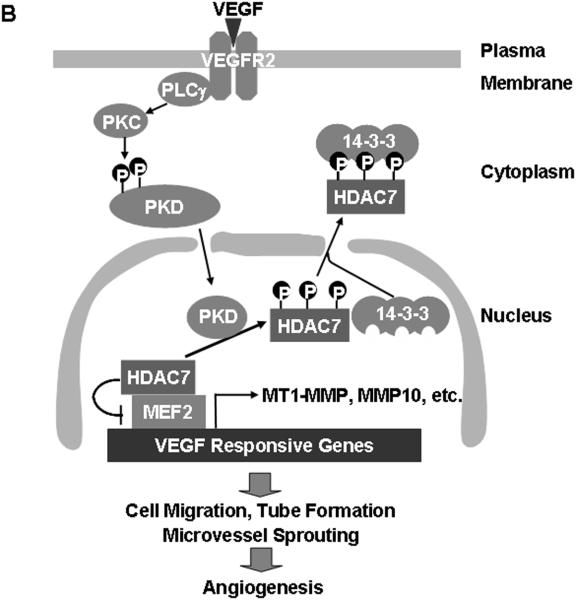
(A) Mouse aorta rings were isolated form mice and infected Ad-LacZ (control), Ad-HA-HDAC7-S/A or Ad-GFP-PKD1-KN, and then the aorta ring assay for ex vivo angiogenesis was performed. The representative images were shown (n=4). (B) Schema for potential role and pathways of PKD1 and HDAC7 for VEGF-induced angiogenesis (see the text in detail).
Discussion
The major findings of this study are that VEGF stimulates PKD1-dependent HDAC7 phosphorylation and cytoplasmic accumulation in ECs, and that PKD1-HDAC7 pathway is involved in VEGF-induced MT1-MMP and MMP10 expression, EC migration, tube formation and microvessel sprouting. Since MT1-MMP gene expression are implicated in angiogenesis in vivo 29, our data suggest that PKD1-HDAC7 pathway is likely to play an important role in VEGF signaling and angiogenesis in physiological and pathological conditions.
Acetylation of chromatin proteins and transcription factors is part of a complex signaling system that is largely involved in the control of gene expression 14, 15. Histone acetyltransferases and HDACs act in an opposing manner to control the acetylation state of nucleosomal histones. Class IIα HDACs including HDAC4, HDAC5, HDAC7 and HDAC9 have been shown to act as signal-responsive repressors of cardiac hypertrophy, skeletal muscle differentiation and bone development 31-33. We recently reported that HDAC5 was involved in VEGF signaling and gene expression 34. In addition to HDAC5, HDAC7 has been implicated in regulation of vascular integrity because HDAC7 deficient mice died in embryonic stages due to vascular leakage 17. HDAC7 down-regulates MMP10 expression in unstimulated ECs, and MMP10 induction plays a role in endothelial capillary-like tube formation 17. However, the role of HDAC7 in VEGF signaling remains unclear. The present study, we showed that VEGF promotes phosphorylation of three serine 178/344/479 residues in HDAC7, which have been shown to be the docking sites for the 14-3-3 chaperone protein 35. Indeed, VEGF stimulated the association of HDAC7 and 14-3-3 in a phosphorylation-dependent manner because HDAC7 phosphorylation-defective mutant (HDAC7-S/A) failed to bind to 14-3-3 in ECs in response to VEGF (Supplemental data S3). Binding of 14-3-3 to HDAC7 disrupts its association with MEF2 transcriptional factors and triggers HDAC7 cytoplasmic accumulation, thus freeing MEF2 to activate subordinate genes that may govern EC growth and migration (Figure 6B). In consistent with this notion, we observed that VEGF induced cytoplasmic accumulation of HDAC7 translocated in response to VEGF stimulation in ECs, and increased MEF2 transcriptional activation. Mutation of these serine sites to alanine (HDAC7-S/A mutant) blocked VEGF-induced nucleo-cytoplasmic shuttling, which is consistent with previous report showing that all three phosphoserine sites are required for the localization of HDAC7 35. Furthermore, HDAC7-S/A mutant inhibited VEGF-stimulated increase in MT1-MMP and MMP10 expression, and EC migration and tube formation (Figure 6B). These findings suggest that HDAC7 is a transcriptional repressor for genes such as MT1-MMP and MMP10 that are involved in angiogenesis. The genes repressive action of endogenous HDAC7 is overcome by VEGF-stimulated PLCγ/PKC/PKD1 signaling pathway that culminate in cytoplasmic accumulation of HDAC7. Of note, it has been reported that knockdown of HDAC7 by siRNA in ECs also disrupts the process of tube formation 18, suggesting that HDAC7 may play a fine tuning mediator for gene expression implicated in the complex processes of angiogenesis.
Several protein kinases have been identified to be responsible for phosphorylation and nuclear export of class IIa HDACs 15. Among them, PKD1, a downstream mediator of PLCγ/PKC pathway 13, has emerged as a key regulator for HDAC5 and HDAC7 in several cell types. We and others have shown that PKD1 is involved in regulation of HDAC5 phosphorylation and nuclear export in cardiac myocytes and ECs 34, 36. PKD1 also regulates HDAC7 phosphorylation and nuclear exclusion as well as gene expression in T cells and B cells 28, 37. In this study, we provide strong evidence shown that HDAC7 is a PKD1 substrate in VEGF signaling. First, we found that HDAC7 phosphorylation and cytoplasmic accumulation was mediated through VEGF-stimulated VEGFR2-PLCγ-PKC pathway, the same pathway for VEGF activation of PKD1 that we have revealed previously 13. Furthermore, the inhibition of PKD1 by the pharmacological inhibitor, siRNA and dominant-negative mutant blocked VEGF-induced HDAC7 phosphorylation cytoplasmic accumulation. In contrast, overexpression of PKD1-WT, which increases its kinase activity, enhanced HDAC7 phosphorylation and cytoplasmic accumulation even in the absence of VEGF stimulation. Concomitantly, the inhibition of PKD1 also abolished VEGF-induced MT1-MMP and MMP10 expression and VEGF-mediated microvessel sprouting, indicating that PKD1 plays an essential role in these processes.
Class IIa HDAC family members HDAC4, HDAC5, HDAC7 and HDAC9 are all expressed in HUVECs manifested by RT-PCR analysis (supplemental data S5). While HDAC4 and HDAC9 were mainly localized in cytosol in COS7 cells and in ECs, HDAC5 and HDAC7 were primarily localized in the nucleus of the unstimulated cells. Constitutive active mutant PKD1-S/E (Ser744/748 were replaced with glutamate) stimulated both HDAC5 and HDAC7 nuclear export in COS7 cells and ECs (Supplemental data S6 and S7). Moreover, both HDAC5 and HDAC7 are involved in MEF2-dependent transcription in VEGF signaling 16. But the physiological relevance of having the two enzymes with similar functions is not clear. Our preliminary observations suggest that both HDAC5 and HDAC7 regulates MEF2-dependent gene NA4R1 (an orphan nuclear receptor, also called NUR77) in VEGF signaling 16, while HDAC7 but not HDAC5 suppresses MEF2-dependent gene MMP10 in ECs. It is possible that HDAC5 and HDAC7 may regulate different genes through collaborating with other transcriptional co-factors, such as HDAC3 38, a member in class I HDAC family. In addition, it has recently been shown that HDAC5 oligomerizes with HDAC4 39, so it is also possible that HDAC5 and HDAC7 may interact. Further studies are needed to clarify the redundancy and the specificity of HDAC5-dependent and HDAC7-dependent pathways.
In summary, our studies have demonstrated that VEGF stimulates HDAC7 phosphorylation and cytoplasmic accumulation in ECs through VEGFR2/PLCγ/PKC/PKD1 signal pathway. Moreover, our experiments provided evidence of a critical role for PKD1 and HDAC7 in VEGF-induced MT1-MMP and MMP10 expression, EC migration and in vitro angiogenesis. Thus, the present findings reveal a critical role of HDAC7 as a PKD1 substrate in VEGF signaling, and this discovery may implicate PKD1 and HDAC7 in mediating angiogenesis in vivo. As such, it could help us develop new strategies to control physiological and pathological angiogenesis.
Acknowledgements
This work was supported by grants RO1 HL-080611 (to Z.G.J.) and RO1 DK62985 (to H.-Y. K.) from the National Institutes of Health, Thomas R. Lee Career Development Award 1-06-CD-13 from American Diabetes Association and Grant-In-Aid 0755916T from American Heart Association Grant-In-Aid 0755916T (to Z.G.J.).
Footnotes
Note: During the revision of this manuscript, Wang et al. published a report 40, consistent with our data, showing that HDAC7 regulates VEGF-induced endothelial cell migration.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. Embo J. 2001;20:2768–78. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–7. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- 6.Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem Soc Trans. 2003;31:20–4. doi: 10.1042/bst0310020. [DOI] [PubMed] [Google Scholar]
- 7.Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc Natl Acad Sci U S A. 2005;102:1076–81. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–30. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 9.Valverde AM, Sinnett S-J, Van L-J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci U S A. 1994;91:8572–6. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johannes FJ, Prestle J, Eis S, Oberhagemann P, Pfizenmaier K. PKCu is a novel, atypical member of the protein kinase C family. J Biol Chem. 1994;269:6140–8. [PubMed] [Google Scholar]
- 11.Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem. 2005;280:13205–8. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- 12.Avkiran M, Rowland AJ, Cuello F, Haworth RS. Protein kinase d in the cardiovascular system: emerging roles in health and disease. Circ Res. 2008;102:157–63. doi: 10.1161/CIRCRESAHA.107.168211. [DOI] [PubMed] [Google Scholar]
- 13.Wong C, Jin ZG. Protein kinase C-dependent protein kinase D activation modulates ERK signal pathway and endothelial cell proliferation by vascular endothelial growth factor. J Biol Chem. 2005;280:33262–9. doi: 10.1074/jbc.M503198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKinsey TA, Olson EN. Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. J Clin Invest. 2005;115:538–46. doi: 10.1172/JCI24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- 16.Ha CH, Wang W, Jhun BS, Wong C, Hausser A, Pfizenmaier K, McKinsey TA, Olson EN, Jin ZG. Protein Kinase D-dependent Phosphorylation and Nuclear Export of Histone Deacetylase 5 Mediates Vascular Endothelial Growth Factor-induced Gene Expression and Angiogenesis. J Biol Chem. 2008;283:14590–9. doi: 10.1074/jbc.M800264200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang S, Young BD, Li S, Qi X, Richardson JA, Olson EN. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell. 2006;126:321–34. doi: 10.1016/j.cell.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 18.Mottet D, Bellahcene A, Pirotte S, Waltregny D, Deroanne C, Lamour V, Lidereau R, Castronovo V. Histone deacetylase 7 silencing alters endothelial cell migration, a key step in angiogenesis. Circ Res. 2007;101:1237–46. doi: 10.1161/CIRCRESAHA.107.149377. [DOI] [PubMed] [Google Scholar]
- 19.Dequiedt F, Kasler H, Fischle W, Kiermer V, Weinstein M, Herndier BG, Verdin E. HDAC7, a thymus-specific class II histone deacetylase, regulates Nur77 transcription and TCR-mediated apoptosis. Immunity. 2003;18:687–98. doi: 10.1016/s1074-7613(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 20.Kasler HG, Verdin E. Histone deacetylase 7 functions as a key regulator of genes involved in both positive and negative selection of thymocytes. Mol Cell Biol. 2007;27:5184–200. doi: 10.1128/MCB.02091-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ha CH, Bennett AM, Jin ZG. A novel role of vascular endothelial cadherin in modulating c-Src activation and downstream signaling of vascular endothelial growth factor. J Biol Chem. 2008;283:7261–70. doi: 10.1074/jbc.M702881200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Jhun BS, Ha CH, Jin ZG. Molecular Mechanisms of Ghrelin-Mediated Endothelial Nitric Oxide Synthase Activation. Endocrinology. 2008 doi: 10.1210/en.2008-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao C, Li X, Lam M, Liu Y, Chakraborty S, Kao HY. CRM1 mediates nuclear export of HDAC7 independently of HDAC7 phosphorylation and association with 14-3-3s. FEBS Lett. 2006;580:5096–104. doi: 10.1016/j.febslet.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 24.Blacher S, Devy L, Burbridge MF, Roland G, Tucker G, Noel A, Foidart JM. Improved quantification of angiogenesis in the rat aortic ring assay. Angiogenesis. 2001;4:133–42. doi: 10.1023/a:1012251229631. [DOI] [PubMed] [Google Scholar]
- 25.Masson VV, Devy L, Grignet-Debrus C, Bernt S, Bajou K, Blacher S, Roland G, Chang Y, Fong T, Carmeliet P, Foidart JM, Noel A. Mouse Aortic Ring Assay: A New Approach of the Molecular Genetics of Angiogenesis. Biol Proced Online. 2002;4:24–31. doi: 10.1251/bpo30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hausser A, Link G, Bamberg L, Burzlaff A, Lutz S, Pfizenmaier K, Johannes FJ. Structural requirements for localization and activation of protein kinase C mu (PKC mu) at the Golgi compartment. J Cell Biol. 2002;156:65–74. doi: 10.1083/jcb.200110047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hausser A, Storz P, Martens S, Link G, Toker A, Pfizenmaier K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat Cell Biol. 2005;7:880–6. doi: 10.1038/ncb1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dequiedt F, Van Lint J, Lecomte E, Van Duppen V, Seufferlein T, Vandenheede JR, Wattiez R, Kettmann R. Phosphorylation of histone deacetylase 7 by protein kinase D mediates T cell receptor-induced Nur77 expression and apoptosis. J Exp Med. 2005;201:793–804. doi: 10.1084/jem.20042034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–7. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen TL, Park AY, Alcaraz A, Peng X, Jang I, Koni P, Flavell RA, Gu H, Guan JL. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005;169:941–52. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci U S A. 2000;97:14400–5. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–88. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, Karsenty G, Olson EN. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119:555–66. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 34.Ha CH, Wang W, Jhun BS, Wong C, Hausser A, Pfizenmaier K, McKinsey TA, Olson EN, Jin ZG. Protein kinase D-dependent phosphorylation and nuclear export of histone deacetylase 5. J Biol Chem. 2008 doi: 10.1074/jbc.M800264200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kao HY, Verdel A, Tsai CC, Simon C, Juguilon H, Khochbin S. Mechanism for nucleocytoplasmic shuttling of histone deacetylase 7. J Biol Chem. 2001;276:47496–507. doi: 10.1074/jbc.M107631200. [DOI] [PubMed] [Google Scholar]
- 36.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24:8374–85. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews SA, Liu P, Spitaler M, Olson EN, McKinsey TA, Cantrell DA, Scharenberg AM. Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Mol Cell Biol. 2006;26:1569–77. doi: 10.1128/MCB.26.4.1569-1577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gregoire S, Xiao L, Nie J, Zhang X, Xu M, Li J, Wong J, Seto E, Yang XJ. Histone deacetylase 3 interacts with and deacetylates myocyte enhancer factor 2. Mol Cell Biol. 2007;27:1280–95. doi: 10.1128/MCB.00882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Backs J, Backs T, Bezprozvannaya S, McKinsey TA, Olson EN. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol Cell Biol. 2008;28:3437–45. doi: 10.1128/MCB.01611-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S, Li X, Parra M, Verdin E, Bassel-Duby R, Olson EN. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci U S A. 2008;105:7738–43. doi: 10.1073/pnas.0802857105. [DOI] [PMC free article] [PubMed] [Google Scholar]