Abstract
Nicotine alters cognitive processes that include working memory and long-term memory. Trace fear conditioning may involve working memory during acquisition while also allowing the assessment of long-term memory. The present study used trace fear conditioning in C57BL/6 mice to investigate the effects of acute nicotine, chronic nicotine, and withdrawal of chronic nicotine on processes active during acquisition and recall 24 hours later and examine the nicotinic acetylcholine receptor subtypes (nAChRs) involved in withdrawal-deficits in trace fear conditioning. During training, acute nicotine (0.09 mg/kg) enhanced, but chronic nicotine (6.3 mg/kg/day, 13 days) and withdrawal of chronic nicotine (6.3 mg/kg/day, 12 days) had no significant effect on acquisition of trace conditioning. At recall, acute treatment enhanced conditioning while chronic nicotine had no effect and withdrawal of chronic nicotine resulted in deficits. Antagonist precipitated withdrawal was used to characterize the nAChRs involved in the withdrawal deficits. The low-affinity nAChR antagonist MLA (1.5, 3, 9 mg/kg) had no effect on trace fear conditioning, but the high-affinity nAChR antagonist DHβE (3 mg/kg) precipitated deficits in trace fear conditioning if administered at training or training and testing, but not if administered at testing alone. The β2 nAChR subunit is involved in the withdrawal effects as withdrawal of chronic nicotine produced deficits in trace fear conditioning in wildtype but not in β2 knockout mice. Thus, nicotine alters processes involved in both acquisition and long-term memory of trace-fear conditioning, and high-affinity β2 subunit containing nAChRs are critically involved in the effects of nicotine withdrawal on trace fear conditioning.
Keywords: Working Memory, Learning, Addiction, Cognition, Hippocampus
Introduction
Nicotine addiction, primarily in the form of cigarette smoking, is responsible for massive health and financial costs to both the individual and society (CDC, 2005; Rice, 1999). Great progress has been made in developing smoking cessation therapies; however, even with current cessation therapies most smokers fail to quit (Schnoll & Lerman, 2006). Part of the reason smokers fail to quit may be that nicotine withdrawal produces deficits in learning and memory. Nicotine withdrawal-induced deficits in learning-related processes may be particularly detrimental because, in addition to the fact that removal of these symptoms by reinstatement of drug use could serve as a negative reinforcer for smoking, these symptoms could result in deficits in the learning of adaptive behaviors that facilitate smoking cessation (Brega et al., 2008; Gutkin et al., 2006).
Nicotine withdrawal produces deficits in a number of tasks involving working memory and long-term memory. For instance, abstinence from smoking produces deficits in working memory, as demonstrated in the N-Back task (Jacobsen et al., 2005; Mendrek et al., 2006; Xu et al., 2005), serial letter recall (Blake & Smith, 1997), and visuospatial working memory (Sacco et al., 2005). Additionally, nicotine withdrawal selectively produces deficits in contextual fear conditioning, without affecting delay fear conditioning (Davis & Gould, 2007; Davis et al., 2005; Gould, 2006; Portugal & Gould, 2007; Portugal et al., 2008). Both working memory and contextual fear conditioning critically involve the hippocampus (Kim et al., 1993; Logue et al., 1997; McEchron et al., 1998; Phillips & LeDoux, 1992; Yoon et al., 2008). Thus, it may be that nicotine withdrawal selectively produces deficits in hippocampus-dependent processes.
Trace fear conditioning involves the association of a conditioned stimulus (CS) and an unconditioned stimulus (US), but the two stimuli are temporally separated by a trace interval. Temporal separation of the stimuli engages the hippocampus (Misane et al., 2005). Lesions to the dorsal hippocampus produce deficits in trace fear conditioning (Bangasser et al., 2006; Burman et al., 2006; Fendt et al., 2005; McEchron et al., 1998; Quinn et al., 2002; Quinn et al., 2008; Rogers et al., 2006; Trivedi & Coover, 2006). Additionally, inhibition of neuronal signaling (Misane et al., 2005; Quinn et al., 2005; Seo et al., 2008; Wanisch et al., 2005), signal transduction pathways (Runyan & Dash, 2004; Villarreal & Barea-Rodriguez, 2006), and protein synthesis in the hippocampus produce deficits in trace fear conditioning (Weitemier & Ryabinin, 2004). Further, in humans trace fear conditioning activates the hippocampus (Büchel et al., 1999; Knight et al., 2004). In addition to requiring hippocampal integrity, trace fear conditioning is thought to necessitate working memory to maintain a memory trace of the CS during conditioning. Trace fear conditioning can be disrupted by tasks that interfere with working memory (Carter et al., 2003) or attention (Han et al., 2003) and strength of trace fear conditioning is associated with subjects’ awareness of the CS-US association (Knight et al., 2006; Weike et al., 2007). Thus, trace fear conditioning depends upon the hippocampus and may involve working memory. These properties of trace fear conditioning combined with the fact that trace fear conditioning is easily performed in many species, including humans (Büchel et al., 1999; Hamm & Weike, 2005; Han et al., 2003; Knight et al., 2004; Knight et al., 2006; Weike et al., 2007; Weike et al., 2008), suggests that trace fear conditioning may be a good model in which to investigate the effects of acute nicotine, chronic nicotine and withdrawal of chronic nicotine on hippocampus-dependent learning.
To investigate the effects of nicotine on trace fear conditioning, we compared the effects of acute nicotine, chronic nicotine, and withdrawal from chronic nicotine on trace fear conditioning in mice, examining changes that occur during training and changes that occur at testing 24 hours later. The acute (0.09 mg/kg) and chronic (6.3 mg/kg/day, 14 days) doses of nicotine were chosen because they produce similar plasma nicotine levels and those levels are within the range reported in smokers (Benowitz, 1988; Davis et al., 2005; Henningfield & Keenan, 1993). In addition, the effects of nicotine on behavior may depend on the nAChRs subtypes involved. Nicotinic acetylcholine receptors are grouped into two classes, low-affinity nAChRs, which are homomeric and consist of the α7 subunit and high-affinity nAChRs which are heteromeric and consist of a combination of α and β subunits (Changeux & Taly, 2008; Hogg & Bertrand, 2003; McGehee, 1999). Thus, to characterize the nicotinic acetylcholine receptor (nAChR) subtypes involved in the effects of withdrawal of chronic nicotine on trace fear conditioning, we attempted to precipitate withdrawal deficits by administering low-affinity or high-affinity nAChR antagonists to mice receiving chronic nicotine treatment. The precipitated withdrawal studies were followed by withdrawal studies in β2 nAChR subunit KO mice.
Materials and Methods
Subjects
Subjects of this study were 257, 8–12 week old male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) and 30 male β2 KO and littermate WT mice. β2 KO and WT mice, bred from heterozygous mice provided by Dr. Beaudet (Baylor College of Medicine), were originally backcrossed onto a C57BL/6J background, for details see (Xu et al., 1999). Mice were housed in groups of four in standard colony cages, maintained on a 12h light/dark cycle with lights on at 7:00 am, and allowed ad libitum access to food and water. All procedures were approved by the Temple University Animal Care and Use Committee.
Materials
Apparatus
Training of trace fear conditioning was conducted in conditioning chambers (model 307AW, Med Associates, St. Albans, VT) housed in sound attenuating boxes. The chamber floors were composed of 18 stainless steel bars connected to a shock generator and scrambler (Med-Associates) through which a 2 second, 0.57 mA footshock US was administered. Speakers attached to the right wall of each chamber provided a 30 second, 85 dB white noise CS. Ventilation fans, providing background noise (69 dB), were mounted on the right wall of each sound-attenuating box. Stimulus administration was controlled by a computer running Med-PC software (Med-Associates).
Testing of trace fear conditioning was conducted in conditioning chambers situated in sound attenuating boxes located in a different room than that used for training. The testing chambers were distinct from the training chambers and had white plastic floors, stainless steel sides, and Plexiglas panels for the front, rear and lid. Additionally, a novel olfactory cue (artificial vanilla extract) was applied to paper toweling placed below each of the chambers. Ventilation fans mounted on the right wall of the sound attenuating chambers provided background noise. A Grason-Stradler noise generator (model 901B, West Concord, MA) generated the white noise CS through 3-inch speakers mounted on the left side of each of the conditioning chambers.
Drugs
Nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MS) was administered both chronically and acutely. Chronic nicotine was administered through osmotic minipumps (model 1002; Alzet, Cupertino, CA) at a dose of 6.3 mg/kg/day (freebase), at a rate of 0.25 ul/h. Dosing of chronic nicotine administration was based on previous studies showing deficits in contextual fear conditioning following spontaneous (Davis & Gould, 2007; Davis et al., 2007) and precipitated nicotine withdrawal (Portugal et al., 2008). Acute nicotine was administered at 0.09 mg/kg ip (freebase) 5 minutes prior to training and testing of trace fear conditioning. Dosing of acute nicotine was based on studies showing enhancement of both trace (Davis & Gould, 2007; Gould et al., 2004) and contextual fear conditioning by acute nicotine (Davis et al., 2005; Gould & Lewis, 2005; Gould & Lommock, 2003; Gould & Stephen Higgins, 2003; Gould & Wehner, 1999; Gould, 2003; for review see Gould, 2006) and studies showing that 10 minutes after ip administration this dose produces plasma nicotine levels similar to those produced by the chronic nicotine dose, which are also comparable to those reported in smokers (Davis et al., 2005).
Dihydro-Beta-erythroidine (DHβE, Sigma-Aldrich), a high-affinity nAChR antagonist, was administered subcutaneously at 3 mg/kg, 25 minutes prior to training and/or testing of trace fear conditioning. Methyllycaconitine (MLA, Sigma-Aldrich), a low-affinity nAChR antagonist, was administered subcutaneously at 1.5, 3, and 9 mg/kg, 25 minutes prior to training and testing of trace fear conditioning. Dosing for DHβE and MLA was based on previous studies (Davis & Gould, 2006; Portugal et al., 2008). All drugs were dissolved in physiological saline and acute administrations were given at a volume of 10 ml/kg.
Procedure
Surgical
On day 1, osmotic minipumps were implanted subcutaneously via an inter-scapular incision, as described in (Davis et al., 2005). Incisions were closed with wound clips. In chronic nicotine and saline treated mice, pumps were left in through training and testing, which occurred on days 13 & 14, respectively. In chronic nicotine and saline withdrawn mice, pumps were removed on day 12, 24 hours before training. Mice were anesthetized with isoflurane (Abbott Laboratories, North Chicago, IL) and surgical procedures were conducted under sterile conditions.
Behavioral
Behavioral procedures were based on previous studies from our lab (Davis & Gould, 2007; Gould et al., 2004). Acute drugs were administered 5 (nicotine) or 25 (MLA & DHβE) minutes prior to the start of training and testing. All training or testing only drug injections were matched with saline injections on the opposite day. Training of trace fear conditioning was conducted during a single 16 minute training session wherein the mice were presented 5 CS-US pairings separated by a variable inter-trial-interval (90–120 seconds). CS-US parings consisted of a 30 second, 85 dB white-noise CS, followed by a 30 second trace interval, after which a 2 second 0.57 mA footshock US was presented. The training session began with the activation of the house light and terminated 30 seconds following the last US presentation. During training, baseline freezing (the first 120 seconds in the chamber) and freezing during the five trace-intervals was recorded. Acquisition of the trace-CS-US association during training was quantified by observing freezing during the 30 second trace-interval, resulting in 3 observations per CS-US presentation, and a total of 15 observations per session; this measure is based on Rogers and colleagues (2006). To ensure sufficient statistical power, for analysis each subject’s trace-interval freezing was grouped into a single bin. Twenty-four hours following training, mice were tested for cued freezing. First, mice were placed in the testing chambers and observed three minutes for freezing to an altered context, then the CS was presented and mice were observed three minutes for cued freezing.
During both training and testing, mice were observed for freezing with a time sampling procedure wherein one second observations were taken at ten second intervals (Gould & Wehner, 1999). Freezing was defined as a lack of movement excepting respiration (Blanchard & Blanchard, 1969).
Experimental Design
To compare the effects of acute nicotine, chronic nicotine, and withdrawal from chronic nicotine on trace fear conditioning, we administered acute saline or nicotine, chronic saline or nicotine, or withdrew chronic saline or nicotine from C57BL/6 mice (n = 8–11). To determine if low-affinity nAChRs are involved in nicotine withdrawal effects on trace conditioning we attempted to precipitate withdrawal effects in chronic nicotine-treated mice with the low-affinity nAChR antagonist MLA (Alkondon et al., 1992). First, to rule out general drug effects, we administered MLA at a range of doses to mice treated with chronic saline (n = 8–9). Then to determine if MLA could precipitate nicotine withdrawal-induced deficits, we administered MLA across the same dose range to mice treated with chronic nicotine (n = 12–14), and compared conditioning to a chronic saline treated control group. To determine if high-affinity nAChRs were involved in withdrawal deficits in trace conditioning, we attempted to precipitate deficits with the high-affinity antagonist DHβE (Williams & Robinson, 1984). First, to rule out general effects of DHβE on trace conditioning, we administered DHβE (3mg/kg) at training, testing, or both training and testing to chronic saline treated mice (n = 9–12). Then, we administered DHβE at training, testing, or both training and testing to chronic nicotine treated mice (n = 9–14) and compared these groups to a chronic saline treated group. By administering DHβE at either training or testing we were able to determine if withdrawal affected processes underlying acquisition or retrieval of trace conditioning, administration at both training and testing was included as a control for state-dependent effects. DHβE precipitated deficits in chronic nicotine treated mice. However, DHβE antagonizes a number of high-affinity nAChR subtypes, such as α4β2, α3β4, α3β2, and α4β4 receptors (Harvey & Luetje, 1996; Williams & Robinson, 1984). Thus, to determine if the β2 nAChR subunit was involved in nicotine withdrawal effects on trace fear conditioning, we withdrew chronic nicotine or chronic saline from β2 nAChR subunit KO mice and WT littermates (n = 7–8).
Analysis
Data were analyzed with either a 1-way or 2-way ANOVA. Homogeneity of variance was tested with the Levene statistic (Cohen, 2001). Tukey post hoc tests were conducted on data sets meeting the homogeneity of variance assumption. Games-Howell post hoc tests were conducted on data sets not meeting the homogeneity of variance assumption (Maxwell & Delaney, 2003). Differences between groups meeting p < 0.05 are reported as significant. Analyses were conducted with SPSS 13 (SPSS Inc. Chicago, IL).
Results
Effects of Acute Nicotine, Chronic Nicotine, and Withdrawal from Chronic Nicotine on Trace Conditioning
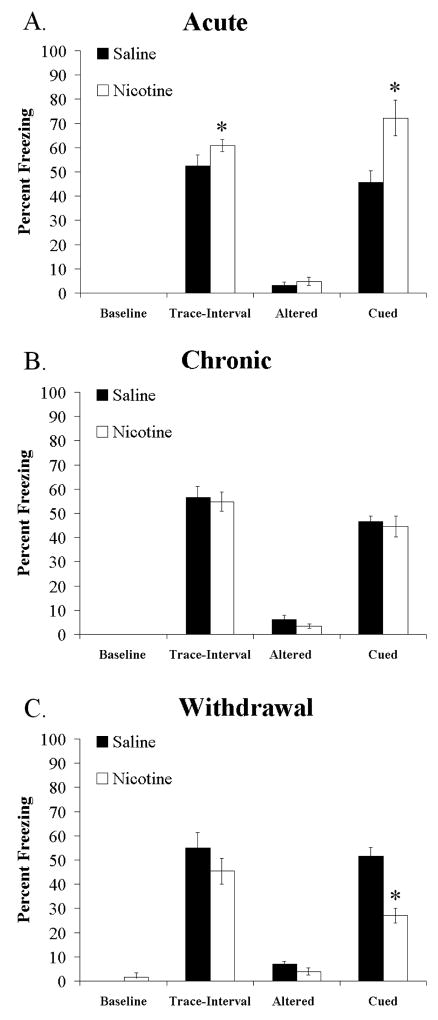
The effects of acute nicotine (Figure 1A), chronic nicotine (Figure 1B), and withdrawal from chronic nicotine (Figure 1C) on trace fear conditioning were examined with a 2-way ANOVA (Drug × Administration). The drug conditions were either saline or nicotine, and the administration conditions were either acute, chronic or withdrawal of chronic. There was no effect of drug or administration and no interaction on baseline freezing or freezing to an altered context. However, there was a significant effect of administration [F (2,50) = 9.682, p < 0.05], and a significant drug administration interaction [F (2,50) = 3.657, p < 0.05] on freezing during the trace-interval at training. Planned comparisons, between drug conditions in the different administration conditions showed that acute nicotine enhanced freezing compared to acute saline during the trace-interval, while chronic nicotine and chronic saline did not differ, and withdrawal of chronic saline and withdrawal of chronic nicotine were not significantly different. Additionally, there was a significant effect of administration [F (5,51) = 11.048, p < 0.05] and a significant interaction between drug and administration [F (5,51) = 16.927, p < 0.05] on cued freezing at testing. Tukey’s post hoc analysis revealed that acute nicotine treated mice froze significantly more to the CS presentation than all other groups, and that chronic-nicotine withdrawn mice froze significantly less to the CS than all other groups (Figure 1A, C). Thus, during training, acute nicotine enhanced freezing during the trace interval, while chronic nicotine and withdrawal from chronic nicotine had no significant effect; and at recall 24 hours later, mice treated with acute nicotine had enhanced trace fear conditioning, chronic nicotine had no effect, and withdrawal of the same dose of chronic nicotine produced deficits in trace conditioning.
Figure 1.
Effects of acute nicotine, chronic nicotine, and withdrawal of chronic nicotine on trace fear conditioning. Acute nicotine (0.09 mg/kg) enhances trace-interval and cued freezing, but has no effect on baseline or altered freezing (A). Chronic nicotine (6.3 mg/kg/day) has no effect on baseline, trace-interval, altered, or cued freezing (B). Withdrawal of chronic nicotine (6.3 mg/kg/day, 24 hours prior to training) produces deficits in cued freezing, but has no effect on baseline, trace-interval, or altered freezing (C). Significant difference (p < 0.05) from saline treated groups denoted with (*), data are reported as mean ± standard error of the mean.
Effects of MLA on Trace Fear Conditioning in Chronic Nicotine and Chronic Saline treated Mice
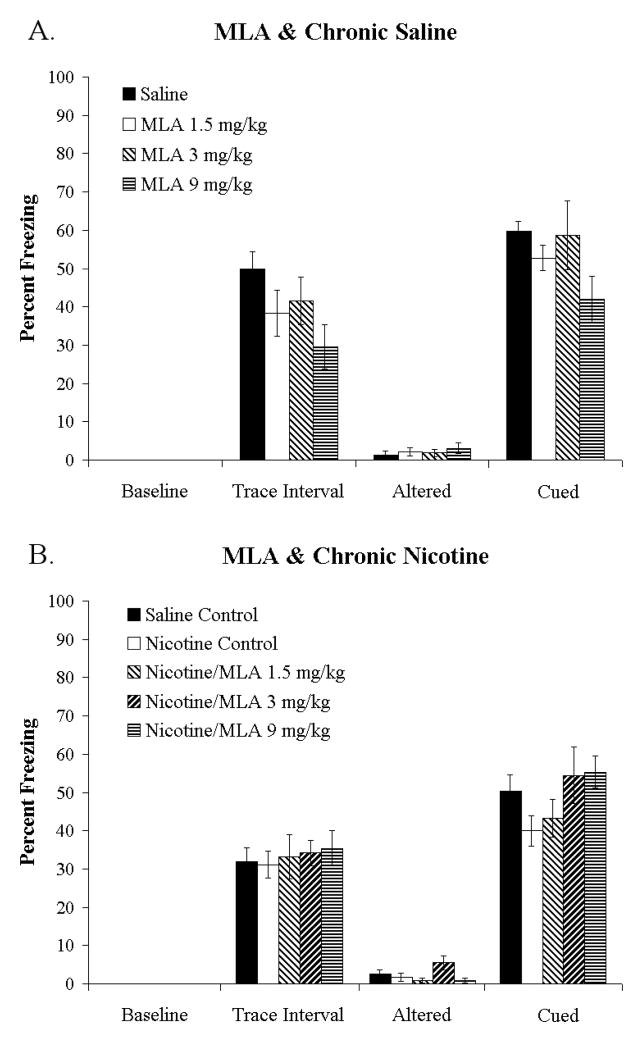
To determine if α7 nAChRs are involved in the effects of withdrawal of chronic nicotine on trace conditioning, we attempted to precipitate withdrawal deficits in chronic nicotine treated animals with the low-affinity nAChR antagonist MLA. First, to rule out effects of MLA on trace fear conditioning, MLA was administered to chronic saline treated mice. A 1-way ANOVA showed no significant effects of 0, 1.5, 3, or 9 mg/kg MLA on baseline, trace-interval, altered, or cued freezing (Figure 2A). Second, to determine if MLA precipitated withdrawal deficits in trace fear conditioning, the same doses were administered to chronic nicotine treated mice, with a chronic saline treated group included for comparison. A 1-way ANOVA showed no significant effects of MLA on baseline, trace-interval, or cued freezing but a significant effect on altered freezing [F (4, 57= 2.619, p < 0.05], however, post hoc analysis revealed no significant differences (Figure 2B). Together these findings suggest that low-affinity nAChRs are not critically involved in the effects of chronic nicotine withdrawal on trace fear conditioning. Somatic nicotine withdrawal symptoms have been precipitated by MLA at 1, 3, and 7.5 mg/kg in mice treated chronically with 24 mg/kg/day of nicotine for 14 days (Damaj et al., 2003), thus our results are not due to use of an ineffective dose of MLA.
Figure 2.
Effects of MLA on trace fear conditioning in chronic saline and chronic nicotine treated mice. MLA (1.5, 3, 9 mg/kg) did not alter baseline, trace-interval, altered, or cued freezing in chronic saline (A) or chronic nicotine (B) treated mice. Thus, the low-affinity nAChR antagonist MLA failed to precipitate nicotine withdrawal-induced deficits in trace fear conditioning. Data are reported as mean ± standard error of the mean.
Effects of DHβE on trace fear conditioning in chronic nicotine and chronic saline treated mice
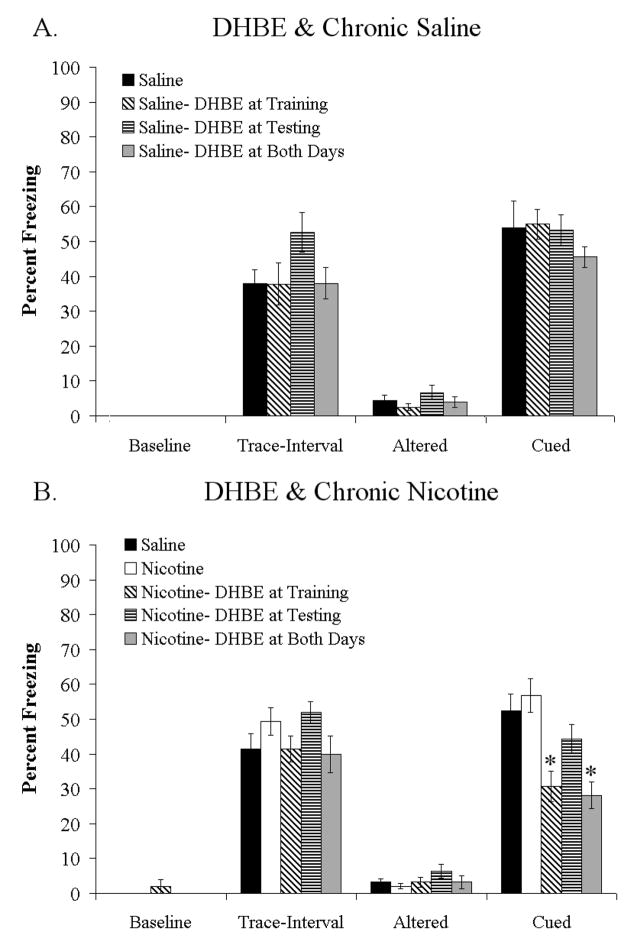
To determine if high-affinity nAChRs are involved in the effects of withdrawal of chronic nicotine on trace conditioning, we attempted to precipitate withdrawal deficits in chronic nicotine treated animals with the high-affinity nAChR antagonist DHβE. First, we administered DHβE to chronic saline treated mice during trace fear conditioning. A 1-way ANOVA showed no effect of DHβE (3 mg/kg) if administered at training, testing, or both training and testing on baseline, trace-interval, altered, or cued freezing (Figure 3A). Second, we administered DHβE (3 mg/kg) to chronic nicotine treated mice prior to training, testing, or both training and testing of trace fear conditioning, with a chronic saline treated group included for comparison. A 1-way ANOVA showed a significant effect of DHβE on cued freezing [F (3,38) = 10.162, p < 0.05], but not on baseline, trace-interval, or altered freezing (Figure 3B). Post-hoc analysis showed that chronic nicotine treated mice administered DHβE at training or at both training and testing showed deficits in trace conditioning compared to chronic saline treated controls and chronic nicotine treated mice that did not receive DHβE. Mice treated with DHβE only at testing did not differ from saline treated controls. Thus, DHβE precipitated deficits in trace conditioning in chronic nicotine treated mice, but had no effect on trace conditioning in chronic saline treated mice. Additionally, DHβE precipitated deficits if administered prior to training or prior to both training and testing of trace conditioning. These results suggest that high-affinity nAChRs may be involved in processes underlying the withdrawal deficits, and that antagonism of these receptors during chronic nicotine administration produces deficits in processes related to acquisition and/or consolidation of trace conditioning.
Figure 3.
Effects of DHβE on trace fear conditioning in chronic saline and chronic nicotine treated mice. The high-affinity nAChR antagonist DHβE (3 mg/kg) had no effect on baseline, trace-interval, altered, or trace cued freezing in chronic saline treated mice if administered at training, testing, or both training and testing (A). However, in chronic nicotine treated mice DHβE (3 mg/kg) precipitated deficits in cued freezing if administered prior to training or prior to both training and testing, but had no effect on baseline, trace-interval, or altered freezing (B). Significant difference (p < 0.05) from saline treated groups denoted with (*), data are reported as mean ± standard error of the mean.
Effects of spontaneous nicotine withdrawal in β2 nAChR subunit KO mice
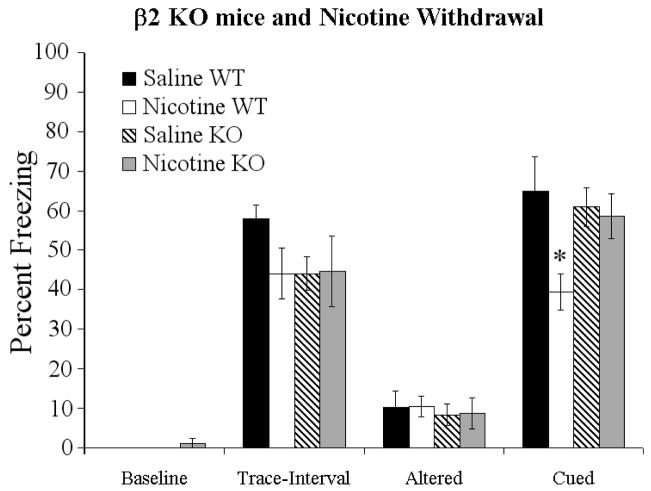
To determine if β2 subunit containing nAChRs were involved in chronic nicotine withdrawal-induced deficits in trace fear conditioning, we withdrew either chronic saline or chronic nicotine from β2 subunit KO mice and from WT mice. Trace fear conditioning data were analyzed with a two-way ANOVA, comparing effects of genotype and drug condition. There were no main effects or interactions on measures of baseline, trace-interval, or altered freezing, and there was no main effect of drug or genotype on trace conditioning. However, there was a significant drug × genotype interaction on trace conditioning [F (3,26) = 4.24, p < 0.05] (Figure 4). Post-hoc analysis showed that nicotine withdrawal produced deficits in cued freezing in WT mice but did not produce deficits in KO mice. Thus, the β2 nAChR subunit is critical for the development of nicotine withdrawal deficits in trace fear conditioning.
Figure 4.
Nicotine withdrawal in β2 KO mice. Withdrawal of chronic nicotine (6.3 mg/kg/day, 24 hours prior to training) produced deficits in cued freezing in WT mice, but did not affect baseline, trace-interval, or altered freezing. However, in β2 nAChR subunit KO mice, withdrawal of chronic nicotine had no effect on cued freezing, baseline freezing, or altered freezing. Significant difference (p < 0.05) from saline treated groups denoted with (*) data are reported as mean ± standard error of the mean.
Discussion
The primary findings of this study are threefold. First, acute nicotine, chronic nicotine, and withdrawal of chronic nicotine, at doses that produce matched plasma nicotine levels (Davis et al., 2005), have different effects on trace fear conditioning assessed 24 hours after acquisition. Specifically, acute administration enhances trace conditioning; chronic administration has no effect, which suggests the development of tolerance; and withdrawal of chronic nicotine produces deficits in trace conditioning. Importantly, the doses of nicotine used produce plasma nicotine concentrations within the range reported in smokers (Benowitz, 1988; Davis et al., 2005; Henningfield & Keenan, 1993); thus, these findings may model what occurs in smokers. Second, high-affinity, β2 subunit containing nAChRs are critical for the development of nicotine withdrawal-induced deficits in trace fear conditioning. The high-affinity nAChR (e.g., α4β2 nAChR) antagonist DHβE precipitated deficits in trace fear conditioning in chronic nicotine-treated mice, while the low-affinity nAChR (e.g., α7 nAChR) antagonist MLA did not. Further supporting a role for high-affinity nAChRs in withdrawal deficits in trace fear conditioning, β2 nAChR subunit KO mice did not show deficits in trace fear conditioning following withdrawal of chronic nicotine unlike WT mice. Third, nicotine withdrawal disrupts acquisition and/or consolidation of trace fear conditioning. DHβE precipitated deficits if administered at training or at both training and testing of trace fear conditioning but not if administered only at testing, which suggests that deficits occur in processes related to acquisition of trace fear conditioning, but not in processes related to retrieval of trace fear conditioning.
Effects of nicotine on trace fear conditioning could occur through enhancement of working memory, which is thought to support acquisition of the trace-CS-US relationship during trace conditioning (Carter et al., 2003; Han et al., 2003). Alternately, effects of nicotine on trace fear conditioning could reflect changes in processes associated with long-term memory formation. Enhancement of working memory may be reflected in increased freezing during the trace-interval at training, whereas enhancement of long-term memory effects would be evident as increases in cued freezing 24 hours post training. Our finding that freezing during the trace interval during training is enhanced by acute nicotine suggests that acute nicotine may enhance working memory, while chronic nicotine had no effects on trace-interval freezing suggesting that tolerance developed to the effects of nicotine during acquisition. Withdrawal of chronic nicotine did not produce significant deficits in trace-interval freezing at training. Thus, it may be that while acute nicotine enhances working memory in this task, chronic nicotine results in tolerance reflected by lack of enhancement.
Previous studies have found that in non-spatial working memory tasks acute nicotine enhances working memory but with chronic treatment tolerance develops to this effect and withdrawal produces deficits in working memory (Ernst et al., 2001; Jacobsen et al., 2004; Jacobsen et al., 2005; Jacobsen et al., 2006; Jacobsen et al., 2007; Kumari et al., 2003; Mendrek et al., 2006; Rusted & Trawley, 2006; Rycroft et al., 2006; Spinelli et al., 2006; Xu et al., 2006). The results of the current study are consistent with these findings. However, in tasks examining spatial working memory, as assessed with the radial arm maze, a different pattern emerges; both acute nicotine and chronic nicotine enhance performance and some studies report that the enhancement remains after removal of chronic nicotine (Levin et al., 1990; Levin et al., 1992; Levin et al., 1993; Levin et al., 1996; Levin et al., 1999). These findings suggest that the cognitive processes underlying spatial versus nonspatial tasks may involve neural substrates that are differentially affected by nicotine.
While both trace fear conditioning and radial arm maze depend on the hippocampus, effects of nicotine on these tasks may depend on different parts of the hippocampus. Effects of nicotine on radial arm maze depend upon nicotinic activity in the ventral hippocampus (Felix & Levin, 1997; Kim & Levin, 1996; Levin et al., 1999). Whereas trace fear conditioning depends largely on the medial prefrontal cortex (Blum et al., 2006; McLaughlin et al., 2002; Quinn et al., 2008; Runyan & Dash, 2004; Runyan et al., 2004) and dorsal hippocampus (Bangasser et al., 2006; Burman et al., 2006; Fendt et al., 2005; McEchron et al., 1998; Quinn et al., 2002; Quinn et al., 2008; Rogers et al., 2006; Trivedi & Coover, 2006; Yoon & Otto, 2007); although some studies have implicated the ventral hippocampus in trace fear conditioning as well (Rogers et al., 2006; Yoon & Otto, 2007). Thus, it is possible that nAChRs within the dorsal hippocampus mediate the effects of nicotine on trace fear conditioning. If this is the case then one might expect chronic nicotine treatment to differentially alter nAChRs in the dorsal hippocampus versus the ventral hippocampus. Indeed, the effects of chronic nicotine on nAChRs in these two brain areas are different. Abdulla and colleagues (1996) administered nicotine once a day for 10 days and found upregulation of nAChRs in the dorsal, but not in the ventral hippocampus. In addition, it is possible that the effects of nicotine on these tasks involve different nAChR subtypes.
The present study examined the nAChRs involved in the effects of withdrawal from chronic nicotine on trace fear conditioning and found that the high-affinity nicotinic antagonist DHβE precipitates nicotine withdrawal deficits in trace fear conditioning. This is consistent with a number of studies examining different symptoms of nicotine withdrawal. For instance, in chronic nicotine-treated rodents, DHβE precipitates increases in brain reward threshold, produces deficits in 5-choice serial reaction time task performance, and produces deficits in contextual fear conditioning (Epping-Jordan et al., 1998; Kenny & Markou, 2005; Portugal & Gould, 2008; Shoaib & Bizarro, 2005; Skjei & Markou, 2003; Stoker et al., 2008; Watkins et al., 2000). Additionally, the present finding that DHβE precipitates deficits in trace fear conditioning if administered at training but not at testing is consistent with a previous report that nicotine withdrawal disrupts acquisition, but not recall of contextual fear conditioning (Portugal et al., 2008). While some studies report that DHβE precipitates somatic nicotine-withdrawal symptoms (Damaj, 2003; Malin, 1998), other studies have failed to find this effect (Epping-Jordan, 1998; Stoker, 2008; Watkins, 2000a). However, DHβE is an antagonist for multiple high-affinity nAChR sub-types (Williams & Robinson, 1984; Harvey & Leutje, 1996; Harvey et al, 1996), and it seems that precipitation of somatic withdrawal symptoms occurs through DHβE’s action on β4 subunit-containing nAChRs and not on β2 subunit-containing nAChRs (Salas et al, 2007). Thus, it may be that the β4 subunit is involved in somatic withdrawal symptoms, while the β2 subunit is more involved in cognitive and affective withdrawal symptoms. The involvement of β2 containing nAChRs in nicotine withdrawal-associated cognitive deficits is supported by the present finding that spontaneous nicotine withdrawal-induced deficits in trace conditioning do not occur in β2 KO mice and by a recent report that β2 KO mice fail to show nicotine withdrawal-induced deficits in contextual fear conditioning (Portugal et al., 2008).
The role of high-affinity nAChRs in nicotine withdrawal deficits in trace fear conditioning contrasts with the effects of the low-affinity nicotinic antagonist MLA in chronic nicotine treated animals. While MLA can precipitate somatic withdrawal signs in chronic nicotine treated rodents (Damaj, 2003; Salas, 2007), MLA does not precipitate increases in brain reward threshold or deficits in 5-choice serial reaction time task performance (Markou, 2001; Shoaib, 2005). These findings are consistent with the present finding that MLA does not precipitate nicotine withdrawal deficits in trace fear conditioning. Collectively, these findings and those reviewed in the previous paragraph suggest that high-affinity β2 subunit-containing nAChRs are critical to the development of cognitive and affective nicotine withdrawal symptoms, while low-affinity nAChRs may be critical to the development of somatic withdrawal symptoms. Establishing the nAChR subtypes involved in withdrawal symptoms is only a first step in understanding nicotine withdrawal. The opponent-process theory may explain the behavioral effects of nicotine as administration transitions from acute to chronic and from chronic to withdrawal.
The opponent-process theory predicts that a biological system reacts to perturbation (A-process) by counteracting the perturbation (B-process) and maintaining homeostasis (Solomon & Corbit, 1973). In the case of drugs of abuse, the A-process typically has a positive/rewarding effect. This A-process pushes the system away from its inherent homeostatic set point. To maintain homeostasis, the system reacts with a B-process (opponent-process), which counteracts the initial effects of the drug (A-process), returning the system to equilibrium or baseline (see Figure 5). Thus, if a drug has a positive hedonic effect, the system upon which it acts should react in a negative way, minimizing the effects of the drug and maintaining homeostasis. These effects could occur through multiple mechanisms; for instance, drug receptors could be inactivated, reducing drug effects, or a competing system could increase activity resulting in a net decrease of drug effect. The opponent-process theory also describes the time course of these processes (Solomon & Corbit, 1974). The initial drug effect (A-process) occurs quickly, while the B-process (opponent-process) has a slower onset and typically a slower offset. In the absence of the A-process, the B-process (opponent-process) remains, resulting in negative symptoms. In other words, during chronic drug administration, tolerance develops through the onset of the B-process and discontinuation of drug administration produces withdrawal symptoms.
Figure 5.
Nicotine, cognition, and the opponent-process theory. The opponent process theory explains the effects of nicotine on cognitive processes. Deflection from baseline reflect either an improvement (+) in cognition or a deficit (−). Acute nicotine enhances learning (A-process), but during chronic nicotine treatment, adaptation occurs resulting in an opponent-process (B-process) that negates the enhancing effects of nicotine on cognition. Withdrawal of chronic nicotine treatment removes the A-process, leaving the opponent-process and revealing cognitive deficits.
The effects of nicotine on cognition and affect fit well with the opponent-process theory. This statement holds true for trace fear conditioning, various working memory dependent tasks in humans (Ernst et al., 2001; Jacobsen et al., 2004; Jacobsen et al., 2005; Jacobsen et al., 2006; Jacobsen et al., 2007; Kumari et al., 2003; Mendrek et al., 2006; Xu et al., 2005; Xu et al., 2006), contextual fear conditioning in mice (Davis et al., 2005) and for nicotine’s effects on brain reward threshold (Epping-Jordan et al., 1998; Huston-Lyons & Kornetsky, 1992; Johnson et al., 2008; Stoker et al., 2008; Watkins et al., 2000). As demonstrated in the present study, for example, acute nicotine administration enhances trace fear conditioning; chronic administration leads to the development of tolerance, suggesting an opponent process minimizes the cognitive enhancing effects of nicotine; and withdrawal of chronic nicotine removes the cognitive enhancing effects of nicotine but not the opponent-process, revealing a deficit. Further, the fact that DHβE precipitates deficits in trace conditioning and the fact that β2 KO mice do not display withdrawal deficits suggest that high-affinity β2-containing nAChRs are critically involved in the cognitive deficits associated with nicotine withdrawal. Since the acute, enhancing effects of nicotine are also mediated by these receptors (Davis & Gould, 2007), it seems likely that β2-containing nAChRs mediate the A-process and in chronic nicotine treated mice DHβE blocks the A-process, revealing the B-process. In β2 KO mice, there is no A-process and therefore no B-process develops with chronic nicotine treatment. The opponent-process theory has been applied to habitual behavior associated with nicotine use (Solomon & Corbit, 1973; Ternes, 1977), psychostimulant drugs (Koob et al., 1997), opiates (Koob et al., 1989), and the rewarding effects of nicotine (Gutkin et al., 2006), but to our knowledge this is the first application of opponent-process theory to the cognitive effects of nicotine. A better understanding of the opponent-process (B-process) responsible for the withdrawal effects of nicotine on cognition will aid in the development of new smoking cessation therapies.
Acknowledgments
The authors would like to acknowledge grant support from the National Institute on Drug Abuse (DA017949 TG) and the National Cancer Institute and National Institute on Drug Abuse (P5084718 PI: Caryn Lerman Ph.D); additionally, Jonathan D. Raybuck was supported by a NIH/NIDA training grant (T32DA07237).
Abbreviations
- nAChR
Nicotinic Acetylcholine Receptor
- MLA
Methyllycaconitine
- DHβE
Dihydro-beta-erythroidine
- KO
Knockout
- WT
Wildtype
- US
Unconditioned Stimulus
- CS
Conditioned Stimulus
- ANOVA
Analysis of Variance
References
- Abdulla FA, Bradbury E, Calaminici MR, Lippiello PM, Wonnacott S, Gray JA, Sinden JD. Relationship between up-regulation of nicotine binding sites in rat brain and delayed cognitive enhancement observed after chronic or acute nicotinic receptor stimulation. Psychopharmacology. 1996;124:323–331. doi: 10.1007/BF02247437. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Molecular pharmacology. 1992;41:802–808. [PubMed] [Google Scholar]
- Bangasser DA, Waxler DE, Santollo J, Shors TJ. Trace conditioning and the hippocampus: the importance of contiguity. The Journal of neuroscience. 2006;26:8702–8706. doi: 10.1523/JNEUROSCI.1742-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. The New England journal of medicine. 1988;319:1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- Blake J, Smith A. Effects of Smoking and Smoking Deprivation on the Articulatory Loop of Working Memory. Human Psychopharmacology. 1997;12:250–264. [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of comparative and physiological psychology. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17:341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- Brega AG, Grigsby J, Kooken R, Hamman RF, Baxter J. The impact of executive cognitive functioning on rates of smoking cessation in the San Luis Valley Health and Aging Study. Age and ageing. 2008;37:521–525. doi: 10.1093/ageing/afn121. [DOI] [PubMed] [Google Scholar]
- Burman MA, Starr MJ, Gewirtz JC. Dissociable effects of hippocampus lesions on expression of fear and trace fear conditioning memories in rats. Hippocampus. 2006;16:103–113. doi: 10.1002/hipo.20137. [DOI] [PubMed] [Google Scholar]
- Büchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. The Journal of neuroscience. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC CFDCAP. Annual smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 1997–2001. MMWR Morbidity and mortality weekly report. 2005;54:625–628. [PubMed] [Google Scholar]
- Carter RM, Hofstotter C, Tsuchiya N, Koch C. Working memory and fear conditioning. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1399–1404. doi: 10.1073/pnas.0334049100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J, Taly A. Nicotinic receptors, allosteric proteins and medicine. Trends in molecular medicine. 2008;14:93–102. doi: 10.1016/j.molmed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Cohen BH. Explaining Psychological Stastistics. New York: Wiley; 2001. [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. The Journal of pharmacology and experimental therapeutics. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology. 2006;184:345–352. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Atomoxetine reverses nicotine withdrawal-associated deficits in contextual fear conditioning. Neuropsychopharmacology. 2007;32:2011–2019. doi: 10.1038/sj.npp.1301315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology. 2007;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. The Journal of neuroscience. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. The Journal of neuroscience. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE, London ED. Effect of nicotine on brain activation during performance of a working memory task. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4728–4733. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix R, Levin ED. Nicotinic antagonist administration into the ventral hippocampus and spatial working memory in rats. Neuroscience. 1997;81:1009–1017. doi: 10.1016/s0306-4522(97)00224-8. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS, Koch M. Lesions of the dorsal hippocampus block trace fear conditioned potentiation of startle. Behavioral neuroscience. 2005;119:834–838. doi: 10.1037/0735-7044.119.3.834. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine produces a within-subject enhancement of contextual fear conditioning in C57BL/6 mice independent of sex. Integrative physiological and behavioral science. 2003;38:124–132. doi: 10.1007/BF02688830. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Molecular neurobiology. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Lewis MC. Coantagonism of glutamate receptors and nicotinic acetylcholinergic receptors disrupts fear conditioning and latent inhibition of fear conditioning. Learning & memory. 2005;12:389–398. doi: 10.1101/lm.89105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behavioral neuroscience. 2003;117:1276–1282. doi: 10.1037/0735-7044.117.6.1276. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Stephen Higgins J. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiology of learning and memory. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behavioural brain research. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Feiro O, Moore D. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behavioural brain research. 2004;155:167–173. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Gutkin BS, Dehaene S, Changeux J. A neurocomputational hypothesis for nicotine addiction. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1106–1111. doi: 10.1073/pnas.0510220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO, Weike AI. The neuropsychology of fear learning and fear regulation. International journal of psychophysiology. 2005;57:5–14. doi: 10.1016/j.ijpsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Han CJ, O’Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R, Koch C, Anderson DJ. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13087–13092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Luetje CW. Determinants of competitive antagonist sensitivity on neuronal nicotinic receptor beta subunits. The Journal of neuroscience. 1996;16:3798–3806. doi: 10.1523/JNEUROSCI.16-12-03798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. Journal of consulting and clinical psychology. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Bertrand D. Regulating the regulators: the role of nicotinic acetylcholine receptors in human epilepsy. Drug news & perspectives. 2003;16:261–266. doi: 10.1358/dnp.2003.16.5.829313. [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacology, biochemistry, and behavior. 1992;41:755–759. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, D’Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biological psychiatry. 2004;55:850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology. 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Mencl WE, Gelernter J. C957T polymorphism of the dopamine D2 receptor gene modulates the effect of nicotine on working memory performance and cortical processing efficiency. Psychopharmacology. 2006;188:530–540. doi: 10.1007/s00213-006-0469-1. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Hollander JA, Kenny PJ. Decreased brain reward function during nicotine withdrawal in C57BL6 mice: Evidence from intracranial self-stimulation (ICSS) studies. Pharmacology, biochemistry, and behavior. 2008;90:409–415. doi: 10.1016/j.pbb.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. The Journal of neuroscience. 2005;25:6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behavioral neuroscience. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Kim JS, Levin ED. Nicotinic, muscarinic and dopaminergic actions in the ventral hippocampus and the nucleus accumbens: effects on spatial working memory in rats. Brain research. 1996;725:231–240. doi: 10.1016/0006-8993(96)00213-2. [DOI] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. The Journal of neuroscience. 2004;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of awareness in delay and trace fear conditioning in humans. Cognitive, affective & behavioral neuroscience. 2006;6:157–162. doi: 10.3758/cabn.6.2.157. [DOI] [PubMed] [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacology, biochemistry, and behavior. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neuroscience and biobehavioral reviews. 1989;13:135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, ffytche DH, Mitterschiffthaler MT, Das M, Zachariah E, Vythelingum GN, Williams SCR, Simmons A, Sharma T. Cognitive effects of nicotine in humans: an fMRI study. NeuroImage. 2003;19:1002–1013. doi: 10.1016/s1053-8119(03)00110-1. [DOI] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Persistence of chronic nicotine-induced cognitive facilitation. Behavioral and neural biology. 1992;58:152–158. doi: 10.1016/0163-1047(92)90399-o. [DOI] [PubMed] [Google Scholar]
- Levin ED, Christopher NC, Weaver T, Moore J, Brucato F. Ventral hippocampal ibotenic acid lesions block chronic nicotine-induced spatial working memory improvement in rats. Brain research. Cognitive brain research. 1999;7:405–410. doi: 10.1016/s0926-6410(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Levin ED, Kim P, Meray R. Chronic nicotine working and reference memory effects in the 16-arm radial maze: interactions with D1 agonist and antagonist drugs. Psychopharmacology. 1996;127:25–30. doi: 10.1007/BF02805971. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lee C, Rose JE, Reyes A, Ellison G, Jarvik M, Gritz E. Chronic nicotine and withdrawal effects on radial-arm maze performance in rats. Behavioral and neural biology. 1990;53:269–276. doi: 10.1016/0163-1047(90)90509-5. [DOI] [PubMed] [Google Scholar]
- Levin E, Briggs S, Christopher N, Rose J. Chronic nicotinic stimulation and blockade effects on working memory. Behavioural pharmacology. 1993;4:179–182. [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behavioral neuroscience. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. Designing Experiments and Analyzing Data: A Model Comparison Perspective. Mahwah, NJ: Erlbaum; 2003. [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McGehee DS. Molecular diversity of neuronal nicotinic acetylcholine receptors. Annals of the New York Academy of Sciences. 1999;868:565–577. doi: 10.1111/j.1749-6632.1999.tb11330.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Skaggs H, Churchwell J, Powell DA. Medial prefrontal cortex and pavlovian conditioning: trace versus delay conditioning. Behavioral neuroscience. 2002;116:37–47. [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addictive behaviors. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misane I, Tovote P, Meyer M, Spiess J, Ogren SO, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15:418–426. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Bupropion dose-dependently reverses nicotine withdrawal deficits in contextual fear conditioning. Pharmacology, biochemistry, and behavior. 2007;88:179–187. doi: 10.1016/j.pbb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Genetic variability in nicotinic acetylcholine receptors and nicotine addiction: Converging evidence from human and animal research. Behavioural brain research. 2008;193:1–16. doi: 10.1016/j.bbr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. Beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiology of learning and memory. 2008;89:106–113. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learning & memory. 2008;15:368–372. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Rice DP. Economic costs of substance abuse, 1995. Proceedings of the Association of American Physicians. 1999;111:119–125. doi: 10.1046/j.1525-1381.1999.09254.x. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Hunsaker MR, Kesner RP. Effects of ventral and dorsal CA1 subregional lesions on trace fear conditioning. Neurobiology of learning and memory. 2006;86:72–81. doi: 10.1016/j.nlm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Dash PK. Intra-medial prefrontal administration of SCH-23390 attenuates ERK phosphorylation and long-term memory for trace fear conditioning in rats. Neurobiology of learning and memory. 2004;82:65–70. doi: 10.1016/j.nlm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. The Journal of neuroscience. 2004;24:1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusted JM, Trawley S. Comparable effects of nicotine in smokers and nonsmokers on a prospective memory task. Neuropsychopharmacology. 2006;31:1545–1549. doi: 10.1038/sj.npp.1300965. [DOI] [PubMed] [Google Scholar]
- Rycroft N, Hutton SB, Rusted JM. The antisaccade task as an index of sustained goal activation in working memory: modulation by nicotine. Psychopharmacology. 2006;188:521–529. doi: 10.1007/s00213-006-0455-7. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, Jatlow PI, Wexler BE, George TP. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Archives of general psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Lerman C. Current and emerging pharmacotherapies for treating tobacco dependence. Expert opinion on emerging drugs. 2006;11:429–444. doi: 10.1517/14728214.11.3.429. [DOI] [PubMed] [Google Scholar]
- Seo D, Pang M, Shin M, Kim H, Choi J. Hippocampal NMDA receptors are necessary for auditory trace fear conditioning measured with conditioned hypoalgesia in rats. Behavioural brain research. 2008;192:264–268. doi: 10.1016/j.bbr.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Bizarro L. Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology. 2005;178:211–222. doi: 10.1007/s00213-004-2004-6. [DOI] [PubMed] [Google Scholar]
- Skjei KL, Markou A. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology. 2003;168:280–292. doi: 10.1007/s00213-003-1414-1. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. Journal of abnormal psychology. 1973;81:158–171. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychological review. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Ballard T, Feldon J, Higgins GA, Pryce CR. Enhancing effects of nicotine and impairing effects of scopolamine on distinct aspects of performance in computerized attention and working memory tasks in marmoset monkeys. Neuropharmacology. 2006;51:238–250. doi: 10.1016/j.neuropharm.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology. 2008;54:1223–1232. doi: 10.1016/j.neuropharm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternes JW. An opponent process theory of habitual behavior with special reference to smoking. NIDA research monograph. 1977:157–185. [PubMed] [Google Scholar]
- Trivedi MA, Coover GD. Neurotoxic lesions of the dorsal and ventral hippocampus impair acquisition and expression of trace-conditioned fear-potentiated startle in rats. Behavioural brain research. 2006;168:289–298. doi: 10.1016/j.bbr.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Villarreal JS, Barea-Rodriguez EJ. ERK phosphorylation is required for retention of trace fear memory. Neurobiology of learning and memory. 2006;85:44–57. doi: 10.1016/j.nlm.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Wanisch K, Tang J, Mederer A, Wotjak CT. Trace fear conditioning depends on NMDA receptor activation and protein synthesis within the dorsal hippocampus of mice. Behavioural brain research. 2005;157:63–69. doi: 10.1016/j.bbr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. The Journal of pharmacology and experimental therapeutics. 2000;292:1053–1064. [PubMed] [Google Scholar]
- Weike AI, Schupp HT, Hamm AO. Fear acquisition requires awareness in trace but not delay conditioning. Psychophysiology. 2007;44:170–180. doi: 10.1111/j.1469-8986.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- Weike AI, Schupp HT, Hamm AO. In dubio pro defensio: initial activation of conditioned fear is not cue specific. Behavioral neuroscience. 2008;122:685–696. doi: 10.1037/0735-7044.122.3.685. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Subregion-specific differences in hippocampal activity between Delay and Trace fear conditioning: an immunohistochemical analysis. Brain research. 2004;995:55–65. doi: 10.1016/j.brainres.2003.09.054. [DOI] [PubMed] [Google Scholar]
- Williams M, Robinson JL. Binding of the nicotinic cholinergic antagonist, dihydro-beta-erythroidine, to rat brain tissue. The Journal of neuroscience. 1984;4:2906–2911. doi: 10.1523/JNEUROSCI.04-12-02906.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, Ernst M, London ED. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biological psychiatry. 2005;58:143–150. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Simon S, Brody AL, Jarvik M, Rodriguez P, Ernst M, London ED. Effects of acute smoking on brain activity vary with abstinence in smokers performing the N-Back task: a preliminary study. Psychiatry research. 2006;148:103–109. doi: 10.1016/j.pscychresns.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, De Biasi M. Multiorgan autonomic dysfunction in mice lacking the beta2 and the beta4 subunits of neuronal nicotinic acetylcholine receptors. The Journal of neuroscience. 1999;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Otto T. Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiology of learning and memory. 2007;87:464–475. doi: 10.1016/j.nlm.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Yoon T, Okada J, Jung MW, Kim JJ. Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learning & memory. 2008;15:97–105. doi: 10.1101/lm.850808. [DOI] [PMC free article] [PubMed] [Google Scholar]