Abstract
The ability of bacterial pathogens to inhibit apoptosis in eukaryotic cells during infection is an emerging theme in the study of bacterial pathogenesis. Prevention of apoptosis provides a survival advantage because it enables the bacteria to replicate inside host cells. Bacterial pathogens have evolved several ways to prevent apoptosis by protecting the mitochondria and preventing cytochrome c release, by activating cell survival pathways, or by preventing caspase activation. This review summarizes the most recent work on bacterial anti-apoptotic strategies and suggests new research that is necessary to advance the field.
Bacterial pathogens and apoptosis
The ultimate goal of all pathogens is to establish a site, or a replicative niche, in the host where the pathogen can multiply. Bacterial pathogens possess unique traits that provide a survival advantage upon infection of the host. Some pathogens manipulate the immune response, whereas others attempt to avoid recognition. Some bacteria induce apoptosis in host cells. The ability of certain bacteria to prevent apoptosis has recently emerged as a new theme in pathogenesis.
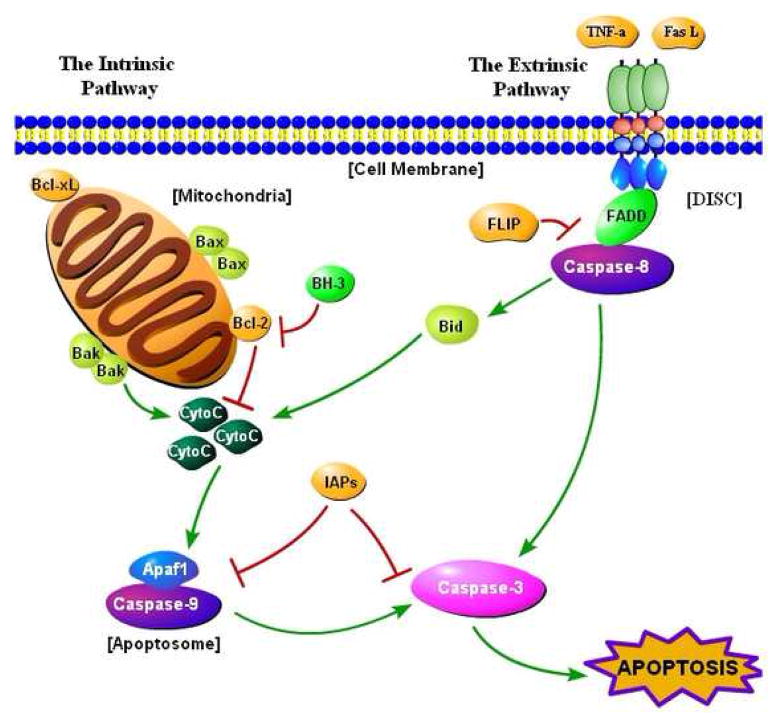
Apoptosis, or programmed cell death, is a natural phenomenon that occurs regularly in multicellular organisms [1,2]. Apoptosis is characterized by DNA fragmentation, chromatin condensation, cytoplasmic shrinkage, and cell death without lysis or damage to neighboring cells. Apoptosis, which is activated by intrinsic and extrinsic pathways (Figure 1), differs from other forms of cell death [3,4].
Figure 1.
The apoptosis pathway. Apoptosis is activated by intrinsic and extrinsic pathways. In the intrinsic pathway, certain apoptotic stimuli alter the normal status of the Bcl-2 family of proteins (Box 1), which leads to permeabilization of the mitochondrial membrane. Under normal circumstances, the pro-survival proteins of the Bcl-2 family protect the mitochondrial membrane. Once cytochrome c (CytoC) is released into the cytosol, it binds to the apoptosome [1], a complex of proteins made up of the apoptosis activating factor-1 (Apaf1) protein and the initiator caspase-9. A morphological change in the apoptosome results from cytochrome c binding, which causes caspase-9 to become activated. Caspase-9 activates the effector caspase-3, leading to apoptosis [1]. Caspase-3 is known as the ‘executioner caspase’, because it activates or cleaves various protein targets, which is detrimental to the cell and results in death [1]. Activation of caspase-9 and caspase-3 are normally inhibited by a family of proteins known as the inhibitor of apoptosis proteins (IAPs), and XIAP (X-linked IAP) is the most potent IAP [1]. In the extrinsic pathway, ligands such as Fas ligand (FasL) or tumor necrosis factor α (TNF-α) bind to death receptors on the membrane of the host cell. Trimerization of the death receptors follows, forming what is known as the death-inducing signaling complex (DISC), which includes the Fas-associated death domain (FADD) adaptor protein, the Flice-like inhibitory protein (FLIP), and procaspase-8 [2]. Caspase-8 is activated, which in turn directly activates caspase-3 [1]. In addition, caspase-8 activates the pro-apoptotic protein Bid, which stimulates the intrinsic pathway to enhance the apoptotic signal, because Bid activation eventually leads to cytochrome c release [1].
Bacteria, viruses [5] and parasites [6] can either induce or prevent apoptosis to augment infection. Many bacterial pathogens that cause apoptosis target immune cells such as macrophages [7] and neutrophils [8] because these cells would otherwise kill the pathogens [9,10]. Bacterial induction of apoptosis has been studied extensively, and several comprehensive reviews on pro-apoptotic bacterial pathogens are available [10,11]. However, new research has shown that many bacterial pathogens can in fact prevent apoptosis during infection. This research grew out of the observations that some organisms induce cell death in one cell type but not so in others (see below). It is becoming more evident that the ability to inhibit apoptosis during infection provides the bacterial pathogen with a survival tool in vivo. Given that there are now many examples of apoptosis inhibition during bacterial infection (Table 1, Figure 2), we review the various ways that bacterial pathogens inhibit apoptosis. Bacterial pathogens can be grouped into three classes based on the mechanisms employed to inhibit apoptosis. Here, we describe these three mechanisms and provide examples of bacterial pathogens that employ each method. The diversity of bacterial pathogens that inhibit apoptosis and the multiple mechanisms they have evolved underscore the importance of this trait to bacterial pathogenesis.
Table 1.
Classification of bacteria that inhibit apoptosis
| Pathogens grouped by class | Cell typea | Proposed or demonstrated mechanism | Refs |
|---|---|---|---|
| Protection of mitochondria | |||
| Chlamydia sp. | Hep2, HeLa | Inhibits and degrades pro-apoptotic proteins | [15–20] |
| Neisseria sp. | HeLa, UEC | Prevents cytochrome c release | [21,22,25–27,29] |
| Activation of cellular pathways | |||
| Salmonella enterica | HeLa, IEC-6 | Activates PI3/Akt pathway | [30] |
| Anaplasma phagocytophilum | Neutrophils | Activates p38 MAPK, ERK, PI3/Akt, NF-κB pathways | [32–34] |
| Ehrlichia chaffeensis | THP-1 | Activates NF-κB and upregulates pro-survival genes | [35] |
| Rickettsia rickettsii | HUVEC | Prevents cytochrome c release | [36,37] |
| Wolbachia | Neutrophils | Prevents caspase-3 activation | [38,39] |
| Bartonella sp. | Mono Mac 6, HUVEC | Activates NF-κB pathway, induces cIAP-1, cIAP-2 expression | [40–42] |
| Helicobacter pylori | MKN45 | Induces cIAP-2 expression through NF- κB activation | [53] |
| Porphyromonas gingivalis | GEC | Activates PI3/Akt pathway | [55] |
| Listeria monocytogenes | J774 | Activates PI3/Akt and NF-κB pathways | [56] |
| Interaction with caspases | |||
| Shigella flexneri | HeLa, T84 | Inhibits caspase-3 activation despite cytochrome c release | [44] |
| Legionella pneumophila | U937 | Activates NF-κB pathway and upregulates pro-survival genes | [46–48] |
| Further investigation required | |||
| Mycoplasma fermentans | U937 | Inhibits TNFα-induced apoptosis | [57,58] |
| Brucella suis | THP-1 | Upregulates pro-survival genes | [59] |
| Escherichia coli K1 | THP-1, RAW 264.7 | Upregulates pro-survival genes | [60] |
| Coxiella burnetii | HeLa, THP-1 | Prevents cytochrome c release | [61,62] |
HeLa, UEC, IEC-6, Hep2, T84 and GEC are epithelial cell lines. MKN45 is a gastric adenocarcinoma cell line. Mono Mac 6, THP-1 and U937 are monocytic cell lines. UVEC are primary human umbilical vein endothelial cells. J774 and RAW 264.7 are macrophage cell lines.
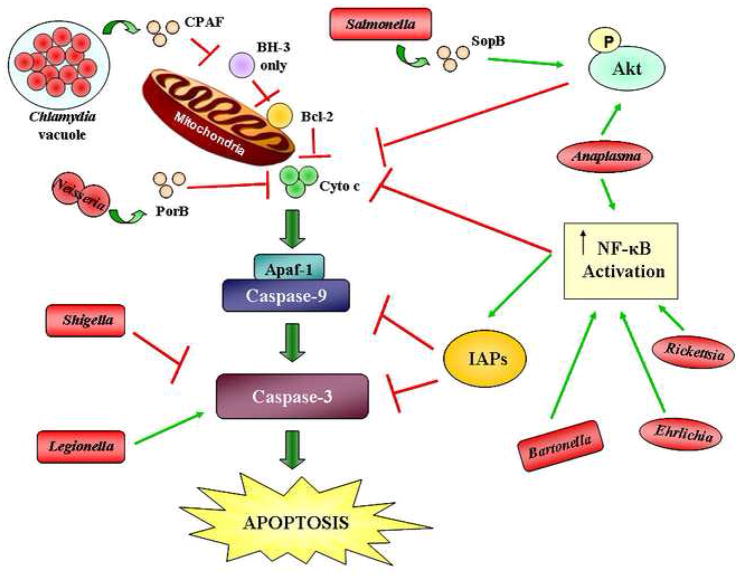
Figure 2.
Mechanisms by which bacterial pathogens inhibit apoptosis at different points along the apoptotic pathway. Chlamydia secretes the chlamydial proteasome-like activity factor (CPAF) to inhibit and degrade the pro-apoptotic proteins with one BH3 domain. These pro-apoptotic proteins inhibit the pro-survival Bcl-2 proteins upon activation. The outer membrane protein porin PorB of Neisseria meningitidis prevents cytochrome c (CytoC) release. Salmonella secretes the effector SopB through a type III secretion system, resulting in the activation of the phosphatidylinositol 3-kinase/Akt (PI3K/Akt) pathway. This pathway prevents cytochrome c release. Anaplasma also activates the PI3K/Akt pathway in addition to activating nuclear factor kappa B (NF-κB). NF-κB prevents the release of cytochrome c and activates the inhibitor of apoptosis proteins (IAPs). Bartonella, Ehrlichia, and Rickettsia activate NF-κB as well. Shigella inhibits caspase-3 activation. Legionella directly activates caspase-3 to enhance infection, but inhibits apoptosis through NF-κB. Red lines indicate inhibition in the pathway while green arrows indicate activation. The bacterial proteins that specifically participate in apoptosis inhibition are shown, where known. The shapes of the bacteria, all shown in red, represent their morphology. P, phosphate.
Protection of the mitochondria and prevention of cytochrome c release
One mechanism utilized by Chlamydia and Neisseria to prevent apoptosis is the use of a secreted product that prevents cytochrome c release. This mechanism has been studied extensively in Chlamydia [12–14]. Infected epithelial cells were exposed to either staurosporine (STS) or ultraviolet (UV) irradiation to determine if the bacteria can prevent apoptosis induced by these stimuli. Initially, it was discovered that Chlamydia trachomatis inhibits the pro-apoptotic host proteins Bax and Bak and prevents them from permeabilizing the mitochondrial membrane. Cytochrome c release and subsequent caspase activation are inhibited in infected cells [12]. Further research indicated that Chlamydia targets pro-apoptotic proteins with a BH3 domain (i.e. Bik, Puma and Bim) for degradation [15–17]. The chlamydial proteasome-like activity factor (CPAF), a protease that is secreted by C. trachomatis, was identified as the bacterial product required for the degradation of the pro-apoptotic proteins [18]. In addition, Chlamydia could also upregulate the inhibitor of apoptosis proteins (IAPs) when tumor necrosis factor α (TNF-α) was used as an inducer of the extrinsic pathway of apoptosis [19]. It is unknown if both CPAF and upregulation of the IAPs are required for the anti-apoptotic activity of Chlamydia, and this should be addressed. Moreover, protein interaction studies of CPAF and BH3 domain proteins might shed light on how Chlamydia specifically targets these host factors.
In addition to blocking apoptosis, Chlamydia-infected cells continue to undergo DNA synthesis and mitosis up to 40 h post-infection, which aids in establishing a persistent infection [20]. Preventing apoptosis would help ensure that the eukaryotic cell continues to divide in the presence of apoptotic signals from the host (see Concluding Remarks, below). Obligate intracellular pathogens like Chlamydia rely on the survival of the host cell. Therefore, it is important not only to inhibit apoptosis, but also to ensure that the cell maintains the division cycle given that cell cycle arrest often leads to apoptosis. It would be interesting to determine if Chlamydia still prevents apoptosis in non-dividing cells. Identification of bacterial proteins required for maintaining mitosis and evaluation of whether these proteins are required for or complement apoptosis inhibition should be performed to further our understanding of apoptosis inhibition and persistent infection.
Neisseria also inhibits apoptosis by preventing cytochrome c release [21,22]. Neisseria meningitidis uses its outer membrane protein porin PorB to prevent apoptosis by targeting it to the mitochondria, which results in inhibition of cytochrome c release. When Neisseria-infected HeLa cells are incubated with STS, mitochondrial integrity remains, there is no cytochrome c release, and a normal mitochondrial membrane potential is maintained [22]. In addition, when purified PorB is incubated with host cells for 24 h, PorB enters the cell and prevents STS-induced apoptosis. Purified PorB localizes to the mitochondrial membrane and PorB co-immunoprecipitates with the mitochondrial protein voltage-dependent anionic channel (VDAC), which is a component of the mitochondrial permeability transition pore (PT). The authors suggested that PorB interacts directly with the mitochondrial PT or indirectly with the PT by binding to VDAC, and that this interaction stabilizes the mitochondria in the presence of STS [21]. Since this publication, however, additional studies on the PT and the role of VDACs [23,24] suggest that VDACs might not be important for mitochondrial membrane permeabilization during cell death. Therefore, PorB localizes to some other protein in the PT and how PorB specifically interacts with the PT to prevent STS-induced apoptosis remains to be determined.
The PorB homolog in Neisseria gonorrhoeae, porin IB, also translocates to the mitochondria, but one group found that it causes apoptosis in Jurkat T cells and HeLa cells [25]. However, Massari et al. [21] speculated that differences in cell types or porin purification explain the discrepancies between the results. In addition, serum deprivation was used, which makes the cells more sensitive to stress and apoptotic stimuli. Massari et al. repeated the experiments with PorB and porin IB using both serum-deprived and normal experimental conditions, and found the same results as he described for PorB. They concluded that N. gonorrhoeae has the same ability to stabilize the mitochondria through porin IB as N. meningitidis does with PorB [21,22].
Other groups have found that porin IB might function differently to inhibit apoptosis. N. gonorrhoeae increases the expression of the host anti-apoptotic genes bfl-1, cIAP-2 and cox-2 through nuclear factor kappa B (NF-κB) activation [26,27]. Bfl-1 interacts with pro-apoptotic proteins such as Bid and Bax to inhibit cytochrome c release, cIAP-2 prevents caspase-3 activation, and Cox-2 activates the phosphatidylinositol 3-kinase/Akt pathway (PI3K/Akt) [26]. This pathway results in Akt phosphorylation, which in turn prevents pro-apoptotic proteins from permeabilizing the mitochondrial membrane [28]. NF-κB activation is required for the induced expression of the pro-survival genes, and an NF-κB activation inhibitor prevents upregulation of the genes [27]. Finally, apoptosis is also inhibited in N. gonorrhoeae-infected human fallopian tube epithelial cells when tumor necrosis factor α (TNF-α) is used to induce apoptosis [29], indicating that Neisseria can also inhibit the extrinsic pathway, and therefore could possess several mechanisms to inhibit apoptosis.
Future research will be needed to address the varying results between the two species of Neisseria. Perhaps N. meningitidis also requires NF-κB activation. It is also possible that despite the close relatedness of the two species, these organisms use quite different strategies to inhibit apoptosis. Identification of the eukaryotic proteins that bind to PorB and porin IB is required to determine if these porins function differently to inhibit apoptosis. Experiments in which the porins are switched between the two species of Neisseria could be performed. In any event, Neisseria and Chlamydia are prototypes of bacteria that inhibit apoptosis by preventing cytochrome c release through the secretion of a bacterial product.
Activation of cell survival pathways
The second mechanism by which bacteria inhibit apoptosis is through exploitation of the cell survival pathways naturally present in the host. Salmonella, Anaplasma, Ehrlichia, Rickettsia, Wolbachia and Bartonella are examples of bacteria with this ability. Salmonella enterica serovar Typhimurium inhibits camptothecin-induced apoptosis in HeLa and rat small intestine epithelial cells by activating the PI3K/Akt pathway [30]. This pathway prevents cytochrome c release, which inhibits activation of the caspase cascade. Akt phosphorylation and activation during infection occurs due to secretion of a type III secretion system (T3SS) effector, SopB. A ΔsopB mutant is unable to prevent camptothecin-induced apoptosis, and Akt is not activated in cells infected with this mutant [30]. Future studies on SopB should determine if this Salmonella protein functions like eukaryotic proteins that activate the PI3K/Akt pathway, for example, Cox-2. These studies will help to further the understanding of the role of this bacterial protein in apoptosis inhibition, and could enable identification of other bacterial proteins that function in a similar manner. Finally, it is interesting to note that Salmonella induces cell death in macrophages [31], which enables the bacteria to escape immune cells. Therefore, Salmonella infection of a eukaryotic cell leads to different outcomes depending on which cell type is infected.
Anaplasma phagocytophilum prevents apoptosis in neutrophils [32–34]. No inducer of apoptosis is required because neutrophils isolated from human donors spontaneously undergo apoptosis by 24 h in cell culture, and A. phagocytophilum infection of these neutrophils prevents the spontaneous apoptosis normally observed [32]. Protein and gene expression studies showed that the p38 mitogen-activated protein kinase (MAPK) pathway is activated by the bacteria during infection. p38 MAPK activation leads to the inhibition of caspase-3 activation, but other signaling pathways might also be involved [32]. Not only is p38 MAPK upregulated, but the extracellular signal-regulated kinase (ERK), PI3K/Akt and NF-κB pathways are also upregulated [33]. A bacterial product has not been identified for this upregulation of cell-survival pathways, but active infection is required because heat-killed bacteria do not prevent neutrophil apoptosis as efficiently as live bacteria [33]. Identifying a bacterial protein required for apoptosis inhibition could shed light on which of the above pathways are essential for apoptosis inhibition. In addition, A. phagocytophilum can inhibit the extrinsic pathway of apoptosis by preventing caspase-8 cleavage [34], and therefore ensures its survival by blocking both pathways of apoptosis.
Three other obligate intracellular pathogens inhibit apoptosis by activating cell survival pathways. First, Ehrlichia chaffeensis inhibits apoptosis in the human monocyte cell line THP1 through upregulation of NF-κB, as determined using microarray analysis. In addition, other pro-survival genes such as bcl-2 and other bcl-2-related genes are also induced during infection (Box 1) [35]. Further analysis is needed to verify the microarray data and to identify the E. chaffeensis proteins required for apoptosis inhibition. Next, the obligate intracellular pathogen Rickettsia rickettsii prevents apoptosis in endothelial cells in an NF-κB-dependent manner, which results in the upregulation of pro-survival proteins, the downregulation of pro-apoptotic proteins, and a lack of cytochrome c release and caspase activation [36,37]. It has not been determined exactly how infection leads to NF-κB activation, or what bacterial products are required to inhibit apoptosis. Future studies identifying the bacterial protein required for NF-κB activation will not only be important to understand the strategy used by R. rickettsii, but will enable comparisons with other bacterial pathogens that depend on NF-κB activation for the inhibition of apoptosis. Finally, Wolbachia is an endosymbiont of filarial nematode parasites, and inhibits apoptosis in these nematodes [38]. The presence of Wolbachia is important during parasitic infections in humans. Interestingly, the Wolbachia surface protein inhibits apoptosis in human neutrophils. Only the lack of caspase-3 activation was reported [39], and additional studies are clearly needed to determine how the surface protein is anti-apoptotic. The Wolbachia surface protein could serve as a means to identify the proteins required for apoptosis inhibition in Anaplasma and Ehrlichia through homology studies because these organisms are closely related.
Box 1. The Bcl-2 family of proteins
The Bcl-2 family of proteins is defined by the presence of at least one Bcl-2 homology (BH) domain. The pro-survival proteins (Bcl-2, Bcl-XL, Mcl-1, Bfl-1/A1, Bcl-W and Boo/Diva) comprise the Bcl-2 subfamily [63,64]. Bcl-2, Bcl-XL and Bcl-W have a hydrophobic domain on the carboxyl terminus that targets the proteins to the cytoplasmic face of the outer mitochondrial membrane, the endoplasmic reticulum and the nuclear envelope. Bcl-2 is an integral membrane protein whereas Bcl-XL and Bcl-W become tightly associated with these membranes only after a cytotoxic signal [63]. The pro-apoptotic proteins are broken down into two subfamilies: the Bax subfamily in which the proteins have multiple BH domains (Bax, Bak, Bok and Bcl-XS) and the BH3-only subfamily, which comprise proteins with a single BH3 domain (Bid, Bad, Bmf, Bim, Bik, Puma, Hrk and Noxa) [63,64]. Both subfamilies of pro-apoptotic proteins are required for apoptosis initiation. The BH3-only proteins are direct antagonists and act by binding to and inhibiting the pro-survival proteins in response to apoptotic signals. Once the BH3-only proteins neutralize Bcl-2, Bcl-XL and Bcl-W, the pro-apoptotic Bax and Bak proteins form heterodimers and homodimers within the mitochondrial membrane, which results in the release of cytochromec and other pro-apoptotic factors [63,64].
It is intriguing to note that not all obligate intracellular pathogens inhibit apoptosis in a similar manner. As mentioned above, Chlamydia utilizes a different strategy to prevent apoptosis during infection. One key difference might be because Chlamydia remains inside a vacuole during infection whereas other obligate intracellular pathogens, such as Rickettsia, replicate in the cytoplasm of the host cell. Perhaps future studies will determine if this difference correlates with the differences seen between apoptosis inhibition for these organisms.
Bartonella henselae activates NF-κB, leading to increased expression of cIAP-1 and cIAP-2 and inhibition of caspase-3 activation and apoptosis [40]. Mitochondrial integrity and the presence or absence of cytochrome c release have not been tested. The B. henselae outer membrane adhesin A (BadA) was identified as the bacterial product required for the inhibition of apoptosis [40]. However, B. henselae and Bartonella quintana also inhibit apoptosis in vascular endothelial cells through the BepA protein, which is secreted through the type IV secretion system [41]. Although infection activates NF-κB, BepA translocates to the plasma membrane and increases the cyclic adenosine monophosphate (cAMP) levels in the cell, which results in the anti-apoptosis activity. cAMP is known to increase the expression of cIAP-2, activate protein kinase A (PKA) to inhibit the pro-apoptotic Bad protein, and activate the ERK and p38 MAPK pathways [41]. It was not determined if cIAP-2, PKA, ERK or p38 MAPK were affected by the BepA-dependent increase in cAMP levels. The differences in results between the two studies might be attributed to differences in cell types used, but future studies should determine if one or both of the Bartonella proteins are required for protection. B. henselae and B. quintana cause human infections in which angioproliferative lesions can occur. Interestingly, Bartonella vinsonii, Bartonella elizabethae and Bartonella clarridgeiae are not associated with disease and do not inhibit apoptosis in endothelial cells in the presence of actinomycin D, a chemical inducer of apoptosis [42]. This observation further illustrates how bacterial pathogens have evolved to inhibit apoptosis to establish a replicative niche inside the host. Bartonella, along with Salmonella, Anaplasma, Ehrlichia, Wolbachia and Rickettsia, have found ways to inhibit apoptosis through the exploitation of the host cell survival pathways that normally function to block apoptosis. This strategy of apoptosis inhibition is an indirect but effective approach to prevent apoptosis during infection.
Interaction with cellular caspases
Unlike the two previous strategies, Shigella flexneri uses a novel tactic to inhibit apoptosis. S. flexneri inhibits apoptosis in epithelial cells but causes cell death in macrophages, which is a dichotomous trait it shares with Salmonella [43]. However, S. flexneri is able to prevent apoptosis in epithelial cells downstream of cytochrome c release [44]. Upon infection of HeLa cells and T84 cells, S. flexneri inhibits STS-induced apoptosis by preventing the activation of caspase-3. Cytochrome c release and caspase-9 activation occur in the presence of STS in infected HeLa cells; however, caspase-3 activation is prevented. When STS is not used, cytochrome c release is not detected, indicating that the bacteria do not damage the mitochondria upon normal infection. A ΔmxiE mutant of S. flexneri is unable to prevent STS-induced apoptosis in infected HeLa cells. MxiE is a transcriptional activator that induces the expression of 17 Shigella genes, whose gene products are secreted into the cytoplasm of the host cell through the T3SS [44]. The MxiE-regulated genes required to inhibit apoptosis remain to be identified. S. flexneri might block apoptosis by targeting the activated form of caspase-9 or the inactive form of caspase-3 to prevent caspase-3 activation. In addition, preliminary analysis with TRAIL (TNF-α-related apoptosis-inducing ligand), an inducer of the extrinsic pathway, has shown that S. flexneri is able to prevent apoptosis induced via this pathway (C. Faherty, unpublished). This suggests that S. flexneri inhibits both pathways of apoptosis by inhibiting caspase-3 activation. It has also been reported that the Shigella effector IpgD, which is homologous to the Salmonella effector SopB (see above), activates the PI3K/Akt pathway like Salmonella [45]. Although the activation of this pathway might have pro-survival affects upon infection, it is not the primary mechanism of apoptosis inhibition during Shigella infection. IpgD activates the PI3K/Akt pathway during the first hour of infection, whereas SopB induces a more sustained activation of the pathway for four hours [30,45]. Finally, a ΔipgD mutant is still able to prevent STS-induced apoptosis [39].
Legionella pneumophila seems to exploit the activation of caspase-3 during intracellular replication in macrophages while at the same time preventing apoptosis until the late stages of infection [46]. L. pneumophila utilizes the Dot/Icm type IV secretion system to activate caspase-3, which in turn enables evasion of the endosomal-lysosomal pathway through an unidentified mechanism [46]. L. pneumophila is then free to grow inside a replicative niche derived from the rough endoplasmic reticulum [46]. The caspase-3 activation is independent of the intrinsic or extrinsic pathways of apoptosis. During late stages of infection, L. pneumophila escapes into the cytoplasm of the host cell, and then apoptosis facilitates bacterial escape and egress [46]. Apoptosis inhibition during early infection despite caspase-3 activation is achieved through the activation of NF-κB, which is also dependent on the Dot/Icm system [47]. Furthermore, the bacterial effector SdhA, which is secreted by the Dot/Icm system, is required for apoptosis inhibition [48]. L. pneumophila provides a fascinating example of a bacterial pathogen that exploits caspase-3 activation for replication while at the same time inhibiting cell death, until the bacteria no longer need the cell.
Shigella and Legionella are the first examples of bacteria that affect caspase-3 activation. The parasite Toxoplasma gondii also inhibits apoptosis by targeting caspase-3 [49], and it will be interesting to see if S. flexneri uses the same strategy as T. gondii. The consequence of this form of protection is that the host cells are damaged when certain apoptotic stimuli are present, because cytochrome c release indicates that the mitochondria are no longer intact. Shigella is a facultative intracellular pathogen and, therefore, it might not require prolonged apoptosis inhibition for survival in vivo. This situation is different from obligate intracellular pathogens such as Chlamydia in that these organisms require longer apoptosis inhibition for their longer replication cycle inside the host cell. Nevertheless, the ability of pathogens to inhibit apoptosis at the point of caspase-3 activation seems to be ideal for protecting the host cells from both pathways of apoptosis.
Concluding remarks and future directions
An emerging theme in bacterial pathogenesis is the ability of bacteria to inhibit apoptosis in eukaryotic cells to establish a replicative niche inside the host. Bacterial pathogens employ at least three different mechanisms to inhibit apoptosis. Mitochondrial permeabilization and subsequent cytochrome c release are important events in apoptosis, and also serve as a means to differentiate the various mechanisms used to inhibit apoptosis. Bacterial pathogens can secrete proteins to protect the mitochondria, upregulate cell survival pathways to prevent cytochrome c release, or ignore this step and focus attention on the caspases to inhibit apoptosis. Related organisms such as A. phagocytophilum and E. chaffeensis use similar methods to inhibit apoptosis, whereas other related organisms such as N. meningitidis and N. gonorrhoeae utilize different mechanisms to inhibit apoptosis. Clearly, the method employed by each bacterial strain does not have to be the same, and each method probably represents the most efficient means of inhibiting apoptosis for that pathogen in that particular cell type.
Natural selection has a key role in determining how each pathogen inhibits apoptosis. As the host evolves to eliminate a bacterial infection, the pathogen must itself evolve to counteract this change to maintain the replicative niche inside the host. It would be constructive if future research could identify key factors that dictate the choice of strategy. Does it depend on which cell type the pathogen infects? Does it depend on how the pathogen enters that cell type? Does it depend on whether the bacteria replicate inside a vacuole or in the cytoplasm? Do homologous proteins in different organisms prevent apoptosis in the same manner? Do all obligate intracellular pathogens inhibit apoptosis? Answers to these questions will further our understanding of apoptosis inhibition as a facet of bacterial pathogenesis and could lead to novel therapeutics. For example, an inhibitor that targets CPAF in Chlamydia could enable the host cell to undergo apoptosis in response to infection and might halt persistent infection. This inhibitor could therefore complement antibiotic therapy. As new pathogens are discovered, the ability to inhibit apoptosis should be considered as a feature of pathogenicity of the organism. More importantly, known pathogens should also be re-examined, especially given that apoptosis inhibition was only recently reported for S. flexneri.
There are two recurrent themes in bacterial pathogens inhibiting apoptosis. First, many pathogens rely on NF-κB activation to inhibit apoptosis. The Gram-negative bacterial cell surface component lipopolysaccharide (LPS) activates the NF-κB pathway during infection [50]. Because NF-κB activation has many pro-survival effects on the host cell, as outlined here, activation of the NF-κB pathway by LPS might be a simple explanation of how most bacteria inhibit apoptosis. Binnicker et al. [27] addressed this aspect in their research with N. gonorrhoeae. When purified lipooligosaccharide (LOS) was applied to the surface of host cells, there was no increase in the expression of the pro-survival gene bfl-1. In addition, infection of host cells with an LOS mutant resulted in the same induced expression of bfl-1 as that seen in wild-type bacteria. The authors speculated that different bacterial products could promote the formation of different Rel/NF-κB complexes during activation, which would interact with different transcription factors and regulatory proteins to regulate the transcription of a completely distinct set of genes [27]. In addition, live extracellular L. pneumophila and formalin-killed bacteria do not trigger NF-κB activation, further suggesting that the anti-apoptotic phenotype induced by pathogens through NF-κB activation is not mediated by LPS [47]. Understanding how NF-κB activation affects the ability of a pathogen to prevent apoptosis needs to be further elucidated.
The second recurrent theme is the fact that most of these studies use immortalized cell lines and chemical inducers of apoptosis, especially in the studies involving epithelial cells. The chemical inducers enable researchers to apply an apoptotic stimulus in culture conditions to study apoptosis inhibition. Most immortalized cell lines already have a pro-survival phenotype. Positive controls, that is, uninfected cells treated with the stimulus, enable researchers to demonstrate that the bacterial pathogen does in fact inhibit apoptosis during infection. These strong stimuli can overcome the pro-survival phenotype of the immortalized cell line. However, many of these stimuli are not physiologically relevant. Therefore, researchers should attempt to use more physiologically relevant conditions, particularly after initial observations are made. For instance, the use of primary human cells from the relevant site of infection should be performed to confirm observations of apoptosis inhibition. Moreover, mimicking apoptotic stimuli that these cells would normally encounter in vivo should also be considered. For example, TNF-α is secreted in response to many infections [51], and it induces the extrinsic pathway of apoptosis. In addition, transmigration of polymorphonuclear (PMN) leukocytes induces apoptosis in T84 intestinal epithelial cells [52]. PMN transmigration occurs during Shigella and Neisseria infections. Therefore, mimicking physiologically relevant conditions is not only possible, but important for researchers to enable an appropriate understanding of apoptosis inhibition during infection.
Another significant area of research will be to determine if the ability to inhibit apoptosis is associated with the carcinogenic potential of the bacterial pathogen. Helicobacter pylori [53] and B. henselae inhibit apoptosis, and these organisms cause gastric carcinoma and bacillary angiomatosis, respectively. Furthermore, Chlamydia seems to stimulate mitosis in infected cells in addition to inhibiting apoptosis. Do certain organisms have the potential to cause cancer through apoptosis inhibition? Some viruses with the ability to inhibit apoptosis do cause cancer. An example is the Epstein–Barr virus, which promotes anti-apoptosis in B lymphocytes and can cause Burkitt’s lymphoma [54]. Drawing connections between apoptosis inhibition and the development of cancer will be important for understanding the full potential of bacterial pathogens.
Clearly, apoptosis inhibition is an emerging theme in bacterial pathogenesis. Prevention of apoptosis enables bacteria to replicate and survive in an environment that is otherwise detrimental to the pathogen. This review classifies the different mechanisms of apoptosis inhibition into three main categories, but other methods might exist. We are only starting to understand the mechanisms employed by bacteria to inhibit apoptosis and the importance of such survival strategies to the pathogens. A clear understanding of the molecular basis of apoptosis inhibition is needed. With all the outstanding questions that remain, future research could reveal that bacterial inhibition of apoptosis is far more important than we currently suspect.
Acknowledgments
We thank the members of the Maurelli laboratory for their input in discussions regarding the inhibition of apoptosis by bacterial pathogens. We thank the Uniformed Services University and the Henry M. Jackson Foundation for the Val Hemming Fellowship awarded to C.S.F. Additional funding is provided by the National Institutes of Allergy and Infectious Diseases grant AI24656.
Footnotes
The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reed JC. Warner-Lambert/Parke-Davis Award lecture: Mechanisms of apoptosis. Am J Pathol. 2000;157:1415–1429. [Google Scholar]
- 2.Hyer ML, et al. The FLIP-side of Fas signaling. Clin Cancer Res. 2006;12:5929–5931. doi: 10.1158/1078-0432.CCR-06-2098. [DOI] [PubMed] [Google Scholar]
- 3.Fink SL, Cookson BT. Apoptosis, pyroptosis and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroemer G, et al. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 5.Hay S, Kannourakis G. A time to kill: viral manipulation of the cell death program. J Gen Virol. 2002;83:1547–1564. doi: 10.1099/0022-1317-83-7-1547. [DOI] [PubMed] [Google Scholar]
- 6.Bruchhaus I, et al. Protozoan parasites: programmed cell death as a mechanism of parasitism. Trends Parasitol. 2007;23:376–383. doi: 10.1016/j.pt.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, et al. Inhibition of MAPK and NF-kappa B pathways is necessary for rapid apoptosis in macrophages infected with Yersinia. J Immunol. 2005;174:7939–7949. doi: 10.4049/jimmunol.174.12.7939. [DOI] [PubMed] [Google Scholar]
- 8.Blomgran R, et al. Uropathogenic Escherichia coli triggers oxygen-dependent apoptosis in human neutrophils through the cooperative effect of type 1 fimbriae and lipopolysaccharide. Infect Immun. 2004;72:4570–4578. doi: 10.1128/IAI.72.8.4570-4578.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi SD, et al. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc Natl Acad Sci U S A. 2003;100:10948–10953. doi: 10.1073/pnas.1833375100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grassmé H, et al. Molecular mechanisms of bacteria induced apoptosis. Apoptosis. 2001;6:441–445. doi: 10.1023/a:1012485506972. [DOI] [PubMed] [Google Scholar]
- 11.Bannerman DD, Goldblum SE. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J Physiol Lung Cell Mol Physiol. 2003;284:L899–L914. doi: 10.1152/ajplung.00338.2002. [DOI] [PubMed] [Google Scholar]
- 12.Zhong Y, et al. Inhibition of staurosporine-induced activation of the proapoptotic multidomain Bcl-2 proteins Bax and Bak by three invasive chlamydial species. J Infect. 2006;53:408–414. doi: 10.1016/j.jinf.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 13.Miyairi I, Byrne GI. Chlamydia and programmed cell death. Curr Opin Microbiol. 2006;9:102–108. doi: 10.1016/j.mib.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Häcker G, et al. Apoptosis in infectious disease: how bacteria interfere with the apoptotic apparatus. Med Microbiol Immunol. 2006;195:11–19. doi: 10.1007/s00430-005-0239-4. [DOI] [PubMed] [Google Scholar]
- 15.Fischer SF, et al. Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins. J Exp Med. 2004;200:905–916. doi: 10.1084/jem.20040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao Y, et al. Chlamydia trachomatis infection inhibits both Bax and Bak activation induced by staurosporine. Infect Immun. 2004;72:5470–5474. doi: 10.1128/IAI.72.9.5470-5474.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong F, et al. Degradation of the proapoptotic proteins Bik, Puma, and Bim with Bcl-2 domain 3 homology in Chlamydia trachomatis-infected cells. Infect Immun. 2005;73:1861–1864. doi: 10.1128/IAI.73.3.1861-1864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pirbhai M, et al. The secreted protease factor CPAF is responsible for degrading pro-apoptotic BH3-only proteins in Chlamydia trachomatis-infected cells. J Biol Chem. 2006;281:31495–31501. doi: 10.1074/jbc.M602796200. [DOI] [PubMed] [Google Scholar]
- 19.Rajalingam K, et al. IAP-IAP complexes required for apoptosis resistance of C. trachomatis-infected cells. PLoS Pathog. 2006;2:e114. doi: 10.1371/journal.ppat.0020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greene W, et al. Chlamydia-infected cells continue to undergo mitosis and resist induction of apoptosis. Infect Immun. 2004;72:451–460. doi: 10.1128/IAI.72.1.451-460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massari P, et al. Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc Natl Acad Sci U S A. 2000;97:9070–9075. doi: 10.1073/pnas.97.16.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massari P, et al. Neisserial PorB is translocated to the mitochondria of HeLa cells infected with Neisseria meningitidis and protects cells from apoptosis. Cell Microbiol. 2003;5:99–109. doi: 10.1046/j.1462-5822.2003.00257.x. [DOI] [PubMed] [Google Scholar]
- 23.Galluzzi L, Kroemer G. Mitochondrial apoptosis without VDAC. Nat Cell Biol. 2007;9:487–489. doi: 10.1038/ncb0507-487. [DOI] [PubMed] [Google Scholar]
- 24.Baines CP, et al. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller A, et al. Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. EMBO J. 2000;19:5332–5343. doi: 10.1093/emboj/19.20.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binnicker MJ, et al. Infection of human urethral epithelium with Neisseria gonorrhoeae elicits an upregulation of host anti-apoptotic factors and protects cells from staurosporine-induced apoptosis. Cell Microbiol. 2003;5:549–560. doi: 10.1046/j.1462-5822.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 27.Binnicker MJ, et al. Gonococcal porin IB activates NF-kappaB in human urethral epithelium and increases the expression of host antiapoptotic factors. Infect Immun. 2004;72:6408–6417. doi: 10.1128/IAI.72.11.6408-6417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy SG, et al. Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morales P, et al. Infection of human fallopian tube epithelial cells with Neisseria gonorrhoeae protects cells from tumor necrosis factor alpha-induced apoptosis. Infect Immun. 2006;74:3643–3650. doi: 10.1128/IAI.00012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knodler LA, et al. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem. 2005;280:9058–9064. doi: 10.1074/jbc.M412588200. [DOI] [PubMed] [Google Scholar]
- 31.Fink SL, Cookson BT. Caspase-1 dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 32.Choi KS, et al. Anaplasma phagocytophilum delay of neutrophil apoptosis through the p38 mitogen-activated protein kinase signal pathway. Infect Immun. 2005;73:8209–8218. doi: 10.1128/IAI.73.12.8209-8218.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HC, Goodman JL. Anaplasma phagocytophilum causes global induction of antiapoptosis in human neutrophils. Genomics. 2006;88:496–503. doi: 10.1016/j.ygeno.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Ge Y, Rikihisa Y. Anaplasma phagocytophilum delays spontaneous human neutrophil apoptosis by modulation of multiple apoptotic pathways. Cell Microbiol. 2006;8:1406–1416. doi: 10.1111/j.1462-5822.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang JZ, et al. Survival strategy of obligately intracellular Ehrlichia chaffeensis: novel modulation of immune response and host cell cycles. Infect Immun. 2004;72:498–507. doi: 10.1128/IAI.72.1.498-507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joshi SG, et al. Nuclear factor kappa B protects against host cell apoptosis during Rickettsia rickettsii infection by inhibiting activation of apical and effector caspases and maintaining mitochondrial integrity. Infect Immun. 2003;71:4127–4136. doi: 10.1128/IAI.71.7.4127-4136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joshi SG, et al. NF-kappaB activation suppresses host cell apoptosis during Rickettsia rickettsii infection via regulatory effects on intracellular localization or levels of apoptogenic and anti-apoptotic proteins. FEMS Microbiol Lett. 2004;234:333–341. doi: 10.1016/j.femsle.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 38.Pannebakker BA, et al. Parasitic inhibition of cell death facilitates symbiosis. Proc Natl Acad Sci U S A. 2007;104:213–215. doi: 10.1073/pnas.0607845104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bazzocchi C, et al. Wolbachia surface protein (WSP) inhibits apoptosis in human neutrophils. Parasite Immunol. 2007;29:73–79. doi: 10.1111/j.1365-3024.2006.00915.x. [DOI] [PubMed] [Google Scholar]
- 40.Kempf VA, et al. Bartonella henselae inhibits apoptosis in Mono Mac 6 cells. Cell Microbiol. 2005;7:91–104. doi: 10.1111/j.1462-5822.2004.00440.x. [DOI] [PubMed] [Google Scholar]
- 41.Schmid MC, et al. A translocated bacterial protein protects vascular endothelial cells from apoptosis. PLoS Pathog. 2006;2:e115. doi: 10.1371/journal.ppat.0020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirby JE, Nekorchuk DM. Bartonella-associated endothelial proliferation depends on inhibition of apoptosis. Proc Natl Acad Sci U S A. 2002;99:4656–4661. doi: 10.1073/pnas.072292699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki T, et al. A novel caspase-1/toll-like receptor 4-independent pathway of cell death induced by cytosolic Shigella in infected macrophages. J Biol Chem. 2005;280:14042–14050. doi: 10.1074/jbc.M414671200. [DOI] [PubMed] [Google Scholar]
- 44.Clark CS, Maurelli AT. Shigella flexneri inhibits staurosporine-induced apoptosis in epithelial cells. Infect Immun. 2007;75:2531–2539. doi: 10.1128/IAI.01866-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pendaries C, et al. PtdIns5P activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. EMBO J. 2006;25:1024–1034. doi: 10.1038/sj.emboj.7601001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abu-Zant A, et al. Incomplete activation of macrophage apoptosis during intracellular replication of Legionella pneumophila. Infect Immun. 2005;73:5339–5349. doi: 10.1128/IAI.73.9.5339-5349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abu-Zant A, et al. Anti-apoptotic signaling by the Dot/Icm secretion system of L. pneumophila. Cell Microbiol. 2007;9:246–264. doi: 10.1111/j.1462-5822.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 48.Laguna RK, et al. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci U S A. 2006;103:18745–18750. doi: 10.1073/pnas.0609012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keller P, et al. Direct inhibition of cytochrome c-induced caspase activation in vitro by Toxoplasma gondii reveals novel mechanisms of interference with host cell apoptosis. FEMS Microbiol Lett. 2006;258:312–319. doi: 10.1111/j.1574-6968.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- 50.Pålsson-McDermott, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung HC, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le’Negrate G, et al. Sustained polymorphonuclear leukocyte transmigration induces apoptosis in T84 intestinal epithelial cells. J Cell Biol. 2000;150:1479–1488. doi: 10.1083/jcb.150.6.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanai A, et al. Helicobacter pylori induces antiapoptosis through nuclear factor-kappaB activation. J Infect Dis. 2003;188:1741–1751. doi: 10.1086/379629. [DOI] [PubMed] [Google Scholar]
- 54.Clemens MJ. Epstein-Barr virus: Inhibition of apoptosis as a mechanism of cell transformation. Int J Biochem Cell Biol. 2006;38:164–169. doi: 10.1016/j.biocel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 55.Yilmaz O, et al. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect Immun. 2004;72:3743–3751. doi: 10.1128/IAI.72.7.3743-3751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mansell A, et al. Internalin B activates nuclear factor-kappa B via Ras, phosphoinositide 3-kinase, and Akt. J Biol Chem. 2001;276:43597–43605. doi: 10.1074/jbc.M105202200. [DOI] [PubMed] [Google Scholar]
- 57.Gerlic M, et al. Mycoplasma fermentans inhibits tumor necrosis factor alpha-induced apoptosis in the human myelomonocytic U937 cell line. Cell Death Differ. 2004;11:1204–1212. doi: 10.1038/sj.cdd.4401482. [DOI] [PubMed] [Google Scholar]
- 58.Gerlic M, et al. The inhibitory effect of Mycoplasma fermentans on tumour necrosis factor (TNF)-alpha-induced apoptosis resides in the membrane lipoproteins. Cell Microbiol. 2007;9:142–153. doi: 10.1111/j.1462-5822.2006.00774.x. [DOI] [PubMed] [Google Scholar]
- 59.Gross A, et al. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect Immun. 2000;68:342–351. doi: 10.1128/iai.68.1.342-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sukumaran SK, et al. Inhibition of apoptosis by Escherichia coli K1 is accompanied by increased expression of BclXL and blockade of mitochondrial cytochrome c release in macrophages. Infect Immun. 2004;72:6012–6022. doi: 10.1128/IAI.72.10.6012-6022.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voth DE, et al. Coxiella burnetti inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect Immun. 2007;75:4263–4271. doi: 10.1128/IAI.00594-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luhrmann A, Roy CR. Coxiella burnetti inhibits activation of host cell apoptosis through a mechanism that involves preventing cytochrome c release from the mitochondria. Infect Immun. 2007;75:5282–5289. doi: 10.1128/IAI.00863-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 64.Roset R, et al. Role of Bcl-2 family members on apoptosis: what we have learned from knock-out mice. Front Biosci. 2007;12:4722–4730. doi: 10.2741/2421. [DOI] [PubMed] [Google Scholar]