Abstract
Social behavior changes dramatically during primate adolescence. However, the extent to which testosterone and other gonadal hormones are necessary for adolescent social behavioral development is unknown. In this study, we determined that gonadectomy significantly impairs social dominance in naturalistic settings and changes reactions to social stimuli in experimental settings. Rhesus macaques were castrated (n = 6) or sham operated (n = 6) at age 2.4 years, group-housed for 2 years, and ethograms were collected weekly. During adolescence the gonadally intact monkeys displayed a decrease in subordinate behaviors and an increase in dominant behaviors, which ultimately related to a rise in social status and rank in the dominance hierarchy. We measured monkey’s reactions to emotional faces (fear, threat, neutral) of conspecifics of three ages (adult, peer, infant). Intact monkeys were faster to retrieve a treat in front of a threatening or infant face, while castrated monkeys did not show a differential response to different emotional faces or ages. No group difference in reaction to an innate fear-eliciting object (snake) was found. Approach and proximity responses to familiar versus unfamiliar conspecifics were tested, and intact monkeys spent more time proximal to a novel conspecific as compared to castrates who tended to spend more time with a familiar conspecific. No group differences in time spent with novel or familiar objects were found. Thus, gonadectomy resulted in the emergence of significantly different responses to social stimuli, but not non-social stimuli. Our work suggests that intact gonads, which are needed to produce adolescent increases in circulating testosterone, impact social behavior during adolescences in primates.
Keywords: Social dominance, sex hormones, testosterone, puberty, fear, object novelty, social novelty, social brain
Introduction
Human adolescence represents a period of increased incidence of antisocial behavior, risk-taking, anxiety, mood disorders and increased chance of expressing the first schizophrenia symptoms (particularly social withdrawal). Thus, a greater understanding of the factors involved in normal and abnormal social development in adolescence may help to maximize the chance of a successful transition into normal adulthood. Gonadal hormones, including testosterone, are known to play key roles in brain development, mood, behavior and cognition and can modulate symptoms of psychiatric disorders (Akhondzadeh et al., 2006; Angold & Costello, 2006; Halari et al., 2004; Ko et al., 2006; Toufexis, Myers, & Davis, 2006). Much research on the neurobiological and behavioral impact of sex steroids has focused either on the effects of testosterone during early fetal/neonatal development or during adulthood (Cochran & Perachio, 1977; Huhtaniemi, Nevo, Amsterdam, & Naor, 1986; Mazur, 1976; Rose, Holaday, & Bernstein, 1971). However, adolescence is a critical transition period between childhood and adulthood, which encompasses significant increases in sex steroids along with unique reproductive, neural and behavioral changes (Blakemore & Choudhury, 2006; Casey, Jones, & Hare, 2008; Yurgelun-Todd, 2007). Our work focuses on how sex steroids may be directly involved in the behavioral and/or neurobiological changes during adolescent development. This is our first report, from a series of experiments, which is aimed at testing how gonadectomy impacts the neurobiology of behavior in the adolescent male rhesus macaque.
Adolescents are more susceptible to drug abuse, have a greater propensity for risk-taking and spend more time in social interactions than children or adults (Spear, 2000). Structural imaging studies indicate both progressive and regressive changes occur during adolescence in specific brain regions involved in social development, especially the prefrontal cortex and amygdala (Giedd et al., 1999; Gogtay et al., 2004; Schumann et al., 2004; Sowell, Thompson, Holmes, Jernigan, & Toga, 1999). In functional imaging studies, adolescents showed increased activity of social brain networks as compared to adults, including anterior cingulate, orbital frontal, medial prefrontal and posterior superior temporal cortex (Blakemore, den Ouden, Choudhury, & Frith, 2007; Monk et al., 2003; Moriguchi, Ohnishi, Mori, Matsuda, & Komaki, 2007). In this study, we postulate that sex hormones are involved in orchestrating changes in social brain networks and test if gonadectomy would result in differences in social behavior during adolescence.
Testosterone, aside from its traditional roles in the development of sexual circuitry early in life, has organizational effects on sex-specific behavior as late as puberty in male rats and hamsters (Primus & Kellogg, 1989, 1990; Schulz, Menard, Smith, Albers, & Sisk, 2006; Sisk, Schulz, & Zehr, 2003). Monkeys have more similar endocrine systems and comparable anatomy of frontal brain regions to humans, in addition to having complex social relationships and cognitive abilities, which makes monkeys ideal models for studying complex developmental phenomenon in humans (Plant, 2006). Successful adolescent development of primates depends on the emergence of appropriate social behavior. Like humans, adolescent male rhesus macaques undergo considerable social changes during the transition from juvenile to adult. In the wild, pubertal male monkeys usually leave their natal group at around 3 years of age and must emigrate to a new social group in order to socialize and breed (Vessey & Meikle, 1987). For males, sexual maturity occurs at about 3.5 – 4 years of age (Van Wagenen & Simpson, 1954) and significant increases in testosterone occur during this time (Gordon, Rose, & Bernstein, 1976). Correlational studies have reported associations between testosterone levels and social dominance in adolescent chimpanzees and rhesus macaques (Anestis, 2006; Rose, 1978). Social dominance is an important predictor of establishment in a new group and male reproductive success (Vessey & Meikle, 1987). Thus, gonadally produced testosterone may represent a particularly important influence on eventual reproductive success in rhesus macaques via its effect on social dominance. However, since these are correlational studies, the extent to which the increase in testosterone and other gonadal hormones drive the maturation in social behavior and change in social dominance rank in adolescence is not clear, as it is possible that greater social competency and stature leads to an increase in the production of gonadal hormones. The purpose of this study was to determine if gonadectomy just prior to puberty leads to specific changes in social behavior as compared to non-social behavior. Over a period of two years, we monitored natural changes in social behavior between an intact and castrated group of monkeys, and we also tested the monkey’s responses to social (emotional facial expressions and novel conspecifics), as compared to non-social stimuli (fear-provoking stimuli and novel objects).
Methods
Subjects
All animal procedures used in this study conformed to the National Institute of Health Guide for the Care and Use of Laboratory Animals (DHEW Publication 80-23, Revised 1985, Office of Science and Health Reports, DRR/ NIH, Bethesda, MD). A total of 12 male rhesus macaques (Macaca mulatta) were chosen from those born at the National Institute of Health Animal Center’s (NIHAC) primate field station as history of maternal social status, matriline rank, birth and health records and experimental history are available for these juveniles. The field station consists of approximately 5 acres of land with a large pond, many trees and approximately 100 monkeys from three different matrilines. The 12 monkeys (n = 6 gonadectomized group and n = 6 intact group) were taken from the field station at approximately 27 months of age and were moved to enclosed housing. We received 8 monkeys in the first year of the study and 4 additional monkeys in the second year of the study. This was due to constraints in acquiring monkeys which is determined by the size of the birth cohort, the number of males and the number of other projects that are being conducted. Two equally sized separate social groups of monkeys were established. Pairs (one monkey to be gonadectomized and one monkey to remain intact) were assigned to group housing with four monkeys in each social group in year 1, six monkeys in each social group in year 2 and one social group of four monkeys in year 3. Groups were housed in one of several large indoor/outdoor habitats ranging from the smallest (3’7” by 5’ by 8’10”) to the largest (33’ by 4’4” by 10’) based on housing availability. Monkeys had water and primate chow available ad libitum, and were supplemented with treats (i.e., fresh fruit, peanuts) several times per week. All monkeys weighed between 3.2 and 5.39 kg, had basal morning testosterone levels of less than 0.06 ng/mL and showed no other signs of puberty, such as canine teeth or descended testes, at the beginning of the study. We selected male animals who were closely matched for age (born within 40 days) for a given birth cohort. At age 29 months (~2.4 years), half of the monkeys from each social group were selected at random for gonadectomy (n = 6) or a sham operation (n = 6) with the constraint the resulting groups were comparable with regard to average matriline rank, maternal rank and body weight. As a rough estimate, one monkey year equates to about 4 human years, although many aspects of monkey postnatal development are relatively accelerated or delayed when comparing across species. Table 1 shows the experimental groups did not differ systematically on any of these factors at the start of the study.
Table 1.
The list of subjects showing matriline rank assigned to each surgical condition as well as weight (kg) at the beginning of the experiment.
| Subject | Matriline Rank |
Maternal Rank |
Social Group |
Data of Birth | Date of surgery |
Weight (kg) |
Condition |
|---|---|---|---|---|---|---|---|
| 1 | 2 (Mid) | Mid | 1 | 29-Apr-03 | 20-Sep-05 | 4.80 | Gonadectomized |
| 2 | 3 (Low) | Mid | 1 | 02-May-03 | 20-Sep-05 | 4.78 | Gonadectomized |
| 3 | 1 (High) | High | 1 | 02-Apr-04 | 28-Sep-06 | 4.80 | Gonadectomized |
| 4 | 2 (Mid) | Low | 2 | 25-Apr-03 | 21-Sep-05 | 4.30 | Gonadectomized |
| 5 | 2 (Mid) | Mid-Low | 2 | 27-Apr-03 | 21-Sep-05 | 5.39 | Gonadectomized |
| 6 | 2 (Mid) | Low | 2 | 16-Apr-04 | 28-Sep-06 | 4.00 | Gonadectomized |
| 7 | 3 (Low) | Low | 1 | 05-Apr-03 | 20-Sep-05 | 4.81 | Intact |
| 8 | 2 (Mid) | Low | 1 | 02-May-03 | 20-Sep-05 | 4.97 | Intact |
| 9 | 1 (High) | Low | 1 | 01-Apr-04 | 28-Sep-06 | 4.20 | Intact |
| 10 | 1 (High) | High | 2 | 21-Apr-03 | 21-Sep-05 | 4.62 | Intact |
| 11 | 2 (Mid) | High | 2 | 28-Apr-03 | 21-Sep-05 | 4.06 | Intact |
| 12 | 2 (Mid) | Mid | 2 | 24-Apr-04 | 28-Sep-06 | 3.20 | Intact |
Surgeries
Animals were sedated with 6 mg/kg telazol IM and placed on isoflurane anesthesia. A midline incision was made between the inguinal rings to allow access to both scrotal tunics. The tunic was opened, testicle exteriorized, double ligated and removed. After hemostatis was ensured, a neuticle was placed and the tunic was closed with 4.0 PDS. Sterilized neuticles (CTI Corporation, 0.75 inches, natural shape) were surgically implanted in the castrated monkeys to control for any obvious visual differences in the gross outward appearance of the genitalia resulting from castration. The surgical procedure was repeated to the opposite testicle and the skin incision was closed with 4-0 PDS via subcuticular pattern. The animals were recovered and returned to individual holding cages for three days until the cuts were healed and then they were returned to their social groups. Post surgical antibiotics and analgesics were administered. The sham control animals underwent the same anesthesia administration and recovery at the same exact time (yoked to their control) but did not have any further surgical manipulations performed.
Endocrine Measurements
Blood samples were drawn every 6–8 weeks beginning at age 28 months, one month prior to surgery, and continuing to the conclusion of the study. Samples were taken at two different daily times, 9:30 am and 11:00 pm, to track the diurnal variations in testosterone in addition to the monthly and seasonal variations. The 11:00 pm blood draws were initiated at age 38 months for eight animals (gonadectomized n = 4, intact n = 4), and at age 30 months for four animals (gonadectomized n = 2, intact n = 2). All samples were drawn under ketamine anesthesia (0.05 mg/kg body weight). Approximately 3 mL of blood was drawn in an EDTA lined vacuum tube from all animals on the same day within a 30-minute window and animals were weighed at the time of the blood draw. Samples were centrifuged and plasma was stored at −80°C. Plasma concentrations of testosterone, estradiol and leutinizing hormone (LH) were measured by radio immuno-assay at the Yerkes National Primate Research Center Biomarkers Core Lab at Emory University.
Social Dominance (Ethograms)
Behavior in the home cage social groups was observed by trained raters for 25 minute sessions approximately once per week per cage for the duration of the study (from age 30 months to 50 months). Observations were made between 10:00 am and 4:00 pm and the monkeys were acclimated to the presence of the observers for several minutes before observations began. Ethograms were used to categorize behavior into 24 different categories (e.g., Winslow, Noble, Lyons, Sterk & Insel, 2003) and over seventy ethograms were collected from each social group over the course of this study. In the 25 minute observation period for each ethogram, all monkeys within each social group were observed using a time sampling procedure and thus all the time periods were sampled evenly for each subject and the behavior from the castrates and the intact animals were collected under identical conditions.
To measure social dominance, social behaviors were classified as dominant or subordinate. We selected behaviors based upon previous reviews of social dominance in macaques (e.g., Altman, 1962) and dominant behaviors included initiating a mount, displacement, and chasing. Conversely, subordinate behaviors included receiving a mount, avoiding, and fleeing from another monkey. Social dominance was defined as the difference between the total number of observed dominant behaviors and subordinate behaviors for each monkey in each observation session. These difference scores were then averaged across two time periods in order to compare the dominance hierarchy before and after the pubertal increase in testosterone. The first time period, “pre-puberty,” corresponded to age 30 months to 36 months and the second, “post-puberty,” corresponded to age 39 months to 50 months. Thirty-nine months was considered the time point at which puberty began since this was the first month intact monkeys expressed nighttime plasma testosterone levels that were significantly higher than that of gonadectomized monkeys. Changes in social behavior were also determined from the ethogram data by calculating the average frequency of dominant and subordinate behaviors during the pre-pubertal and post-pubertal period for each monkey.
Emotional face task and snake task
Monkeys were tested in a Wisconsin general testing apparatus (WGTA) during the emotional face task. To perform tasks in the WGTA, the monkey was placed in a cage (24” × 28” × 30”) adjacent to a white plastic tray containing 3 wells in which small food rewards can be placed (board- 38” across, wells 2” apart). The experimenter sat behind a divider and viewed the monkey indirectly through a video camera screen. Between trials, an opaque screen was lowered between the monkey and the testing tray. All animals were acclimated to the testing apparatus over months.
At age 43 months (after the onset of puberty) the latency to retrieve a food treat placed in front of photographs of novel monkey faces was tested. Twenty-eight photographs of novel monkeys were used. The photographs were obtained from the NIMH primate core archives, and the Yerkes and Oregon primate center websites. Photographs were chosen that showed monkeys facing as forward as possible for eye contact and then converted to a grayscale image and equated for size and contrast. Images were classified into three age groups: baby, peer or adult and three different emotional valences: neutral, fear grimace, or threat. There were therefore ten classifications of photographs presented: Adult Neutral, Adult Fear, Adult Threat, Peer Neutral, Peer Fear, Peer Threat, Baby Neutral, Baby Fear, Baby Threat and Control. The control stimuli consisted of two different solid grey shapes the approximate size of the faces in the test photographs (3” × 5”). A mini-marshmallow was placed in front of each photograph and after the screen was raised, the monkey’s latency to retrieve the reward was measured. If the monkey failed to retrieve the treat within one minute, the trial was terminated and a score of 60 seconds was recorded. All monkeys successfully retrieved a treat from in front of the control stimuli 20 times during training. All sessions were videotaped and the difference scores for response latencies were calculated by subtracting the average latency to respond to the control stimuli from the average latency to respond to each category of photograph listed above for each monkey.
Monkeys were also tested for their reaction to different objects unrelated to faces in the WGTA, including an innately fear eliciting rubber snake. At approximately 44 months of age, the latency to retrieve a treat placed in front of ten different objects was measured. The non-snake objects were small toys without any discernable facial features and small enough to be grasped single-handedly by the monkey. The size of each toy approached but did not exceed 6.35 cm × 6.35 cm by 6.35 cm (height × width × length). The toys had been used by our group in prior studies but were novel to the monkeys on day one and were not expected to produce a fear reaction. The series of single objects were presented to each monkey over three days with each monkey being tested with each object on each day. The food reward was placed in front of each object and the trial ended after the reward was retrieved or 60 seconds elapsed. The order of object presentation was random with the constraint that the rubber snake was not presented either first or last. Testing was conducted over three consecutive days and all sessions were videotaped and response latencies were scored. Response latencies to the rubber snake and to the toy objects were measured and analyzed by three-way ANOVA [with the independent variables, testing day (1, 2 or 3), valence of the object (neutral or fear), and experimental condition of the monkey (castrated or intact)]. To be consistent to what was done for the emotional face task difference scores were calculated for each monkey by subtracting the average latency to respond to the other nine objects from the latency to respond to the rubber snake.
Social novelty test
The novelty tests occurred between the ages of 52 and 53 months. The social novelty test took place in a large wire cage (3’7” by 5’ by 8’10”) in a separate room from the home cage. Each monkey was removed from their home cage and placed alone in a large testing cage containing two smaller holding cages at opposite ends of the testing cage, four meters apart. The smaller cages were similar to one another and allowed the monkey within the cages to view and interact with the test monkey through the bars of the cage. The subject monkey was left alone in the cage for an initial 15 minute acclimatization period. After acclimatization, a familiar monkey from the subject’s home cage was introduced to one of the holding cages placed at the end of the test area. After a 15 minute period spent with the familiar monkey, a novel monkey from a different housing colony and age-matched to the subject was introduced to the second holding cage located at the opposite end of the test area. This marked the beginning of a final 15 minute period which the subject could choose to spend time closer to the familiar or closer to the novel monkey at opposite ends of the test area. The position of the novel and familiar monkeys were counterbalanced between groups. A video camera placed above the test cage was used to record the movement and the location of the subject in the test area. The test area was divided into eight equal sized quadrants and a quadrant proximate to the novel monkey and a quadrant proximate to the familiar monkey were demarcated. The latency of time to approach the novel and familiar monkey quadrant (secs), the total number of approaches and the total duration of time spent in each of these quadrants (secs) was measured.
Object novelty test
The object novelty test took place in a set of three adjacent holding cages that were located in the home cage. The walls of these holding cages were arranged to allow free movement between each cage. The test subject was allowed to enter these cages and acclimatize for a 15 minute period. After acclimatization, a novel play object # 1 was attached by a chain to the wall at one end of the cages. The test monkey was once again allowed to freely explore the three compartments for 15 mins. After this familiarization period, novel play object # 2 was attached to the side wall at the opposite end of the cages. The monkey was then allowed to freely explore the three compartments and interact with the two objects for a further 15 mins. During this period, the latency of time to initially approach each object (secs) as well as the total number of times each object was approached, and the total duration of time (secs) spent touching the novel object (#2) and familiar object (#1) were measured.
Results
Weight and Endocrine Measures
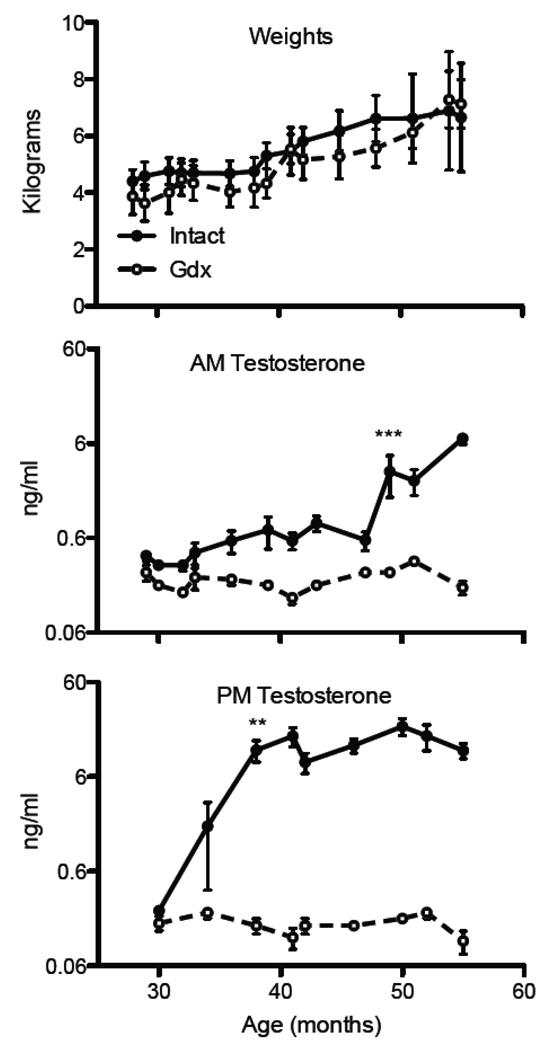
Total body weight measurements taken from 4 months of age and continuing throughout the study showed no significant differences between the intact and castrate monkeys at any point although a slightly lower mean group weight for the castrated monkeys was found at the middle time points (Figure 1, top panel). To account for diurnal hormone fluctuations, testosterone was measured from blood plasma taken at 9:00AM (Figure 1, middle panel) and 11:00PM (Figure 1, bottom panel). For intact monkeys, average testosterone levels at night were approximately 10 fold higher than morning levels (t (5) = 8.74, p < 0.001, paired t-test). Morning testosterone levels in intact animals were not significantly different from that of gonadectomized animals before surgery, (t (10) = 1.86, p > 0.05). After surgery, the average morning testosterone levels in the intact monkeys were two to three times higher than the gonadectomized monkeys until age 49 months when it significantly increased to 12 to 30 times higher than the gonadectomized monkeys (t(10) = 8.02, p < 0.001, comparing group difference scores before 49 months vs after 49 months). Night time testosterone levels were comparable among the subset of monkeys from which we collected data before surgery (Figure 1, bottom panel), however testosterone levels were over 70 times higher in the intact group relative to the gonadectomized group at 38 months, t (10) = 3.87, p = 0.002. Although no significant differences in weight were observed between groups, a significant correlation was found between weight and morning testosterone levels for intact animals (r = 0.84, p < 0.04). No significant correlations between weight and testosterone levels were found for gonadectomized animals.
Figure 1.
Weight and testosterone levels throughout adolescence. The top panel shows body weights (kg) did not differ significantly between the intact animals (filled circles) and gonadectomized animals (unfilled circles) at any time point. The middle panel shows morning testosterone levels were significantly different between groups beginning at 49 months of age (*** p < 0.001). Night testosterone levels were significantly different between the intact group as compared to the gonadectomized group beginning at 38 months age (** p < 0.01). Data represent mean ± SEM.
To control for any possible changes in estradiol due to pubertal castration, we measured serum estradiol. Estradiol levels were below the normal assay range (estradiol < 5.00 pg/mL) at all times sampled for both the castrate and intact groups. In order to confirm that gonadectomy was complete and that hypothalamic response was normal, Lutenizing hormone (LH) was measured once at 9:30 am and once at 11:00 pm when the monkeys were approximately 3 years old. LH was significantly higher in gonadectomized animals than intact animals both in the morning (4.0 ± 0.8 ng/ml vs. 0.4 ± 0.1 ng/ml; p = 0.001, unpaired t-test) and at night (8.0 ± 2.0 ng/ml vs. 0.9 ± 0.2 ng/ml; p = 0.006, unpaired t-test).
Social Dominance
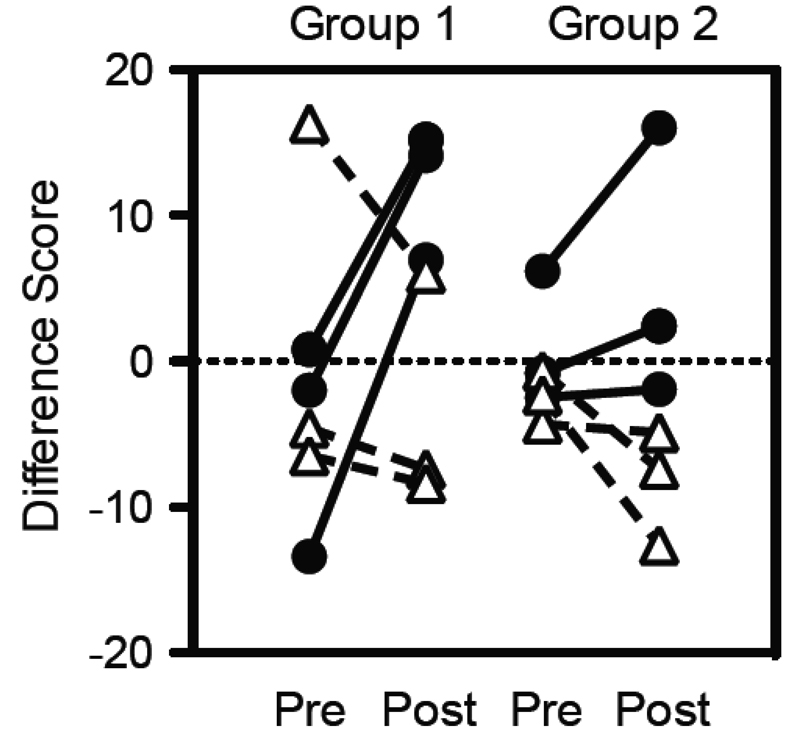
Social dominance (defined as the difference between the number of observed dominant and subordinate behaviors) was compared before and after the onset of puberty We defined the beginning of puberty as the first time point where testosterone differed significantly (after 38 months of age, Figure 1) and avoided using any behavioral measures from the previous two months in either calculation as testosterone levels were beginning to rise (months 36–38). In both social groups, each intact monkey showed an increase in dominance score while each gonadectomized monkey showed little change in dominance score. Each monkey’s individual score was used as a ranking system to define the social hierarchy of each social group. In the first group (Figure 2, Group 1), the dominant monkey (alpha) was gonadectomized and was subsequently superceded in rank by the second and third place monkeys, both of whom were intact monkeys and so experienced the normal rise in testosterone in adolescence. The most subordinate member in this social group, an intact monkey, also slightly superceded the original alpha gonadectomized monkey by the end of the study. The remaining two gonadectomized monkeys also lost rank to the originally subordinate intact monkey, resulting in the three intact monkeys occupying the first three positions in the social hierarchy and the three gonadectomized monkeys occupying the bottom three subordinate positions. In the second social group (Figure 2, Group 2), the dominant monkey, an intact, was able to maintain his status, and the closely ranked remaining monkeys separated as the dominance hierarchy was established across puberty. As is clear in the figure, this separation resulted in each intact monkey attaining the highest three dominant positions while the gonadectomized monkeys occupied the lowest three positions. Overall, we demonstrated that intact monkeys tended to increase their social status and castrated monkeys tended to decrease in their social status over the two year observation period.
Figure 2.
Social dominance difference scores (defined as the difference between the total number of observed dominant and subordinate behaviors per monkey per ethogram session) before puberty (Pre) and after puberty (Post). In both cohorts, all but one intact animal (Intact ●) increased their dominance ranking while the gonadectomized animals (Gdx Δ) lost ranking or stayed equivalent over time.
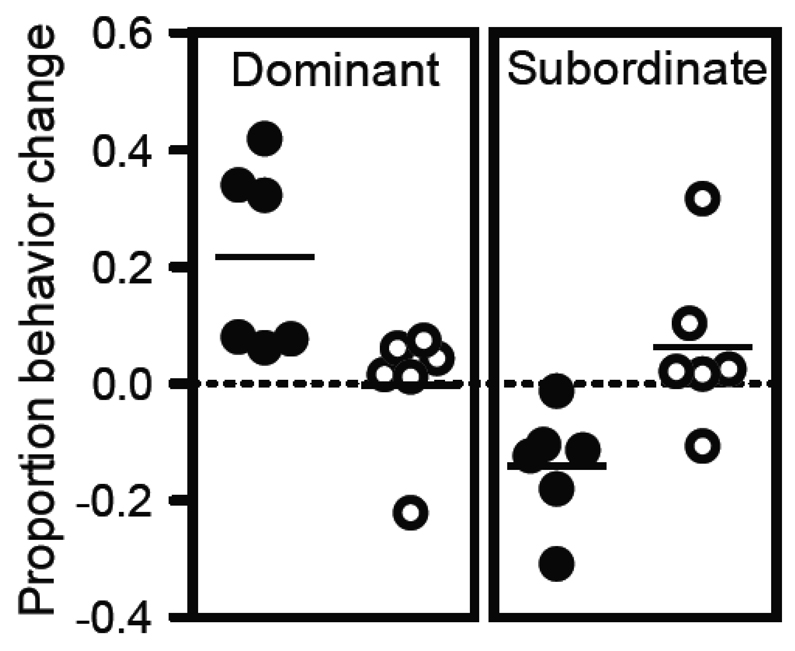
We also found the intact and gonadectomized groups displayed opposite changes in the amount of dominant and subordinate behavior across the study. Intact monkeys significantly increased their proportion of dominant behaviors compared to gonadectomized monkeys (Figure 3 left panel; t (10) = 2.75, p = 0.02, unpaired t-test). In contrast, gonadectomized monkeys significantly increased their proportion of subordinate behaviors compared to intact monkeys (Figure 3, right panel; t (10) = 2.88, p = 0.016, unpaired t-test). These behavioral changes are consistent with the increases and decreases in social rank described above and confirms that social dominance reflects not only the total number of behaviors displayed but also the relative proportion of dominant or subordinate interactions.
Figure 3.
Changes in the average frequency of dominant and subordinate behaviors were measured relative to the change in total social behaviors for each monkey. The left panel shows intact animals (Intact ●) had a significantly higher ratio of change in dominant behavior frequency to change in social behavior frequency than gonadectomized animals (Gdx ○) (p = 0.02). The right panel shows the ratio between the change in subordinate behavior frequency and change in social behavior frequency was significantly larger for gonadectomized animals (p = 0.02).
Correlations between testosterone, matriline rank, maternal social status and social dominance during the post-pubertal period among the intact monkeys were also calculated to determine the strength of the relationship between these variables. The results of this analysis are presented in Table 2.
Table 2.
Correlations (Pearson r) between maternal social status, matriline rank, morning and evening testosterone, and social dominance among intact monkeys (p-values are displayed in parenthesis).
| Maternal rank |
Matriline rank | Testo AM |
Testo PM |
Social Dominance |
|
|---|---|---|---|---|---|
| Maternal rank | -- | +.32(0.54) | −.10(0.85) | +.37(0.47) | −.05(0.92) |
| Matriline rank | +.32(0.54) | -- | −.14(0.79) | −.75(0.09) | − .85(0.03) |
| Testosterone AM | −.10(0.85) | −.14(0.79) | -- | −.12(0.82) | +.52(0.28) |
| Testosterone PM | +.37(0.47) | −.75(0.09) | −.12(0.82) | -- | +.71(0.11) |
| Social Dominance | −.05(0.92) | − .85(0.03) | +.52(0.28) | +.71(0.11) | -- |
The only statistically significant correlation among the intact group was a strong correlation between matriline rank and social dominance (r = -0.85, p < 0.05), indicating that individuals born in more dominant matrilines were also more dominant among their own social group. However, matriline rank was not strongly correlated with social dominance among the gonadectomized group (r = 0.05). The highest correlation among gonadectomized monkeys was between matriline rank and morning testosterone r = +0.68 (p > 0.05). There were two non-significant trends between testosterone and social dominance, and testosterone and matriline rank among intact monkeys (r = +0.71 and −0.74), indicating higher levels of testosterone were related to greater social dominance scores and higher matriline rank (lower number).
Emotional face task and snake task
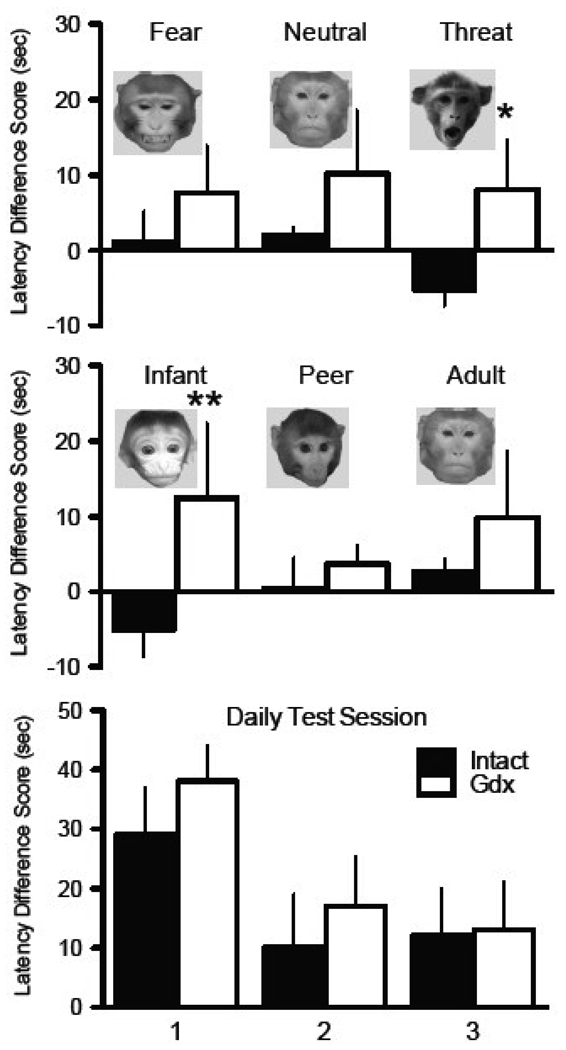
Intact and gonadectomized monkeys differed in their response to photographs of threatening faces and of baby monkey faces. In general, castrated animals had longer latencies to retrieve the food reward than intact monkeys, regardless of age or expression of the face in the photograph (Figure 4, top and middle panels; F (1, 8) = 8.1, p = 0.006). In post-hoc comparisons, we found that monkey faces with threatening (fear-eliciting) expressions elicited a greater latency to retrieve a food treat in gonadectomized animals as compared to intact animals (Effect size = 1.37, Figure 4, top panel, p = 0.03). The latency to retrieve a treat in front of neutral and fear-expressing (submissive look) faces were not significantly different (Effect size= 0.77 and 0.56 respectively, both p > 0.05). Age of monkey face also influenced responses, where gonadectomized monkeys were slower to retrieve a treat in the presence of the infant monkey faces (Effect size = 1.15, Figure 4, middle panel, p = 0.04). Gonadectomized monkeys were also about twice as slow to take a treat in front of an adult monkey face, but this difference did not reach statistical significance (Effect size = 0.61, p > 0.05). Both groups had a similar latency in the presence of a peer monkey face (Effect size = 0.45, p > 0.05).
Figure 4.
Latency to retrieve a food reward from in front of a photograph of a novel monkey or a rubber snake relative to the latency to retrieve a reward from in front of a blank picture or neutral object are plotted (means and SEMs). The top panel shows gonadectomized animals (Gdx, white bars) were significantly slower than intacts (Intacts, black bars) to respond to pictures of monkeys with threatening facial expressions (* p < 0.05). The middle panel shows gonadectomized animals were significantly slower to respond to pictures of infant monkeys than intact animals (** p < 0.01). The bottom panel shows there was no significant differences in response time to a rubber snake between groups, and responses for both groups got faster after the first testing day.
When the latency to retrieve a food reward in front of an object was measured the average latency of all monkeys to reach for a treat in the presence of a rubber snake was about three times longer as compared to the average latency to reach for a treat in presence of other toy objects (F = 22.16, p < 0.0001). Also, the greatest increase in latency produced by the presence of the snake occurred on the first day of the testing as compared to the second and third day (F = 6.03, p < 0.01). However, there was no effect of experimental group (castrates versus intacts, all effect sizes < 0.2), nor was there an interaction between treatment group and day or object type (fear evoking versus non-fear evoking, all F < 2.4, all p > 0.10). While the gonadectomized group tended to take slightly longer to retrieve the treat on all testing days, no significant differences between the castrated group and intact group in their average latency to retrieve a food reward in front of a snake was found when expressed as a difference score (Effect size = 0.38, F = 0.44, p = 0.51, Figure 4 bottom panel). We did detect a main effect of test day with significantly longer latencies on Day 1 (F = 3.25, p = 0.05), but no interaction effect was detected suggesting equal rates of habituation over the three days in the castrates and intacts (F = 0.08, p = 0.91) although there was substantial variation in monkey response times on days 2 and 3.
Social and Object Novelty
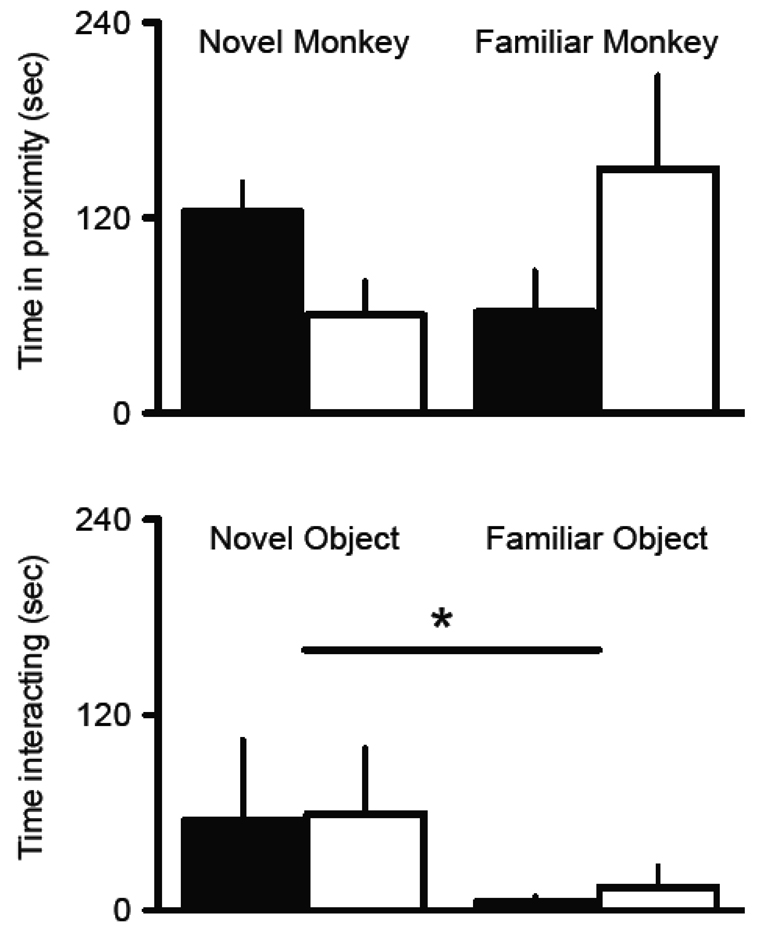
Intact and gonadectomized monkeys also differed in their response to a novel monkey. Gonadectomized monkeys spent more time in the familiar quadrant than the novel quadrant during the social novelty test, while the intact monkeys displayed the opposite tendency (Figure 5, top panel). This interaction was significant, F (1, 10) = 6.09, p < 0.05. Post-hoc LSD tests revealed that intact monkeys spent more time, on average, with the novel monkey than the gonadectomized group, effect size = 1.30, p = 0.04. The amount of time spent with the familiar monkey while higher in the castrates, was not significantly different between groups, effect size = 0.86, p = 0.19. The results of the number of approaches as well as the latency to make the initial contact were consistent with the duration of contact measure. In general, the gonadectomized group took longer to approach the novel monkey [29.95 (22.48) vs 9.97 (4.51)] seconds) and they made slightly fewer approaches [5.67 (1.41) vs 6.00 (1.46)] than the intact group. Conversely, they were faster to approach the familiar monkey [25.02 (10.26) vs 59.75 (20.52) seconds] and they made more approaches [7.33 (1.41) vs 5.50 (1.73)] than the intact group.
Figure 5.
The average total duration of time (sec) each group spent in the proximate quadrant with the novel and familiar monkey (top panel) and the novel and familiar object (bottom panel) during the final test session are plotted (means and SEM). The intact group (black bar) spent significantly more time with the novel intruder than the gonadectomized group (white bar) (* p < 0.05). There were no significant differences between groups in the object novelty test, however both groups spent significantly more time with the novel object compared to the familiar object (* p < 0.05).
The results of the object novelty test found that both groups, on average, spent more time with the novel object than the familiar object (Figure 5, bottom panel). Thus, the main effect of object novelty status was significant, effect size = 1.12, F (1, 10) = 5.50, p = 0.04. However, there were no significant main effects when comparing the intact and castrated group, effect size = 0.04, F < 1.0, which suggests that the reaction to novel objects is similar among these groups, unlike the reaction to a novel monkey. Both groups were also faster to approach the novel object and made more approaches to it than the familiar object. There were no differences between groups on either of these measures, p > 0.05.
Discussion
In our study, the onset of puberty in intact male monkeys, as marked by a significant increase in nocturnal testosterone just after 3 years of age, resulted in a rise in social dominance rank and significantly impacted reactions to social stimuli, such as fearful faces and preference for novel monkeys. However, reactions to non-social stimuli did not appear to be different between the gonadally intact and castrated monkeys during adolescence.
We found pubertal elevations in nocturnal testosterone in developing male monkeys precedes detectable pubertal changes in morning testosterone by about 10 months, which agrees with previous studies showing that nocturnal plasma testosterone levels can become elevated as early as 2.5 years of age (Rose, 1978). The developmental delay in increased morning testosterone levels has also been found by other studies in rhesus macaques (Plant, 1985) and adolescent males of other species including humans (Wu, Borrow, Nicol, Elton, & Hunter, 1989). Additionally, an increase in body weight is commonly associated with puberty onset, however. no significant differences in total body weight between the intact and gonectomized groups were found at any stage of this study. Therefore, gonad function cannot be the primary driving force behind pubertal changes in body size. Instead, some other factor independent of circulating testosterone may drive overall increases in body weight at this time, such as growth hormone. Furthermore, our observations suggest the changes in the social behavior of the intact males (discussed below) are not due to differences in body size or other physical cues such as size of the scrotal sac (as this was controlled for in our study).
In the current study, intact monkeys increased their dominant behavior and decreased their subordinate behavior relative to the gonadectomized monkeys. Intact monkeys also increased their social dominance rank relative to gonadectomized monkeys. These results suggest that gonadal hormones, such as testosterone, help an individual gain or maintain social status in rhesus macaques. Previous experiments have given mixed results on the relationship between testosterone and social dominance in monkeys (Bouissou, 1983). For example, Cochran found that treating gonadectomized adult rhesus macaques with the androgen receptor agonist dihydrotestosterone propionate resulted in an increase in dominance ranking for treated males over untreated males (Cochran & Perachio, 1977). However, other studies failed to find a correlation between dominance ranking and fecal testosterone levels (Barrett, Shimizu, Bardi, & Mori, 2002). And in at least one study, an agonadal adult male rhesus macaque rose in social dominance after placement in several different heterosexual colonies, becoming the alpha male on multiple occasions and exhibiting typical age- and status-specific sexual and social behavior (Bernstein, Gordon, & Peterson, 1979), which suggests that other factors apart from circulating gonadal hormones also influence social rank such as maternal social status (e.g., Dixon & Nevison, 1997).
Because information regarding maternal rank as well as three generations of matriline rank were available from the NIMH primate core, the present study was also able to report on the influence of maternal and matriline rank on measured testosterone levels and social behavior. A significant correlation was found between matriline rank and social dominance after puberty, indicating that individuals from high ranking families were more dominant, and providing evidence that genetic background may contribute to social behavior in these monkeys. However the correlation between matriline rank and social dominance was absent among the gonadectomized monkeys, which strongly suggests the effect of matriline rank on social behavior is dependent on the presence of gonadal hormones such as testosterone. Strong (albeit non-significant) correlations between matriline rank and testosterone, and testosterone and social dominance are also consistent with the suggestion that matriline rank exerts its influence on social behavior via gonadal hormones. Nevertheless, other factors such as genetic background or early rearing may also play a role in determining position within the social hierarchy.
Recent studies have found that adolescence is associated with unique behavioral and functional brain changes. For example, adolescent vervet monkeys show more impulsive behavior toward intruders than adult or juvenile monkeys (Fairbanks, 2001; Fairbanks et al., 2004), while adolescent humans have a higher level of amygdala activation when viewing face stimuli than children or adults (Hare et al., 2008). The amygdala undergoes considerable structural change during adolescence (Schumann et al., 2004) and faces evoke potent firing in over half of the neurons in this region (Gothard et al., 2007). Thus, we expected reactions to emotional faces and unfamiliar conspecifics to change in our adolescent monkeys as well. The emotional face task we used required the monkey to interact with the challenge stimulus in order to receive a food reward. The behavioral responses of the gonadectomized group were not affected by either the age or the expression of the photographed faces, while the intact animals were faster to take the reward when placed in front of an infant face or a face displaying an open-mouth. The similar response of intact monkeys to infant faces and open-mouth expressions probably reflects the fact that both infants and the open-mouth gaze are social stimuli which evoke fear-like behavior in adolescent male monkeys (Chamove, Harlow, & Mitchell, 1967; Sackett, 1966), and thus may signify an impending threat. Male monkeys would be severely punished by a mature mother female if they approached or attempted to interact with an infant monkey and the open-mouth gaze is a salient social threat from other monkeys. It should also be mentioned that reactions to fear-grimaces are likely to differ from the human response to a fearful face (typically evoking fear in us) since when another monkey is fear expressing (fear grimace) this is a sign of submission.
To determine if there was a more generalized effect of gonadectomy on reactions to fear-eliciting stimuli, we also tested the monkey’s reaction to a food reward placed in front of a rubber snake. Snakes are a fear-eliciting stimulus to monkeys (Mineka, Davidson, Cook & Keir, 1984), and monkeys with lesioned amygdale are less fearful of the snake and retrieve food treats faster than non-lesioned monkeys (Amaral, Capitanio, Machado, Mason, & Mendoza, 1997; Prather et al., 2001). In our study, the intact and the gonadectomized group both took longer to retrieve the food reward in the presence of the snake compared to the other objects, but there was no clear evidence the two treatment groups differed. This result contrasts with the significant difference we observed in response to the face stimuli, and appears to indicate some fear-related regions of the brain such as the amygdala are functioning normally in the ‘snake task’ among the two groups. So rather than a general change in amygdala function, the results of the face task may reflect more subtle hormone sensitive amygdalar changes which result in different reactions to social cues. Other research has indicated that sex hormones such as estrogen can change the reward value of faces in a relatively gender- and species-specific manner. For example, female rhesus will press a button to look longer at male rhesus faces during peaks in their estrogen cycle, but not female faces or faces of humans or chimpanzees (Lacreuse, Martin-Malivel, Lange & Hendon, 2007). Rises in testosterone, similar to the effect of estrogen, may have also increased the ability of intact monkeys to make differential responses to emotional faces of conspecifics.
We also tested whether the intact and castrate groups differed in their response to a novel conspecific. We found that intact monkeys spent more time in close proximity to a novel male, compared to the gonadectomized monkeys. In contrast, the results of an object novelty test did not reveal any significant group differences in novelty-seeking in general. Both groups spent more time with the novel toy than the familiar toy. These results are consistent with the suggestion that sex hormones increase interest in, or reduce fear towards, novel conspecifics. Other research has found knocking out the estrogen receptor (either alpha or beta) in female mice reduces the preference for novel conspecifics in a similarly designed social discrimination task using rodents (Choleris et al., 2006; Lacreuse et al., 2007). Given that testosterone in the brain can be converted to estrogen by aromatase, the present results may also reflect the action of sex steroids on estrogen receptors through aromatization of testosterone to estrogen. Indeed, monkeys, rats and humans express estrogen receptor alpha in brain areas implicated in social behavior, including amygdala and prefrontal cortical areas, which would allow direct hormone modulation of social behavior (Montague et al., 2008; Perlman, Matsumoto et al., 2005; Perlman, Webster, Kleinman & Weickert, 2004; Wang et al., 2004). Further work is needed to address whether gonadectomy produced changes in the neurobiology of these regions implicated in social behavior.
The present results have implications for our understanding of social impairments in people, which are also associated with a decreased response to or absence of sex hormones. Decreased testosterone levels, such as that due to Klinefelter’s syndrome, is associated with increased distress during social encounters and less assertiveness (van Rijn, Swaab, Aleman & Kahn, 2008). Testosterone may also support normal social behavior during adolescence in humans. Lower levels of salivary testosterone or decreases throughout the day in adolescent boys (and girls) are associated with higher anxiety and problems with attention and depression (Granger et al., 2003). The present results also suggest that altered sex hormonal levels during puberty could negatively impact an individual’s ability to respond appropriately in a challenging social situation, such as being confronted by an unfamiliar person. Sex hormones have also been linked to psychiatric disorders that are characterized by alterations in social behavior including schizophrenia (Akhondzadeh et al., 2006; Halari et al., 2004), anxiety (Toufexis et al., 2006) and depression (Angold & Costello, 2006). In other work, we have found that patients with schizophrenia may be unable to respond normally to the pubertal surge in sex steroids due to the presence of lower or truncated estrogen receptor alpha in the prefrontal cortex (Perlman, Tomaskovic-Crook et al., 2005; Weickert et al., 2008). Thus, it may be that schizophrenia develops in part due to a blunted ability of the brain to respond to testosterone, albeit in a different fashion than gonadectomy which eliminates testosterone. One implication of our current study is if patients have a blunted response to sex hormones, this may contribute to some of their deficits in social functioning. For example, schizophrenia is associated with a deficit in or altered brain activity when recognizing angry or fearful facial expressions (Morris, Weickert, & Gur et al., 2007; Turetsky et al., 2007), a result which may correspond to the failure to respond differentially to emotional facial expressions among gonadectomized monkeys.
Adolescence is a period in which individuals undergo fairly dramatic changes in their social behavior yet few studies have reported significant behavioral changes that are specific to social cognition and which cannot be explained by general changes in anxiety or attention (Blakemore, 2008; Spear, 2000). Our study shows for the first time that the reactions to social stimuli in adolescent male rhesus macaques are sensitive to gonadectomy. Gonadectomy changed behavioral responses to social cues, such as threatening facial expressions and novel conspecifics, but it did not appear to affect the fear response to snakes or interest in novel toys. These results are consistent with growing evidence that increases in sex hormones during adolescence or menstruation are associated with increases in attention to social cues as well as social behavior. This is relevant to our understanding of deficits in social behavior among people with schizophrenia, which often emerges during or shortly after adolescence. Furthermore, it sets the stage for future research to determine the molecular changes which are responsible for the development of normal social behavior in sex hormone–sensitive brain areas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhondzadeh S, Rezaei F, Larijani B, Nejatisafa AA, Kashani L, Abbasi SH. Correlation between testosterone, gonadotropins and prolactin and severity of negative symptoms in male patients with chronic schizophrenia. Schizophr Res. 2006;84(2–3):405–410. doi: 10.1016/j.schres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Altmann SA. A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Ann NY Acad Sc. 1962;102:338–435. doi: 10.1111/j.1749-6632.1962.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Capitanio JP, Machado CJ, Mason WA, Mendoza SP. The role of the amygdaloid complex in rhesus monkey social behavior. Society for Neurosciences Abstracts. 1997;23:570. [Google Scholar]
- Anestis SF. Testosterone in juvenile and adolescent male chimpanzees (Pan troglodytes): effects of dominance rank, aggression, and behavioral style. Am J Phys Anthropol. 2006;130(4):536–545. doi: 10.1002/ajpa.20387. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ. Puberty and depression. Child Adolesc Psychiatr Clin N Am. 2006;15(4):919–937. doi: 10.1016/j.chc.2006.05.013. ix. [DOI] [PubMed] [Google Scholar]
- Barrett GM, Shimizu K, Bardi M, Mori A. Fecal testosterone immunoreactivity as a non-invasive index of functional testosterone dynamics in male Japanese macaques (Macaca fuscata) Primates. 2002;43(1):29–39. doi: 10.1007/BF02629574. [DOI] [PubMed] [Google Scholar]
- Bernhardt PC, Dabbs JM, Jr, Fielden JA, Lutter CD. Testosterone changes during vicarious experiences of winning and losing among fans at sporting events. Physiol Behav. 1998;65(1):59–62. doi: 10.1016/s0031-9384(98)00147-4. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP, Peterson M. Role Behavior of an Agonadal Alpha-Male Monkey in a Heterosexual Group. Folia Primatol (Basel) 1979;32:263–267. [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9(4):267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry. 2006;47(3–4):296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive Affective Neuroscience. 2007;2:130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A, Shelley G, Mazur A, Tharp G, Kittok R. Testosterone, and winning and losing in human competition. Horm Behav. 1989;23(4):556–571. doi: 10.1016/0018-506x(89)90042-1. [DOI] [PubMed] [Google Scholar]
- Bouissou MF. Androgens, aggressive behaviour and social relationships in higher mammals. Horm Res. 1983;18(1–3):43–61. doi: 10.1159/000179778. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamove A, Harlow HF, Mitchell G. Sex differences in the infant-directed behavior of preadolescent rhesus monkeys. Child Dev. 1967;38(2):329–335. [PubMed] [Google Scholar]
- Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, et al. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5(7):528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Cochran CA, Perachio AA. Dihydrotestosterone propionate effects on dominance and sexual behaviors in gonadectomized male and female rhesus monkeys. Hormones and Behavior. 1977;8(2):175–187. doi: 10.1016/0018-506x(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Dixson AF, Nevison CM. The socioendocrinology of adolescent development in male rhesus monkeys (Macaca mulatta) Horm Behav. 1997;31(2):126–135. doi: 10.1006/hbeh.1997.1374. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA. Individual differences in response to a stranger: social impulsivity as a dimension of temperament in vervet monkeys (Cercopithecus aethiops sabaeus) J Comp Psychol. 2001;115(1):22–28. doi: 10.1037/0735-7036.115.1.22. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Jorgensen MJ, Huff A, Blau K, Hung YY, Mann JJ. Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. Am J Primatol. 2004;64(1):1–17. doi: 10.1002/ajp.20057. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Pompili A, d'Onofrio A, Cifariello A, Tavares MC, Tomaz C. Working memory for emotional facial expressions: Role of the estrogen in young women. Psychoneuroendocrinology. 2008;33(7):964–972. doi: 10.1016/j.psyneuen.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon TP, Rose RM, Bernstein IS. Seasonal rhythm in plasma testosterone levels in the rhesus monkey (Macaca mulatta): a three year study. Horm Behav. 1976;7(2):229–243. doi: 10.1016/0018-506x(76)90050-7. [DOI] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol. 2007;97(2):1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: individual differences and developmental effects. Dev Psychopathol. 2003;15(2):431–449. [PubMed] [Google Scholar]
- Gur RE, Loughead J, Kohler CG, Elliott MA, Lesko K, Ruparel K, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64(12):1356–1366. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- Halari R, Kumari V, Mehrotra R, Wheeler M, Hines M, Sharma T. The relationship of sex hormones and cortisol with cognitive functioning in Schizophrenia. J Psychopharmacol. 2004;18(3):366–374. doi: 10.1177/026988110401800307. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi IT, Nevo N, Amsterdam A, Naor Z. Effect of postnatal treatment with a gonadotropin-releasing hormone antagonist on sexual maturation of male rats. Biol Reprod. 1986;35(3):501–507. doi: 10.1095/biolreprod35.3.501. [DOI] [PubMed] [Google Scholar]
- Ko YH, Joe SH, Cho W, Park JH, Lee JJ, Jung IK, et al. Estrogen, cognitive function and negative symptoms in female schizophrenia. Neuropsychobiology. 2006;53(4):169–175. doi: 10.1159/000093780. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Martin-Malivel J, Lange HS, Herndon JG. Effects of the menstrual cycle on looking preferences for faces in female rhesus monkeys. Anim Cogn. 2007;10(2):105–115. doi: 10.1007/s10071-006-0041-8. [DOI] [PubMed] [Google Scholar]
- Ledoux JE. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York: Simon & Schuster; 1996. [Google Scholar]
- Mazur A. Effects of testosterone on status in primate groups. Folia Primatol (Basel) 1976;26(3):214–226. doi: 10.1159/000155752. [DOI] [PubMed] [Google Scholar]
- Mazur A, Lamb TA. Testosterone, status, and mood in human males. Horm Behav. 1980;14(3):236–246. doi: 10.1016/0018-506x(80)90032-x. [DOI] [PubMed] [Google Scholar]
- Mineka S, Davidson M, Cook M, Keir R. Observational conditioning of snake fear in rhesus monkeys. J Abnorm Psychol. 1984;93(4):355–372. doi: 10.1037//0021-843x.93.4.355. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Montague D, Weickert CS, Tomaskovic-Crook E, Rothmond DA, Kleinman JE, Rubinow DR. Oestrogen receptor alpha localisation in the prefrontal cortex of three mammalian species. J Neuroendocrinol. 2008;20(7):893–903. doi: 10.1111/j.1365-2826.2008.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Mori T, Matsuda H, Komaki G. Changes of brain activity in the neural substrates for theory of mind during childhood and adolescence. Psychiatry Clin Neurosci. 2007;61(4):355–363. doi: 10.1111/j.1440-1819.2007.01687.x. [DOI] [PubMed] [Google Scholar]
- Morris RW, Weickert CS, Loughland C. Current Opinion in Psychiatry. 2009;22(2):140–146. doi: 10.1097/YCO.0b013e328324f895. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Matsumoto M, Beltaifa S, Hyde TM, Saunders RC, Webster MJ, et al. Expression of estrogen receptor alpha exon-deleted mRNA variants in the human and non-human primate frontal cortex. Neuroscience. 2005;134(1):81–95. doi: 10.1016/j.neuroscience.2005.03.055. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Tomaskovic-Crook E, Montague DM, Webster MJ, Rubinow DR, Kleinman JE, et al. Alteration in estrogen receptor alpha mRNA levels in frontal cortex and hippocampus of patients with major mental illness. Biol Psychiatry. 2005;58(10):812–824. doi: 10.1016/j.biopsych.2005.04.047. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor alpha messenger RNA levels in the amygdala of patients with major mental illness. Biol Psychiatry. 2004;56(10):844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta) Endocrinology. 1985;116(4):1341–1350. doi: 10.1210/endo-116-4-1341. [DOI] [PubMed] [Google Scholar]
- Plant TM. The male monkey as a model for the study of the neurobiology of puberty onset in man. Mol Cell Endocrinol. 2006;254–255:97–102. doi: 10.1016/j.mce.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, et al. Increased social fear and decreased fear of objects in monkeys with neonatal amygdale lesions. Neuroscience. 2001;106(4):653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22(6):633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Horm Behav. 1990;24(3):311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Rose RM. Changes in testosterone and behavior during adolescence in the male rhesus monkey. Psychosomatic medicine. 1978;40(1):60–70. doi: 10.1097/00006842-197802000-00007. [DOI] [PubMed] [Google Scholar]
- Rose RM, Holaday JW, Bernstein IS. Plasma testosterone, dominance rank and aggressive behaviour in male rhesus monkeys. Nature. 1971;231(5302):366–368. doi: 10.1038/231366a0. [DOI] [PubMed] [Google Scholar]
- Sackett GP. Monkeys reared in isolation with pictures as visual input: evidence for an innate releasing mechanism. Science. 1966;154(755):1468–1473. doi: 10.1126/science.154.3755.1468. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Menard TA, Smith DA, Albers HE, Sisk CL. Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Horm Behav. 2006;50(3):477–483. doi: 10.1016/j.yhbeh.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Schulz KM, Zehr JL. Puberty: A Finishing School for Male Social Behavior. Annals of the New York Academy of Sciences. 2003;1007(1):189–198. doi: 10.1196/annals.1286.019. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Horm Behav. 2006;50(4):539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr Res. 2007;94(1–3):253–263. doi: 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S, Swaab H, Aleman A, Kahn RS. Social behavior and autism traits in a sex chromosomal disorder: Klinefelter (47XXY) syndrome. J Autism Dev Disord. 2008;38(9):1634–1641. doi: 10.1007/s10803-008-0542-1. [DOI] [PubMed] [Google Scholar]
- Van Wagenen G, Simpson ME. Testicular development in the rhesus monkey. Anat Rec. 1954;118(2):231–251. doi: 10.1002/ar.1091180209. [DOI] [PubMed] [Google Scholar]
- Vessey SH, Meikle DB. Factors affecting social behavior and reproductive success of male rhesus monkeys. [Review] International Journal of Primatology. 1987;8(3):281–292. [Google Scholar]
- Wang J, Cheng CM, Zhou J, Smith A, Weickert CS, Perlman WR, et al. Estradiol alters transcription factor gene expression in primate prefrontal cortex. J Neurosci Res. 2004;76(3):306–314. doi: 10.1002/jnr.20076. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Miranda-Angulo AL, Wong J, Perlman WR, Ward SE, Radhakrishna V, et al. Variants in the estrogen receptor alpha gene and its mRNA contribute to risk for schizophrenia. Hum Mol Genet. 2008;17(15):2293–2309. doi: 10.1093/hmg/ddn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharm. 2003;28(5):910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Wu FC, Borrow SM, Nicol K, Elton R, Hunter WM. Ontogeny of pulsatile gonadotrophin secretion and pituitary responsiveness in male puberty in man: a mixed longitudinal and cross-sectional study. J Endocrinol. 1989;123(2):347–359. doi: 10.1677/joe.0.1230347. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;17(2):251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]