Abstract
Background
Progressive cardiomyopathy is a common cause of death in Duchenne muscular dystrophy (DMD), presumably secondary to fibrosis of the myocardium. The posterobasal and left lateral free wall of the left ventricle (LV) are initial sites of myocardial fibrosis pathologically. The purposes of this study were to assess whether cardiac magnetic resonance imaging (CMRI), utilizing late gadolinium enhancement (LGE), could identify fibrosis in selective areas of the myocardium, and to assess the relationship of the presence and extent of fibrosis to LV function.
Methods
The cardiology databases at Primary Children's Medical Center and Cincinnati Children's Hospital Medical Center were reviewed to identify patients with DMD who had undergone a CMRI within the last 2 years. Age, LV ejection fraction, LV mass, presence and location of LGE were documented. Volumes were measured using MASS (Medis, Inc.) to calculate ejection fraction and mass. LGE images were acquired and when positive, manual and customized computer assisted sizing of the areas of late gadolinium enhancement were performed on all slices. Normal function was defined as LV ejection fraction >54%.
Results
A total of 74 patients with DMD had complete data sets (median age 13.7 years, range 7.7 − 26.4). Twenty-four patients (32%) had LGE involving the posterobasal region of the LV in a sub-epicardial distribution. Those patients with more involvement had spread to the inferior and left lateral free wall with progressive transmural fibrous replacement. There was relative sparing of the interventricular septum and right ventricle. Patients with LGE were significantly older than those without (mean age16.4 years vs 12.9 years, p<0.001). LGE was positively associated with BSA-adjusted LV mass, LV end-diastolic volume, LV end-systolic volume, and RV end-systolic volume but inversely correlated with ejection fraction of the LV (p<0.001) and RV (p = 0.004).
Conclusions
LGE by CMRI is able to detect fibrosis in selective regions of myocardium in patients with DMD. Unfavorable LV remodeling, with a corresponding decreased ejection fraction, is associated with the presence of LGE. Serial studies are warranted to determine if LGE precedes a decrease in function, and if early medical management is useful in preventing progression once LGE is documented.
Keywords: Duchenne muscular dystrophy, Magnetic Resonance Imaging, cardiomyopathy
Introduction
Duchenne muscular dystrophy (DMD), an X-linked recessive disorder, is caused by a mutation in the gene that encodes dystrophin. Dystrophin is a large cytoskeletal protein present in skeletal and cardiac muscle cells that connects the sarcomere to the external basement membrane, and is thought to play a role in membrane stabilization, force transduction, and organization of membrane specializations [1]. Skeletal muscle involvement in DMD results in progressive skeletal muscle weakness with loss of ambulation typically by the early second decade of life. Cardiac muscle involvement, manifest as dilated cardiomyopathy, is also present in up to 90% of DMD patients, and at least 20% [2] die of heart failure, thought now to be the leading cause of death in this population [3].
Cardiac pathologic changes in DMD most commonly begin in the posterobasal aspect of the left ventricular (LV) free wall, a process that has been demonstrated pathologically, [3, 4] [5] echocardiographically, [6]and by cardiac magnetic resonance imaging (CMRI). [7] Histologically, specimens demonstrate evidence of fatty replacement of myocytes and myocardial fibrosis in the affected areas of the myocardium. Although this process begins in the posterobasal region of the left ventricular free wall, myocardial fibrosis progresses to involve other regions of the left ventricle ultimately resulting in a generalized dilated cardiomyopathy [3].
Decreased left ventricular function often goes unrecognized in DMD patients due to lack of classic symptoms, masked by the DMD patients’ baseline lack of physical activity [8]. Although change in cardiac function can be demonstrated by noninvasive imaging during early adolescence [9] [10], cardiac evaluation in this patient population is center specific, and often does not occur until late, when there is evidence of overt congestive heart failure. Because a significant proportion of these patients will die secondary to cardiac decompensation, a reliable method of early detection of myocardial involvement would be useful in guiding potential prophylactic therapies, such as corticosteroids and afterload reducing agents [11]. We sought to assess whether CMRI, utilizing late gadolinium enhancement (LGE), could identify fibrosis in selective areas of myocardium, and to assess the relationship of the presence and extent of fibrosis to LV function.
Methods
The Institutional Review Boards at both the University of Utah School of Medicine and Cincinnati Children's Hospital Medical Center approved this retrospective study. The CMRI databases were used to identify all patients undergoing CMRI from October 2005 to September 2007 at each institution with a diagnosis of DMD. The primary indication for CMRI was evaluation of cardiac function. Criteria for inclusion were as follows: any patient with DMD (established by genetic testing) having CMRI for clinical reasons; received contrast with late gadolinium enhanced images acquired; informed consent for the procedure given by the patient, parent or legal guardian. Criteria for exclusion were: Patients with a pacemaker or a history of arrhythmias not controlled with medications and patients who could not cooperate during the CMRI. The medical records were reviewed for patient age, diagnosis, body surface area, and date of study.
Magnetic Resonance Imaging
Studies were performed using a GE Medical Systems (Milwaukee, Wisconsin) 1.5-T Signa LX Echospeed, (Utah and Cincinnati), or Siemens Medical Solutions (Malvern, Pennsylvania 1.5-T Magnetom Avanto (Utah) or 3T Trio (Cincinnati) systems. Choice of scanner was based on clinical availability only. Torso or cardiac-phased array coils were chosen according to body size. All studies were performed utilizing breath-holding when possible, to limit motion artifact. The following imaging protocol was employed at both centers: 1) three-plane localizer; 2) Electrocardiogram-triggered segmented k-space fast-spoiled gradient-recalled cine sequences (SSFP) in two- and four-chamber planes; 3) Electrocardiogram-triggered SSFP sequences in the short axis plane with 12 contiguous slabs perpendicular to the long axis of the left and right ventricle from base to apex (slice thickness 6−8mm, interslice space 0−2mm); 4) Inversion-recovery prepared gradient-echo sequence 10−20 minutes after intravenous bolus of 0.2-mmol/kg gadolinium based contrast in the short axis and 4-chamber planes.
The images were transferred to an independent computer workstation for analysis using commercially available software (MASS, Medis, Leiden, The Netherlands). By use of visual inspection of the cine loops, the end-diastolic and end-systolic frames were chosen. The endocardial borders of the LV and right ventricle (RV) were outlined at end-diastole (EDV) and end-systole (ESV) at all levels from base to apex. Summing these volumes allowed determination of the LV and RVEDV and LV and RVESV. Ejection fraction for each ventricle was calculated by the following equation EF = EDV – ESV / EDV. The epicardial borders of the LV and RV were outlined at end-diastole for determination of ventricular mass. Ventricular mass in grams was obtained by multiplying wall volume by the specific density of the myocardium (1.05 g/cm3). The ventricular septum was considered a part of the left ventricle. [12] Patients found to have LGE underwent customized computer assisted sizing of the regions of LGE in each slice using a semi-automatic thresholding algorithm. This algorithm uses the histogram of pixel intensities within the myocardium to identify scar as pixels that have a signal intensity more than 2−3 standard deviations higher than regions of normal tissue. The number of standard deviations was chosen for each patient to ensure that scar sizes reflected visual assessment of the scar tissue. Summing the areas yielded a total volume which was converted to mass and expressed as a percent of the total mass of the LV.
Statistical Methods
Data are reported as means and standard deviations. Continuous variables were compared by presence or absence of late gadolinium enhancement using a t-test. Unadjusted linear regression was used to assess the association between the delayed enhancement and MRI indices of cardiac size and function. The second, adjusted model was built to control for age. Statistical analysis was performed using Stata 9.1 Software package (StataCorp, TX). A p value < 0.05 was considered statistically significant.
Results
One hundred and sixty nine patients with DMD underwent cardiac MRI at the two institutions during the time course considered. Seventy-four patients met all inclusion and no exclusion criteria for the study. Patient demographic data are listed in Table 1. The entire DMD population had a mean age of 13.7 ± 4.1 years (range 7.7 − 26.4) and BSA 1.39 ± 0.29 m2 (range 0.85 − 2.0m2). Twelve out of 74 MRI measurements included adults (≥18 years) with the remainder being children. Nine patients required sedation for their MRI and had sequences performed with free-breathing.
Table 1.
Patients Characteristics and CMRI Indices of cardiac size and function (n=74)
| All (n=74) | Presence LGE (n=24) | Absence LGE (n=50) | p value | |
|---|---|---|---|---|
| Gender (M/F) | 74/0 | 24/0 | 50/0 | NA |
| BSA (m2) | 1.39 ± 0.29 | 1.47 ± 0.26 | 1.35 ± 0.30 | 0.099 |
| Age at MRI (yrs) | 14.2 ± 4.2 | 16.6 ± 4.4 | 13.0 ± 3.6 | <0.001 |
| RV ESV Indexed (ml/m2) | 23.0 ± 9.7 | 27.2 ± 13.6 | 21.0 ± 6.4 | 0.009 |
| RV EDV Indexed (ml/m2) | 60.9 ± 15.6 | 61.4 ± 20.1 | 60.6 ± 13.1 | 0.840 |
| RV EF (%) | 61.5 ± 12.7 | 55.5 ± 13.5 | 64.4 ± 11.3 | 0.004 |
| LV EDV Indexed (ml/m2) | 76.3 ± 29.5 | 94.4 ± 42.5 | 67.7 ± 14.5 | <0.001 |
| LV ESV Indexed (ml/m2) | 36.3 ± 26.8 | 56.5 ± 38.2 | 26.6 ± 9.4 | <0.001 |
| LV Mass Indexed (g/m2) | 53.7 ± 12.5 | 59.0 ± 16.9 | 51.2 ± 8.9 | 0.012 |
| LV EF (%) | 55.4 ± 13.8 | 42.6 ± 13.6 | 61.5 ± 8.8 | <0.001 |
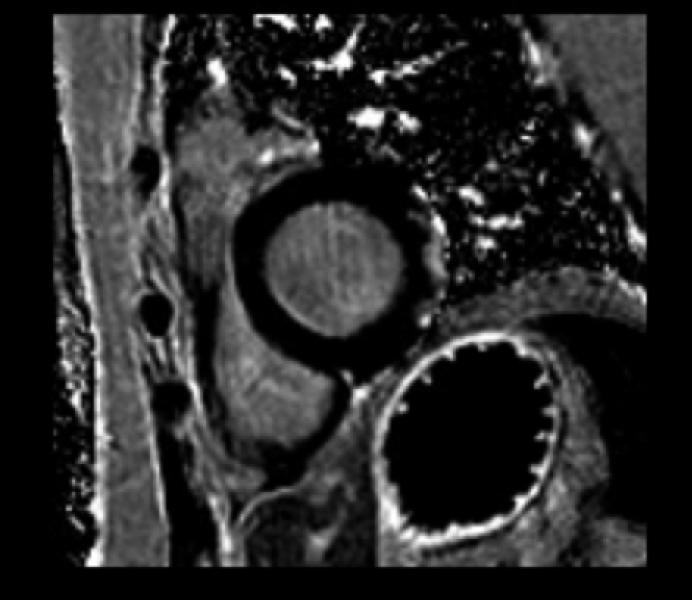
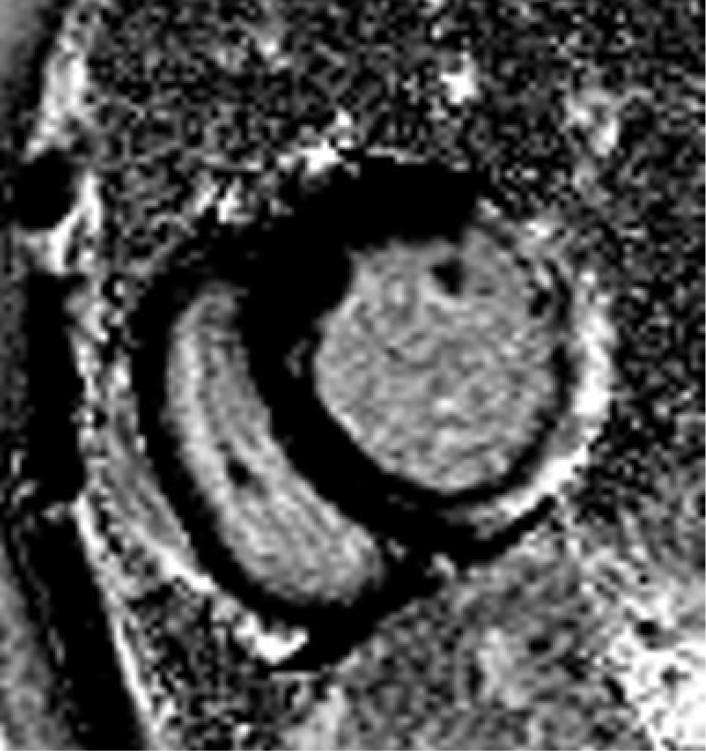
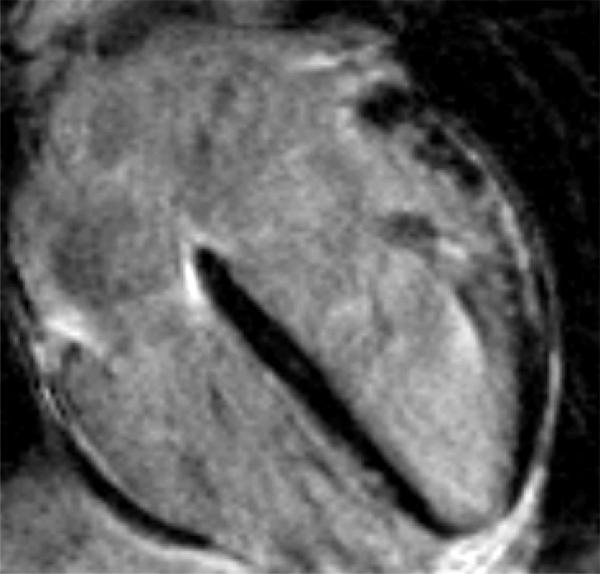
The DMD population was divided into two groups based on the presence or absence of LGE. Of the 50 patients without LGE, 44 (88%) had normal function, and 6 patients had decreased LV EF. All 6 patients with decreased EF and 15 with normal function had a dilated LV. The subgroup of patients with LGE was significantly older (16.4 years vs 12.9 years) than those without. Two of 24 patients (8%) felt to be positive for LGE were not quantifiable using our customized sizing due to poor signal to noise ratio. All patients with LGE had involvement in the basal inferolateral LV free wall in the sub-epicardial area (Figure 1), and as the percentage of LV involvement increased, there was progression to the basal inferior region and basal anterolateral region. Concomitant with the spreading from the posterobasal region was progressive transmural fibrous replacement instead of the typical sub-epicardial involvement (Figure 2). There was relative sparing of the interventricular septum and right ventricle (Figure 3). Patients with more LGE mass involved showed significant regional wall motion abnormalities evidenced by decrease wall thickening.
The proportion of the LV mass with LGE ranged from 8.1 − 43% (mean 21.9 ± 11.1%). Unadjusted linear regression analysis demonstrated a significant positive correlation between LGE and indexed LV EDV, and LV ESV (Table 2). There was a significant negative correlation between LGE and LV EF (Table 2). Patients who were older were more likely to have LGE, therefore, after controlling for age, an adjusted linear regression was performed. LGE correlates with increasing LV EDV and ESV after adjustment for age and shows a trend to significance for function.
Table 2.
Relationship between LGE mass Indexed (g/m2) and CMRI Indices of cardiac size and function (n=22)
| Unadjusted regression | Age-Adjusted regression | |||
|---|---|---|---|---|
| Outcome MRI Indices | Coefficient ± SE | p value | Coefficient ± SE | p value |
| LV EF (%) | −0.74 ± 0.34 | 0.041 | −0.69 ± 0.35 | 0.065 |
| LV EDV Indexed (ml/m2) | 3.19 ± 0.94 | 0.003 | 3.25 ± 0.99 | 0.004 |
| LV ESV Indexed (ml/m2) | 2.48 ± 0.90 | 0.012 | 2.53 ± 0.95 | 0.016 |
| RV EF (%) | 0.45 ±0.36 | 0.231 | 0.56 ±0.36 | 0.138 |
| RV EDV Indexed (ml/m2) | 0.52 ± 0.54 | 0.347 | 0.54 ± 0.57 | 0.349 |
| RV ESV Indexed (ml/m2) | 0.05 ± 0.38 | 0.889 | 0.02 ±0.40 | 0.955 |
SE-standard error
Discussion
LGE performed by CMRI is able to detect fibrosis in selective regions of myocardium in patients with DMD. Unfavorable LV remodeling, with a corresponding decrease in EF, is seen in the presence of LGE.
Dilated cardiomyopathy is increasingly recognized as the cause of death in DMD with the general understanding that this phenomenon occurs during the second decade of life. Data from clinical studies however, suggest that heart disease is well underway long before symptoms appear. [13]CMRI can be used to assess ventricular size and function as well as to evaluate myocardial tissue characteristics. Specifically, cardiac MRI is used to evaluate myocardial fibrosis using LGE, which is present in dystrophinopathy patients. [7, 14] Silva et al demonstrated LGE in a small population (n = 7) of 10 patients with Duchenne and Becker muscular dystrophy by CMRI. The patients without LGE had normal function and 3/7 with LGE had decreased function. There was no correlation with the amount of fibrosis nor did they attempt to relate this to the age of their patients.
Our study involved significantly more patients and identified LGE in a larger subgroup, all of whom had DMD. We showed LGE was present in basal infero-lateral left ventricular free wall in a sub-epicardial distribution and extended to the basal inferior region and basal anterolateral region in a smaller group of patients. These findings are consistent with necropsy studies which showed the posterobasal abnormality spreads to the epicardial third of the contiguous lateral LV free wall with progressive transmural fibrous replacement with relative sparing of the RV and ventricular septum. [4] [15] Based on these observations, the investigators felt that DMD was a unique form of heart disease characterized by a consistent and probably genetically determined predilection for specific regions of the heart, namely the posterobasal and lateral LV walls. It is possible that fibrosis is present throughout the myocardium and only more extensive in the posterobasal and lateral LV wall. If there is undetectable “micro-fibrosis” of the septum and RV, we do not expect these areas play a major role in the global dilated cardiomyopathy [16]. Instead, we feel that if a large enough mass of free wall becomes fibrotic and non-functional, eventually a threshold is reached in which there is global functional decline, similar to that seen in regional ischemia which leads to globally depressed function [17].
Increasing areas of involvement, including transmural spread, was not specific to the oldest patients, suggesting that myocardial fibrosis is not purely age related. In addition, some patients with LGE had normal function, suggesting that myocardial fibrosis may begin before there is a decrease in global LV systolic function, consistent with another recent report [18]. We detected LGE in 3 patients less than 12.5 years of age with normal systolic function. This raises the question of specific genetic mutations playing a role and influencing the development and severity of dilated cardiomyopathy. Different gene mutations may also explain why some patients had adverse LV remodeling and decreased function with no LGE. These findings emphasize the importance of using CMRI to get a more detailed evaluation of the myocardium, specifically identification of LGE and are over and above the normal advantages of CMRI over echocardiography, namely: absence of lung artifact and freedom from poor imaging due to thoracic deformity, common in patients with DMD.
The presence of LGE correlated with LV size and function independent of age. Correlating LGE with function was important since DMD patients have a progressive decrease in LV systolic function that is age-related and well described [19] [9] [20] [21] [22] [10] [23] [24]. In perhaps the largest longitudinal study of this patient population, Nigro et al reported the results of cardiac evaluations in 328 patients with DMD of varying ages who were followed for 3 to 11 years [19]. These investigators found evidence of cardiomyopathy in no patient less than 10 years of age, approximately 30% of patients age 10 to 14 years, 53% of patients 14 to 18 years of age, and nearly 98% of patients over 18 years of age. We found that while our population of relatively young DMD patients had normal LV systolic function as a group, the sub-group with LGE had significantly reduced LV function and increased LV size. The severity of LV enlargement and decreased LV function was more pronounced when LGE involved a greater proportion of the LV myocardium. This suggests a role for identifying myocardial fibrosis at an early age to allow earlier treatment in the hope of delaying the progression or development of dilated cardiomyopathy, as has been demonstrated by Duboc and colleagues with a randomized trial using perindopril. [11] They showed delayed onset and progression of LV dysfunction in the patients who were treated with perindopril compared to controls. Further studies are clearly needed to investigate this in more detail.
Study Limitations
This study is limited by the retrospective design. Not every measurement was available for each data set decreasing the total sample size. The presence of LGE may also affect diastolic function, which was not evaluated in this study. Some of these patients may have been on medication such as afterload reduction, which may impact the measures of LV systolic performance, and this information was not collected.
Conclusions
LGE performed by CMRI is able to detect fibrosis in selective regions of myocardium in patients with DMD. The LV dilates with a corresponding decreased EF when adjusted for age in the presence of LGE. Serial studies are warranted to determine if LGE precedes a decrease in LV function and whether medical management is useful once LGE is documented.
Footnotes
Publisher's Disclaimer: This paper was published in Int J Cardiovasc Imaging. 2009 Jan;25(1):57−63. Link: http://www.springerlink.com/content/r301h97726355257/fulltext.pdf. The original publication is available at springerlink.com
References
- 1.Towbin JA. The role of cytoskeletal proteins in cardiomyopathies. Curr Opin Cell Biol. 1998;10(1):131–9. doi: 10.1016/s0955-0674(98)80096-3. [DOI] [PubMed] [Google Scholar]
- 2.Finsterer J, Stollberger C. The heart in human dystrophinopathies. Cardiology. 2003;99(1):1–19. doi: 10.1159/000068446. [DOI] [PubMed] [Google Scholar]
- 3.Eagle M, et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12(10):926–9. doi: 10.1016/s0960-8966(02)00140-2. [DOI] [PubMed] [Google Scholar]
- 4.Frankel KA, Rosser RJ. The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum Pathol. 1976;7(4):375–86. doi: 10.1016/s0046-8177(76)80053-6. [DOI] [PubMed] [Google Scholar]
- 5.Perloff JK, et al. The distinctive electrocardiogram of Duchenne's progressive muscular dystrophy. An electrocardiographic-pathologic correlative study. Am J Med. 1967;42(2):179–88. doi: 10.1016/0002-9343(67)90017-4. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg SJ, et al. Serial two-dimensional echocardiography in Duchenne muscular dystrophy. Neurology. 1982;32(10):1101–5. doi: 10.1212/wnl.32.10.1101. [DOI] [PubMed] [Google Scholar]
- 7.Silva MC, et al. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J Am Coll Cardiol. 2007;49(18):1874–9. doi: 10.1016/j.jacc.2006.10.078. [DOI] [PubMed] [Google Scholar]
- 8.Heymsfield SB, et al. Sequence of cardiac changes in Duchenne muscular dystrophy. Am Heart J. 1978;95(3):283–94. doi: 10.1016/0002-8703(78)90358-7. [DOI] [PubMed] [Google Scholar]
- 9.Takenaka A, et al. Discrepancy between systolic and diastolic dysfunction of the left ventricle in patients with Duchenne muscular dystrophy. Eur Heart J. 1993;14(5):669–76. doi: 10.1093/eurheartj/14.5.669. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki K, et al. Sequential changes in cardiac structure and function in patients with Duchenne type muscular dystrophy: a two-dimensional echocardiographic study. Am Heart J. 1998;135(6 Pt 1):937–44. doi: 10.1016/s0002-8703(98)70057-2. [DOI] [PubMed] [Google Scholar]
- 11.Duboc D, et al. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45(6):855–7. doi: 10.1016/j.jacc.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz CH. The range of normal values of cardiovascular structures in infants, children, and adolescents measured by magnetic resonance imaging. Pediatr Cardiol. 2000;21(1):37–46. doi: 10.1007/s002469910006. [DOI] [PubMed] [Google Scholar]
- 13.Cardiovascular health supervision for individuals affected by Duchenne or Becker muscular dystrophy. Pediatrics. 2005;116(6):1569–73. doi: 10.1542/peds.2005-2448. [DOI] [PubMed] [Google Scholar]
- 14.Raman SV, et al. Mid-myocardial fibrosis by cardiac magnetic resonance in patients with lamin A/C cardiomyopathy: possible substrate for diastolic dysfunction. J Cardiovasc Magn Reson. 2007;9(6):907–13. doi: 10.1080/10976640701693733. [DOI] [PubMed] [Google Scholar]
- 15.Perloff JK, Henze E, Schelbert HR. Alterations in regional myocardial metabolism, perfusion, and wall motion in Duchenne muscular dystrophy studied by radionuclide imaging. Circulation. 1984;69(1):33–42. doi: 10.1161/01.cir.69.1.33. [DOI] [PubMed] [Google Scholar]
- 16.Aurigemma GP, Zile MR, Gaasch WH. Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation. 2006;113(2):296–304. doi: 10.1161/CIRCULATIONAHA.104.481465. [DOI] [PubMed] [Google Scholar]
- 17.Rosen BD, et al. Lower myocardial perfusion reserve is associated with decreased regional left ventricular function in asymptomatic participants of the multi-ethnic study of atherosclerosis. Circulation. 2006;114(4):289–97. doi: 10.1161/CIRCULATIONAHA.105.588525. [DOI] [PubMed] [Google Scholar]
- 18.Kehr E, et al. Gadolinium-enhanced magnetic resonance imaging for detection and quantification of fibrosis in human myocardium in vitro. Int J Cardiovasc Imaging. 2008;24(1):61–8. doi: 10.1007/s10554-007-9223-y. [DOI] [PubMed] [Google Scholar]
- 19.Nigro G, et al. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26(3):271–7. doi: 10.1016/0167-5273(90)90082-g. [DOI] [PubMed] [Google Scholar]
- 20.Corrado G, et al. Prognostic value of electrocardiograms, ventricular late potentials, ventricular arrhythmias, and left ventricular systolic dysfunction in patients with Duchenne muscular dystrophy. Am J Cardiol. 2002;89(7):838–41. doi: 10.1016/s0002-9149(02)02195-1. [DOI] [PubMed] [Google Scholar]
- 21.de Kermadec JM, et al. Prevalence of left ventricular systolic dysfunction in Duchenne muscular dystrophy: an echocardiographic study. Am Heart J. 1994;127(3):618–23. doi: 10.1016/0002-8703(94)90672-6. [DOI] [PubMed] [Google Scholar]
- 22.Lanza GA, et al. Impairment of cardiac autonomic function in patients with Duchenne muscular dystrophy: relationship to myocardial and respiratory function. Am Heart J. 2001;141(5):808–12. doi: 10.1067/mhj.2001.114804. [DOI] [PubMed] [Google Scholar]
- 23.Melacini P, et al. Cardiac and respiratory involvement in advanced stage Duchenne muscular dystrophy. Neuromuscul Disord. 1996;6(5):367–76. doi: 10.1016/0960-8966(96)00357-4. [DOI] [PubMed] [Google Scholar]
- 24.Backman E, Nylander E. The heart in Duchenne muscular dystrophy: a non-invasive longitudinal study. Eur Heart J. 1992;13(9):1239–44. doi: 10.1093/oxfordjournals.eurheartj.a060343. [DOI] [PubMed] [Google Scholar]