Abstract
Neuregulin-1 (NRG1) and its receptor, ErbB4, have been implicated in schizophrenia at both gene and transcript levels. The present investigation compared NRG1 and ErbB4 protein levels in prefrontal cortical (PFC) cytoplasmic and nuclear fractions among normal, schizophrenic, bipolar and major depressed subjects from the Stanley Consortium. We used immunoblotting procedures to examine potential NRG1 and ErbB4 immunoreactive bands, but specifically quantified NRG1 immunoreactive signals at 42, 48 and 53kDa and ErbB4 immunoreactive signals at 21, 55, 60 and 180kDa. PFC cytoplasmic 53kDa NRG1 protein levels were significantly increased (~20%) in schizophrenic patients relative to each of the other subject groups. We also detected a trend towards diagnostic effects on PFC cytoplasmic full-length (180kDa) ErbB4 protein levels, and post hoc tests revealed that these quantities were significantly increased (~30%) in schizophrenic patients relative to normal and to depressed subjects. In addition, we examined the levels of potential ErbB4 cleavage products at 21, 55 and 60kDa relative to those of full-length ErbB4 in the PFC fractions. We detected trends for diagnostic effects on PFC cytoplasmic 21kDa/180kDa and 55kDa/180kDa ratios, and post hoc tests revealed that these ratios were significantly reduced in schizophrenic patients relative to normal individuals. Our investigation suggests that schizophrenia-associated NRG1 and ErbB4 mRNA elevations also occur at the protein level and may be specific to schizophrenia. We hypothesize that ErbB4 proteolytic processing may also be altered in schizophrenia, yielding altered ratios of functionally distinct forms of ErbB4.
Keywords: Stanley Consortium, Schizophrenia, Immunoblotting, Cytoplasm, Nucleus, Prefrontal Cortex, Neuregulin-1, ErbB4
1. Introduction
Neuregulin-1 (NRG1) and its receptor, ErbB4, have been genetically linked to schizophrenia susceptibility (Harrison and Law 2006;Nicodemus et al. 2006;Norton et al. 2006;Silberberg et al. 2006;Stefansson et al. 2002) and are critical in neurodevelopment (Anton et al. 2004;Ozaki et al. 2000;Sardi et al. 2006). Hence, NRG1 and ErbB4 are appealing molecules to study in schizophrenia, since this disease is hypothesized to have genetic and neurodevelopmental origins (Akbarian et al. 1996;Eastwood and Harrison 2003;Jones et al. 1994). Mice in which NRG1 and ErbB4 expression is reduced exhibit schizophrenia-associated phenotypes (Stefansson et al. 2002), while the prefrontal cortex (PFC) of schizophrenic subjects exhibit NRG1α protein reductions (Bertram et al. 2007). However, isoform-specific increases in PFC NRG1 and ErbB4 mRNA expression have also been observed in these patients (Hashimoto et al. 2004;Law et al. 2006;Law et al. 2007;Silberberg et al. 2006). Thus, it appears changes in brain NRG1 and/or ErbB4 levels may play roles in schizophrenia, although altered ErbB4 activation with no significant changes in NRG1 and ErbB4 protein quantities has recently been shown in patient brains (Hahn et al. 2006).
Only a few studies of brain NRG1 or ErbB4 protein levels have been conducted in schizophrenic subjects. Hahn et al. (2006) examined PFC NRG1 and ErbB4 protein in an aged schizophrenia population, while Bertram et al. (2007) analyzed PFC NRG1α protein though NRG1β may be more abundant in brain (Meyer and Birchmeier 1994; Wen et al. 1994). The present research sought to determine whether previously reported schizophrenia-associated alterations in PFC NRG1 and ErbB4 mRNA levels could be observed at the protein level in PFC tissues from the Stanley Consortium. We selected these tissues for study because schizophrenia-associated PFC ErbB4 transcript alterations were previously found in this cohort (Silberberg et al. 2006) and because these samples have not been examined with respect to NRG1 and ErbB4 protein. This cohort was also chosen for study because it consists of tissues from individuals with affective disorders, in which NRG1 has also recently been implicated (Green et al. 2005). Hence, this cohort would allow us to examine the diagnostic specificity of putative protein changes detected.
In addition, both NRG1 and ErbB4 proteins can be cleaved to produce different-sized fragments (Carpenter 2003;Falls 2003). Since full-length and cleaved NRG1 and ErbB4 can have unique functions in the cytoplasm and in the nucleus (Bao et al. 2003;Bao et al. 2004;Garcia et al. 2000;Hahn et al. 2006;Sardi et al. 2006;Wang et al. 1998), the levels of NRG1 and ErbB4 proteins, along with their cleavage products, may vary in mental illness according to intracellular compartment. Therefore, we examined the quantities of NRG1 and ErbB4 proteins, along with their possible cleavage products, in PFC cytoplasmic and nuclear fractions. To detect potential NRG1-associated and ErbB4-associated proteins, we used immunoblotting procedures with antibodies that can recognize the intracellular portions of NRG1 and ErbB4 and can detect both full-length and potential cleaved forms of these proteins. The focus of this investigation is to determine if PFC NRG1 and/or ErbB4 proteins, whether full-length or cleaved, are altered in schizophrenia and to ascertain whether NRG1/ErbB4 immunoreactive protein levels are abnormal in cytoplasmic or in nuclear fractions of PFC tissues in patients.
2. Materials and Methods
2.1. Human cohort
PFC tissues were derived from the Stanley Consortium, which consists of four diagnostic groups: normal controls (n=15), schizophrenia (n=15), bipolar disorder (n=15), and major depression (n=15) (Table 1). Diagnoses were performed independently by two psychiatrists according to DSM-IV criteria, and antipsychotic exposure was determined as an estimate of lifetime antipsychotic medication intake (fluphenazine milligram equivalents). The groups did not differ according to age [F(3,56)=0.728, p=0.540], pH [F(3,56)=0.611, p=0.611] or postmortem interval (PMI) [F(3,56)=1.857, p=0.147] and have been described previously (Torrey et al. 2000). Experiments were performed without knowledge of subject diagnoses, and diagnostic information was provided only after attainment of results.
Table 1.
Subject diagnostic groups and demographics.
| Factor | Normal | Schizophrenia | Bipolar Disorder |
Major Depression |
|---|---|---|---|---|
| Age (y) | 48.1 (29–68) | 44.5 (25–62) | 42.3 (25–61) | 46.5 (30–65) |
| Brain pH | 6.3 (5.8–6.6) | 6.2 (5.8–6.6) | 6.2 (5.8–6.5) | 6.2 (5.8–6.5) |
| PMI (h) | 23.7 (8–42) | 33.7 (12–61) | 32.5 (13–62) | 27.5 (7–47) |
| AP Exposure (×104 F-mg-Eq) | n.a. | 5.2 (0–20) | 2.1 (0–6) | n.a. |
| Gender | 9M/6F | 9M/6F | 9M/6F | 9M/6F |
| Race | 14C/1AA | 13C/2A | 14C/1AA | 15C |
For continuous variables (i.e. age, brain pH, PMI, AP Exposure), means are shown with ranges in brackets. y, Years; PMI, Post mortem interval; h, Hours; AP, Antipsychotic; F-mg-Eq, Fluphenazine milligram equivalents; M, Male; F, Female; C, Caucasian; AA, African American; A, Asian.
2.2. Nuclear and cytoplasmic protein isolation
Nuclear and cytoplasmic protein extractions were performed as described by Butler et al. (Butler et al. 1999). Frozen PFC tissues were first homogenized in ice-cold buffer 1 (20 mM HEPES, 5 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 1 mM DTT, 5 µg/ml aprotinin, 0.5 mg/ml bacitracin, 5 µg/ml pepstatin A, 5 µg/ml leupeptin, 40 µg/ml PMSF, pH 7.9) using a Dounce homogenizer. Homogenates were then centrifuged at 14 000g (4°C, 10minutes), and supernatants (cytoplasmic fractions) were collected on ice. The remaining nuclear pellets were briefly vortexed in buffer 2 (buffer 1, with 350 mM instead of 5 mM NaCl) and incubated in this solution (4°C, 20minutes) with constant rotation. Buffer 3 (buffer 1 with no NaCl) was then added (final [NaCl]=175 mM), and the resulting mixtures were precipitated on ice (20minutes). The mixtures were centrifuged at 14 000g (4°C, 10minutes), and supernatants (nuclear fractions) were collected on ice. Collected samples were stored at −80°C. Total protein concentrations of both fractions (n=60/cellular compartment or n=120 total) were determined for each subject using the Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA, USA).
2.3. Immunoblotting
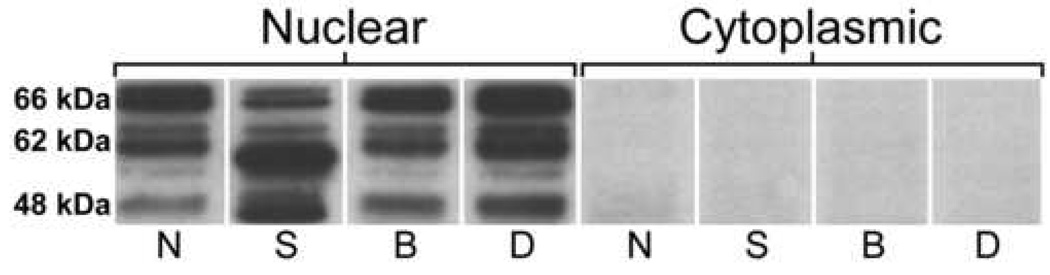
For each sample, 15 µg of total protein, along with a molecular weight ladder (SeeBlue Plus 2, Invitrogen, Carlsbad, CA, USA), were electrophoresed in pre-cast 4–12% bis-tris polyacrylamide NuPAGE gels (Invitrogen) (120V, 2hours), transferred onto nitrocellulose membranes (Invitrogen) (130V, 90minutes), and blocked in tris-buffered saline containing 0.1% Tween-20 (TBS-T) and either 10% normal goat serum (for NRG1 antibody) or 5% skim milk (for NeuN, ErbB4 and β-actin antibodies) (4°C, overnight). Blots were then incubated for 1hour [room temperature (RT)] with one of the following antibodies diluted in TBS-T containing 1% skim milk: anti-NeuN (1:1 000, MAB377, Chemicon, Temecula, CA, USA), anti-NRG1 (1:100, SC-348, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-ErbB4 (1:500, SC-283, Santa Cruz Biotechnology) or anti-β-actin (1:10 000, MAB1501, Chemicon). Since NeuN is a neuronal protein expressed highly in nuclei (Mullen et al. 1992), NeuN protein was examined in PFC cytoplasmic and nuclear isolates to assess purity (Figure 1). Following primary antibody exposure, membranes were washed in TBS-T and incubated for 2hours (RT) with appropriate peroxidase-conjugated secondary antibody in TBS-T. After washing, proteins of interest were detected using the ECL Plus Western Blotting Detection Reagents kit (Amersham Biosciences, Piscataway, NJ, USA).
Figure 1.
Representative sections of immunoblots showing NeuN detection in PFC nuclear but not cytoplasmic fractions of Stanley cohort subjects. Three predominant NeuN immunoreactive bands were detected in PFC nuclear fractions at 48, 62 and 66kDa, as labeled. N, Normal; S, Schizophrenia; B, Bipolar disorder; D, Depressed.
The membranes were then exposed to Kodak Bio-Max films (Eastman Kodak Company, Rochester, NY, USA) for 1–60minutes with longer exposure times used for weaker immunoreactive signals. Developed films were scanned using a Hewlett-Packard Scanjet 8200 (Hewlett-Packard, Palo Alto, CA, USA), and optical densities (O.D.) of detected bands were quantified using ImageJ software (http://rsb.info.nih.gov/ij/, NIH, Bethesda, MD, USA). The specificity of analyzed immunoreaction signals was confirmed in preabsorption experiments in which antibodies were preincubated with a 5-fold excess of their respective epitope-containing peptides or of a non-related peptide. Further controls included: (a) ensuring the optical density of each analyzed band was within the linear range of detection; (b) controlling for gel-to-gel variability by normalizing the O.D. of each analyzed band to that of a corresponding band detected in an a pooled sample (all subjects; termed “internal control”) also run on each gel under identical conditions as samples; (c) controlling for lane-to-lane variability by normalizing the internal control-normalized O.D. of each band to that of a β-actin immunoreaction signal detected in the same lane; (d) running samples in duplicate either in individual gels or whole experimental runs whenever unusual variance was detected.
2.4. Statistical analyses
Statistica software (Statsoft, Tulsa, OK, USA) was used to analyze PFC NRG1 or ErbB4 O.D. levels among diagnostic groups for each immunoreactive band in each cellular fraction. The normalized values were used in the analyses for each detected band. Outliers above or below two standard deviations from the mean value of the diagnostic group were excluded. Pearson correlation tests were used to determine whether continuous variables [i.e. age, pH and postmortem interval (PMI)] correlated significantly with outlier-free data. If correlations were detected, ANCOVA procedures were performed with respect to diagnosis with appropriate covariates. If no correlations were detected, ANOVA procedures were performed. However, to examine the robustness of any ANOVA findings, we performed ANCOVA analyses on identified diagnostically affected immunoreactive bands using both pH and PMI as covariates. LSD post hoc tests were performed when significant differences or a trend towards a significant difference was found by ANCOVA or ANOVA. Pearson correlation tests were used to ascertain whether significant correlations existed between quantities of NRG1 and ErbB4 immunoreactive bands which were significantly affected by diagnosis. Significance was at p≤0.05.
3. Results
3.1. Detected NRG1 and ErbB4 immunoreactive bands
The NRG1 antibody used reacts with a carboxy terminal region present in the intracellular portion of NRG1 isoforms and can recognize full-length NRG1 and intracellular cleavage products (Frenzel and Falls 2001). Among the samples, we detected NRG1 immunoreactive bands ranging from 23–130kDa, which were preabsorbable with the NRG1 peptide but not with an unrelated peptide (Figure 2A). Based on NRG1 molecular weights reported previously, we speculate that the 88–130kDa immunoreactive bands represent full-length NRG1 (Frenzel and Falls 2001;Hahn et al. 2006;Law et al. 2004) while the 48–59kDa immunoreactive bands correspond to cleaved NRG1 intracellular fragments (Bao et al. 2004;Carroll et al. 1997;Frenzel and Falls 2001;Hahn et al. 2006;Law et al. 2004). The smaller immunoreactive bands ranging from 23–42kDa have not been documented in neuronal tissue/cells, but are comparable to NRG1 molecular weights observed in peripheral tissue (Cote et al. 2005;Hirata et al. 2007;Lebrasseur et al. 2003).
Figure 2.
Representive immunoblots showing immunoreactive bands detected (a) by the NRG1 antibody and (b) by the ErbB4 antibody in the internal control. Internal control consists of a pool of all PFC protein samples. In each figure, the first lane displays the resulting immunoreactive bands following no preabsorption (None), the second lane displays the resulting immunoreactive bands following preabsorption with the NRG1 peptide (N) or the ErbB4 peptide (E) and the third lane displays the resulting immunoreactive bands following preabsorption with an unrelated oxytocin peptide (O) used as a negative control. A molecular weight ladder is shown on the left of each figure, and arrowed bands are fragments further analyzed in the investigation.
The ErbB4 antibody also reacts with a carboxy terminal region present in the intracellular portion of ErbB4 and can recognize full-length ErbB4 and intracellular cleavage products of the receptor (Garcia et al. 2000;Hahn et al. 2006;Sardi et al. 2006;Thompson et al. 2007). Among the samples, this antibody detected ErbB4 immunoreactive bands at roughly 180 and 80kDa (Figure 2B), which correspond to full-length ErbB4 and the cleaved ErbB4 intracellular domain, respectively (Elenius et al. 1997;Elenius et al. 1999;Hahn et al. 2006;Plowman et al. 1993;Vecchi et al. 1996). The ErbB4 antibody also detected several immunoreactive bands ranging from 21–62kDa (Figure 2B), which have not been described in human brain. However, we previously detected the 21, 55, 60 and 180kDa immunoreactive bands in non-human primate brain (Thompson et al. 2007). All detected ErbB4 immunoreactive fragments were preabsorbable with the ErbB4 peptide but not with an unrelated peptide (Figure 2B).
3.2. NRG1 intracellular cleavage domain (NRG1-ICD) protein levels are increased in cytoplasmic fractions of the PFC in schizophrenia
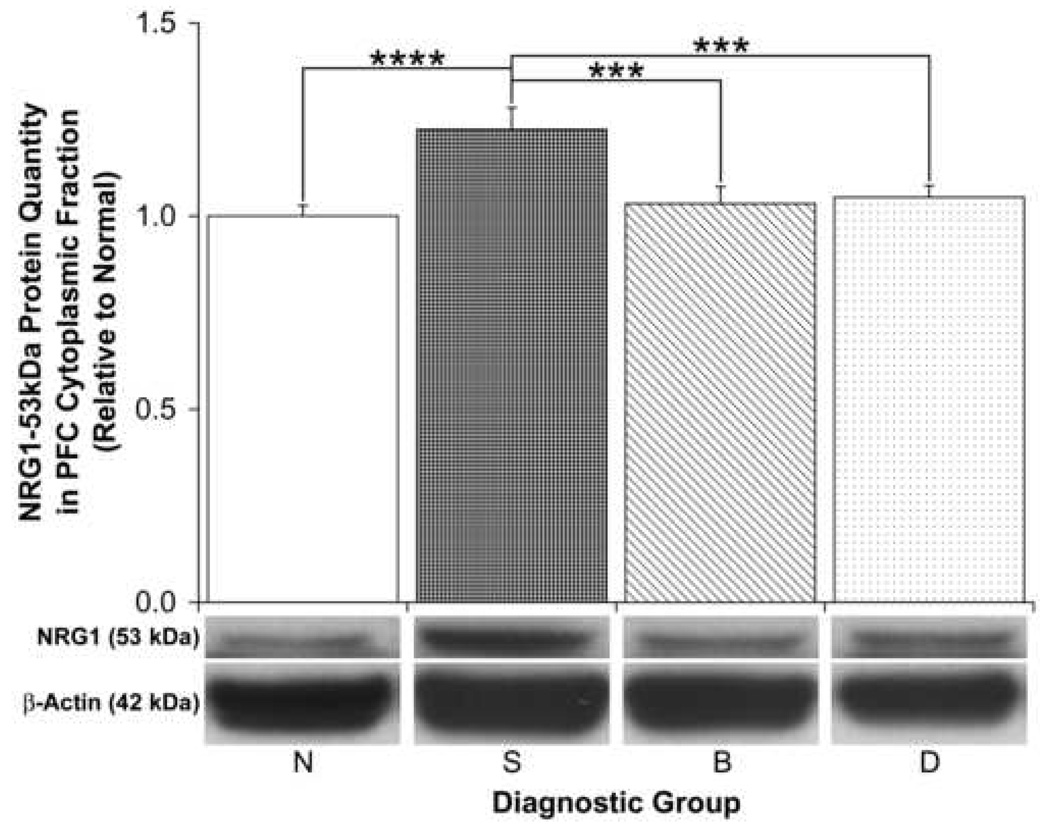
We specifically examined the NRG1 immunoreactive proteins detected at 42, 48 and 53kDa because they were preabsorbable and were consistently present in both PFC compartments of all subjects. Furthermore, the 53kDa fragment detected by the antibody we used has been functionally characterized as the NRG1-ICD (Bao et al. 2003;Bao et al. 2004;Wang et al. 1998). Only the 53kDa NRG1 protein was found to be significantly affected by diagnosis specifically in PFC cytoplasmic fractions [ANOVA: F(3,53)=5.94, p=0.001; Table 2; Figure 3]. We also found this diagnostic effect to be significant by ANCOVA with pH and PMI as covariates [F(3,51)=4.91, p=0.004]. Post hoc tests demonstrated that the NRG1-ICD was significantly increased (>20%) in PFC cytoplasmic fractions of schizophrenic patients relative to normal (p≤0.001), to bipolar (p≤0.01) and to depressed (p≤0.01) subjects (Figure 3). However, no significant differences in nuclear NRG1-ICD levels were detected (Table 2), and no significant differences in the levels of the 48 or 42kDa bands were detected in either cytoplasmic or nuclear fractions of the PFC (Table 2).
Table 2.
ANOVA results for diagnostic effects on NRG1 and ErbB4 immunoreactive band quantities in PFC cytoplasmic and nuclear fractions.
| Analyzed Fragment |
PFC Fraction | F | df1, df2 | p |
|---|---|---|---|---|
| NRG1-53kDa | C | 5.94 | 3, 53 | 0.001 |
| NRG1-48kDaa | C | 0.30 | 3, 51 | 0.824 |
| NRG1-42kDa | C | 0.78 | 3, 55 | 0.510 |
| ErbB4-180kDa | C | 2.65 | 3, 52 | 0.058 |
| ErbB4-60kDa | C | 1.32 | 3, 53 | 0.277 |
| ErbB4-55kDa | C | 0.72 | 3, 52 | 0.547 |
| ErbB4-21Kda | C | 1.28 | 3, 50 | 0.291 |
| NRG1-53kDa | N | 1.43 | 3, 51 | 0.244 |
| NRG1-48kDa | N | 0.18 | 3, 52 | 0.909 |
| NRG1-42kDa | N | 0.17 | 3, 53 | 0.914 |
| ErbB4-180kDab | N | 2.92 | 3, 51 | 0.043 |
| ErbB4-60kDaa | N | 0.51 | 3, 52 | 0.677 |
| ErbB4-55kDab | N | 0.49 | 3, 51 | 0.694 |
| ErbB4-21Kda | N | 0.94 | 3, 53 | 0.429 |
Fragments further analyzed by post hoc tests are bolded.
Covaried for PMI
Covaried for pH; C, Cytoplasm; N, Nucleus.
Figure 3.
Relative PFC cytoplasmic NRG1-53kDa protein quantities among diagnostic groups. Representative sections of immunoblots are shown below the graph. Data are expressed relative to normal (N) subjects, and error bars represent standard errors. S, Schizophrenia; B, Bipolar disorder; D, Depressed (***p ≤ 0.01; ****p ≤ 0.001).
3.3. ErbB4 full-length protein levels are increased in cytoplasmic and nuclear fractions of the PFC in schizophrenia
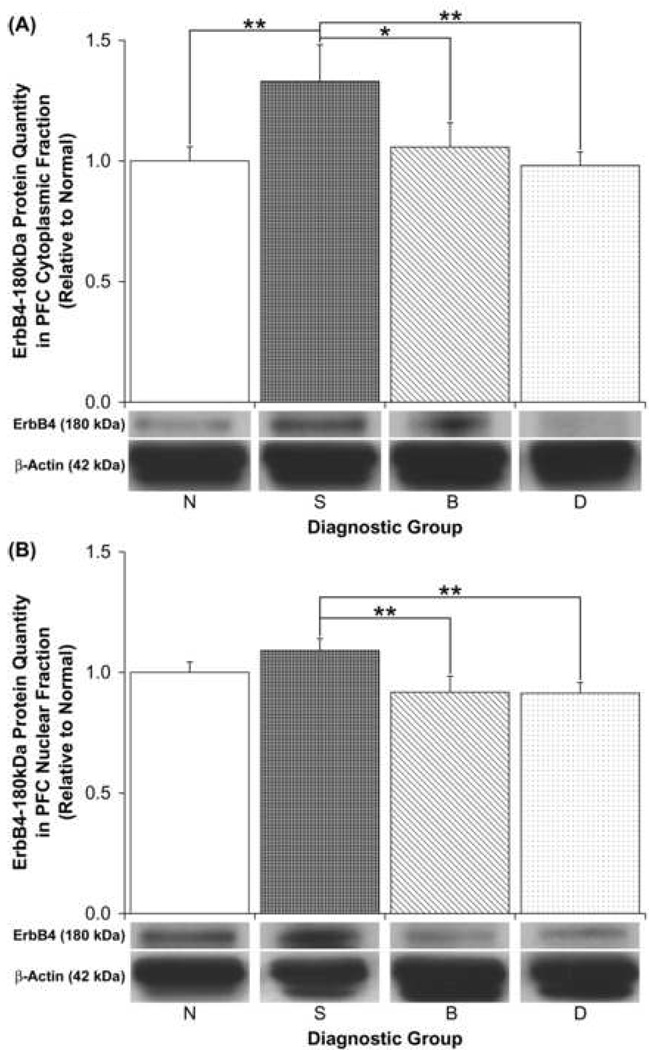
We specifically examined the ErbB4 immunoreactive bands observed at 21, 55, 60 and 180kDa because they were preabsorbable and were consistently present in both PFC compartments of all subjects. These proteins were also studied because they were previously detected with two distinct ErbB4 carboxy terminal antibodies in non-human primate brain (Thompson et al. 2007). We detected a significant effect of diagnosis on PFC cytoplasmic levels of the full-length 180kDa ErbB4 band by ANCOVA using both pH and PMI as covariates [F(3,50)=3.05, p=0.037]. However, only a trend towards a significant effect of diagnosis on PFC cytoplasmic full-length ErbB4 levels was observed when an ANOVA was conducted on these quantities without these covariates [F(3,52)=2.65, p=0.058; Table 2; Figure 4A]. Post hoc tests revealed significant increases (>30%) in PFC cytoplasmic full-length ErbB4 levels in schizophrenic patients relative to normal subjects (p≤0.03) and to depressed patients (p≤0.02) (Figure 4A). A trend for increased PFC cytoplasmic full-length ErbB4 protein quantities was also observed in schizophrenic patients relative to bipolar individuals (ANOVA, p=0.06) (Figure 4A).
Figure 4.
Relative PFC (a) cytoplasmic and (b) nuclear ErbB4-180kDa protein quantities among diagnostic groups. Representative sections of immunoblots are shown below the graphs. Data are expressed relative to normal (N) subjects, and error bars represent standard errors. S, Schizophrenia; B, Bipolar disorder; D, Depressed (*p = 0.058; **p ≤ 0.03).
With respect to PFC nuclear levels of the analyzed ErbB4 immunoreactive bands, we detected a significant effect of diagnosis on full-length ErbB4 protein levels by ANOVA [F(3,51)=2.92, p=0.043; Table 2; Figure 4B] and ANCOVA using both pH and PMI as covariates [F(3,50)=2.74, p=0.05]. Post hoc tests demonstrated that the full-length ErbB4 protein was significantly increased (~20%) in PFC nuclear fractions of schizophrenic patients relative to bipolar patients (p≤0.02) and to depressed (p≤0.02) subjects (Figure 4B). However, no difference between schizophrenic and normal subjects in the nuclear levels of full-length ErbB4 was detected (Figure 4B). No significant diagnostic differences in the levels of the 21, 55 and 60kDa ErbB4 immunoreactive bands were detected by ANOVA in either cytoplasmic or nuclear fractions of the PFC (Table 2), suggesting that the PFC ErbB4 quantity increases we observed were specific to full-length ErbB4.
3.4. Effects of possible confounding variables on the examined NRG1 and ErbB4 proteins
In terms of NRG1, a significant positive correlation was detected between PMI and levels of the 48kDa NRG1 fragment in PFC cytoplasmic fractions (R=0.402, p=0.002). No significant correlations were detected between continuous variables and the levels of the remaining NRG1 immunoreactive bands in the cytoplasm or nucleus (R≤│0.255│, p≥0.06). No significant correlations were detected between lifetime neuroleptic estimates and levels of any of the NRG1 immunoreactive bands in either of the examined PFC fractions when schizophrenic and bipolar subjects were analyzed together or when these subject groups were analyzed separately (R≤│0.511│, p≥0.074).
With respect to ErbB4, significant positive correlations between brain pH and the levels of the 180kDa (R=0.361, p=0.006) and 55kDa (R=0.423, p=0.001) ErbB4 bands and between PMI and the levels of the 60kDa ErbB4 fragment (R=−0.362, p=0.006) were detected in PFC nuclear fractions only. No significant correlations were detected between continuous variables and the remaining ErbB4 immunoreactive bands in either of the examined PFC fractions (R≤│0.248│, p≥0.063). No significant correlations were detected between the lifetime neuroleptic estimates and the levels of any of the ErbB4 immunoreactive bands in either of the examined PFC fractions when schizophrenic and bipolar subjects were analyzed together or when these subject groups were analyzed separately (R≤│0.312│, p≥0.131).
We believe the NRG1 and ErbB4 proteins we selected for analysis do not represent protein degradation fragments for the following three reasons: 1) neuronal cell cultures with no PMIs express similar sized NRG1 proteins (i.e. 42, 48 and 53 kDa) (Bao et al. 2004) and ErbB4 proteins (i.e. 21, 55, 60 and 180 kDa) (unpublished data); 2) the ErbB4 proteins we studied were previously detected in non-human primate brains with minimal PMIs (Thompson et al. 2007); 3) prefrontal cortical quantities of the examined smaller NRG1 and ErbB4 immunoreactive bands rarely correlated with PMI among the entire cohort or in any individual diagnostic group. We speculate the additional fragments detected for NRG1 and ErbB4 represent novel intracellular cleavage products, since the antibodies used recognize intracellular portions of NRG1 and ErbB4, both of which can be cleaved to release intracellular molecules (Bao et al. 2004;Ni et al. 2001;Sardi et al. 2006;Zhang et al. 2006). Alternatively, these smaller immunoreactive fragments may represent NRG1-like or ErbB4-like proteins, though the presence of multiple immunoreactive bands for both NRG1 and ErbB4 is not unexpected (Bao et al. 2004;Carroll et al. 1997;Cote et al. 2005;Frenzel and Falls 2001;Hahn et al. 2006;Hirata et al. 2007;Law et al. 2004;Lebrasseur et al. 2003;Thompson et al. 2007).
3.5. Putative ErbB4 cleavage product levels relative to full-length ErbB4
While the putative full-length NRG1 proteins (88–130kDa) could not be detected reliably in all PFC isolates, full-length ErbB4 protein could be measurably detected in all samples. Since the ErbB4 antibody used reacts with an intracellular region of ErbB4 isoforms, we speculated that detected ErbB4 immunoreactive fragments smaller than 80kDa may represent additional uncharacterized intracellular cleavage products. Thus, we examined whether the quantities of these putative ErbB4 intracellular cleavage products (i.e. at 21, 55 and 60kDa) expressed as ratios of full-length ErbB4 levels (180kDa) varied according to diagnosis in either the cytoplasm or nucleus. While we did not detect a significant diagnostic effect on any of these ratios in either PFC fraction by ANOVA (Table 3), we detected a trend towards a diagnostic effect on both 55kDa/180kDa ErbB4 ratios [F(3,54)=2.75, p=0.052; Table 3; Figure 5A] and 21kDa/180kDa ErbB4 ratios [F(3,53)=2.63, p=0.059; Table 3; Figure 5B] in cytoplasmic fractions by ANOVA. Post hoc tests revealed that PFC cytoplasmic 55kDa/180kDa ratios and PFC cytoplasmic 21kDa/180kDa ratios were significantly reduced specifically in schizophrenic patients relative to normal subjects (p≤0.01 for both ratios; Figures 5A and 5B, respectively).
Table 3.
ANOVA results for diagnostic effects on quantities of potential ErbB4 intracellular cleavage products (i.e. at 60, 55 and 21kDa) expressed as ratios of the 180kDa full-length ErbB4 band in PFC cytoplasmic and nuclear fractions.
| Analyzed ErbB4 Ratios | PFC Fraction | F | df1, df2 | p |
|---|---|---|---|---|
| 60kDa/180kDa | C | 1.80 | 3, 52 | 0.159 |
| 55kDa/180kDa | C | 2.75 | 3, 54 | 0.052 |
| 21kDa/180kDa | C | 2.63 | 3, 53 | 0.059 |
| 60kDa/180kDa | N | 1.37 | 3, 54 | 0.261 |
| 55kDa/180kDa | N | 0.49 | 3, 55 | 0.693 |
| 21kDa/180kDa | N | 1.13 | 3, 51 | 0.346 |
Ratios further analyzed by post hoc tests are bolded. C, Cytoplasm; N, Nucleus.
Figure 5.
Relative PFC cytoplasmic (a) ErbB4 55kDa/180kDa and (b) ErbB4 21kDa/180kDa ratios among diagnostic groups. Data are expressed relative to normal (N) subjects, and error bars represent standard errors. S, Schizophrenia; B, Bipolar disorder; D, Depressed (***p ≤ 0.01).
3.6. Effect of ethnicity and family psychiatric history on NRG1-ICD and full-length ErbB4 protein levels
Since NRG1 and ErbB4 have been associated with schizophrenia at the gene level, we examined the effects of the genetically influenced factors, ethnicity and family psychiatric history, on PFC amounts of NRG1-ICD and full-length ErbB4, which were the only proteins displaying diagnostically specific schizophrenia-associated quantity changes in our study. Race did not affect PFC cytoplasmic NRG1-ICD protein levels [F(2, 54)=2.12, p=0.130] and did not affect PFC full-length ErbB4 protein levels in cytoplasmic [F(2, 53)=1.69, p=0.194] or in nuclear [F(2, 53)=2.18, p=0.123] fractions. However, since Caucasians make up >90% of the Stanley Consortium and hence likely contribute most to our observations, we also analyzed diagnostic effects on PFC NRG1-ICD and full-length ErbB4 protein quantities among Caucasians only. With the exception of the schizophrenia-associated PFC nuclear full-length ErbB4 elevations we observed, our positive findings remained unchanged when non-Caucasians were removed from the analyses. In fact, we detected more robust diagnostically specific schizophrenia-associated alterations in PFC cytoplasmic full-length ErbB4 quantities [ANOVA: F(3, 48)=3.32, p=0.028; post hoc: p≤0.02, schizophrenic relative to normal, bipolar and depressed subjects] and in PFC cytoplasmic 55kDa/180kDa ErbB4 ratios [ANOVA: F(3, 50)=4.06, p=0.012; post hoc: p≤0.001, schizophrenic relative to normal subjects] when we considered Caucasians alone.
With respect to examining the cohort (n=60) according to family psychiatric history, 25 subjects were negative for major psychiatric disorder among 1st or 2nd degree relatives, 19 subjects had definite or probable psychosis among 1st or 2nd degree relatives and 13 subjects had definite or probable depression among 1st or 2nd degree relatives. No information regarding family psychiatric history was available for the remaining 3 subjects. Interestingly, we found that family psychiatric history status significantly affected PFC cytoplasmic full-length ErbB4 protein quantities [F(2, 50)=8.44, p=0.0007] and that subjects with definite or probable psychosis among 1st or 2nd degree relatives displayed significant increases in prefrontal cortical cytoplasmic full-length ErbB4 (≥40%; p≤0.002) relative to those of the other family psychiatric history groups. However, it is important to be cautious when interpreting this finding because the available information on family psychiatric history has not been rigorously assessed in every case.
3.7. Correlation between NRG1 and ErbB4 immunoreactive bands
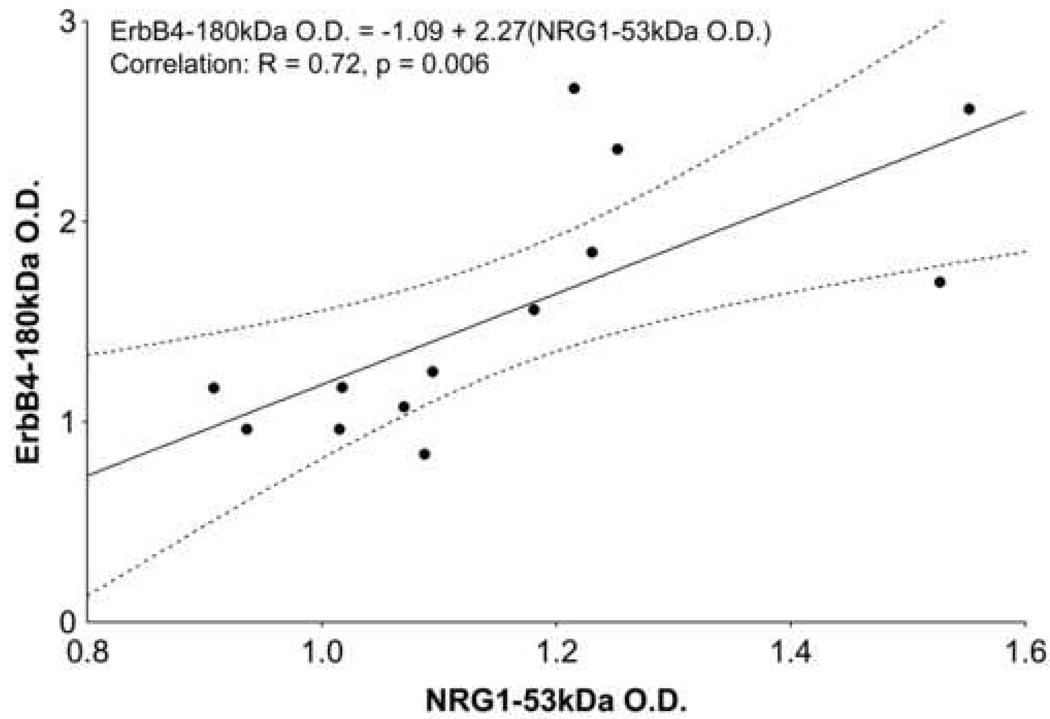
Since schizophrenia-associated elevations were observed in PFC cytoplasmic NRG1-ICD and full-length ErbB4 proteins, Pearson correlation analysis was performed to determine whether the levels of increased full-length ErbB4 and increased NRG1-ICD proteins correlated. The NRG1-ICD levels significantly and positively correlated with full-length ErbB4 levels in PFC cytoplasmic fractions specifically in the schizophrenic group (R=0.72, p=0.006) (Figure 6), but not in normal, bipolar and major depressed groups (R≤│0.441│, p≥0.132).
Figure 6.
Correlation between ErbB4-180kDa and NRG1-53kDa protein quantities in PFC cytoplasmic fractions of schizophrenic patients. A correlation was observed specifically between these fragments exclusively in schizophrenic subjects. Equation of best fit line (solid line), along with correlation coefficient (R) and p-value, is inset. Dots represent data points; dotted line encloses data within 95% confidence.
4. Discussion
We found that both NRG1-ICD (53kDa) and full-length ErbB4 (180kDa) protein levels were elevated in PFC cytoplasmic fractions of schizophrenic patients relative to the other subject groups. These findings appear consistent with previous reports showing isoform-specific increases in PFC NRG1 and ErbB4 mRNA levels in schizophrenia (Hashimoto et al. 2004;Law et al. 2007;Silberberg et al. 2006). Furthermore, none of the analyzed PFC NRG1 and ErbB4 immunoreactive fragments were altered in major depressed or bipolar subjects, suggesting the schizophrenia-associated PFC increases in NRG1-ICD and full-length ErbB4 protein levels were specific to schizophrenia. Hence, our results suggest the mechanism of NRG1 gene dysregulation in schizophrenia may be distinct from that in bipolar disorder since the NRG1 gene has been implicated in bipolar illness as well (Green et al. 2005). We also found a significant positive correlation between NRG1-ICD and full-length ErbB4 levels exclusively in schizophrenic patients, but not in normal subjects. This preliminary observation may suggest that this NRG1 and ErbB4 relationship is unusually constrained in schizophrenic patients due to the disease process.
Our previous finding of schizophrenia-associated NRG1 mRNA increases (Hashimoto et al. 2004;Law et al. 2006) suggests elevated transcription may contribute to the increased PFC cytoplasmic NRG1-ICD levels in schizophrenia. However, the relation between NRG1 mRNA isoforms and the NRG1-ICD we detected is unclear. The elevations in PFC cytoplasmic NRG1-ICD could also indicate enhanced PFC NRG1 intracellular cleavage, which would have implications in NRG1 back signaling-mediated gene expression and cytoskeletal organization in schizophrenia. Specifically, the NRG1-ICD can repress apoptotic protein expression (Bao et al. 2003) as well as bind and promote nuclear translocation of transcription factors like Eos, which enhances postsynaptic density-95 (PSD-95) transcription (Bao et al. 2004). The NRG1-ICD also interacts with LIM kinase 1 in the cytoplasm where this association could regulate LIM kinase 1-mediated actin polymerization (Wang et al. 1998). Hence, the schizophrenia-associated PFC cytoplasmic NRG1-ICD elevations may suggest augmentation of any of these NRG1-ICD-mediated events in the schizophrenic PFC. Alternatively, the elevated cytoplasmic NRG1-ICD levels could suggest defective NRG1-ICD-mediated nuclear translocation in the PFC of schizophrenic subjects, as we did not detect diagnostic changes in PFC nuclear NRG1-ICD levels.
Since we could not reliably measure full-length NRG1 in all subjects, it is difficult to predict whether increased synthesis or intracellular cleavage more likely explains the elevated PFC cytoplasmic NRG1-ICD. In the case of ErbB4, however, we could readily detect full-length ErbB4 and the smaller putative ErbB4 cleavage products. Hence, we were able to show that while PFC cytoplasmic full-length ErbB4 levels were elevated in schizophrenic subjects, PFC cytoplasmic ratios between 55 and 180kDa ErbB4 levels and between 21 and 180kDa ErbB4 quantities were reduced in schizophrenic patients. These findings, together with the previous observations of schizophrenia-associated ErbB4 mRNA increases (Law et al. 2007;Silberberg et al. 2006), may indicate PFC full-length ErbB4 synthesis is elevated with decreased intracellular cleavage of the receptor in schizophrenia.
Interestingly, several NRG1 and ErbB4 immunoreactive bands were found in both cytoplasmic and nuclear fractions of subjects in all diagnostic categories. This observation suggests that many NRG1- and ErbB4-derived proteins may translocate from the cytoplasm into the nucleus and that NRG1 and ErbB4 can regulate gene expression and/or nuclear protein shuttling, as hypothesized previously (Bao et al. 2004;Sardi et al. 2006). In addition, we detected the 180kDa ErbB4 protein in PFC nuclear fractions, supporting suggestions that full-length ErbB4 can enter the nucleus without being cleaved (Carpenter 2003), though the function of full-length ErbB4 in neuronal nuclei is unknown. Since schizophrenic patients had PFC elevations in nuclear full-length ErbB4 compared to bipolar subjects and to depressed patients, the nuclear actions of the 180kDa protein in the PFC may be worthy of further investigation.
If our findings do reflect the schizophrenia-associated NRG1 and ErbB4 mRNA elevations reported previously, then the NRG1-ICD protein we detected would be expected to be derived from the type I isoform (Hashimoto et al. 2004;Law et al. 2006) while the full-length ErbB4 protein we observed would be expected to consist of JMa and/or Cyt1 domains (Law et al. 2007;Silberberg et al. 2006). Based on these assumptions, our observations would support previous evidence of enhanced PFC NRG1-ErbB4 signaling in schizophrenia (Hahn et al. 2006) because the extracellular domains of both NRG1 type I and JMa-containing ErbB4 isoforms can be cleaved to activate membrane-bound ErbB4 and NRG1, respectively (Bao et al. 2003;Carpenter 2003;Falls 2003). In addition, if the 180kDa ErbB4 protein is indeed derived from Cyt1-containing isoforms, which have phosphatidyl inositol 3 kinase (PI3K) sites, our findings may suggest that the increased full-length ErbB4 is associated with alterations in PI3K-regulated processes previously implicated in schizophrenia (Kalkman 2006;Law et al. 2007).
An alternative explanation for the observed concomitant PFC NRG1 and ErbB4 protein elevations in schizophrenia is that these increases are compensatory responses to defective NRG1-ErbB4 signaling. More specifically, if feed-forward NRG1-ErbB4 signaling (i.e. NRG1 to ErbB4) is intact, an up-regulation of both NRG1 type I and JMa-containing ErbB4 proteins would be expected to contribute to greater ErbB4-regulated downstream signaling events, including intracellular cleavage of the receptor. Yet, we found that full-length ErbB4 content increased with no alterations in the quantities of the smaller ErbB4 immunoreactive bands we detected in PFC cytoplasmic fractions of schizophrenic subjects. Moreover, the quantities of two of the detected potential ErbB4 intracellular cleavage fragments (i.e. at 21 and 55kDa) relative to full-length ErbB4 levels were significantly less in PFC cytoplasmic fractions of schizophrenic individuals. These reductions may indicate decreased PFC ErbB4 intracellular cleavage and hence abnormal feed-forward NRG1-ErbB4 signaling in these patients.
One limitation of our study is that we could not distinguish the particular variants of the detected NRG1 and ErbB4 immunoreactive bands due to the “pan” nature of the antibodies implemented. Furthermore, our observations conflict with those of Hahn et al., who showed no alterations in NRG1 or ErbB4 protein quantities in the PFC of schizophrenic patients (Hahn et al. 2006). This discrepancy may be due to the age difference between our study group (mean age ~45y) and their relatively old cohort (mean age ~79y) that could possess aging-associated confounds (e.g. oxidative stress and DNA damage), which can influence protein levels (Lu et al. 2004;Serrano and Klann 2004). In addition, one study reported schizophrenia-associated reductions in PFC NRG1α protein (Bertram et al. 2007). This observation, along with the fact our NRG1 antibody detects α and β isoforms, may suggest the increased NRG1-ICD is not derived from the α isoform. Thus, it appears precise schizophrenia-associated alterations in PFC NRG1 protein quantities may vary according to the NRG1 type measured.
In conclusion, we found that the PFC shows schizophrenia-specific alterations in the quantities of two interacting growth factor molecules. We also demonstrated NRG1 and ErbB4 alterations in distinct PFC intracellular compartments, involving both the NRG1-ICD and full-length ErbB4. These observations, along with the finding of novel NRG1 and ErbB4 immunoreactive bands, add to the complexity of our understanding of NRG1-ErbB4 signaling, particularly with respect to implications in schizophrenia. More studies are required to ascertain how the elevated NRG1 and ErbB4 immunoreactive proteins relate to the increased NRG1 and ErbB4 mRNA in the PFC of schizophrenic patients. Further research is also necessary to examine if the NRG1 and ErbB4 protein elevations in schizophrenia could be compensatory responses to aberrant NRG1-ErbB4 signaling. In addition, the PFC cell types associated with the observed schizophrenia-associated protein elevations need to be determined to understand the type of NRG1-ErbB4 signaling altered (paracrine, juxtacrine and/or autocrine) along with the neuronal circuits affected. These details may allow us to better understand the mechanism of concomitant NRG1 and ErbB4 protein elevations in the PFC as well as the consequences of our findings in relation to schizophrenia pathology and treatment.
Acknowledgment
The authors would like to thank Eva Tomaskovic-Crook for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE, Jr, Jones EG. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry. 1996;53:425–436. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim TW, Mei L, Dai P, Ohlemiller KK, Ambron RT. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat Neurosci. 2004;7:1250–1258. doi: 10.1038/nn1342. [DOI] [PubMed] [Google Scholar]
- Bao J, Wolpowitz D, Role LW, Talmage DA. Back signaling by the Nrg-1 intracellular domain. J Cell Biol. 2003;161:1133–1141. doi: 10.1083/jcb.200212085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram I, Bernstein HG, Lendeckel U, Bukowska A, Dobrowolny H, Keilhoff G, Kanakis D, Mawrin C, Bielau H, Falkai P, Bogerts B. Immunohistochemical evidence for impaired neuregulin-1 signaling in the prefrontal cortex in schizophrenia and in unipolar depression. Ann N Y Acad Sci. 2007;1096:147–156. doi: 10.1196/annals.1397.080. [DOI] [PubMed] [Google Scholar]
- Butler JA, Kallo I, Sjoberg M, Coen CW. Evidence for extensive distribution of oestrogen receptor alpha-immunoreactivity in the cerebral cortex of adult rats. J Neuroendocrinol. 1999;11:325–329. doi: 10.1046/j.1365-2826.1999.00346.x. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Nuclear localization and possible functions of receptor tyrosine kinases. Curr Opin Cell Biol. 2003;15:143–148. doi: 10.1016/s0955-0674(03)00015-2. [DOI] [PubMed] [Google Scholar]
- Carroll SL, Miller ML, Frohnert PW, Kim SS, Corbett JA. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J Neurosci. 1997;17:1642–1659. doi: 10.1523/JNEUROSCI.17-05-01642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote GM, Miller TA, Lebrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp Cell Res. 2005;311:135–146. doi: 10.1016/j.yexcr.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol Psychiatry. 2003;8(769) doi: 10.1038/sj.mp.4001399. 821-769, 831. [DOI] [PubMed] [Google Scholar]
- Elenius K, Choi CJ, Paul S, Santiestevan E, Nishi E, Klagsbrun M. Characterization of a naturally occurring ErbB4 isoform that does not bind or activate phosphatidyl inositol 3-kinase. Oncogene. 1999;18:2607–2615. doi: 10.1038/sj.onc.1202612. [DOI] [PubMed] [Google Scholar]
- Elenius K, Corfas G, Paul S, Choi CJ, Rio C, Plowman GD, Klagsbrun M. A novel juxtamembrane domain isoform of HER4/ErbB4. Isoform-specific tissue distribution and differential processing in response to phorbol ester. J Biol Chem. 1997;272:26761–26768. doi: 10.1074/jbc.272.42.26761. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Frenzel KE, Falls DL. Neuregulin-1 proteins in rat brain and transfected cells are localized to lipid rafts. J Neurochem. 2001;77:1–12. doi: 10.1046/j.1471-4159.2001.t01-1-00132.x. [DOI] [PubMed] [Google Scholar]
- Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EK, Raybould R, Macgregor S, Gordon-Smith K, Heron J, Hyde S, Grozeva D, Hamshere M, Williams N, Owen MJ, O'Donovan MC, Jones L, Jones I, Kirov G, Craddock N. Operation of the schizophrenia susceptibility gene, neuregulin 1, across traditional diagnostic boundaries to increase risk for bipolar disorder. Arch Gen Psychiatry. 2005;62:642–648. doi: 10.1001/archpsyc.62.6.642. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry. 2004;9:299–307. doi: 10.1038/sj.mp.4001434. [DOI] [PubMed] [Google Scholar]
- Hirata M, Sakuma K, Okajima S, Fujiwara H, Inashima S, Yasuhara M, Kubo T. Increased expression of neuregulin-1 in differentiating muscle satellite cells and in motoneurons during muscle regeneration. Acta Neuropathol (Berl) 2007;113:451–459. doi: 10.1007/s00401-007-0198-5. [DOI] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Kalkman HO. The role of the phosphatidylinositide 3-kinase-protein kinase B pathway in schizophrenia. Pharmacol Ther. 2006;110:117–134. doi: 10.1016/j.pharmthera.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5' SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Shannon WC, Hyde TM, Kleinman JE, Harrison PJ. Neuregulin-1 (NRG-1) mRNA and protein in the adult human brain. Neuroscience. 2004;127:125–136. doi: 10.1016/j.neuroscience.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Lebrasseur NK, Cote GM, Miller TA, Fielding RA, Sawyer DB. Regulation of neuregulin/ErbB signaling by contractile activity in skeletal muscle. Am J Physiol Cell Physiol. 2003;284:C1149–C1155. doi: 10.1152/ajpcell.00487.2002. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Distinct isoforms of neuregulin are expressed in mesenchymal and neuronal cells during mouse development. Proc Natl Acad Sci U S A. 1994;91:1064–1068. doi: 10.1073/pnas.91.3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- Nicodemus KK, Luna A, Vakkalanka R, Goldberg T, Egan M, Straub RE, Weinberger DR. Further evidence for association between ErbB4 and schizophrenia and influence on cognitive intermediate phenotypes in healthy controls. Mol Psychiatry. 2006;11:1062–1065. doi: 10.1038/sj.mp.4001878. [DOI] [PubMed] [Google Scholar]
- Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, Spurlock G, Kirov G, Buckland P, Waddington JL, Gill M, Corvin AP, Owen MJ, O'Donovan MC. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Tohyama K, Kishida H, Buonanno A, Yano R, Hashikawa T. Roles of neuregulin in synaptogenesis between mossy fibers and cerebellar granule cells. J Neurosci Res. 2000;59:612–623. doi: 10.1002/(SICI)1097-4547(20000301)59:5<612::AID-JNR4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Plowman GD, Culouscou JM, Whitney GS, Green JM, Carlton GW, Foy L, Neubauer MG, Shoyab M. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci U S A. 1993;90:1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M, Lauderdale S, Webster MJ, Chong VZ, McClintock B, Saunders R, Weickert CS. Widespread expression of ErbB2, ErbB3 and ErbB4 in non-human primate brain. Brain Res. 2007;1139:95–109. doi: 10.1016/j.brainres.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Webster M, Knable M, Johnston N, Yolken RH. The stanley foundation brain collection and neuropathology consortium. Schizophr Res. 2000;44:151–155. doi: 10.1016/S0920-9964(99)00192-9. [DOI] [PubMed] [Google Scholar]
- Vecchi M, Baulida J, Carpenter G. Selective cleavage of the heregulin receptor ErbB-4 by protein kinase C activation. J Biol Chem. 1996;271:18989–18995. doi: 10.1074/jbc.271.31.18989. [DOI] [PubMed] [Google Scholar]
- Wang JY, Frenzel KE, Wen D, Falls DL. Transmembrane neuregulins interact with LIM kinase 1, a cytoplasmic protein kinase implicated in development of visuospatial cognition. J Biol Chem. 1998;273:20525–20534. doi: 10.1074/jbc.273.32.20525. [DOI] [PubMed] [Google Scholar]
- Wen D, Suggs SV, Karunagaran D, Liu N, Cupples RL, Luo Y, Janssen AM, Ben Baruch N, Trollinger DB, Jacobsen VL. Structural and functional aspects of the multiplicity of Neu differentiation factors. Mol Cell Biol. 1994;14:1909–1919. doi: 10.1128/mcb.14.3.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Prentiss L, Heitzman D, Stahl RC, DiPino F, Jr, Carey DJ. Neuregulin isoforms in dorsal root ganglion neurons: effects of the cytoplasmic domain on localization and membrane shedding of Nrg-1 type I. J Neurosci Res. 2006;84:1–12. doi: 10.1002/jnr.20861. [DOI] [PubMed] [Google Scholar]