Abstract
Dietary antioxidants have radioprotective effects after γ-radiation exposure that limit hematopoietic cell depletion and improve animal survival. The purpose of this study was to determine whether a dietary supplement consisting of l-selenomethionine, vitamin C, vitamin E succinate, α-lipoic acid and N-acetyl cysteine could improve survival of mice after proton total-body irradiation (TBI). Antioxidants significantly increased 30-day survival of mice only when given after irradiation at a dose less than the calculated LD50/30; for these data, the dose-modifying factor (DMF) was 1.6. Pretreatment of animals with antioxidants resulted in significantly higher serum total white blood cell, polymorphonuclear cell and lymphocyte cell counts at 4 h after 1 Gy but not 7.2 Gy proton TBI. Antioxidants significantly modulated plasma levels of the hematopoietic cytokines Flt-3L and TGFβ1 and increased bone marrow cell counts and spleen mass after TBI. Maintenance of the antioxidant diet resulted in improved recovery of peripheral leukocytes and platelets after sublethal and potentially lethal TBI. Taken together, oral supplementation with antioxidants appears to be an effective approach for radioprotection of hematopoietic cells and improvement of animal survival after proton TBI.
INTRODUCTION
Proton radiation is an emerging treatment modality that has generated significant interest for its putative capacity to selectively increase radiation dose to the tumor while lowering the dose to non-targeted tissues (1-6). Furthermore, exposure of humans to high-energy protons, largely from solar particle events (SPE), is a major consideration in prolonged space travel (7-10). While the efficacy of proton therapy in comparison to current treatment modalities remains to be determined through randomized controlled clinical trials (1, 2, 6), there is a also a need to further characterize systemic responses to proton radiation in vivo (7, 11).
Total-body exposure to ionizing radiation (TBI) in humans and animals can result in multiple organ dysfunction as a consequence of toxicity to the hematopoietic, gastrointestinal or cerebrovascular systems, depending on the total dose of radiation absorbed (12, 13). There remains a need to develop safe and effective radioprotectors that would be required in the event of a massive radiological accident, a nuclear terrorist attack, or prolonged space travel (12-16). We recently reported that dietary supplementation with a mixture of antioxidants comprised of l-selenomethionine (SeM), vitamin C, vitamin E succinate, α-lipoic acid and N-acetyl cysteine (NAC) was effective as a preventative measure prior to total-body X irradiation or as a treatment after TBI in limiting hematopoietic cell depletion, promoting hematopoietic cell recovery and improving animal survival (17). We have also previously observed that TBI of mice and rats with γ rays, protons or high-atomic number and high-energy (HZE) particles resulted in oxidative stress that could be quantified by decreased serum total antioxidant capacity within 4 h of radiation exposure (18, 19). The observed TBI-induced decrease in serum antioxidant capacity was prevented by dietary supplementation with antioxidants (18, 19). Several studies have characterized the in vivo hematopoietic system response to proton TBI (11, 20-22). To our knowledge, none of the recent studies have examined hematopoietic effects of potentially lethal doses of proton TBI on 30-day survival.
The aim of the current study was to characterize the hematopoietic syndrome after TBI with 1 GeV/nucleon protons and assess the efficacy of dietary antioxidant supplementation in protecting hematopoietic cells and promoting animal survival after proton TBI.
MATERIALS AND METHODS
Animals
Male ICR mice aged 4–5 weeks were purchased from Taconic Farms Inc. (Germantown, NY). Animals were acclimated for 7 days in the Brookhaven National Laboratory (BNL) Animal Facility. Ten animals were housed per cage with ad libitum access to water and food pellets. Animal care and treatment procedures were approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania and BNL.
Upon acclimation, the animals were randomly assigned to the AIN-93G rodent (Control) diet or AIN-93G diet supplemented with SeM (0.06 μg/g diet), α-lipoic acid (85.7 μg/g diet), NAC (171.4 μg/g diet), sodium ascorbate (142.8 μg/g diet), and vitamin E succinate (71.4 μg/ g diet); the diets were obtained from Bio-Serv (Frenchtown, NJ). The levels of SeM, vitamin E and ascorbic acid used in these studies are equivalent on a weight basis to the established maximum level of daily nutrient intake in humans that is likely to pose no risk of adverse effects. The antioxidant combination in the animal diets was formulated to provide the equivalent of 2000 mg/day, 1000 mg/day and 400 μg/day, which represent the upper limits of the established Recommended Dietary Allowances (RDAs) for vitamin C, vitamin E succinate and selenium (23). The control diet (AIN-93G rodent diet) contains vitamin E and selenium at levels in the animal diets that are comparable on a weight basis to the human RDA levels of these compounds. Although there is no published RDA for NAC or α-lipoic acid, these thiol supplements were formulated according to previously determined effective doses in humans, 2400 mg/day and 1200 mg/day, respectively (24, 25), which did not exhibit chronic toxicity.
Irradiation
Total-body irradiation of animals was performed with 1 GeV/nucleon protons [approximate linear energy transfer (LET) of 0.24 keV/μm] at dose rates ranging from 20–70 cGy/min at the NASA Space Radiation Laboratory (NSRL) at BNL. Proton irradiation was carried out within the non-stopping region (Bragg plateau) of the curve for energy deposition as a function of depth. The animals were restrained in plastic holders during radiation exposures and returned to their cages afterward. Animals were returned to the animal facility once their radiation levels were determined to be at background. Sham-irradiated animals were restrained similarly.
Peripheral Leukocyte Count Evaluations
Some animals were killed humanely 4 h after irradiation by CO2 asphyxiation followed by cardiac puncture in a sterile fashion for peripheral complete blood cell (CBC) analyses. Blood was collected in 1.7-ml microcentrifuge tubes containing 20 units of heparin and kept at ambient temperature. A 50-μl whole blood aliquot per animal was diluted with 200 μl 5% BSA in PBS for each sample. The use of 5% BSA was a recommendation from Dr. Suresh Shelat, Director of the Pathology and Medical Laboratories at the Children's Hospital of Philadelphia, where the blood samples were analyzed. BSA in PBS solution is generally used as a diluent, especially when small volumes of blood are expected and not pooled. The BSA acts to stabilize cell membranes and creates an isosmotic medium, having no direct effect on cell counts (personal communication, Dr. Suresh Shelat). Further, compared to humans, animal leukocyte populations are known to have more frequent separation failures. In these situations, histogram and blood film review are required to identify the separation failures, verify the total count, and correct the differential. BSA is reported as a blood cell stabilizer in total blood to reduce the number of “atypical” lymphocytes and aids in the consistency of the morphological evaluation of the peripheral blood (26).
Samples were packaged with ice packs and shipped overnight via commercial courier for CBC analysis 16–20 h later with an ADVIA 2120 Hematology System (Bayern Diagnostic, Dublin, Ireland) at the Children's Hospital of Philadelphia.
Survival Experiment
Animals were maintained on their respective diets until they were killed except where noted. One group of irradiated animals (Control → AO) was fed the control diet until 2 h after radiation exposure, at which time the control diet was exchanged for the antioxidant-supplemented diet, which was maintained for the remainder of the experiment. Animals were evaluated twice daily after irradiation. One week after irradiation, animals were shipped via a courier service to the University of Pennsylvania quarantine facility, where they were kept for the remainder of the experiment. After radiation exposure the animals were carefully monitored for signs of general toxicity: lack of grooming, ataxia, limping, abnormal posture, paralysis, lethargy, weakness, anorexia, tremors, hunched posture, convulsions, labored respiration, bleeding, rough or strained hair coat, eye lesions, sores/wounds and red eyes (or red tears). Animals showing signs of hunched posture, labored breathing and immobility were immediately euthanized because these symptoms are associated with impending death.
Spleen and Bone Marrow Cell Isolation and Quantification
The spleen was dissected, defatted, weighed and flash frozen in liquid nitrogen. Tibiae and femurs were removed and the ends of the bones were bluntly cut. The bone marrow cavity was flushed with PBS using a sterile 22-gauge needle and resuspended to obtain a single cell suspension. Aliquots were counted using a Coulter counter.
ELISA
Blood samples were collected in 1.7-ml microcentrifuge tubes, combined with 20 units of heparin, and centrifuged at 1000 g for 15 min at 4°C. Plasma was then separated from the pellet, kept on ice and frozen at −80°C within 1 h of collection. The concentrations of Flt-3 ligand (Flt-3L) and TGFβ1 in heparin plasma were quantified by a sandwich enzyme immunoassay using the Quantikine ELISA kits (R&D Systems) according to the manufacturer's procedure. Briefly, thawed plasma was crosslinked to monoclonal antibodies specific for Flt-3L or TGFβ1 precoated onto a 96-well plate for 2 h at room temperature, followed by the sandwiching of analyte with enzymelinked polyclonal antibodies specific for Flt-3L or TGFβ1 for another 2 h. Signals were acquired at 450 nm using a spectrophotometric microplate reader after 30 min of color development.
Statistical Analysis
The 30-day survival Kaplan-Meier curves were compared using a log-rank test. The CBC counts were compared between control and antioxidant treatment groups by a Student's t test. The statistical analyses were performed using Prism Version 2.01 (GraphPad Software, San Diego, CA) and SigmaPlot Version 10.0 (Systat Software Inc, San Jose, CA) statistical software. P < 0.05 was regarded as statistically significant.
RESULTS
Thirty-Day Survival of Mice after Total-Body Irradiation
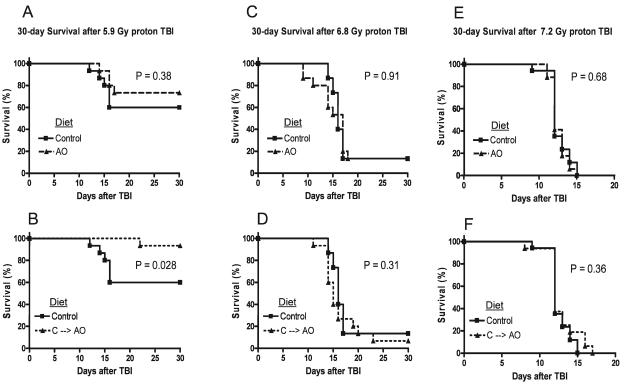
The effects of dietary antioxidant supplementation on survival were determined in mice irradiated with a total-body dose of 5.9, 6.8 and 7.2 Gy 1 GeV/nucleon protons (Fig. 1). When antioxidant supplementation was initiated 7 days prior to TBI and maintained for the duration of the observation period, a small increase in survival was observed when the results were compared to those from animals fed the control diet, although this increase in survival did not reach statistical significance (Fig. 1A). However, Control → AO treatment exhibited a statistically significant survival benefit (Fig. 1B) with a hazard ratio of 0.13 (0.039–0.82). The 30-day survival rate after 5.9 Gy TBI was 60% for animals fed the control diet, 93% for the Control → AO group, and 73% for animals that received antioxidants prior to irradiation. We observed that 5.5 Gy proton TBI was sublethal in all groups (data not shown). A total-body dose of 6.8 Gy resulted in 13.3% survival that was not significantly affected by antioxidant supplementation (Fig. 1C and D, solid lines), while 7.2 Gy TBI was universally lethal in all groups (Fig. 1E and F, solid lines). There was no statistically significant difference in survival between animals that received the antioxidant supplements before or after radiation exposure (Fig. 1C–F).
FIG. 1.
Effect of antioxidants on mouse survival after TBI. Panel A: Male ICR mice were fed the control AIN-93G diet (n = 15) or the AIN-93G diet supplemented with antioxidants (AO, n = 15) for 7 days prior to 5.9 Gy TBI. The animals were maintained on their respective diets and observed for 30 days after TBI. Panel B: Survival of animals fed the control diet was compared to that of animals given the antioxidant diet 2 h after 5.9 Gy TBI and maintained on this diet for the remaining 30 days (C → AO, n = 15). Panels C and D: Survival of mice fed the control diet (n = 15) or the antioxidant-supplemented diet for 7 days prior to (AO, n = 15) or 2 h after (C → AO, n = 15) 6.8 Gy TBI. Panels E and F: Survival of mice fed the control diet (n = 15) or the antioxidant-supplemented diet for 7 days prior to (AO, n = 15) or 2 h after (C → AO, n = 15) 7.2 Gy TBI.
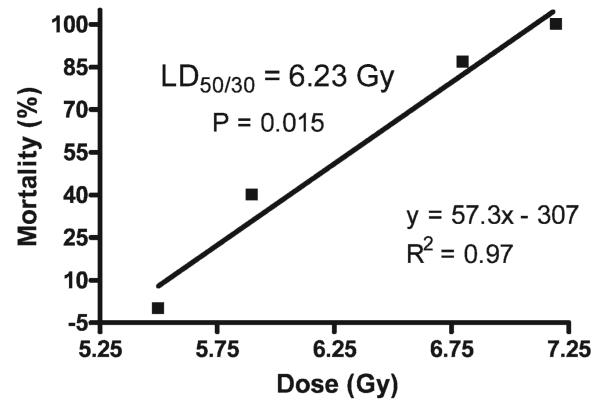
Determination of LD50/30 for 1 GeV/nucleon Proton TBI
The 30-day survivals after 5.5, 5.9, 6.8 and 7.2 Gy TBI were plotted with percentage survival as the dependent variable. Linear regression analysis demonstrated a correlation coefficient of 0.97 and a calculated LD50/30 of 6.23 Gy for male ICR mice (Fig. 2).
FIG. 2.
Calculation of LD50/30 for 1 GeV/nucleon protons in male ICR mice (15 per dose) fed the control AIN-93G diet for 7 days prior to TBI. The 30-day survival after TBI ranging from non-lethal (100% survival, 5.5 Gy) to universally lethal (0% survival, 7.2 Gy) was plotted as a function of radiation dose. Linear regression was used to calculate the dose equivalent of 50% 30-day animal survival.
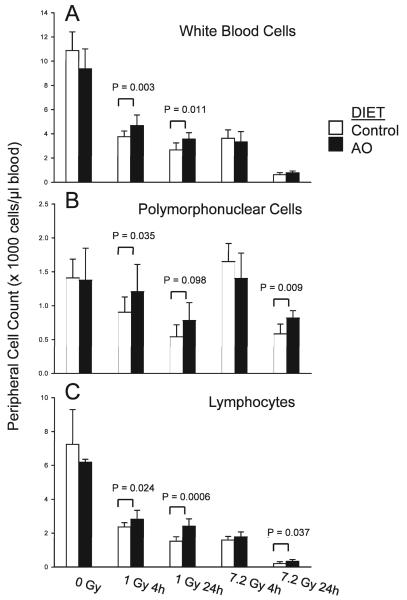
Peripheral Leukocyte Counts 4 and 24 h after 1 and 7.2 Gy Proton TBI
The hematopoietic syndrome ensues within the first 24 h of total-body radiation exposure at doses as low as 1 Gy (27). Peripheral blood cell counts (as well as bone marrow cell depletion and spleen mass) were investigated to evaluate the effects of antioxidant supplementation on the proton TBI-induced hematopoietic syndrome at a low dose associated with the hematopoietic syndrome (1 Gy) and a lethal dose (7.2 Gy).
Whole-blood cell (WBC) counts
There was no difference in WBC counts between nonirradiated animals fed the control or antioxidant-supplemented diet for 7 days (Fig. 3A). At 4 and 24 h after 1 Gy and 7.2 Gy proton TBI, control animals and animals fed the antioxidant supplemented diet had significantly decreased WBC counts compared to nonirradiated animals fed the control diet (Fig. 3A). However, irradiated animals fed the diet supplemented with antioxidants exhibited significantly higher WBC counts at 4 h and 24 h after 1 Gy TBI, whereas antioxidants did not affect WBC counts after 7.2 Gy TBI in a statistically significant manner compared to irradiated animals fed the control diet (Fig. 3A).
FIG. 3.
Effect of prophylactic antioxidant dietary supplementation on peripheral leukocyte counts after low- and high-dose TBI. Male ICR mice were fed the control AIN-93G or the control diet supplemented with antioxidants (AO) for 7 days prior to 1 Gy or 7.2 Gy TBI and were killed at 4 or 24 h after TBI. Panel A: Total white blood cell (WBC) counts, 0 Gy control and 0 Gy AO (n = 4), 1 Gy control 4 h (n = 13), 1 Gy AO 4 h (n = 12), 1 Gy control 24 h (n = 7), 1 Gy AO 24 h (n = 7), 7.2 Gy control 4 h (n = 6), 7.2 Gy AO 4 h (n = 4), 7.2 Gy control 24 h (n = 7), and 7.2 Gy AO 24 h (n = 7). Panel B: PMN cell counts, 0 Gy control and 0 Gy AO (n = 6), 1 Gy control 4 h (n = 13), 1 Gy AO 4 h (n = 9), 1 Gy control 24 h and 1 Gy AO 24 h (n = 5), 7.2 Gy control 4 h (n = 4), 7.2 Gy AO 4 h (n = 4), 7.2 Gy control 24 h (n = 6), and 7.2 Gy AO 24 h (n = 6). Panel C: Lymphocyte counts, 0 Gy control and 0 Gy AO (n = 3–4), 1 Gy control 4 h (n = 10), 1 Gy AO 4 h (n = 10), 1 Gy control 24 h (n = 7), 1 Gy AO 24 h (n = 7), 7.2 Gy control 4 h (n = 5), 7.2 Gy AO 4 h (n = 5), 7.2 Gy control 24 h (n = 8), and 7.2 Gy AO 24 h (n = 7). Each bar represents mean ± SD.
Polymorphonuclear (PMN) cell counts
There was no difference in PMN cell counts between nonirradiated animals fed the control or antioxidant-supplemented diet for 7 days. After 1 Gy proton TBI, animals fed the control diet had significantly decreased PMN cell counts at 4 h and 24 h after radiation exposure. Supplementation with dietary antioxidants resulted in no decrease in PMN cell counts at 4 h after 1 Gy TBI (Fig. 3B). The difference in PMN cell counts at 4 h after 1 Gy proton TBI between animals fed the control and antioxidant diets was statistically significant (Fig. 3B). At 24 h after 1 Gy TBI, there were higher peripheral PMN cell counts in animals fed the antioxidant-supplemented diet compared to irradiated animals fed the control diet; this effect was of borderline statistical significance (P = 0.098). At 4 h after 7.2 Gy proton TBI, there was no change in PMN cell counts in animals fed the control or antioxidant diets compared to unirradiated animals fed the control diet (Fig. 3B). Animals exposed to 7.2 Gy proton TBI had significantly higher PMN cell counts at 4 h after radiation exposure compared to animals fed the control diet and exposed to 1 Gy TBI. Animals fed the diet supplemented with antioxidants exhibited higher PMN cell counts than animals fed the control diet at 24 h after 7.2 Gy TBI (Fig. 3B).
Lymphocyte cell counts
There was no significant difference in peripheral lymphocyte counts between nonirradiated animals fed the control or antioxidant-supplemented diets (Fig. 3C). At 4 and 24 h after 1 Gy proton TBI, there was a significant decrease in peripheral lymphocytes regardless of diet compared to unirradiated animals fed the control diet (Fig. 3C). Nevertheless, dietary supplementation with antioxidants resulted in more peripheral lymphocytes at 4 h and 24 h after 1 Gy TBI compared to irradiated animals fed the control diet (Fig. 3C). At 4 h after 7.2 Gy proton TBI, dietary antioxidant supplementation did not affect the significant decrease in peripheral lymphocytes (Fig. 3C). However, at 24 h after 7.2 Gy TBI, antioxidant-supplemented animals had higher peripheral lymphocyte counts than irradiated animals fed the control diet (Fig. 3C).
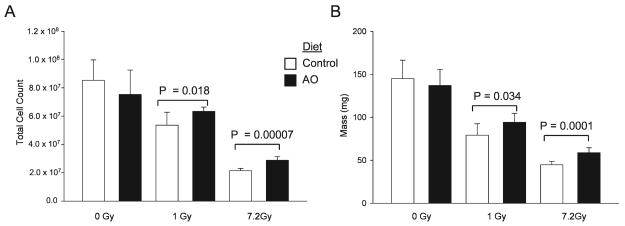
Effect of Antioxidant Dietary Supplements on Radiation-Induced Bone Marrow Cell Depletion and Spleen Mass at 24 h after TBI
Nonirradiated animals fed the control and antioxidant diets had similar bone marrow cell counts (Fig. 4A). At 24 h after 1 Gy and 7.2 Gy proton TBI, there was a significant decrease in bone marrow cell counts in animals fed the control and antioxidant diets compared to nonirradiated animals fed the control diet (Fig. 4A). Dietary supplementation with antioxidants resulted in more cells in the bone marrow of animals exposed to 1 Gy and 7.2 Gy TBI compared to irradiated animals fed the control diet (Fig. 4A).
FIG. 4.
Effect of dietary antioxidant (AO) supplementation on bone marrow cell depletion and spleen mass 24 h after TBI. Male ICR mice were fed the control AIN-93G or the control diet supplemented with antioxidants (AO) for 7 days prior to 1 Gy or 7.2 Gy TBI and were killed at 24 h after TBI. Panel A: Twenty-four hours after TBI, animals were killed, both femurs and tibiae were flushed with PBS, and cell counts were determined with a Coulter Counter. Each group represents n = 5–7. Panel B: Twenty-four hours after TBI, animals were killed, and the spleens were harvested, defatted and weighed. Each group represents n = 7. Each bar represents mean ± SD.
Unirradiated animals had similar spleen mass regardless of diet (Fig. 4B). At 24 h after 1 Gy and 7.2 Gy proton TBI, there was a significant decrease in spleen mass in animals fed the control diet compared to animals fed the antioxidant-supplemented diet (Fig. 4B). Supplementation with antioxidants resulted in significantly higher spleen mass at 24 h after 1 Gy and 7.2 Gy TBI compared to irradiated animals fed the control diet (Fig. 4B).
Effect of Antioxidant Dietary Supplements on Plasma Levels of Flt-3L and TGFβ1 after Proton TBI
Flt-3L blood levels has been suggested as a biomarker of radiation injury to the bone marrow (28, 29). There was no difference in plasma levels of Flt-3L in nonirradiated animals regardless of diet (Fig. 5A). At 4 h after 1 Gy proton TBI, there was a statistically significant increase in plasma Flt-3L levels in animals fed the control diet (Fig. 5A), whereas there was a decrease in Flt-3L levels in animals supplemented with antioxidants that was of borderline statistical significance (Fig. 5A, P = 0.069). Antioxidant supplementation resulted in significantly lower levels of plasma Flt-3L at 4 h after 1 Gy TBI (Fig. 5A). There was no change in plasma Flt-3L levels at 4 h after 7.2 Gy TBI regardless of diet (Fig. 5A) compared to unirradiated animals fed the control diet. Furthermore, there was no difference in Flt-3L levels at 4 h after 7.2 Gy TBI between animals fed the control or antioxidant diet (Fig. 5A).
FIG. 5.
Effect of prophylactic dietary antioxidant supplementation on plasma levels of Flt-3L and TGFβ1 after TBI. Male ICR mice were fed the control AIN-93G diet or the control diet supplemented with antioxidant (AO) for 7 days prior to 1 Gy or 7.2 days prior to 1 Gy or 7.2 Gy TBI and were killed 4 and 24 H after TBI; plasma was separated from peripheral blood and stored at −70°C until further analysis. Panel A: Flt-3L: 0 Gy control and AO (n = 5–7), 1 Gy control and AO 4 h (n = 6–8), 1 Gy control and AO 24 h (n = 6–8), 7.2 Gy control and AO 4 h (n = 8), 7. 2 Gy control and AO 24 h (n = 4). Data are means ± SD. ***P = 0.00008 between 1 Gy control and 1 Gy AO at 4 h. *P = 0.018 between 1 Gy control and 1 Gy AO at 24 h. Panel B: TGFβ1: 0 Gy control and AO (n = 6–8), 1 Gy control and AO 4 h (n = 6–8), 1 Gy control and AO 24 h (n = 6), 7.2 Gy control and AO 4 h (n = 7), 7. 2 Gy control and AO 24 h (n = 3). Data are means ± SD with significant difference accepted as P < 0.05 by Student's t test. **P = 0.0013 between 0 Gy control and 1 Gy control at 4 h. **P = 0.0074 between 0 Gy control and 7.2 Gy control at 4 h. *P = 0.031 between 0 Gy control and 1 Gy AO at 24 h. Data are means ± SD.
At 24 h after 1 Gy proton TBI, there was a significant increase in plasma Flt-3L in animals fed the control (Fig. 5A) and antioxidant (Fig. 5A) diets compared to nonirradiated animals fed the control diet. Animals fed the control diet and exposed to 1 Gy TBI had significantly higher plasma Flt-3L levels at 24 h after exposure compared to similarly irradiated animals whose diets were supplemented with antioxidants (Fig. 5A). Dietary supplementation with antioxidants did not affect the significant increase in plasma Flt-3L levels at 24 h after exposure to 7.2 Gy TBI (Fig. 5A).
TGFβ1 is one of the few negative regulators of hematopoiesis (30-32). Nonirradiated animals had similar levels of plasma TGFβ1 regardless of diet (Fig. 5B). At 4 h after 1 Gy proton TBI, there was a significant increase in plasma TGFβ1 levels in animals fed the control diet compared to nonirradiated animals fed the same diet (Fig. 5B). Dietary antioxidant supplementation completely inhibited the increase in plasma TGFβ1 at 4 h after 1 Gy TBI (Fig. 5B). At 4 h after 7.2 Gy TBI, there was a significant decrease in plasma TGFβ1 in animals fed the control diet compared to nonirradiated animals fed the control diet (Fig. 5B). Dietary antioxidant supplementation completely inhibited the decrease in plasma TGFβ1 at 4 h after 7.2 Gy TBI exposure (Fig. 5B).
Whereas there was no longer a significant difference in plasma TGFβ1 levels at 24 h after 1 Gy TBI between animals fed the control diet and nonirradiated animals fed the same diet (Fig. 5B), 1 Gy TBI animals supplemented with antioxidants were found to have significantly higher levels of TGFβ1 compared to nonirradiated animals fed the control diet (Fig. 5B). However, the difference in plasma TGFβ1 at 24 h after 1 Gy TBI between animals fed the control and antioxidant diet did not reach statistical significance (Fig. 5B).
At 24 h after 7.2 Gy proton TBI, there was no difference in plasma TGFβ1 between irradiated animals fed the control or antioxidant diet and nonirradiated animals fed the control diet (Fig. 5B). Likewise there was no difference in plasma TGFβ1 levels between animals fed the control or antioxidant diet at 24 h after 7.2 Gy TBI (Fig. 5B).
Peripheral Leukocyte Counts in Moribund Lethally Irradiated Animals
At the time of euthanasia, 10–15 days after 7.2 Gy TBI, moribund animals were found to have a severe pancytopenia that was not affected by dietary antioxidants (Table 1).
TABLE 1.
Peripheral Blood Counts in Moribund Animals after 7.2 Gy TBI
| Diet | WBC (×103 cells/μl) |
Lymphocytes (×103 cells/μl) |
PMN (×103 cells/μl) |
Hct (%) |
Hemoglobin (g/dl) |
Platelets (×103/μl) |
|---|---|---|---|---|---|---|
| Control (n = 6) | 0.2 ± 0.2 | 0.1 ± 0.1 | 0.1 ± 0.0 | 8.6 ± 0.6 | 1.8 ± 0.3 | 35 ± 13 |
| Antioxidants (n = 3) | 0.3 ± 0.2 | 0.1 ± 0.1 | 0.1 ± 0.1 | 10.7 ± 1.4 | 2.7 ± 0.6 | 45 ± 26 |
| C → AO (n = 4) | 0.3 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.0 | 8.7 ± 2.3 | 2.3 ± 0.6 | 58 ± 25 |
Note. The data are presented means ± SD.
Recovery of Peripheral Leukocyte Counts in Animals Exposed to a Sublethal Dose of Radiation
At 4 weeks after 5.5 Gy proton TBI, there remained a 52% decrease in lymphocytes, a 220% increase in PMN cells, and a 32% decrease in platelets in animals fed the control diet compared to age-matched nonirradiated animals fed the same diet (Table 2). Dietary antioxidant supplementation prior to irradiation and maintenance of this diet for 4 weeks after TBI did not affect recovery of peripheral leukocytes or platelets at 1 month after TBI (Table 2).
TABLE 2.
Peripheral Blood Cell Count Recovery after 5.5 Gy TBI
| Diet | WBC (×103 cells/μl) |
Lymphocytes (×103 cells/μl) |
PMN (×103 cells/μl) |
Hct (%) |
Hemoglobin (g/dl) |
Platelets (×103/μl) |
|---|---|---|---|---|---|---|
| Control (0 Gy, n = 4) | 9.7 ± 1.4 | 5.4 ± 1.1 | 3.0 ± 1.0 | 55.1 ± 1.5 | 17.2 ± 0.8 | 1522 ± 176 |
| Antioxidants (0 Gy, n = 4) | 10.4 ± 1.3 | 7.5 ± 1.1 | 2.1 ± 0.3 | 56.7 ± 2.0 | 17.7 ± 0.4 | 1502 ± 501 |
| Control (4 weeks, n = 4) | 10.3 ± 3.4 | 2.6 ± 0.8** | 6.6 ± 2.1** | 57.0 ± 2.9 | 17.4 ± 1.0 | 1030 ± 69** |
| Antioxidants (4 weeks n = 5) | 10.4 ± 2.0 | 2.8 ± 0.6** | 6.6 ± 1.6** | 57.2 ± 1.8 | 17.2 ± 0.8 | 1130 ± 130** |
| Control (9 weeks, n = 3) | 7.9 ± 0.1* | 5.9 ± 0.6 | 1.8 ± 0.1* | 52.0 ± 3.0 | 16.5 ± 1.1 | 1311 ± 143 |
| Antioxidants (9 weeks, n = 4) | 9.2 ± 0.8ns | 5.4 ± 1.0 | 2.9 ± 0.9ns | 53.0 ± 1.3 | 16.6 ± 0.4 | 1550 ± 103† |
Notes. The data are means ± SD.
P < 0.05.
P < 0.01 compared to nonirradiated animals fed the control diet.
P < 0.05 compared to age-matched irradiated animals fed the control diet.
Non-significant difference from nonirradiated animals fed the control diet.
At 9 weeks after 5.5 Gy proton TBI, the peripheral total leukocyte, lymphocyte, PMN and platelet cell counts of animals supplemented with dietary antioxidants completely recovered to the levels observed in nonirradiated animals fed the control or antioxidant diets (Table 2). However, at 9 weeks after 5.5 Gy TBI, animals fed the control diet had significantly fewer peripheral total leukocyte and PMN cells compared to nonirradiated age-matched animals fed the control diet (Table 2). The lymphopenia observed immediately after TBI and at 4 weeks after irradiation completely resolved by 9 weeks after exposure in all treatment groups. Conversely, the granulocytosis noted in irradiated animals at 4 weeks after exposure resolved by 9 weeks as lymphocyte counts recovered.
Recovery of Peripheral Blood Counts in Animals Surviving a Potentially Lethal TBI
Eight weeks after 5.9 Gy proton TBI, the total white blood cell count in animals fed the control diet was 63% of the value of nonirradiated animals fed the same diet (Table 3). Animals supplemented with dietary antioxidants prior to TBI and maintained on this diet throughout the recovery period had 91% recovery of total white blood cell counts compared to nonirradiated animals fed the control diet (Table 3). Initiation of dietary antioxidants 2 h after TBI (C → AO) resulted in 68% recovery of peripheral total leukocytes at 8 weeks compared to nonirradiated animals fed the control diet (Table 3). Total peripheral leukocyte counts were significantly higher in animals supplemented with dietary antioxidants prior to radiation exposure compared to animals supplemented with antioxidants after TBI (Table 3) or to irradiated animals fed the control diet (Table 3).
TABLE 3.
Peripheral Blood Cell Count Recovery 8 Weeks after 5.9 Gy TBI
| Diet | WBC (×103 cells/μl) |
Lymphocytes (×103 cells/μl) |
PMN (×103 cells/μl) |
Hct (%) |
Hemoglobin (g/dl) |
Platelets (×103/μl) |
|---|---|---|---|---|---|---|
| Control (0 Gy, n = 4) | 10.4 ± 1.8 | 7.1 ± 1.0 | 2.3 ± 0.7 | 43.5 ± 4.0 | 13.3 ± 1.3 | 1389 ± 83 |
| Control (n = 3) | 6.6 ± 2.0* | 4.0 ± 1.0** | 2.7 ± 0.7 | 45.8 ± 2.0 | 14.4 ± 1.0 | 906 ± 113*** |
| Antioxidants (n = 5) | 9.5 ± 0.5ns | 5.7 ± 0.7ns | 3.3 ± 0.9 | 44.5 ± 3.1 | 13.8 ± 1.1 | 1289 ± 121ns‡ |
| C → AO (n = 6) | 7.1 ± 0.8** | 4.7 ± 0.5** | 2.0 ± 0.4 | 45.4 ± 5.5 | 14.4 ± 1.5 | 1328 ± 205ns† |
Notes. The data are means ± SD.
P < 0.05.
P < 0.001 compared to nonirradiated animals fed the control diet.
P < 0.5 compared to age-matched irradiated animals fed the control diet.
P < 0.01 compared to irradiated animals fed control diet.
Non-significant difference from nonirradiated animals fed control diet.
At 8 weeks after 5.9 Gy, proton-irradiated animals fed the control diet had 56.3% (Table 3) of the peripheral lymphocyte count of nonirradiated age-matched animals fed the same diet (Table 3). Supplementation with antioxidants prior to TBI resulted in peripheral lymphocyte counts at 8 weeks that were 80% of the levels observed in nonirradiated aged-matched animals fed the control diet (Table 3). Initiation of antioxidant supplementation 2 h after TBI resulted in lymphocyte counts that were 66% of those of nonirradiated age-matched animals fed the control diet (Table 3). Lymphocyte counts were higher in animals supplemented with dietary antioxidants prior to radiation exposure compared to animals supplemented with antioxidants after TBI (Table 3) or to irradiated animals fed the control diet (Table 3).
Antioxidant supplementation, whether initiated prior to or after proton TBI, resulted in non-significant differences in platelet counts between irradiated animals and nonirradiated age-matched animals fed the control diet at 8 weeks after TBI (Table 3). However, irradiated animals fed the control diet continued to have significantly lower platelet counts than nonirradiated animals fed the same diet at 8 weeks after TBI (Table 3).
DISCUSSION
We have previously demonstrated the efficacy of dietary supplementation with a mixture of antioxidants comprised of SeM, vitamin C, vitamin E succinate, α-lipoic acid and NAC as a preventative measure prior to TBI with X rays or as a treatment after TBI in limiting hematopoietic cell depletion, promoting hemapoietic cell recovery, and improving animal survival (17). In the present study we aimed to study the radioprotective efficacy of the same dietary antioxidants on similar end points mediated by proton TBI. When administered as a preventative measure prior to TBI, dietary antioxidant supplementation was effective in significantly limiting radiation-induced peripheral leukopenia, neutropenia and lymphopenia at 4 and 24 h after 1 Gy, whereas the antioxidants were less effective against the hematopoietic effects of 7.2 Gy of proton TBI. Supplementation with antioxidants prior to TBI also significantly limited radiation-induced bone marrow cell depletion and the decrease in spleen mass at 24 h after exposure. Furthermore, antioxidant supplementation protected against hematopoietic syndrome-induced animal mortality in a statistically significant manner when given as a treatment after radiation exposure; survival of the irradiated animals increased from 60% in the animals fed the control diet to 93% in the animals fed with the antioxidant diet. For these data, the dose-modifying factor (DMF) for antioxidant therapy (ratio of survival of animals protected by antioxidant therapy to survival of the unprotected animals) was 1.6. The antioxidant diet was less effective in increasing survival when given in a preventative fashion before TBI. Antioxidants were effective in improving animal survival only in ICR mice exposed to a dose below the calculated LD50/30 for 1 GeV/nucleon proton TBI. Preventative antioxidant supplementation was also associated with significant modulation of proton TBI-induced changes in plasma levels of the hematopoietic cytokines Flt-3L and TGFβ1 in a dose- and time-dependent fashion. Last, preventative supplementation with antioxidants was the most effective regimen at increasing the recovery of radiation-induced peripheral leukocyte depletion.
Several recent studies have established the short-term and long-term deleterious effects of sublethal (0.5–4 Gy) proton TBI on various hematopoietic cell parameters (11, 20-22, 33-36). These studies in sum elucidate the potential hematopoietic risk and harm of extended human space travel, particularly in the event of SPEs. It is worthwhile noting that the aforementioned studies used clinically relevant 250 MeV/nucleon protons. To our knowledge, none of these past studies assessed either the effect of potentially lethal doses of protons on hematopoietic cells, organs and animal survival or any countermeasures (preventative or treatment) aside from shielding (16). Older studies did assess the hematopoietic effects of proton TBI in dogs and primates along with shielding or hypoxia as countermeasures (37-42). Our previous in vivo studies with γ rays, protons or HZE particles in mice and rats suggested that dietary supplementation with antioxidants is an effective countermeasure to prevent ionizing radiation-induced decreases in plasma total antioxidant status (a marker of oxidative stress) (18, 19). We therefore hypothesized that dietary antioxidants would confer a protective effect against the deleterious hematopoietic effects of proton TBI in vivo.
The RBE of 250 MeV/nucleon protons (or lower energies) has been estimated to range from 0.9–1.25 depending on the particular end point measured and the energy of the protons used, although a general RBE of 1.1 is conventionally proposed and used (4, 43-47). At 70 GeV/nucleon, the proton RBE was noted to be 1.6–7.6 in Chinese hamster fibroblasts and 1.04–3.8 in lymphoid cells with single-strand DNA breaks as the end point, whereas the RBE was 1.14–1.7 for survival of Chinese hamster cells (48). We sought to investigate the effects of proton TBI on 30-day mortality resulting from the hematopoietic syndrome in ICR mice and to establish the RBE for this end point. The LD50/30 for total-body exposure to X rays in ICR mice was previously estimated to be 7.55 Gy (49). Similarly, we found previously that 8 Gy TBI resulted in 87% mortality at 30 days in the same strain of mice (17). In the present study we calculated the LD50/30 of 1 GeV/nucleon protons to be 6.23 Gy, corresponding to an RBE of 1.21 compared to the results observed for X rays. Our results are in agreement with previous in vivo studies and fall within the accepted 10–20% variance in RBE in the clinical setting and the conventionally accepted value of 1.1 for various proton radiation-induced biological effects (47).
We found that the radioprotective effect of dietary supplementation with antioxidants on animal mortality after proton TBI occurred only at a dose less than the LD50/30, which was 5.9 Gy (equivalent to a dose of 6.5 Gy X rays, assuming an RBE of 1.1). In contrast, our previous results using X rays indicated that antioxidants significantly increased animal survival at a total-body dose of 8 Gy (greater than the LD50/30 of 7.55 Gy). We also noticed another difference in the efficacy of dietary supplementation with antioxidants as a radioprotective countermeasure against hematopoietic injury and death induced by TBI with X rays compared to protons. Whereas antioxidants were effective at increasing animal survival when administration began at 7 days prior to or 2 h after X irradiation, dietary antioxidants were considerably more effective at increasing animal survival after proton TBI when they were administered 2 h after TBI compared to the results observed when the antioxidants were administered both before and after TBI. These data suggest that the antioxidants used in this study could be used safely as supportive therapy after proton TBI. Although the effects of the diets on animal weights were not evaluated in this study, the effects of the antioxidant diet compared to the control AIN-93G diet on animal weights and toxicity parameters were evaluated carefully in previous studies in this laboratory (50). In those studies, no effects on animal weight or other toxic effects were attributed to the antioxidant diet in either short-term or long-term studies involving irradiated or unirradiated animals.
The self-renewal capacity or reconstitution of hematopoietic stem cells (HSC) is dependent on ataxia telangiectasia mutated (ATM)-mediated inhibition of oxidative stress generated by p38 MAPK activity, whereas proliferation of more differentiated hematopoietic progenitor cells is less sensitive to levels of p38 MAPK-derived reactive oxygen species (ROS) (51, 52). Treatment of adult mice lacking the ataxia telangiectasia mutated gene (Atm−/−) with NAC or catalase not only prevents elevation in ROS but also results in partial rescue of bone marrow failure associated with increased chronic p38 MAPK-induced ROS (51, 52). However, it is also known that several hematopoietic cytokines that stimulate growth, differentiation and prevention of apoptosis of progenitor cells, including granulocyte macrophage colony-stimulating factor (GM-CSF), interleukin 3 (IL-3), steel factor (SF), thrombopoietin (TPO) and erythropoietin (Epo), cause rapid increases in ROS levels in quiescent progenitor cells via receptor-mediated signaling cascades (53, 54). Several antioxidants, including NAC, have been shown to abolish or diminish the receptor-mediated signaling of these hematopoietic cytokines (53, 54). In hematopoietic stem and progenitor cells, redox signaling mediated by NADPH oxidase and its regulatory proteins may be an important regulator of the critical balance between self-renewal and differentiation (55).
In the present study with proton TBI, as previously observed for total-body X irradiation, dietary antioxidants were most effective in improving animal survival when administration began 2 h after radiation exposure. Although the signaling cascades common or specific to either photons or protons are not completely known, it is evident that TBI of animals results in an inflammatory state partially mediated by oxidative stress immediately after radiation exposure that can ultimately result in animal death depending on the dose delivered. Furthermore, it remains unknown as to what extent TBI-induced oxidative stress is a necessary physiological response to promote animal survival, e.g. hematopoietic cytokine-induced receptor signaling cascades. When administered 2 h after the proton TBI, the antioxidant diet would not affect the initial oxidative stress mediated by or in response to TBI, but it could have a major effect on persistent oxidative stress induced by the radiation exposure, ultimately resulting in the most effective increase in animal survival after both proton and X irradiation. In our previous studies with mice and rats exposed to γ rays, protons or HZE particles, we found that dietary antioxidant supplementation prior to TBI resulted in prevention of the radiation-induced decrease in serum total antioxidant capacity (a surrogate for oxidative stress) at 4 h after exposure (18, 19). Although this observation is consistent with our hypothesis that TBI depletes serum antioxidant levels or other endogenous antioxidant stores, which accounts for the efficacy of dietary antioxidant supplementation, we fully acknowledge that direct measurement of antioxidants levels is necessary to confirm this mechanism of action.
In the bone marrow, total-body X irradiation at doses of 0.5–6 Gy results in a significant decrease or complete depletion of endogenous vitamin C and vitamin E levels as early as 1 h, with the nadir at 24 h after exposure (56, 57). These radiation-induced changes in endogenous antioxidant vitamin levels are associated with concurrent or delayed increases in markers of oxidative stress in the bone marrow including 4-hydroxynonenal, hexanal and thiobarbituric acid-reactive substances (57). Interestingly, sublethal TBI with 3 Gy resulted in recovery of vitamin levels in the bone marrow at 8 days after exposure, whereas there was no recovery back to normal levels after 6 Gy X irradiation (56). From our studies as well as those of others, antioxidant supplementation prior to or after TBI likely modifies the bone marrow response to radiation exposure.
In the current study we identified another putative means by which antioxidant supplementation affects hematopoietic cell response after TBI, which is the modulation of the hematopoietic cytokines Flt-3L and TGFβ1. Several studies have shown that after exposure to ionizing radiation, blood levels of Flt-3L are a surrogate for the extent of damage to hematopoietic progenitor cells in the bone marrow (28, 29, 58). Furthermore, the concentration of Flt-3L in plasma after irradiation is inversely correlated with PMN cell counts in the peripheral blood (29). Dietary antioxidant supplementation prior to TBI resulted in significantly lower levels of plasma Flt-3L at 4 and 24 h after 1 Gy TBI compared to levels in similarly irradiated animals fed the control diet. These results not only corroborate the protective effect of antioxidants on peripheral PMN cell counts after TBI but also suggest that preventative dietary antioxidant supplementation has a protective effect on bone marrow cell depletion after 1 Gy proton TBI. Interestingly, we observed the protective effects of antioxidants after 1 Gy TBI not only in bone marrow cell counts but also in spleen mass and peripheral PMN cell and lymphocyte counts in a similar fashion and to a similar extent. Furthermore, we observed that antioxidants did not affect the increase in plasma levels of Flt-3L after 7.2 Gy TBI. These data are consistent with the lack of difference in peripheral PMN cell and lymphocyte counts regardless of diet after 7.2 Gy TBI. The extent of peripheral leukocyte depletion after proton TBI observed in our study is consistent with results obtained from previous studies (11, 22). Taken together, preventative dietary antioxidant supplementation is more effective at mitigating proton TBI-induced hematopoietic cell changes at 1 Gy compared to 7.2 Gy.
Proton TBI resulted in significant changes in the plasma levels of TGFβ1 that were affected by preventative dietary antioxidant supplementation in a statistically significant manner. In animals fed the control diet, plasma TGFβ1 levels exhibited a dose-dependent response to TBI in that 1 Gy TBI resulted in significantly increased levels of the hematopoietic cytokine compared to nonirradiated control animals and 7.2 Gy resulted in significantly decreased levels at 4 h after exposure. At 24 h after TBI, TGFβ1 levels returned to the levels in nonirradiated animals in both 1-Gy and 7.2-Gy animals fed the control diet. Antioxidant supplementation resulted in an increase in TGFβ1 plasma levels at 24 h after 1 Gy compared to nonirradiated controls. Plasma levels of TGFβ1 returned to those in nonirradiated animals at 24 h after 7.2 Gy TBI regardless of diet. This suggests that antioxidant supplementation potentially abolished or delayed the endogenous TGFβ1 response. The mechanism and significance of antioxidant modulation of radiation-induced plasma TGFβ1 levels are not known. However, this is likely an important means by which antioxidants also affect bone marrow cell response or recovery after TBI. Although we did not measure plasma levels of this cytokine in our previous study with X rays, we did notice a profound effect of TBI on TGFβ1 mRNA expression in the bone marrow at 4 and 24 h after exposure (17). Our observation that proton TBI results in significant changes in TGFβ1 levels was not observed in the study by Kaijoka et al., who compared levels of this cytokine in proton- and γ-irradiated animals (34). The discordance between these studies likely represents evaluation of cytokine levels at different times. Kaijoka et al. evaluated plasma TGFβ1 at 7 days after TBI (34), and we report that by 24 h the elevation of this cytokine in the circulation returns to levels observed in nonirradiated animals.
In this study we also observed that antioxidant supplementation increased peripheral leukocyte cell recovery when given prior to sublethal or potentially lethal proton TBI. The benefits of antioxidants in improving recovery of hematopoietic cells in the periphery and bone marrow were also observed in our study with X rays (17). Interestingly, despite a lower impact on animal survival, antioxidant supplementation before TBI resulted in the greatest improvement in hematopoietic cell recovery. These results suggest that the end point of animal survival after potentially lethal TBI is affected by many factors, including bone marrow as well as peripheral hematopoietic cell protection and recovery.
This report shows that the effects of proton TBI on hematopoietic end points, including 30-day survival, are not completely similar to the effects observed for total-body X irradiation. Some differences may be related to the higher RBE of protons compared to photons. Dietary antioxidant supplementation may be an effective countermeasure for proton-induced hematopoietic effects. However, additional studies are needed to elucidate the endogenous hematopoietic oxidative stress response to TBI and the impact and ideal timing of exogenous antioxidants on this important regulatory hematopoietic pathway.
ACKNOWLEDGMENTS
We would like to thank Mr. Mathew Baran and Ms Ying-Hui Ko for assistance with the animal experiments. We also thank Ms. Mary-Ann Kershaw, Ms. Kerri Bonti and the staff of the Brookhaven National Laboratory (BNL) Animal Facility for facilitating experiments done at BNL. We also would like to thank Dr. Suresh Shelat and Ms. Susan Shibutani of the Children's Hospital of Philadelphia Hematology Laboratory for assistance with peripheral blood count measurements. This work was supported by a grant from the National Space Biomedical Research Institute (NSBRI) through NASA NCC 9-58 and NIH Training Grant 5T32CA009677.
REFERENCES
- 1.Lundkvist J, Ekman M, Ericsson SR, Jonsson B, Glimelius B. Proton therapy of cancer: potential clinical advantages and cost-effectiveness. Acta Oncol. 2005;44:850–861. doi: 10.1080/02841860500341157. [DOI] [PubMed] [Google Scholar]
- 2.Olsen DR, Bruland OS, Frykholm G, Norderhaug IN. Proton therapy—a systematic review of clinical effectiveness. Radiother. Oncol. 2007;83:123–132. doi: 10.1016/j.radonc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Tsujii H, Tsuji H, Inada T, Maruhashi A, Hayakawa Y, Takada Y, Tada J, Fukumoto S, Tatuzaki H. Clinical results of fractionated proton therapy. Int. J. Radiat. Oncol. Biol. Phys. 1993;25:49–60. doi: 10.1016/0360-3016(93)90144-k. [DOI] [PubMed] [Google Scholar]
- 4.Vargas C, Fryer A, Mahajan C, Indelicato D, Horne D, Chellini A, McKenzie C, Lawlor P, Henderson R, Keole S. Dose–volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:744–751. doi: 10.1016/j.ijrobp.2007.07.2335. [DOI] [PubMed] [Google Scholar]
- 5.Zietman AL. The Titanic and the iceberg: prostate proton therapy and health care economics. J. Clin. Oncol. 2007;25:3565–3566. doi: 10.1200/JCO.2007.11.9768. [DOI] [PubMed] [Google Scholar]
- 6.Brada M, Pijls-Johannesma M, De Ruysscher D. Proton therapy in clinical practice: current clinical evidence. J. Clin. Oncol. 2007;25:965–970. doi: 10.1200/JCO.2006.10.0131. [DOI] [PubMed] [Google Scholar]
- 7.Cucinotta FA. Issues in risk assessment from solar particle events. Radiat. Meas. 1999;30:261–268. doi: 10.1016/s1350-4487(99)00054-2. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy AR, Guan J, Ware JH. Countermeasures against space radiation induced oxidative stress in mice. Radiat. Environ. Biophys. 2007;46:201–203. doi: 10.1007/s00411-007-0105-4. [DOI] [PubMed] [Google Scholar]
- 9.Nachtwey DS, Yang TC. Radiological health risks for exploratory class missions in space. Acta Astronaut. 1991;23:227–231. doi: 10.1016/0094-5765(91)90122-l. [DOI] [PubMed] [Google Scholar]
- 10.Petrov VM. Solar cosmic rays as a specific source of radiation risk during piloted space flight. Adv. Space Res. 2004;34:1390–1394. doi: 10.1016/j.asr.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Kajioka EH, Andres ML, Li J, Mao XW, Moyers MF, Nelson GA, Slater JM, Gridley DS. Acute effects of whole-body proton irradiation on the immune system of the mouse. Radiat. Res. 2000;153:587–594. doi: 10.1667/0033-7587(2000)153[0587:aeowbp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Coleman CN, Blakely WF, Fike JR, MacVittie TJ, Metting NF, Mitchell JB, Moulder JE, Preston RJ, Seed TM, Wong RSL. Molecular and cellular biology of moderate-dose (1–10 Gy) radiation and potential mechanisms of radiation protection: Report of a workshop at Bethesda, Maryland, December 17–18, 2001. Radiat. Res. 2003;159:812–834. doi: 10.1667/rr3021. [DOI] [PubMed] [Google Scholar]
- 13.Mettler FA, Jr., Voelz GL. Major radiation exposure— what to expect and how to respond. N. Eng. J. Med. 2002;346:1554–1561. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- 14.Coleman CN, Stone HB, Moulder JE, Pellmar TC. Modulation of radiation injury. Science. 2004;304:693–694. doi: 10.1126/science.1095956. [DOI] [PubMed] [Google Scholar]
- 15.Moulder JE. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: a review. Int. J. Radiat. Biol. 2004;80:3–10. doi: 10.1080/09553000310001642920. [DOI] [PubMed] [Google Scholar]
- 16.Wilson JW, Cucinotta FA, Shinn JL, Simonsen LC, Dubey RR, Jordan WR, Jones TD, Chang CK, Kim MY. Shielding from solar particle event exposures in deep space. Radiat. Res. 1999;30:361–382. doi: 10.1016/s1350-4487(99)00063-3. [DOI] [PubMed] [Google Scholar]
- 17.Wambi C, Sanzari J, Wan XS, Nuth M, Davis J, Ko YH, Sayers CM, Baran M, Ware JH, Kennedy AR. Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body irradiation. Radiat. Res. 2008;169:384–396. doi: 10.1667/RR1204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan J, Stewart J, Ware JH, Zhou Z, Donahue JJ, Kennedy AR. Effects of dietary supplements on the space radiation-induced reduction in total antioxidant status in CBA mice. Radiat. Res. 2006;165:373–378. doi: 10.1667/rr3523.1. [DOI] [PubMed] [Google Scholar]
- 19.Guan J, Wan XS, Zhou Z, Ware J, Donahue JJ, Biaglow JE, Kennedy AR. Effects of dietary supplements on space radiation-induced oxidative stress in Sprague-Dawley rats. Radiat. Res. 2004;162:572–579. doi: 10.1667/rr3249. [DOI] [PubMed] [Google Scholar]
- 20.Gridley DS, Pecaut MJ, Dutta-Roy R, Nelson GA. Dose and dose rate effects of whole-body proton irradiation on leukocyte populations and lymphoid organs: part I. Immunol. Lett. 2002;80:55–66. doi: 10.1016/s0165-2478(01)00306-6. [DOI] [PubMed] [Google Scholar]
- 21.Gridley DS, Rizvi A, Luo-Owen X, Makinde AY, Coutrakon GB, Koss P, Slater JM, Pecaut MJ. Variable hematopoietic responses to acute photons, protons and simulated solar particle event protons. In Vivo. 2008;22:159–169. [PubMed] [Google Scholar]
- 22.Kajioka EH, Gheorghe C, Andres ML, Abell GA, Folz-Holbeck J, Slater JM, Nelson GA, Gridley DS. Effects of proton and gamma radiation on lymphocyte populations and acute response to antigen. In Vivo. 1999;13:525–533. [PubMed] [Google Scholar]
- 23.Prentice A, Branca F, Decsi T, Michaelsen KF, Fletcher RJ, Guesry P, Manz F, Vidailhet M, Pannemans D, Samartin S. Energy and nutrient dietary reference values for children in Europe: methodological approaches and current nutritional recommendations. Br. J. Nutr. 2004;92(Suppl. 2):S82–S146. doi: 10.1079/bjn20041159. [DOI] [PubMed] [Google Scholar]
- 24.Aitio ML. N-acetylcysteine—passe-partout or much ado about nothing? Br. J. Clin. Pharmacol. 2006;61:5–15. doi: 10.1111/j.1365-2125.2005.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wollin SD, Jones PJ. Alpha-lipoic acid and cardiovascular disease. J. Nutr. 2003;133:3327–3330. doi: 10.1093/jn/133.11.3327. [DOI] [PubMed] [Google Scholar]
- 26.Lunning MA, Zenger VE, Dreyfuss R, Stetler-Stevenson M, Rick ME, White TA, Wilson WH, Marti GE. Albumin enhanced morphometric image analysis in CLL. Cytometry B Clin. Cytom. 2004;57:7–14. doi: 10.1002/cyto.b.10059. [DOI] [PubMed] [Google Scholar]
- 27.Weisdorf D, Chao N, Waselenko JK, Dainiak N, Armitage JO, McNiece I, Confer D. Acute radiation injury: contingency planning for triage, supportive care, and transplantation. Biol. Blood Marrow Transplant. 2006;12:672–682. doi: 10.1016/j.bbmt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Huchet A, Belkacemi Y, Frick J, Prat M, Muresan-Kloos I, Altan D, Chapel A, Gorin NC, Gourmelon P, Bertho JM. Plasma Flt-3 ligand concentration correlated with radiation-induced bone marrow damage during local fractionated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003;57:508–515. doi: 10.1016/s0360-3016(03)00584-4. [DOI] [PubMed] [Google Scholar]
- 29.Bertho JM, Demarquay C, Frick J, Joubert C, Arenales S, Jacquet N, Sorokine-Durm I, Chau Q, Lopez M, Gourmelon P. Level of Flt3-ligand in plasma: a possible new bio-indicator for radiation-induced aplasia. Int. J. Radiat. Biol. 2001;77:703–712. doi: 10.1080/09553000110043711. [DOI] [PubMed] [Google Scholar]
- 30.Scandura JM, Boccuni P, Massague J, Nimer SD. Transforming growth factor beta-induced cell cycle arrest of human hematopoietic cells requires p57KIP2 up-regulation. Proc. Natl. Acad. Sci. USA. 2004;101:15231–15236. doi: 10.1073/pnas.0406771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacGrogan D, Kalakonda N, Alvarez S, Scandura JM, Boccuni P, Johansson B, Nimer SD. Structural integrity and expression of the L3MBTL gene in normal and malignant hematopoietic cells. Genes Chromosomes Cancer. 2004;41:203–213. doi: 10.1002/gcc.20087. [DOI] [PubMed] [Google Scholar]
- 32.Vodovotz Y, Lucia MS, DeLucca AM, Mitchell JB, Kopp JB. Reduced hematopoietic function and enhanced radiosensitivity of transforming growth factor-beta1 transgenic mice. Int. J. Cancer. 2000;90:13–21. [PubMed] [Google Scholar]
- 33.Gridley DS, Pecaut MJ. Whole-body irradiation and long-term modification of bone marrow-derived cell populations by low- and high-LET radiation. In Vivo. 2006;20:781–789. [PubMed] [Google Scholar]
- 34.Kajioka EH, Andres ML, Mao XW, Moyers MF, Nelson GA, Gridley DS. Hematological and TGF-beta variations after whole-body proton irradiation. In Vivo. 2000;14:703–708. [PubMed] [Google Scholar]
- 35.Pecaut MJ, Gridley DS, Nelson GA. Long-term effects of low-dose proton radiation on immunity in mice: shielded vs. unshielded. Aviat. Space Environ. Med. 2003;74:115–124. [PubMed] [Google Scholar]
- 36.Pecaut MJ, Gridley DS, Smith AL, Nelson GA. Dose and dose rate effects of whole-body proton-irradiation on lymphocyte blastogenesis and hematological variables: part II. Immunol. Lett. 2002;80:67–73. doi: 10.1016/s0165-2478(01)00307-8. [DOI] [PubMed] [Google Scholar]
- 37.Aceto H, Jr., Springsteen R, Gee W, Winchell HS, Tobias CA. Erythropoietic response in dogs given sublethal whole-body proton irradiation followed by hypoxic hypoxia. Radiat. Res. 1969;39:101–111. [PubMed] [Google Scholar]
- 38.Kalandarova MP. Comparative characteristics of bone marrow hematopoiesis in dogs following acute irradiation by protons in conditions of partial shielding of a portion of the bone marrow. Radiobiologiia. 1973;13:774–778. in Russian. [PubMed] [Google Scholar]
- 39.Kalandarova MP. Condition of the bone marrow in dogs at late intervals after 2-stage irradiation with high-energy protons under conditions of local protection of the body. Radiobiologiia. 1974;14:781–783. in Russian. [PubMed] [Google Scholar]
- 40.Kalandarova MP. Comparative evaluation of bone marrow hematopoiesis in the dog sternum and iliac bone following nonuniform proton irradiation with different absorbed doses. Radiobiologiia. 1975;15:905–908. in Russian. [PubMed] [Google Scholar]
- 41.Nevskaia GF, Abramova GM, Ginsburg EV, Ishmukhametova DN, Skorik AS. Hematopoiesis in dogs irradiated with protons in lethal doses with screening of the bone marrow. Kosm. Biol. Aviakosm. Med. 1977;11:67–70. in Russian. [PubMed] [Google Scholar]
- 42.Kirk JH, Casey HW, Traynor JE. Summary of latent effects in long term survivors of whole body irradiations in primates. Life Sci. Space Res. 1972;10:165–173. [PubMed] [Google Scholar]
- 43.Ando K, Furusawa Y, Suzuki M, Nojima K, Majima H, Koike S, Aoki M, Shimizu W, Futami Y, Ikeda H. Relative biological effectiveness of the 235 MeV proton beams at the National Cancer Center Hospital East. J. Radiat. Res. (Tokyo) 2001;42:79–89. doi: 10.1269/jrr.42.79. [DOI] [PubMed] [Google Scholar]
- 44.Gueulette J, Bohm L, Slabbert JP, De Coster BM, Rutherfoord GS, Ruifrok A, Octave-Prignot M, Binns PJ, Schreuder AN, Jones DT. Proton relative biological effectiveness (RBE) for survival in mice after thoracic irradiation with fractionated doses. Int. J. Radiat. Oncol. Biol. Phys. 2000;47:1051–1058. doi: 10.1016/s0360-3016(00)00535-6. [DOI] [PubMed] [Google Scholar]
- 45.Gueulette J, Gregoire V, Octave-Prignot M, Wambersie A. Measurements of radiobiological effectiveness in the 85 MeV proton beam produced at the cyclotron CYCLONE of Louvain-la-Neuve, Belgium. Radiat. Res. 1996;145:70–74. [PubMed] [Google Scholar]
- 46.Gueulette J, Slabbert JP, Bohm L, De Coster BM, Rosier JF, Octave-Prignot M, Ruifrok A, Schreuder AN, Wambersie A, Jones DT. Proton RBE for early intestinal tolerance in mice after fractionated irradiation. Radiother. Oncol. 2001;61:177–184. doi: 10.1016/s0167-8140(01)00446-7. [DOI] [PubMed] [Google Scholar]
- 47.Paganetti H. Significance and implementation of RBE variations in proton beam therapy. Technol. Cancer Res. Treat. 2003;2:413–426. doi: 10.1177/153303460300200506. [DOI] [PubMed] [Google Scholar]
- 48.Leont'eva GA, Fomenko BS, Antipov AV. Effect of secondary radiation produced by 70 GeV protons on DNA of mammalian cells. Adv. Space Res. 1984;4:231–235. doi: 10.1016/0273-1177(84)90246-1. [DOI] [PubMed] [Google Scholar]
- 49.Kurishita A, Katoh H, Uehara Y, Uchida A, Mizutani Y, Ono T, Hirose S, Okada S. Post-irradiation treatment with OK432 can prevent radiation-induced bone marrow death. Int. J. Radiat. Biol. 1991;59:711–716. doi: 10.1080/09553009114550621. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy AR, Davis JG, Carlton W, Ware JH. Effects of dietary antioxidant supplementation on the development of malignant lymphoma and other neoplastic lesions in mice exposed to proton or iron-ion radiation. Radiat. Res. 2008;169:615–625. doi: 10.1667/RR1296.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 52.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 53.Iiyama M, Kakihana K, Kurosu T, Miura O. Reactive oxygen species generated by hematopoietic cytokines play roles in activation of receptor-mediated signaling and in cell cycle progression. Cell Signal. 2006;18:174–182. doi: 10.1016/j.cellsig.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Sattler M, Winkler T, Verma S, Byrne CH, Shrikhande G, Salgia R, Griffin JD. Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood. 1999;93:2928–2935. [PubMed] [Google Scholar]
- 55.Piccoli C, D'Aprile A, Ripoli M, Scrima R, Lecce L, Boffoli D, Tabilio A, Capitanio N. Bone-marrow derived hematopoietic stem/progenitor cells express multiple isoforms of NADPH oxidase and produce constitutively reactive oxygen species. Biochem. Biophys. Res. Commun. 2007;353:965–972. doi: 10.1016/j.bbrc.2006.12.148. [DOI] [PubMed] [Google Scholar]
- 56.Umegaki K, Aoki S, Esashi T. Whole body X-ray irradiation to mice decreases ascorbic acid concentration in bone marrow: comparison between ascorbic acid and vitamin E. Free Radic. Biol. Med. 1995;19:493–497. doi: 10.1016/0891-5849(95)00033-t. [DOI] [PubMed] [Google Scholar]
- 57.Umegaki K, Sano M, Suzuki K, Tomita I, Esashi T. Increases in 4-hydroxynonenal and hexanal in bone marrow of rats subjected to total body X-ray irradiation: association with antioxidant vitamins. Bone Marrow Transplant. 1999;23:173–178. doi: 10.1038/sj.bmt.1701531. s. [DOI] [PubMed] [Google Scholar]
- 58.Prat M, Demarquay C, Frick J, Thierry D, Gorin NC, Bertho JM. Radiation-induced increase in plasma Flt3 ligand concentration in mice: evidence for the implication of several cell types. Radiat. Res. 2005;163:408–417. doi: 10.1667/rr3340. [DOI] [PubMed] [Google Scholar]