SUMMARY
Physical force is implicated in many cell functions. However, the molecular mechanisms of force sensing are poorly understood. Here, we show that mechanical extension of p130Cas (Cas) in vitro and in vivo causes phosphorylation by Src family kinases with no apparent change in Src kinase activity and that Cas phosphorylation is involved in stretch-dependent activation of the small GTPase, Rap1. In vitro, we mechanically extended bacterially expressed Cas substrate domain (CasSD) and found a remarkable enhancement of phosphorylation by specific kinases. Using an antibody that recognized extended CasSD in vitro, Cas extension in intact cells was observed in the peripheral regions of spreading cells where higher traction forces are expected and phosphorylated Cas was detected, suggesting that the in vitro extension and phosphorylation of CasSD is relevant to physiological force transduction. Thus, Cas acts as a primary force-sensor through extension of the substrate domain, which primes it for phosphorylation.
INTRODUCTION
Cellular responses to mechanical force underlie many critical functions from normal morphogenesis to carcinogenesis, cardiac hypertrophy, wound healing and bone homeostasis. Recent studies indicate that various signaling pathways are involved in force transduction, including MAP kinases, small GTPases, and tyrosine kinases/phosphatases (Geiger and Bershadsky, 2002; Giannone and Sheetz, 2006; Katsumi et al., 2002; Sawada et al., 2001). A variety of primary force-sensing mechanisms could be postulated, including mechanical extension of cytoplasmic proteins, activation of ion channels, and formation of force-stabilized receptor-ligand bonds (catch bonds) (Vogel and Sheetz, 2006), which would then activate downstream signaling pathways. At a biochemical level, tyrosine phosphorylation levels appear to be linked to mechanically-induced changes controlling many other cellular functions (Giannone and Sheetz, 2006). One protein involved in mechanically-induced phosphorylation-dependent signaling is the Src family kinase substrate, Cas (Crk-associated substrate), which is involved in various cellular events such as migration, survival, transformation, and invasion (Defilippi et al., 2006). Stretch-dependent tyrosine phosphorylation of Cas by Src family kinases (SFKs) occurs in detergent-insoluble cytoskeletal complexes and is involved in force-dependent activation of the small GTPase, Rap1 (Tamada et al., 2004). Rap 1 is activated by distinct types of guanine nucleotide exchange factors coupled with various receptors or second messengers and plays an important role in a number of signaling pathways including integrin signaling (Hattori and Minato, 2003).
Cas substrate domain, which is located in the center of Cas, is flanked by the amino-terminal SH3 and the carboxy-terminal Src-binding domains. These amino- and carboxy-terminal domains are involved in Cas localization at focal adhesions while the substrate domain itself is not (Nakamoto et al., 1997), suggesting that these flanking domains anchor Cas molecules to the cytoskeletal complex and that the substrate domain could be extended upon cytoskeleton stretching. Furthermore, Cas substrate domain has fifteen repeats of a tyrosine-containing motif (YxxP) (Mayer et al., 1995) and multiple sequence repeats are found in molecules with mechanical functions such as titin (Rief et al., 1997).
Cell stretching could increase tyrosine phosphorylation by: 1) directly activating the kinase, 2) inactivating the phosphatase, 3) mechanically bringing the kinase to the substrate, or 4) enhancing the susceptibility of the substrate to phosphorylation. To test between these possibilities, we have analyzed the mechanisms of stretch-dependent enhancement of Cas phosphorylation. In intact cells, Cas phosphorylation by c-Src is significantly increased by cell stretching with no detectable change in c-Src kinase activity. Cas phosphorylation mediates physiological force transduction through stretch-dependent activation of Rap1 in intact cells. With in vitro protein extension experiments, we find that phosphorylation of CasSD by specific kinases is increased upon extension. Further, an antibody that recognizes extended CasSD in vitro preferentially recognizes Cas molecules at the periphery of late spreading cells where higher traction forces are predicted and Cas is phosphorylated, indicating that the in vitro extension and phosphorylation of CasSD is relevant to force transduction through Cas phosphorylation in intact cells. Thus, we suggest that Cas serves as a direct mechano-sensor where force induces a mechanical extension of the substrate domain that primes it for phosphorylation. We propose that such ‘substrate priming’ is a general mechanism for force transduction.
RESULTS
Cell Stretching Enhances SFK-dependent Phosphorylation of Cas without a Detectable Increase in Src Kinase Activity
We first examined whether the phosphorylation of Cas increased upon intact cell stretching, using the cell stretching system that we developed (Sawada et al., 2001). Cells were cultured on a stretchable substrate (collagen-coated silicone) and the substrate was stretched uniformly and biaxially (10% in each dimension), and held stretched. To analyze the primary responses to cell stretching, samples were prepared from the cells lysed shortly (1 min) after stretching. Immunoblotting using an anti-phospho-Cas antibody (pCas-165) that specifically recognizes multiple phosphorylated YxxP motifs in the substrate domain (Fonseca et al., 2004) revealed a stretch-dependent increase in tyrosine phosphorylation of Cas in HEK293 cells (Figure 1A). When the selective SFK inhibitor, CGP77675 (Missbach et al., 1999) (Novartis Pharma AG, Switzerland), was added prior to stretching, stretch-dependent tyrosine phosphorylation of Cas was inhibited (Figure 1A). Furthermore, stretch-dependent phosphorylation of Cas was greatly attenuated in SYF cells that lacked the major SFKs, c-Src, c-Yes, and Fyn (Klinghoffer et al., 1999), and was restored in SYF cells stably expressing c-Src (Figure 1B), c-Yes or Fyn (data not shown). Thus, stretching intact cells increased tyrosine phosphorylation of Cas by SFKs.
Figure 1. SFK- and Stretch-dependent Tyrosine Phosphorylation of Cas In Vivo.
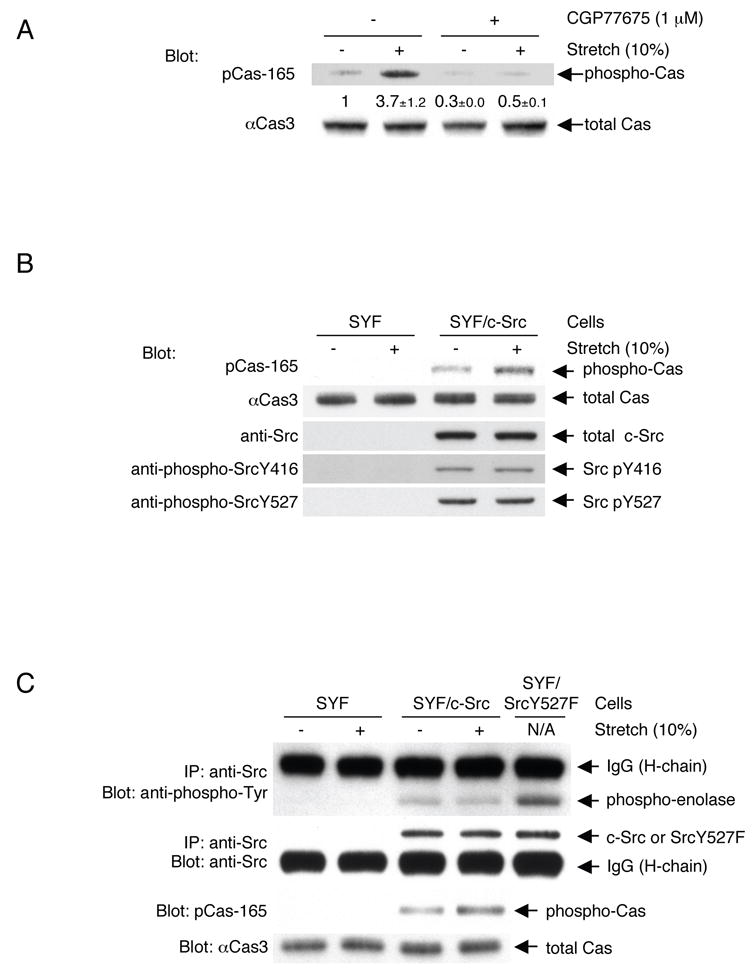
(A) Stretch-dependent tyrosine phosphorylation of Cas in intact cells.
2 x 105 HEK293 cells on the collagen (Type I)-coated stretchable silicone dish were treated with either CGP77675 (1 μM) or its vehicle (0.01% DMSO) and were either stretched (biaxially, 10% in each dimension) or left unstretched. 1 min after stretching or without stretching, the cells were solubilized with 1 x SDS sample buffer containing 20 mM DTT and analyzed for Cas phosphorylation by anti-phospho-Cas (pCas-165) and anti-Cas (αCas3) immunoblotting. Quantification of phosphorylation (phospho-Cas / total Cas) was scaled with unstretched control set at 1 and noted below the pCas-165 blot with s.d. (n = 4).
(B) Cell stretching increases Src-dependent phosphorylation of Cas without apparent change in phosphorylation levels of activating and inhibiting tyrosines of c-Src. 4 x 105 SYF cells (triple knock-out cells of c-src, c-yes, and fyn) or SYF cells stably expressing c-Src were either stretched or left unstretched. 1 min after stretching or without stretching, cells were solubilized with SDS sample buffer and equivalent portions of each sample were subjected to SDS-PAGE followed by pCas-165, αCas3, anti-Src, anti-phospho-Src Y416, and anti-phospho-Src Y527 immunoblotting.
(C) Cell stretching increases Src-dependent phosphorylation of Cas without apparent change in Src kinase activity.
4 x 105 SYF cells or SYF cells stably expressing c-Src were either stretched or left unstretched. 1 min after stretching or without stretching, cells were lysed and subjected to immunoprecipitation followed by an in vitro kinase assay using acid-treated enolase as a substrate. Src kinase activity was analyzed by measuring the phosphorylation of enolase with anti-phospho-tyrosine immunoblotting (top panel). Immunoprecipitated Src, i.e. Src protein in the kinase reaction, was quantified by anti-Src immunoblotting (second panel). Equivalent small portions of each lysate was mixed with SDS sample buffer and subjected directly to SDS-PAGE followed by pCas-165 and αCas3 immunoblotting to analyze for Cas phosphorylation (third and fourth panels). Kinase reactions for the lane 3 and 4 samples appeared not to be saturated because the sample prepared from SYF/SrcY527F cells (SYF cells expressing SrcY527F, the highly active mutant form of c-Src) cultured on a plastic plate following the same protocol gave more phosphorylation of enolase (lane 5). The intense bands above enolase (top panel) and below Src (second panel) represent IgG (heavy chain) from the anti-Src antibody.
To determine if stretch-dependent increases in Cas phosphorylation correlated with SFK activation, the levels of c-Src phosphorylation at either activating or inhibiting tyrosine residue (Y416 and Y527, respectively) were examined in SYF cells stably expressing c-Src, either stretched or left unstretched. We observed no changes in phosphorylation levels of those tyrosines (pY416 and pY527) (Figure 1B, lanes 3 and 4). Since the levels of pY416 and pY527 indicate Src kinase inhibition and activation, respectively (Thomas and Brugge, 1997), cell stretching did not appear to affect c-Src activity while Cas phosphorylation significantly increased. This was further confirmed by an in vitro kinase assay of immunoprecipitated c-Src (Figure 1C). Thus, stretching intact cells increased tyrosine phosphorylation of Cas by c-Src without detectable enhancement of c-Src kinase activity.
Tyrosine Phosphorylation of Cas Is Involved in Stretch-dependent Rap1 Activation
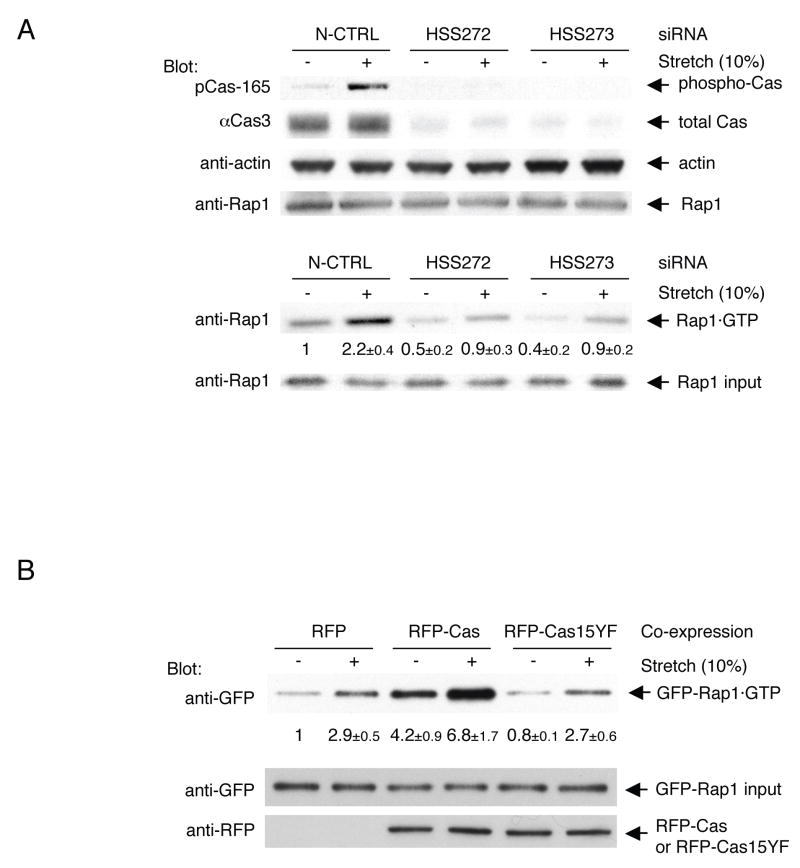
To explore the role of Cas in physiological force transduction pathways, we analyzed the involvement of Cas in the stretch-dependent activation of Rap1 in cells (Sawada et al., 2001). When the level of Cas protein and phosphorylated Cas was selectively decreased by small interfering RNA (siRNA) in HEK293 cells (Figure 2A, upper panel), Rap1 activity in cells, either stretched or unstretched, was significantly attenuated (Figure 2A, lower panel). Thus, Cas plays a significant role in the stretch-dependent activation of Rap1 in intact cells. However, there is likely more than one pathway for Rap1 activation, considering the fold decrease of Rap1 activity (~50%) in Cas knock-down cells (Figure 2A) as well as the stretch-dependent Rap1 activation observed in Cas-deficient fibroblasts (data not shown).
Figure 2. Significant Role of Cas Phosphorylation in Physiological Force Transduction.
(A) Cas is involved in stretch-dependent Rap1 activation in intact cells.
RNAi experiments were performed as described in Experimental Procedures. siRNA used were Stealth RNAi Negative Control Med GC (N-CTRL: lanes 1 and 2), BCAR1-HSS114272 (HSS272: lanes 3 and 4), and BCAR1-HSS114273 (HSS273: lanes 5 and 6) (Invitrogen). 24 h after transfection, HEK293 cells were either stretched or left unstretched. To determine the level of Cas expression and phosphorylation, cells were solubilized with SDS sample buffer 1 min after stretching or without stretching and equivalent portions of each sample were subjected to SDS-PAGE followed by anti-phospho-Cas (pCas-165), anti-Cas (αCas3), anti-Rap1, and anti-actin immunoblotting (upper panel). To measure stretch-dependent Rap1 activity, cells were solubilized with lysis buffer for GST pull-down assay (see Experimental Procedures) 5 min after stretching or without stretching. Rap1 was quantified by anti-Rap1 immunoblotting. Rap1 activity (Rap1•GTP / Rap1 input) was scaled with the unstretched control set at 1 and noted below the Rap1•GTP blot with s.d. (n = 4) (lower panel). The data shown in Figure 2A (upper and lower panels) was obtained with siRNA transfection performed at the same time.
(B) Significant role of tyrosine phosphorylation of Cas in stretch-dependent Rap1 activation.
RFP, RFP-Cas or RFP-Cas15YF was co-transfected with GFP-Rap1 into HEK293 cells (1 x 105 / dish). 24 h after transfection, cells were either stretched or left unstretched. 5 min after stretching or without stretching, cells were solubilized and subjected to the GST pull-down assay. GFP-Rap1 was quantified by anti-GFP immunoblotting. GFP-Rap1 activity (GFP-Rap1•GTP / GFP-Rap1 input) was scaled with the unstretched RFP-transfected cells set at 1 and noted below the GFP-Rap1•GTP blot with s.d. (n = 4).
To further examine the role of phosphorylation of Cas in stretch-dependent Rap1 activation, we overexpressed Cas together with Rap1 in HEK293 cells. Upon co-expression of monomeric red fluorescent protein (RFP)-tagged wild-type Cas (RFP-Cas) with green fluorescent protein (GFP)-tagged Rap1 (GFP-Rap1), stretch-dependent activity of GFP-Rap1 was enhanced over the cells co-expressing RFP alone or RFP-Cas15YF that had all fifteen YxxP motifs in the substrate domain mutated to FxxP (Figure 2B). The fold increase of Rap1 activity by cell stretching appeared to be smaller in the case of RFP-Cas-expressing cells probably due to the less efficient incorporation of ‘overexpressed’ Cas into physiological signaling complexes. However, we conclude that tyrosine phosphorylation of Cas is responsible for a significant fraction of the stretch-dependent Rap1 activation.
In Vitro Extension of CasSD
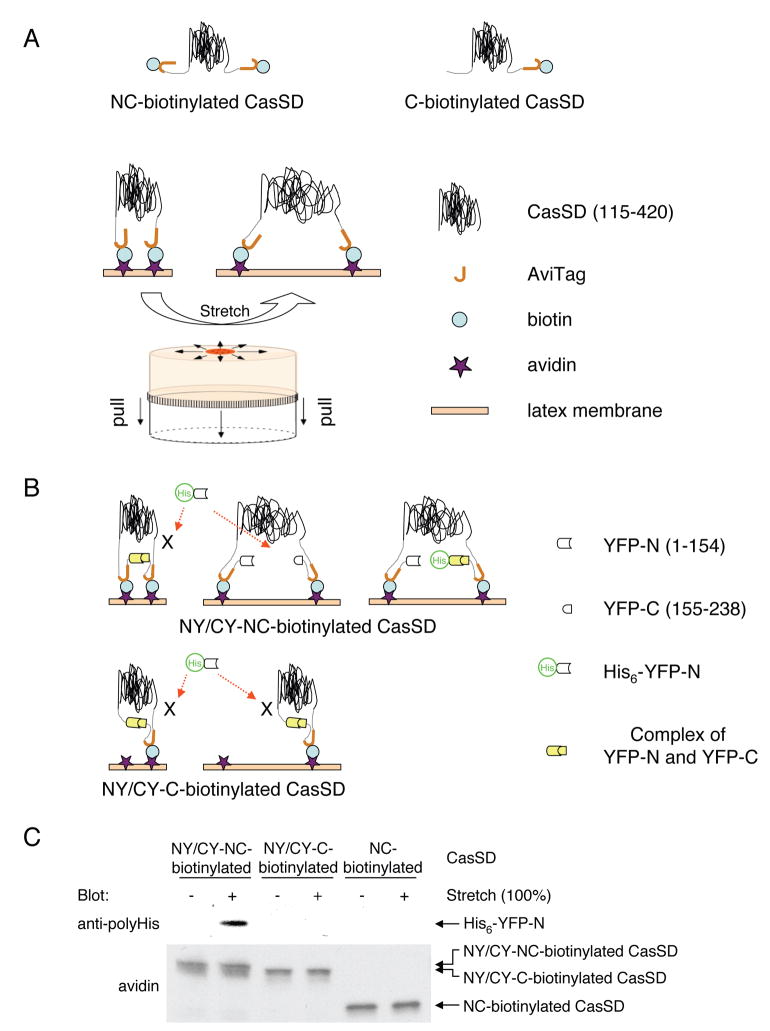
Because kinase activation did not appear to be the primary mechanism regulating Cas phosphorylation in response to cell stretching (Figure 1B, C), we tested whether the mechanical extension of Cas substrate domain modulated its susceptibility to phosphorylation by SFKs. To eliminate the involvement of any extraneous molecules, we performed biochemical analysis, using an in vitro protein extension (IPE) system. In that system, bacterially expressed Cas substrate domain protein, CasSD (Cas115-420), was biotinylated on both amino- and carboxy-terminals (designated NC-biotinylated CasSD, Figure 3A, top) and was bound to avidin covalently immobilized on a latex substrate (Figure 3A). After stretching of the latex membrane (Figure 3A), biochemical analyses were performed.
Figure 3. In Vitro Protein Extension (IPE) System.
(A) Scheme of NC-biotinylated CasSD, C-biotinylated CasSD, and the process of mechanical extension of CasSD in the IPE system.
(B) Schematic description of YFP amino-terminal swapping.
(C) His6-YFP-N binds to extended NY/CY-NC-biotinylated CasSD, but not to NY/CY-C-biotinylated CasSD or NC-biotinylated CasSD.
Biotinylated CasSD proteins, either extended or unextended on latex membrane, were incubated with His6-YFP-N in the buffer containing 1% Triton X-100 and 1% BSA. After washing, bound complex was solubilized and subjected to SDS-PAGE followed by anti-polyHistidine immunoblotting or avidin affinity blotting.
To determine if stretching of the latex membrane actually extended NC-biotinylated CasSD, we developed the yellow fluorescent protein (YFP) amino-terminal swapping assay based on the interaction between the amino- and carboxy-terminal regions of YFP. In this assay, CasSD extension was detected by the separation of YFP components attached to the ends of CasSD, causing the binding of an exogenous YFP component. When the two halves of a split YFP, YFP-N and YFP-C, were fused to the amino- and carboxy-terminal ends of NC-biotinylated CasSD, respectively (NY/CY-NC-biotinylated CasSD, Figure 3B), we observed yellow fluorescence in both NY/CY-NC-biotinylated CasSD-expressing bacteria and the purified protein, as expected (Hu et al., 2002). When we added purified His6-YFP-N to bind to YFP-C in NY/CY-NC biotinylated CasSD (Figure 3B, top), His6-YFP-N binding was not observed without latex membrane stretching (Figure 3C, lane 1). However, we observed His6-YFP-N binding upon stretching (Figure 3C, lane 2). Furthermore, His6-YFP-N did not bind to NY/CY-C-biotinylated CasSD (the unextendable mono-biotinylated control, Figure 3B, bottom) or NC-biotinylated CasSD (extendable, but with no YFP component, Figure 3A, top) even following stretching (Figure 3C, lanes 3–6). Using His6-YFP-N together with YFP-C fused to glutathione S-transferase (GST) in a GST pull-down experiment, we found that YFP-N bound to YFP-C under the same buffer conditions used in the YFP amino-terminal swapping assay (data not shown). Thus, stretching of the latex membrane separated the YFP halves in NY/CY-NC-biotinylated CasSD and allowed His6-YFP-N to bind, indicating the extension of CasSD (Figure 3B, top).
Extension-dependent Phosphorylation of CasSD by Recombinant Tyrosine Kinases In Vitro
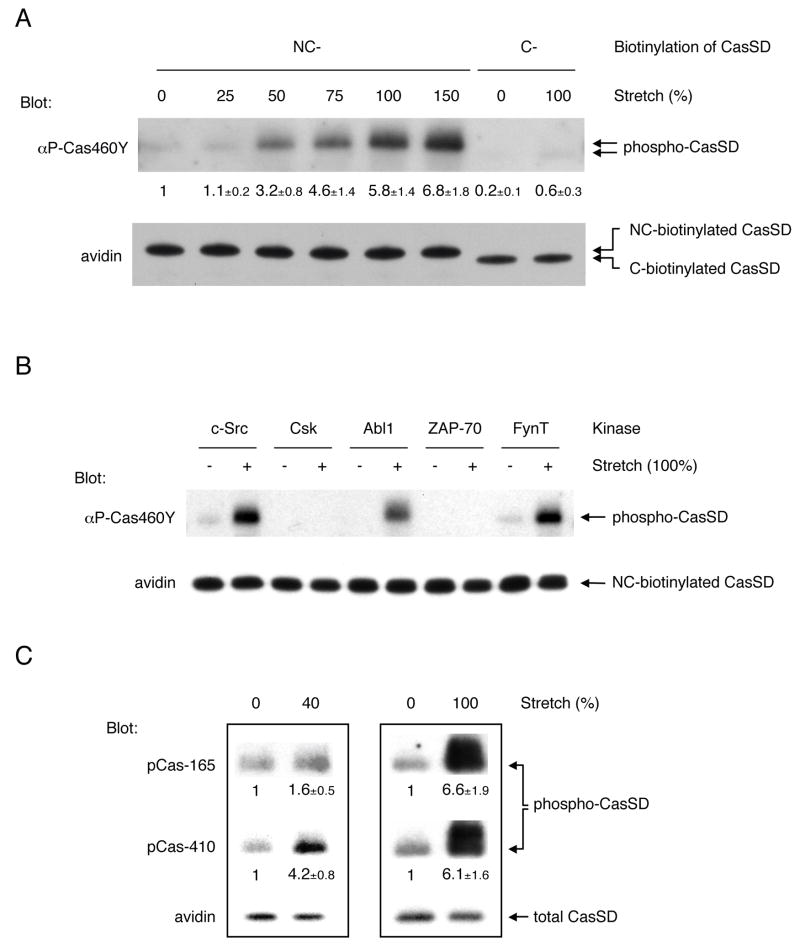
Since CasSD could be extended by the IPE system, we examined the effect of extension on tyrosine phosphorylation of CasSD by recombinant active c-Src. While the level of phosphorylation was low without stretching (Figure 4A, lane 1), CasSD phosphorylation increased in proportion to the magnitude of latex membrane stretching (25%, 50%, 75%, 100% and 150%) (Figure 4A, lanes 2–6). An unextendable mono-biotinylated CasSD (C-biotinylated CasSD, see Figure 3A, top) was poorly tyrosine phosphorylated either with or without stretching (Figure 4A, lanes 7 and 8). To test if c-Src kinase activity was modulated in the IPE experiments, we added acid-treated enolase to the kinase mixture at the time of kinase reaction and measured its phosphorylation. In the same reaction that gave an extension-dependent increase in CasSD phosphorylation, neither the level of enolase phosphorylation nor the phosphorylation levels of Y416 and Y527 of c-Src kinase was affected by stretching (data not shown). These results indicated that extension-dependent tyrosine phosphorylation of CasSD resulted from CasSD extension and not from an increase in the kinase activity of recombinant c-Src.
Figure 4. Extension-dependent Phosphorylation of CasSD by Tyrosine Kinases In Vitro.
(A) CasSD is tyrosine phosphorylated by recombinant c-Src in an extension-dependent manner.
NC-biotinylated or C-biotinylated CasSD was either extended or left unextended on latex membrane, incubated with recombinant c-Src for 2 min, washed, solubilized, and analyzed for tyrosine phosphorylation by anti-phospho-Cas (αP-Cas460Y) immunoblotting and avidin affinity blotting. The magnitude of the latex membrane stretching is described as the % change of length in each dimension. Quantification of phosphorylation of CasSD was scaled with unextended NC-biotinylated CasSD set at 1 and noted below the anti-phospho-Cas blot with s.d. (n = 4).
(B) Kinase specificity of extension-dependent tyrosine phosphorylation of CasSD. NC-biotinylated CasSD was either extended (100%) or left unextended and then incubated with recombinant c-Src, Csk, Abl1, ZAP-70 or FynT for 2 min at room temperature. Tyrosine phosphorylation of CasSD was analyzed as in A.
(C) Extension-dependent phosphorylation of CasSD by c-Src measured by two different anti-phospho-Cas antibodies.
Samples were prepared as in A except for the extent of the latex membrane stretching (40% in the left panel and 100% in the right panel). Equivalent portions of each sample were subjected to SDS-PAGE followed by pCas-165 and pCas-410 immunoblotting and avidin affinity blotting. Quantification of phosphorylation of CasSD was scaled with unextended NC-biotinylated CasSD set at 1 and noted below the anti-phospho-Cas blots with s.d. (n = 4).
We also asked whether or not other kinases phosphorylated CasSD in an extension-dependent manner in IPE experiments. Neither the non-SFK tyrosine kinase, Csk (C-terminal Src kinase), nor ZAP-70 phosphorylated NC-biotinylated CasSD even after stretching (Figure 4B). However, in the same kinase reaction protocol, both Csk and ZAP-70 were able to phosphorylate their known substrates, acid-treated enolase (Bougeret et al., 1993) and the cytoplasmic fragment of human erythrocyte band 3 (cdb3) (Isakov et al., 1996), respectively (data not shown). On the other hand, a known Cas kinase, Abl (Mayer et al., 1995) and another SFK, FynT phosphorylated CasSD in an extension-dependent manner (Figure 4B). Thus, extension-dependent phosphorylation of CasSD in vitro is caused in a kinase-specific manner, and not by a non-specific effect of the IPE system.
Although neither the force needed for CasSD extension nor its effect on individual YxxP motifs in CasSD is known, the IPE experiments revealed that different extents of extension induced the phosphorylation of different regions. When we used two different anti-phospho-Cas antibodies (pCas-165 and pCas-410) that had different, though not strictly specific, binding preferences for YxxPs in Cas substrate domain (Shin et al., 2004) to measure the in vitro CasSD phosphorylation, pCas-410 immunoblotting gave significantly greater fold increase than pCas-165 blots by 40% latex membrane stretching (Figure 4C, left panel). However, pCas-165 and pCas-410 blots showed a similar fold increase by 100% stretching (Figure 4C, right panel). These results suggest that the pCas-410 sites are more efficiently exposed and phosphorylated than pCas-165 sites by smaller extent of CasSD extension.
αCas1, an Antibody That Recognizes Extended CasSD
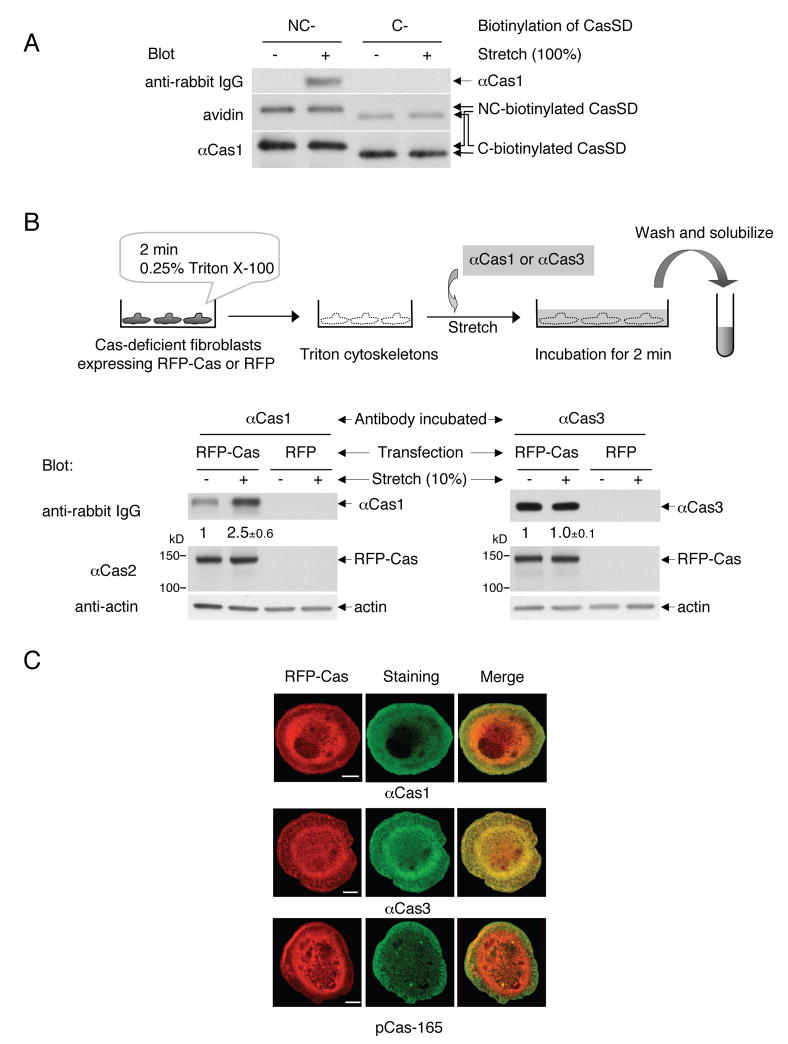
In order to test if Cas was extended in regions of cell traction forces, we utilized an antibody, αCas1, which was raised against a peptide sequence in Cas substrate domain (Sakai et al., 1994) (Figure. S1A). We found that αCas1 recognized the extended NC-biotinylated CasSD and not the unextended control, C-biotinylated CasSD in the IPE system (Figure 5A). Further, αCas1 bound to SDS-denatured CasSD regardless of its phosphorylation state (Figure S1B) as well as full-length Cas in the SDS-denatured cell lysates (Figure S1C). Thus, αCas1 binding appeared to require the exposure of its epitope in Cas substrate domain by either extension or denaturation.
Figure 5. Extension of Cas In Situ and In Vivo.
(A) αCas1 recognizes extended CasSD in vitro.
NC-biotinylated or C-biotinylated CasSD was either extended (100%) or left unextended in the IPE system. After blocking, CasSD proteins were incubated with αCas1, washed, and solubilized with SDS sample buffer containing 0.12 M DTT. Equivalent portions of each sample were analyzed for quantification of bound αCas1 by anti-rabbit IgG immunoblotting. The amount of NC-biotinylated and C-biotinylated CasSD in each sample was quantified by avidin affinity blotting and αCas1 immunoblotting. Note that the difference in the relative signal intensity between avidin and αCas1 blots is consistent with the molar ratio of biotinylation (NC-biotinylated CasSD : C-biotinylated CasSD = 2 : 1).
(B) Stretch-dependence of αCas1 and αCas3 binding to Cas in Triton cytoskeletons. Triton cytoskeletons were prepared from Cas-deficient fibroblasts transfected with RFP-Cas or RFP alone, either stretched or left unstretched, and incubated with either αCas1 or αCas3 as shown in the diagram. Quantification of bound antibody by anti-rabbit IgG immunoblotting was scaled with unstretched control set at 1 and noted with s.d. (n = 4).
(C) αCas1 preferentially binds to Cas where higher traction forces are expected in vivo. Cas-deficient fibroblasts expressing RFP-Cas were plated, fixed after 20 min, and then stained with αCas1, αCas3 or pCas-165. Confocal images are shown for RFP-Cas (left, red channel), immunostaining (center, green channel) and merged (right). Scale bars represent 10 μm.
Extension of Cas in Triton Cytoskeletons
Using αCas1, we examined whether Cas was extended by stretching Triton cytoskeletons where tyrosine phosphorylation of Cas was observed (Tamada et al., 2004). When we stretched Triton cytoskeletons from Cas-deficient fibroblasts expressing RFP-Cas, we observed a significant increase in αCas1 binding (Figure 5B, lower panel, lanes 1 and 2). Triton cytoskeletons from Cas-deficient fibroblasts expressing RFP alone did not bind αCas1 (Figure 5B, lower panel, lanes 3 and 4). Further, another anti-Cas antibody, αCas3, the epitope of which did not involve the substrate domain (Figure S1A) (Sakai et al., 1994), did not change its binding to Cas in Triton cytoskeletons upon stretching (Figure 5B, lower panel, lanes 5 and 6). These results indicate that the extension of Cas substrate domain is enhanced by cytoskeleton stretching.
Cas Is Extended at the Sites of High Traction Forces Where Cas Is Phosphorylated In Vivo
Cas extension was difficult to observe in intact cells, since the cell stretching system could not fit onto a total internal reflection fluorescence (TIRF) or confocal microscope. Further, the stretchable substrate (silicone) had high background fluorescence. Therefore, we looked at αCas1 immunostaining of intact cells during the late phase of spreading on collagen-coated glass coverslips (20 min after plating), when the fast movement of actin cytoskeletons at the periphery is observed (Dubin-Thaler et al., 2004) and the forces required for continuous spreading are generated (Giannone et al., 2004). In RFP-Cas-expressing Cas-deficient fibroblasts, we found that αCas1 staining primarily co-localized with RFP-Cas in the peripheral regions (Figure 5C, top). An anti-phospho-Cas antibody (pCas-165) also exhibited a peripheral staining in the late spreading cells (Figure 5C, bottom), confirming that Cas extension correlated with phosphorylation. These staining patterns did not appear to be artifacts of antibody staining, since αCas3 staining always co-localized with RFP-Cas (Figure 5C, middle). Considering the specificity of αCas1 and αCas3 in immunoblotting (Figure S1C), both αCas1 and αCas3 staining most likely represent the distribution of their Cas epitopes, and not the crossreaction with other cellular protein(s). Indeed, we observed only faint background staining in untransfected Cas-deficient fibroblasts with either αCas1 or αCas3 (data not shown).
These in situ (cytoskeleton stretching) (Figure 5B) and in vivo (intact cell spreading) (Figure 5C) results together with the observed preference of αCas1 binding for extended CasSD in vitro (Figure 5A) suggest that the in vitro extension of CasSD causes the conformational change of CasSD that is relevant to the force-dependent conformational change of Cas protein in vivo. Therefore, the extension-dependent phosphorylation of CasSD in vitro (Figure 4) appears relevant to the force-dependent phosphorylation of Cas in vivo (Figures 1, 2, and 5C).
DISCUSSION
Tyrosine Phosphorylation of Cas Is Involved in Physiological Force Transduction
Cas appears to act as a force transducer in vivo, since the knock-down of Cas expression by siRNA significantly attenuated stretch-dependent Rap1 activity (Figure 2A) and overexpression of wild-type Cas but not co-expression of the phosphorylation-defective Cas mutant (Cas15YF) enhanced stretch-dependent Rap1 activity (Figure 2B). Phosphorylation by SFK is critical since stretch-dependent Cas phosphorylation was inhibited by the SFK inhibitor, CGP77675 (Figure 1A), and attenuated in SYF cells (Figure 1B, C). These findings conform to our previous observation of stretch-dependent Cas phosphorylation by SFK in cytoskeletal complexes (Triton cytoskeletons) (Tamada et al., 2004) and indicate that tyrosine phosphorylation of Cas upon cell stretching constitutes a significant pathway for stretch-dependent Rap1 activation in intact cells (Sawada et al., 2001).
Possible Mechanisms for Stretch-increased Cas Phosphorylation
Although Src was shown to be mechanically activated using genetically engineered reporters (Wang et al., 2005), it is not clear how endogenous c-Src is activated or if it is indirectly activated by force. We observed that Src-dependent phosphorylation of Cas was significantly increased by stretching in c-Src-expressing SYF cells without causing Src kinase activation (Figure 1B, C). These findings suggest that activation of the kinase is not primarily responsible for stretch-dependent increase of Cas phosphorylation in vivo. Since the SFK inhibitor greatly attenuated the stretch-dependent increase of Cas phosphorylation (Figure 1A), stretch-dependent alteration of phosphatase activity is also unlikely to be the cause. Further, the tyrosine phosphatase inhibitor, sodium orthovanadate did not inhibit stretch-dependent tyrosine phosphorylation of Cas in Triton cytoskeletons (Tamada et al., 2004).
It is unlikely that stretching causes the spatial interaction between the kinase and the substrate, considering the constraints that such a mechanism places on the geometry of the cytoskeleton (Tamada et al., 2004). Since a mechanical modification of the substrate (Cas) was sufficient to increase Cas phosphorylation in vitro, we have focused on the analysis of that possibility.
Extension of Cas by Cellular Force
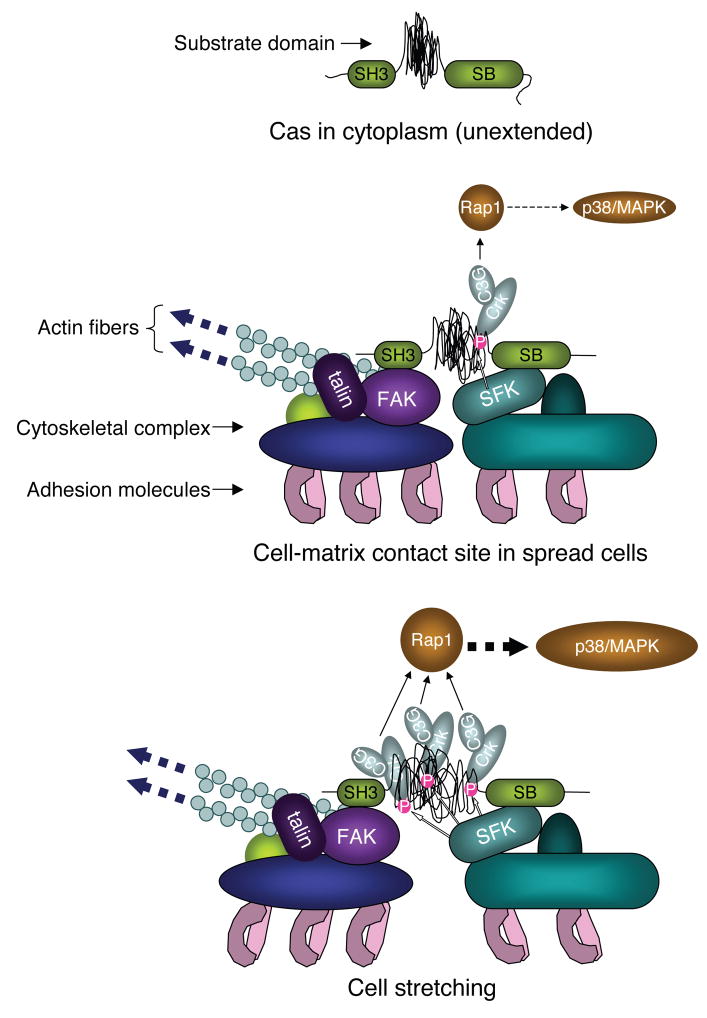
Both the amino-terminal SH3 and carboxy-terminal Src-binding domains are required for Cas localization at focal adhesions (Nakamoto et al., 1997) where cellular force is expected to be concentrated and force-dependent signaling involving tyrosine phosphorylation occurs (Geiger and Bershadsky, 2002; Tamada et al., 2004). Since different proteins are known to associate with SH3 and Src-binding domains of Cas (Defilippi et al., 2006), an individual Cas molecule would be anchored to the cytoskeleton-adhesion complex via two distinct sites. In addition, the SH3 domain of Cas binds to FAK (focal adhesion kinase) (Harte et al., 1996). FAK has a FERM (erythrocyte band 4.1-ezrin-radixin-moesin) domain commonly found in actin-binding proteins (Lee et al., 2004), associates with an actin-binding protein, talin (Chen et al., 1995), and is involved in the dynamic variation in tyrosine phosphorylation within focal adhesions (Ballestrem et al., 2006). Thus, we speculate that the amino-terminal anchor of Cas to the focal adhesion complex is more closely linked to the actin cytoskeleton than the carboxy-terminal anchor and that Cas is subjected to traction forces generated by the actin cytoskeleton (Figure 6).
Figure 6. Model of Extension of Cas and Signaling at Cell-matrix Contact Sites.
The top and middle panels represent a Cas molecule with unextended configuration of substrate domain in the cytoplasm and with moderate extension of substrate domain at the cell-matrix contact site of spread cells, respectively. The bottom panel represents the extension-dependent phosphorylation of Cas substrate domain by SFK and enhancement of its downstream signaling. SH3 and SB represent the SH3 and the Src-binding domains of Cas, respectively.
Although the structures of the SH3 and the serine-rich domains of Cas were reported (Briknarova et al., 2005; Wisniewska et al., 2005), no structural analysis of the substrate domain has been reported and the structure prediction algorithms (available at the Network Protein Sequence Analysis site) do not give a clear prediction for the structure of Cas substrate domain. We speculate that the intramolecular interactions within the substrate domain constrain its conformation in the absence of traction force and that traction force is required to expose YxxP sites to kinases.
Because our model centers on extension of Cas, the magnitude of force needed for extension is a concern. The force per integrin molecule in the adhesion site was estimated to be on the order of 1 pN (Balaban et al., 2001; Jiang et al., 2003), an order of magnitude below the force needed to reversibly unfold single domains of proteins such as spectrin by AFM (atomic force microscope) (Fisher et al., 1999). However, it has been shown that the protein unfolding force depends exponentially on the loading rate (Carrion-Vazquez et al., 1999) and at a low loading rate, proteins can be unfolded by forces even orders of magnitude below the forces required for unfolding at a high loading rate (Merkel et al., 1999). Further, extension of Cas may not require the force needed to cause ‘unfolding’, i.e. linearization of a mechanically stable distinct structure. Thus, extension of Cas probably can occur by physiological forces at focal contact sites (order of a few pN). Further details of force-dependent extension and phosphorylation of Cas substrate domain in vitro as well as in vivo are under study.
Physiological Role of Extension and Phosphorylation of Cas in Force Transduction
Several different experimental approaches indicate that Cas extension plays a role in the direct sensing of traction forces in vivo. In vitro, extension of NC-biotinylated CasSD remarkably enhanced its phosphorylation by exogenous c-Src, FynT, or Abl1 (Figure 4). Extension of CaSD was confirmed by measuring the separation of two halves of YFP linked to the ends of the CasSD (Figure 3B). Stretch-dependent phosphorylation of Cas in cytoskeletons and in intact cells further supports the idea that it is involved in physiological force sensing. Antibody binding to epitopes exposed by extension in regions of higher traction forces shows that Cas is extended in vivo. Although many force-dependent effects are observed within cells, extension of Cas appears to be a primary force-sensing process and not part of a secondary force-response pathway, since extension-dependent phosphorylation of CasSD by active kinases was observed in vitro where any extraneous biochemical interactions or signaling pathways were completely eliminated. While extracellular matrix proteins also respond to force by unfolding (Oberhauser et al., 2002) and exhibit different functional effects (Zhong et al., 1998), we show here that a cytoplasmic protein, Cas, has a gain of function upon cell stretching in terms of increase in phosphorylation and activation of Crk/C3G-Rap1 signaling.
A much greater percentage extension is required to observe the increase of in vitro phosphorylation of CasSD (Figure 4A, lanes 2–6) than the percentage of cell stretching to observe an increase of in vivo Cas phosphorylation (Figures 1 and 2). In vivo, cytoskeletal filaments will not stretch significantly and cytoskeletal networks are believed to be strain-hardened by cell-generated traction forces; therefore, molecular complexes at ‘stress-bearing’ sites will be greatly extended upon even mild cell stretching. Moreover, cell traction forces will pre-extend the cytoskeleton-bound Cas molecules in spread cells even without stretching (Figure 6, middle). Thus, 10% stretching of intact cells can cause more than 10% extension of ‘unextended’ Cas (Figure 6, top), since traction forces are concentrated at cell-matrix contact sites (Geiger and Bershadsky, 2002). In addition, αCas1 immunostaining shows that Cas is extended in the high traction force regions of cells where Cas is phosphorylated (Figure 5C).
In other studies, shear stress increases Cas phosphorylation by SFK in vascular endothelial cells (Okuda et al., 1999). Since shear stress is known to modulate the cell contractility (Chien et al., 2005) in which Cas has been shown to play a role (Tang and Tan, 2003), extension of Cas caused by the increased cell contractility might result in shear stress-dependent phosphorylation. Thus, local extension of Cas is likely to be involved in the local response to various types of ‘mechanical stress’ and can possibly account for the versatile function of Cas (Defilippi et al., 2006).
Substrate Priming as a General Mechanism of Cell Signaling
Enhancement of a substrate’s susceptibility to phosphorylation by mechanical extension is designated as extension-dependent ‘substrate priming.’ The transduction of cell forces into a biochemical signal by mechanical substrate priming could be highly flexible and dynamic. The extent of substrate extension in vivo will depend upon the extent of strain produced locally in the cell, resulting in a graded extent of substrate phosphorylation and, consequently, gradations in the magnitude of downstream signaling events. Substrate priming by mechanical force might be generally involved in kinase signaling, particularly in light of our observation that a number of other cytoskeletal proteins are tyrosine phosphorylated in a stretch-dependent manner (Tamada et al., 2004) and since substrate conformation is a critical determinant in phosphorylation of other SFK substrates (Cooper et al., 1984). Thus, we suggest that substrate priming by localized protein extension provides a simple mechanism for sensing the level of force on a cell as well as the location at which force is applied.
EXPERIMENTAL PROCEDURES
Antibodies
Polyclonal antibodies against Cas protein (αCas1, αCas2 and αCas3) were described previously (Sakai et al., 1994). Polyclonal anti-phospho-Cas, pCas-165 and pCas-410, a polyclonal anti-phospho-SrcY416, and a polyclonal anti-phospho-SrcY527 antibodies were purchased from Cell Signaling. The polyclonal anti-phospho-Cas antibody, αP-Cas460Y (Miyake et al., 2005), was used for in vitro experiments with CasSD. Monoclonal anti-GFP (JL-8) and anti-RFP (anti-DsRed) antibodies were purchased from Clontech and BD Pharmingen, respectively. Monoclonal anti-Src (GD11) and anti-polyHistidine antibodies were purchased from Upstate Biotechnology and Sigma, respectively. Polyclonal anti-actin and anti-Rap1 antibodies were purchased from Santa Cruz Biotech.
Cells and DNA Plasmid Transfection (transient and stable)
Human embryonic kidney (HEK) 293 cells, Cas-deficient fibroblasts (Huang et al., 2002), and SYF cells that lack c-Src, c-Yes, and Fyn were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (100 IU/ml, and 100 μg/ml) at 37 °C and 5% CO2. DNA plasmid transfection was performed with Fugene 6 (Roche) according to the manufacture’s protocol. To isolate stably transfected cell lines, pPUR that carried a puromycin-resistant gene (Clontech) was co-transfected and clones were selected using puromycin (Clontech).
Immunoprecipitation and In Vitro Kinase Assay of Src
To measure the Src kinase activity in SYF cells and stable transfectant cells derived from SYF cells, Src was immunoprecipitated and an in vitro kinase assay was performed using acid-treated enolase as a substrate. Phosphorylation of enolase was analyzed by anti-phospho-tyrosine immunoblotting. Details of the in vitro kinase assay of immunoprecipitated Src are described in the Supplemental Data section.
RNA Interference (RNAi) Experiments
To decrease the endogenous expression of Cas protein, two different siRNAs, BCAR1-HSS114272 and BCAR1-HSS114273 (Stealth™ RNAi, Invitrogen), were transfected into HEK293 cells (1 x 105 / dish) using Lipofectamine™ RNAiMAX according to the manufacturer’s protocol (180 pmole RNAi and 9 μl Lipofectamine RNAiMAX /dish) (Invitrogen). 6 h after transfection, culture medium was replaced with fresh DMEM containing 10% FBS. 24 h after transfection, cells were either stretched or left unstretched and subjected to biochemical analyses.
Quantification of Rap1 Activity
A GST pull-down assay was performed to measure the Rap1 activity using GST-RalGDS-RBD that preferentially bound to Rap1·GTP (Sakakibara et al., 2002). To measure the Rap1 input, equivalent portions of each lysate were directly subjected to SDS-PAGE followed by immunoblotting.
Kinases and Substrates
Recombinant c-Src, FynT, Abl1, Csk, and ZAP-70 were purchased from Invitrogen. Specific activity of each kinase was determined by an in vitro kinase assay using poly (Glu, Tyr) 4:1 (for c-Src, FynT, Csk and ZAP-70) or Abl1 substrate (for Abl1) (Invitrogen). Enolase (rabbit muscle) was purchased from Sigma. Bacterially expressed cdb3 was used as a substrate to measure the ZAP-70 activity.
Preparation of Biotinylated Proteins
Various forms of biotinylated CasSD were prepared using Biotin AviTag™ technology (Avidity). The Biotin AviTag sequence consists of 15 residues (GLNDIFEAQKIEWHE), which is specifically and efficiently biotinylated by the protein biotin ligase, Bir A. Biotinylated AviTag-fused proteins were obtained by co-expression with BirA in bacteria (BL21 Star™, Invitrogen) cultured in NZCYM medium containing d-biotin (50 μM, Research Organics) at the time of IPTG induction. The molar ratio of biotin to AviTag-fused protein was confirmed to be 2: 1 in NC-biotinylated CasSD and NY/CY-NC-biotinylated CasSD, and 1: 1 in C-biotinylated CasSD and NY/CY-C-biotinylated CasSD. Details of biotinylated protein preparation are given in the Supplemental Data section.
For chimeric proteins of biotinylated CasSD and YFP components (NY/CY-NC-biotinylated CasSD and NY/CY-C-biotinylated CasSD), yellow fluorescence was observed and estimated in bacteria and in solution by quantitative fluorescence microscopy. Solutions containing NY/CY-NC-biotinylated CasSD or NY/CY-C-biotinylated CasSD were as fluorescent as solutions containing bacterially expressed full-length YFP at the same concentration (0.8 μM).
Plasmids
Plasmids used in this work are described in the Supplemental Data section.
Stretching of Intact Cells
Cells plated on collagen (type I; Sigma-Aldrich)-coated stretchable silicone dishes (Sawada et al., 2001) were either stretched biaxially (and kept stretched) or left unstretched in our cell stretching system (Sawada et al., 2001; Tamada et al., 2004).
Covalent Avidin-coating of Latex Membrane and Preparation of Biotinylated Proteins Specifically Bound to Avidin-coated Latex Membrane
Avidin (Neutravidin) was covalently immobilized onto the surface of latex membrane by introducing the amine-reactive groups using Friedal-Crafts chemistry and biotinylated proteins were bound to the immobilized avidin. Details of preparation of biotinylated protein bound to latex membrane are described in the Supplemental Data section.
In Vitro Protein Extension (IPE) System
A biotinylated protein-bound latex membrane set in an adjustable tension ring was placed on a lubricated round-shaped glass stage and stretched biaxially and uniformly by pulling down the tension ring (Figure 3A, bottom). Magnitude of the latex membrane stretching was described as % change of length in each dimension. For example, 100% stretching represented twofold expansion in each dimension. To recover the protein for analysis, the protein-bound membrane (Figure 3A, bottom) was incubated with 1 x SDS sample buffer containing 0.12 M DTT at 95 °C for 5 min. Using amine-reactive, photocleavable biotin analog (NHS-PC-LC-Biotin, PIERCE) (Sawada and Sheetz, 2002), we confirmed that this procedure recovered the majority (>95%) of biotinylated proteins bound to the immobilized avidin.
YFP Amino-terminal Swapping Assay
NY/CY-NC-biotinylated, NY/CY-C-biotinylated (Figure 3B) or NC-biotinylated CasSD (Figure 3A) bound to avidin-coated latex membrane was prepared as described above. After stretching of latex membrane (100%) or without stretching, biotinylated CasSD proteins were washed 2 times with 1% BSA, 1% Triton X-100 in PBS and incubated with 2 μM His6-YFP-N in 1% BSA, 1% Triton X-100 in PBS containing 1 mM DTT for 10 min at room temperature. The bound protein complex on the latex membrane was washed 4 times with 1% BSA, 1% Triton X-100 in PBS and 2 times with 1% Triton X-100 in PBS, recovered with 1 x SDS sample buffer containing 0.12 M DTT. The samples were subjected to SDS-PAGE followed by anti-polyHistidine immunoblotting and avidin affinity blotting.
In Vitro Extension and Phosphorylation of CasSD
NC-biotinylated or C-biotinylated CasSD bound to avidin-coated latex membrane was prepared as described above. After stretching of latex membrane or without stretching, biotinylated CasSD proteins were washed 3 times with 0.25% Triton X-100, 2% BSA in buffer A (20 mM Hepes pH 7.5, 150 mM NaCl, 4 mM MgCl2, 1 mM DTT, 1 mM PMSF, 20 μg/ml aprotinin, 0.5 mM EGTA) and 3 times with 0.1% BSA in buffer A, and incubated with recombinant kinases (specific activity of each kinase used: 700 pmol/min phosphate transfer) in 350 μl of kinase reaction buffer (20 mM Hepes pH 7.5, 0.9 mM ATP, 0.1% BSA, 140 mM NaCl, 10 mM MgCl2, 3 mM MnCl2, 0.5 mM EGTA, 20 μg/ml aprotinin, 1.5 mM DTT, 1.5 mM Na3VO4, 0.03% Brij-35) for 2 min at room temperature. After kinase reaction, biotinylated CasSD proteins were washed 3 times with ice-cold 1% Triton X-100 in PBS containing 1 mM Na3VO4, recovered and solubilized by incubation with 1 x SDS sample buffer containing 0.12 M DTT at 95 °C for 5 min. Tyrosine phosphorylation of CasSD was determined by anti-phospho-Cas immunoblotting and avidin affinity blotting.
In Vitro Binding of αCas1 to Extended CasSD
NC-biotinylated or C-biotinylated CasSD bound to avidin-coated latex membrane was either extended (100%) or left unextended in PBS containing 1% Triton X-100, 2% BSA, 5% FBS, 1 mM DTT, 20 μg/ml aprotinin, and 0.5 mM EGTA. After 10 min, CasSD proteins on the latex surface were washed 3 times and incubated for 30 min with PBS containing 2% BSA, 5% FBS, 1 mM DTT, 20 μg/ml aprotinin, and 0.5 mM EGTA to block non-specific binding, and then incubated for 2 min with αCas1 diluted at 1:1200 in the same buffer. After 6 washes with PBS containing 0.1% Tween-20 and 1 mM DTT, bound proteins were solubilized with 1x SDS sample buffer containing 0.12 M DTT and subjected to SDS-PAGE followed by anti-rabbit IgG or αCas1 immunoblotting and avidin affinity blotting.
Binding of Two Different Anti-Cas Antibodies ( αCas1 and αCas3) to Triton Cytoskeletons
Triton cytoskeletons were prepared from Cas-deficient fibroblasts transiently expressing RFP-Cas or RFP alone as described previously (Sawada and Sheetz, 2002). After 2 washes with buffer A containing 2% BSA and 5% FBS, the buffer was replaced with the buffer A containing 0.5 mM ATP, 2% BSA, 5% FBS, and either αCas1 or αCas3 (1: 400 dilution) and Triton cytoskeletons were either stretched or left unstretched. After 2 min of incubation, samples were washed 2 times with buffer A containing 2% BSA and 5% FBS and 4 times with buffer A, solubilized with 1x SDS sample buffer containing 20 mM DTT, and subjected to SDS-PAGE followed by anti-rabbit IgG, αCas2 and anti-actin immunoblotting (Figure 5B, upper panel).
Immunofluorescence Staining, Fluorescence Microscopy, and Image Display
20 min after being plated on collagen (Type-I)-coated coverslips, Cas-deficient fibroblasts expressing RFP-Cas were washed with PBS, fixed with 3.7% formaldehyde in PBS, permeabilized with 0.1% Triton X-100 in PBS, stained using αCas1, αCas3 or pCas-165 as a primary antibody (1: 400 dilution for αCas1, αCas3 and 1:100 dilution for pCas-165) and Alexa Fluor 488 anti-rabbit IgG as a secondary antibody, and then viewed with a confocal microscope (Olympus IX-81 with FV500 system). Image intensity from the green channel (immunofluorescence with αCas1, αCas3 or pCas-165) and the red channel (RFP-Cas) was displayed with the contrast enhanced by setting the highest intensity in each image at the maximum value of the dynamic range and the background (cell-free area) at zero in ImageJ, a free open source Java imaging platform (http://rsb.info.nih.gov/ij).
Statistical Analysis
Statistical analysis was performed with the paired Student’s t-test and P < 0.05 was defined as significant.
Supplementary Material
Acknowledgments
We thank S. Ohkubo, T. Mandai, M. Cull, M. Galbmillion, and M.L. Bushey for assisting in the construction of the IPE system, P. S. Low, S. K. Hanks, T. Yamamoto, and M. Matsuda for the plasmids, J. M. Fernandez, M. Edidin, T. D. Perez, A. Sakakibara, C. D. Hu, H. Takayanagi, T. Miyazaki, T. Tezuka, M. Saitoh, K. Takeda, H. Ichijo and K. Nakamura for helpful discussions and consistent support. This work was supported by NIH Grant R01 EB001480.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Ballestrem C, Erez N, Kirchner J, Kam Z, Bershadsky A, Geiger B. Molecular mapping of tyrosine-phosphorylated proteins in focal adhesions using fluorescence resonance energy transfer. J Cell Sci. 2006;119:866–875. doi: 10.1242/jcs.02794. [DOI] [PubMed] [Google Scholar]
- Bougeret C, Rothhut B, Jullien P, Fischer S, Benarous R. Recombinant Csk expressed in Escherichia coli is autophosphorylated on tyrosine residue(s) Oncogene. 1993;8:1241–1247. [PubMed] [Google Scholar]
- Briknarova K, Nasertorabi F, Havert ML, Eggleston E, Hoyt DW, Li C, Olson AJ, Vuori K, Ely KR. The serine-rich domain from Crk-associated substrate (p130Cas) is a four-helix bundle. J Biol Chem. 2005;280:21908–21914. doi: 10.1074/jbc.M501258200. [DOI] [PubMed] [Google Scholar]
- Carrion-Vazquez M, Oberhauser AF, Fowler SB, Marszalek PE, Broedel SE, Clarke J, Fernandez JM. Mechanical and chemical unfolding of a single protein: a comparison. Proc Natl Acad Sci U S A. 1999;96:3694–3699. doi: 10.1073/pnas.96.7.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan JL. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- Chien S, Li S, Shiu YT, Li YS. Molecular basis of mechanical modulation of endothelial cell migration. Front Biosci. 2005;10:1985–2000. doi: 10.2741/1673. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Esch FS, Taylor SS, Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984;259:7835–7841. [PubMed] [Google Scholar]
- Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Dubin-Thaler BJ, Giannone G, Dobereiner HG, Sheetz MP. Nanometer analysis of cell spreading on matrix-coated surfaces reveals two distinct cell states and STEPs. Biophys J. 2004;86:1794–1806. doi: 10.1016/S0006-3495(04)74246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher TE, Oberhauser AF, Carrion-Vazquez M, Marszalek PE, Fernandez JM. The study of protein mechanics with the atomic force microscope. Trends Biochem Sci. 1999;24:379–384. doi: 10.1016/s0968-0004(99)01453-x. [DOI] [PubMed] [Google Scholar]
- Fonseca PM, Shin NY, Brabek J, Ryzhova L, Wu J, Hanks SK. Regulation and localization of CAS substrate domain tyrosine phosphorylation. Cell Signal. 2004;16:621–629. doi: 10.1016/j.cellsig.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell. 2002;110:139–142. doi: 10.1016/s0092-8674(02)00831-0. [DOI] [PubMed] [Google Scholar]
- Giannone G, Dubin-Thaler BJ, Dobereiner HG, Kieffer N, Bresnick AR, Sheetz MP. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–223. doi: 10.1016/j.tcb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Harte MT, Hildebrand JD, Burnham MR, Bouton AH, Parsons JT. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- Hattori M, Minato N. Rap1 GTPase: functions, regulation, and malignancy. J Biochem (Tokyo) 2003;134:479–484. doi: 10.1093/jb/mvg180. [DOI] [PubMed] [Google Scholar]
- Hu CD, Chinenov Y, Kerppola TK. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell. 2002;9:789–798. doi: 10.1016/s1097-2765(02)00496-3. [DOI] [PubMed] [Google Scholar]
- Huang J, Hamasaki H, Nakamoto T, Honda H, Hirai H, Saito M, Takato T, Sakai R. Differential regulation of cell migration, actin stress fiber organization, and cell transformation by functional domains of Crk-associated substrate. J Biol Chem. 2002;277:27265–27272. doi: 10.1074/jbc.M203063200. [DOI] [PubMed] [Google Scholar]
- Isakov N, Wange RL, Watts JD, Aebersold R, Samelson LE. Purification and characterization of human ZAP-70 protein-tyrosine kinase from a baculovirus expression system. J Biol Chem. 1996;271:15753–15761. doi: 10.1074/jbc.271.26.15753. [DOI] [PubMed] [Google Scholar]
- Jiang G, Giannone G, Critchley DR, Fukumoto E, Sheetz MP. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 2003;424:334–337. doi: 10.1038/nature01805. [DOI] [PubMed] [Google Scholar]
- Katsumi A, Milanini J, Kiosses WB, del Pozo MA, Kaunas R, Chien S, Hahn KM, Schwartz MA. Effects of cell tension on the small GTPase Rac. J Cell Biol. 2002;158:153–164. doi: 10.1083/jcb.200201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. Embo J. 1999;18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Bellin RM, Walker DL, Patel B, Powers P, Liu H, Garcia-Alvarez B, de Pereda JM, Liddington RC, Volkmann N, et al. Characterization of an actin-binding site within the talin FERM domain. J Mol Biol. 2004;343:771–784. doi: 10.1016/j.jmb.2004.08.069. [DOI] [PubMed] [Google Scholar]
- Mayer BJ, Hirai H, Sakai R. Evidence that SH2 domains promote processive phosphorylation by protein-tyrosine kinases. Curr Biol. 1995;5:296–305. doi: 10.1016/s0960-9822(95)00060-1. [DOI] [PubMed] [Google Scholar]
- Merkel R, Nassoy P, Leung A, Ritchie K, Evans E. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature. 1999;397:50–53. doi: 10.1038/16219. [DOI] [PubMed] [Google Scholar]
- Missbach M, Jeschke M, Feyen J, Muller K, Glatt M, Green J, Susa M. A novel inhibitor of the tyrosine kinase Src suppresses phosphorylation of its major cellular substrates and reduces bone resorption in vitro and in rodent models in vivo. Bone. 1999;24:437–449. doi: 10.1016/s8756-3282(99)00020-4. [DOI] [PubMed] [Google Scholar]
- Miyake I, Hakomori Y, Misu Y, Nakadate H, Matsuura N, Sakamoto M, Sakai R. Domain-specific function of ShcC docking protein in neuroblastoma cells. Oncogene. 2005 doi: 10.1038/sj.onc.1208523. [DOI] [PubMed] [Google Scholar]
- Nakamoto T, Sakai R, Honda H, Ogawa S, Ueno H, Suzuki T, Aizawa S, Yazaki Y, Hirai H. Requirements for localization of p130cas to focal adhesions. Mol Cell Biol. 1997;17:3884–3897. doi: 10.1128/mcb.17.7.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser AF, Badilla-Fernandez C, Carrion-Vazquez M, Fernandez JM. The mechanical hierarchies of fibronectin observed with single-molecule AFM. J Mol Biol. 2002;319:433–447. doi: 10.1016/S0022-2836(02)00306-6. [DOI] [PubMed] [Google Scholar]
- Okuda M, Takahashi M, Suero J, Murry CE, Traub O, Kawakatsu H, Berk BC. Shear stress stimulation of p130(cas) tyrosine phosphorylation requires calcium-dependent c-Src activation. J Biol Chem. 1999;274:26803–26809. doi: 10.1074/jbc.274.38.26803. [DOI] [PubMed] [Google Scholar]
- Rief M, Gautel M, Oesterhelt F, Fernandez JM, Gaub HE. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science. 1997;276:1109–1112. doi: 10.1126/science.276.5315.1109. [DOI] [PubMed] [Google Scholar]
- Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. Embo J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara A, Ohba Y, Kurokawa K, Matsuda M, Hattori S. Novel function of Chat in controlling cell adhesion via Cas-Crk-C3G-pathway-mediated Rap1 activation. J Cell Sci. 2002;115:4915–4924. doi: 10.1242/jcs.00207. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Nakamura K, Doi K, Takeda K, Tobiume K, Saitoh M, Morita K, Komuro I, De Vos K, Sheetz M, Ichijo H. Rap1 is involved in cell stretching modulation of p38 but not ERK or JNK MAP kinase. J Cell Sci. 2001;114:1221–1227. doi: 10.1242/jcs.114.6.1221. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Sheetz MP. Force transduction by Triton cytoskeletons. J Cell Biol. 2002;156:609–615. doi: 10.1083/jcb.200110068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin NY, Dise RS, Schneider-Mergener J, Ritchie MD, Kilkenny DM, Hanks SK. Subsets of the major tyrosine phosphorylation sites in Crk-associated substrate (CAS) are sufficient to promote cell migration. J Biol Chem. 2004;279:38331–38337. doi: 10.1074/jbc.M404675200. [DOI] [PubMed] [Google Scholar]
- Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Tang DD, Tan J. Role of Crk-associated substrate in the regulation of vascular smooth muscle contraction. Hypertension. 2003;42:858–863. doi: 10.1161/01.HYP.0000085333.76141.33. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- Wisniewska M, Bossenmaier B, Georges G, Hesse F, Dangl M, Kunkele KP, Ioannidis I, Huber R, Engh RA. The 1.1 A resolution crystal structure of the p130cas SH3 domain and ramifications for ligand selectivity. J Mol Biol. 2005;347:1005–1014. doi: 10.1016/j.jmb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.