Abstract
Obesity is associated with an increased incidence and severity of asthma, as well as other lung disorders, such as pulmonary hypertension. Adiponectin (APN), an antiinflammatory adipocytokine, circulates at lower levels in the obese, which is thought to contribute to obesity-related inflammatory diseases. We sought to determine the effects of APN deficiency in a murine model of chronic asthma. Allergic airway inflammation was induced in APN-deficient mice (APN−/−) using sensitization without adjuvant followed by airway challenge with ovalbumin. The mice were then analyzed for changes in inflammation and lung remodeling. APN−/− mice in this model develop increased allergic airway inflammation compared with wild-type mice, with greater accumulation of eosinophils and monocytes in the airways associated with elevated lung chemokine levels. Surprisingly, APN−/− mice developed severe pulmonary arterial muscularization and pulmonary arterial hypertension in this model, whereas wild-type mice had only mild vascular remodeling and comparatively less pulmonary arterial hypertension. Our findings demonstrate that APN modulates allergic inflammation and pulmonary vascular remodeling in a model of chronic asthma. These data provide a possible mechanism for the association between obesity and asthma, and suggest a potential novel link between obesity, inflammatory lung disease, and pulmonary hypertension.
Keywords: asthma, obesity, pulmonary hypertension
CLINICAL RELEVANCE
The research presented in this article provides a mechanism that might explain the increased incidence of asthma in the obese. Furthermore, the data suggest a novel mechanism by which obesity may influence the development of pulmonary hypertension in the presence of inflammatory lung disease.
According to the Centers of Disease Control, currently more than 30% of the adult population in the United States is considered obese (1). Obesity is associated with a higher incidence of hypertension, diabetes, cardiovascular disease, and pulmonary disorders, such as asthma and pulmonary hypertension (2–5). Recent experimental evidence suggests that obesity may directly contribute to the pathogenesis of these disorders through the metabolic and immune activity of adipose tissue (6). Consistent with this, adipocytes secrete multiple bioactive mediators (adipocytokines), which may influence energy homeostasis, inflammation and tissue remodeling (6–8). Adiponectin (APN), the most abundant adipocytokine, has a wide range of metabolic, antiinflammatory, and antiproliferative activities (9). Of note, individuals with obesity have low plasma APN levels, suggesting that decreased APN levels may contribute to the increased inflammatory state in obesity. Supporting this hypothesis, mice with a deletion of the APN gene (APN−/−) are predisposed to inflammatory diseases, such as diabetes and atherosclerosis (9–11), and develop enhanced organ remodeling and vascular smooth muscle cell (SMC) proliferation in disease (9–11).
Asthma is a complex syndrome, broadly defined by inflammation of the airways associated with airways hyperresponsiveness (AHR) and mucus hypersecretion (12). In addition, chronic asthma is associated with remodeling of the airways and associated vasculature (13, 14). There is accumulating evidence in the literature that obesity can increase both the incidence and severity of asthma in patients, and is associated with worse asthma outcomes (2, 15–19). Studies have demonstrated that adipose tissue expresses multiple adipocytokines, which may influence airway inflammation and airway remodeling (7, 20). In addition, experimental studies in animals have also supported a link between obesity and asthma (3, 21, 22). Taken together, these data suggest that obesity may actually contribute to the pathogenesis of asthma; however, the mechanisms for this interaction are not well defined.
There is also an increased incidence of pulmonary hypertension in the obese, with a prevalence of 5% in people with a body mass index greater than 30 kg/m2 (23). This association is thought to relate partially to the obesity–hypoventilation syndrome (a syndrome notable for a combination of obesity, sleep-disordered breathing, and hypercapnia) and obstructive sleep apnea; however, the risk for pulmonary hypertension in the obese seems to be independent of these diagnoses (23–25). Even more provocative are autopsy data from patients with obesity, which demonstrate a high incidence (72%) of pulmonary hypertensive changes (including extensive muscularization of medium and small pulmonary arteries) in this population (26, 27). Although these data strongly suggest an association between obesity and pulmonary hypertension, the exact mechanisms linking these disorders remain unknown.
Recent studies have suggested that APN can influence the development of lung inflammation and, possibly, pulmonary hypertension. In a murine model of acute asthma, treatment of mice with APN attenuated airway inflammation and AHR (21). More recently, it was demonstrated that APN is detectable in the airways of normal mice at levels 5-fold less than serum. Furthermore, APN-deficient (APN−/−) mice develop spontaneous emphysema, and lung macrophages from these mice express higher levels of inflammatory mediators than macrophages from wild-type mice (28). Potential effects of APN on the pulmonary vasculature have also recently been recognized. Male apolipoprotein E–deficient mice on a high-fat diet develop pulmonary hypertension associated with lower APN levels compared with wild-type mice (29). Treatment of these mice with the peroxisome proliferator–activated receptor-γ activator, rosiglitazone, resulted in higher plasma APN levels and complete regression of pulmonary hypertension and pulmonary artery remodeling. These data suggest that lower APN levels in obesity could directly affect the severity of inflammation in asthma and influence pulmonary vascular disease. Interestingly, pulmonary hypertension and pulmonary arterial remodeling have recently been reported in murine models of allergic airway inflammation (30–33), and inflammation is now recognized as an important stimulus for the development of pulmonary hypertension (30, 32–34). However, whether obesity influences the development of pulmonary hypertension in the setting of inflammatory lung disease has not been studied. Here, we investigated the effects of APN deficiency in a murine model of chronic allergic airway inflammation, and demonstrate that APN modulates allergic airway inflammation and pulmonary vascular remodeling.
MATERIALS AND METHODS
Mouse Experiments
APN−/− mice were backcrossed seven generations onto a C57BL/6 background (35). Wild-type C57BL/6 control mice were obtained from Taconic (Hudson, NY), so we cannot fully rule out subtle differences in the phenotype of these mice arising from genetic differences. Mice were used at 6 to 8 weeks of age, and were age and sex matched for all experiments. There were no baseline differences in weight (data not shown), as previously reported (28). All protocols for mouse experiments were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital.
Asthma Model
Acute allergic airway inflammation was induced in mice as previously described (36). Briefly, mice were immunized with two intraperitoneal injections of 10 μg of chicken ovalbumin (OVA) (Sigma-Aldrich, St. Louis, MO) bound to 1 mg of alum (Sigma-Aldrich) in 0.5 ml PBS on Days 1 and 7. Starting on Day 14, mice were challenged by aerosol nebulization with 10 mg/ml OVA in PBS or PBS alone (control mice) for 20 minutes daily for 3 days. Chronic allergic airway inflammation was induced in mice using a modified protocol (37). Briefly, mice were immunized with three intraperitoneal injections of 50 μg of OVA in 0.1 ml PBS on Days 1, 4, and 7. Starting on Day 12, mice were challenged by intranasal injection with 20 μg OVA in 30 μl PBS or PBS alone (control mice) weekly for 4 weeks. Mice were harvested for analysis 24 hours after the last challenge.
Hypoxia Experiments
Mice were exposed for 3 weeks to 10% normobaric hypoxia in a specially designed cage rack, as previously described (38).
Mouse Harvest and Analysis
Bronchoalveolar lavage (BAL) and harvest of the lungs were performed as previously described (36). Cells recovered from the BAL fluid were stained with fluorescently labeled antibodies to CD4, CD8, and CD69 (BD Biosciences, San Jose, CA).
Histological Analyses
For histopathologic examination, lungs were flushed free of blood, inflated with 10% buffered formalin to 25 cm H2O of pressure, and prepared and evaluated as previously described (36). Sections of paraffin-embedded lungs were prepared and stained with hematoxylin and eosin, Masson trichrome, and Picrosirius red. Sections were also stained with antibodies to α-smooth muscle actin (Abcam, Cambridge, MA), and proliferating cell nuclear antigen (Cell Signaling, Danvers, MA), according to the manufacturers' recommended protocols. Transferase biotin-dUTP nick end labeling (TUNEL) assay was performed using the Apoptag Plus Peroxidase in situ apoptosis kit (Millipore, Billerica, MA). The number of TUNEL+ nuclei per 20× field was counted in three random fields for each section. The slides were evaluated by light microscopy, and the amount of inflammation and vascular remodeling was assessed by a researcher blinded to the genotype of the animals. The evaluator assessed the inflammatory infiltrate around the airways and the amount of vascular obliteration on three whole lung sections of the left lung from each mouse evaluated. Each set of three sections was given a score of 0–4 for inflammation (0 = no inflammation; 1 = < 25% of airways with inflammation; 2 = 25–50% of airways with inflammation; 3 = 50–75% of airways with inflammation; 4 = > 75% of airways with inflammation) and 0–4 for vascular remodeling (0 = no remodeling; 1 = < 25% of medium-sized pulmonary arteries obliterated; 2 = 25–50% of medium-sized pulmonary arteries obliterated; 3 = 50–75% of medium-sized pulmonary arteries obliterated; 4 = greater than 75% of medium-sized pulmonary arteries obliterated). In addition, measurements were made of the vascular wall thickness (expressed as a percentage of the vessel diameter) for the preacinar vessels in each lung section, as previously described (38). Finally, collagen types I and III were stained with Picrosirius red. Sections were incubated for 90 minutes in 0.1% Sirius red F3BA (Polyscience Inc., Warrington, PA) in saturated picric acid. Staining with Sirius red was analyzed by polarization microscopy with image analysis software (Image-Pro Plus; MediaCybernetics, Bethesda, MD).
Airway Hyperresponsiveness
Airway resistance and dynamic lung compliance were measured invasively using a whole-body plethysmograph (Buxco, Wilmington, NC), as previously described (36).
Hemodynamic Studies
Mice were anesthetized with ketamine (100 mg/kg) and fentanyl (250 μg/kg) intraperitoneally, then intubated and mechanically ventilated (10 μl/g, 110 bpm; FiO2 = 1). Pancuronium (2 mg/kg) was administered intraperitoneally, and a PE-10 polyethylene catheter was placed in the left carotid artery for continuous heart rate and systemic arterial pressure monitoring. Then, a 1F high-fidelity pressure catheter (SPR-1000; Millar Instruments Inc., Houston, TX) was advanced into the right ventricle via the jugular vein to measure right ventricular systolic pressure (RVSP) as an estimate of pulmonary arterial systolic pressure. All signals were recorded and analyzed using a data acquisition system (PowerLab with Chart; AD Instruments, Colorado Springs, CO). At the end of the study, an arterial blood sample was taken while the animal was breathing room air, and the partial pressure of oxygen was measured using a Chiron Diagnostic Blood Gas Machine (Siemens, Deerfield, IL).
Cell Culture and Stimulation
Murine bone marrow–derived macrophages were prepared as previously reported (39). The cells were incubated in standard medium containing 2% FCS with or without 10 μg/ml of mouse recombinant APN (BioVendor, Candler, NC) for 18 hours. The bone marrow–derived macrophages were then stimulated with a combination of IL-4 (50 ng/ml) and TNF-α (100 ng/ml) (R&D Systems, Minneapolis, MN) for 6 hours. After the treatment, RNA was isolated for quantitative RT-PCR.
Quantification of Gene and Protein Expression
RNA was purified from the lung and analyzed by quantitative RT-PCR, as previously described (36). Primer sequences used were selected using the Massachusetts General Hospital PrimerBank (http://pga.mgh.harvard.edu/primerbank/). Supernatants from BAL fluid were collected and then used undiluted in commercial ELISA kits for CCL11 and CCL24 (R&D Systems), according to the manufacturer's protocol. BAL and serum were collected, diluted 1:1,000 and 1:10,000, respectively, and used in a commercial ELISA kit to measure the protein levels of mouse APN (B-Bridge International, Mountain View, CA).
Hydroxyproline Assay
Hydroxyproline was assayed as previously described (40).
Statistical Analysis
Results are shown as mean (±SEM) values. Two groups were compared using Student's t test. Between-group comparison of means was performed by repeated-measures ANOVA. A P value of less than 0.05 was regarded as indicative of a significant difference.
RESULTS
APN Deficiency Does Not Affect Acute Allergic Airway Inflammation
To investigate the potential role of APN in regulating allergic airway inflammation, we used APN−/− and wild-type mice in an allergen challenge model of acute asthma, which uses OVA immunization with adjuvant (alum) and OVA aerosol challenges to induce allergic airway inflammation (36). Prominent airway inflammation occurred in OVA-challenged APN−/− mice and wild-type mice, whereas PBS-challenged mice had normal-appearing lungs without inflammation. There was no difference in T-cell recruitment, eosinophil recruitment, mucus cell number, or AHR between the OVA-challenged wild-type and APN−/− mice (data not shown). Thus, APN deficiency did not influence the development of airway inflammation in this model of acute asthma.
APN Deficiency Exacerbates Chronic Allergic Airway Inflammation
Previous data have demonstrated that serum APN levels decrease in mice that are acutely challenged with OVA compared with mice challenged with PBS (21). Thus, the absence of an effect of APN deficiency in the acute model of asthma may be a result of down-regulation of APN in wild-type mice. In addition, the effects of obesity on inflammation have mainly been studied in the context of chronic diseases, such as diabetes and atherosclerosis. Thus, we reasoned that a model of chronic low-level inflammation would be a better means to differentiate effects of APN deficiency on allergic airway inflammation. Therefore, we used a murine model of chronic asthma that induces mild chronic airway inflammation and remodeling (37). Wild-type mice and APN−/− mice were immunized with OVA (without adjuvant) and then exposed to a weekly intranasal OVA challenge. Unlike in the previous study using this model (37), we only used four weekly challenges rather than nine weekly challenges to limit the intensity of inflammation. We analyzed serum APN levels in wild-type mice after 2 and 4 weeks of OVA challenges, and found that APN levels were the same as in naive wild-type mice, and did not decrease during the induction of inflammation as seen in an acute asthma model (21) (APN level: baseline = 5.6 ± 0.6 μg/ml; 2 weeks = 5.3 ± 0.2 μg/ml; and 4 weeks = 5.4 ± 0.5 μg/ml; n = 4 samples per group). Levels of APN were undetectable in APN−/− mice.
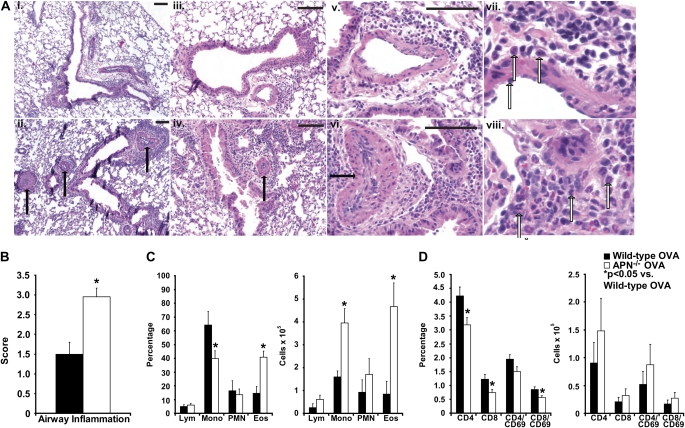
After four weekly OVA challenges, wild-type mice developed mild eosinophilic airway inflammation (Figure 1A [panels i, iii, and vii] and Figure 1B), and mild pulmonary vascular muscularization (Figure 1A, panel v). In contrast, APN−/− mice had markedly more eosinophilic airway inflammation (Figure 1A [panels ii, iv, and viii] and Figure 1B), and perivascular eosinophil accumulation associated with obliteration of small- and medium-sized pulmonary vessels (Figure 1A, panel vi). Analysis of cells isolated from BAL fluid from OVA-immunized and -challenged mice demonstrated a nearly threefold increase in the percentage of eosinophils, and a twofold-lower percentage of monocytes/macrophages in the airways of APN−/− mice compared with wild-type mice (Figure 1C). Total cell numbers of both eosinophils and monocytes/macrophages were fivefold and 2.5-fold greater, respectively, in the BAL fluid from APN−/− mice than from wild-type mice (Figure 1C). Flow cytometry of BAL cells for T-cell subsets and activated T cells (indicated by CD69 positivity) demonstrated a lower percentage of CD4+ and CD8+ T cells in the BAL fluid from APN−/− mice, but the total number of T-cell subsets and activated T cells did not differ between genotypes (Figure 1D). These data demonstrate that APN−/− mice display greater eosinophilic airway inflammation than wild-type mice in this chronic asthma model.
Figure 1.
Increased chronic airway inflammation in adiponectin (APN)-deficient (−/−) mice. (A) Hematoxylin and eosin–stained lung sections of wild-type (top panels: i, 100× magnification; iii, 200× magnification; v, 400× magnification) and APN−/− mice (bottom panels: ii, 100× magnification; iv, 200× magnification; vi, 400× magnification) harvested after 4 weeks of ovalbumin (OVA) challenges. Extensively remodeled vessels are indicated with closed arrows. Scale bars, 100 μm. Further enlarged views of lungs from wild-type mice (vii) and APN−/− mice (viii) demonstrating eosinophils around the airways (open arrows). (B) Blinded histologic scoring of inflammation (scale of 0–4 [0 = normal, 4 = severe]) from wild-type and APN−/− mice harvested after 4 weeks of OVA challenges (*P < 0.001 compared with wild-type mice; n = 10 mice per group from two experiments). (C) Percentage and number of lymphocytes (Lym), monocytes/macrophages (Mono), neutrophils (PMN), and eosinophils (Eos) in the bronchoalveolar lavage (BAL) fluid of wild-type and APN−/− mice (*P < 0.05 compared with wild-type mice; n = 7 mice per group from two experiments). (D) Percentage and number of CD4+, CD8+, CD4+/CD69+, and CD8+/CD69+ lymphocytes in the BAL of wild-type and APN−/− mice (*P < 0.05 compared with wild-type mice; n = 13 mice per group from three experiments).
APN Deficiency Decreases Dynamic Lung Compliance
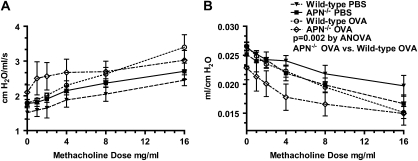
As a result of airway inflammation, mice develop AHR in response to increasing doses of inhaled methacholine. This can be assessed using changes in airway resistance (reflecting AHR in large airways) and dynamic lung compliance (reflecting AHR in small airways). Baseline airways resistance in mice immunized with OVA and challenged with PBS was not different between wild-type and APN−/− mice (1.59 ± 0.14 cm H2O/ml/s versus 1.51 ± 0.18 cm H2O/ml/s, respectively), and increased in a similar manner in response to inhaled methacholine (Figure 2A). Baseline dynamic lung compliance was also not different between PBS-challenged wild-type and APN−/− mice (0.026 ± 0.000 ml/cm H2O versus 0.025 ± 0.002 ml/cm H2O, respectively). However, compliance decreased more in response to inhaled methacholine in the PBS-challenged APN−/− mice compared with wild-type mice (Figure 2B; P = 0.028 by repeated-measures ANOVA). As seen in prior studies (37), there was an increase in airways resistance in response to methacholine inhalation in both wild-type and APN−/− mice challenged with OVA compared with mice challenged with PBS (Figure 2A). Wild-type mice challenged with OVA also had a decrease in dynamic lung compliance in response to methacholine compared with PBS-challenged mice. However, there was only a nonsignificant trend (P = 0.079 by repeated-measures ANOVA) toward a decrease in compliance in APN−/− mice challenged with OVA compared with APN−/− mice challenged with PBS (Figure 2B). There was also a greater decrease in dynamic lung compliance in response to methacholine in APN−/− mice than in wild-type mice challenged with OVA (P = 0.002 by repeated-measures ANOVA). Airway resistance was not different between the genotypes, although there was a trend toward higher resistance in the APN−/− mice (P = 0.11 by repeated-measures ANOVA). In summary, we found that dynamic compliance is decreased in APN−/− mice compared with wild-type mice both at baseline and in response to methacholine challenge in both PBS- and OVA-challenged mice.
Figure 2.
Decreased dynamic lung compliance in APN−/− mice. Airway resistance (A) and dynamic lung compliance (B) in wild-type and APN−/− mice in response to methacholine inhalation, measured after 4 weeks of OVA or PBS challenges (P = 0.11 by ANOVA for resistance between wild-type and APN−/− OVA-challenged mice; P = 0.002 by ANOVA for compliance between wild-type and APN−/− OVA-challenged mice; n = 10 mice per group from two experiments).
APN Deficiency Increases Chemokine Secretion in the Lung
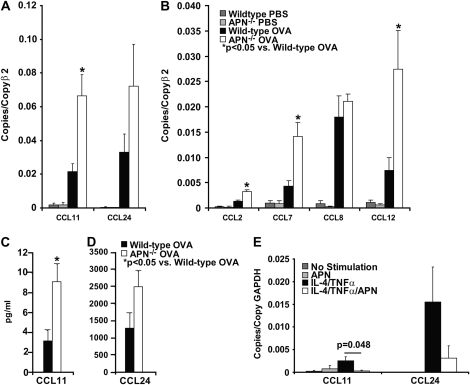
We analyzed lung expression of a panel of lung cytokine and chemokines by quantitative RT-PCR of RNA isolated from the lungs of APN−/− and wild-type mice used in this model of chronic asthma. There was no difference in cytokine or chemokine RNA levels in PBS-challenged wild-type and APN−/− mice, although there was a nonsignificant trend for increased TNF-α levels in the APN−/− mice (data not shown; P = 0.08). After OVA immunization and four weekly OVA challenges, cytokine (IL-4, IL-13, IL-6, and TNF-α) and chemokine (CCL11/eotaxin-1, CCL24/eotaxin-2, CCL2/monocyte chemoattractant protein (MCP)-1, CCL7/MCP-3, CCL8/MCP-2, CCL12/MCP-5) levels increased in both genotypes compared with PBS-challenged mice (Figures 3A and 3B, and data not shown). However, there were threefold-higher levels of CCL11 RNA transcripts, and a trend toward higher CCL24 RNA transcripts in the lungs of APN−/− mice than in wild-type mice (Figure 3A). In addition, RNA levels of the monocyte-active chemokines, CCL2, CCL7, and CCL12, were nearly threefold higher in APN−/− mice than in wild-type mice (Figure 3B). There was no difference in RNA levels of CCL8, IFN-γ, IL-4, IL-5, IL-13, IL-6, and TNF-α between the genotypes (data not shown). We also found threefold-higher levels of CCL11 protein, and a trend toward higher CCL24 protein in BAL samples from APN−/− mice than from wild-type mice (Figures 3C and 3D). These data suggest that increased chemokine production in APN−/− mice in this model of chronic asthma leads to increased recruitment of eosinophils and monocytes into the airways.
Figure 3.
Increased chemokine production in APN−/− mice. (A and B) Chemokine RNA expression in lungs from APN−/− mice and wild-type mice harvested after 4 weeks of OVA or PBS challenges (*P < 0.05 compared with wild-type mice; n = 10 mice per group from two experiments). (C and D) CCL11 and CCL24 protein measured by ELISA in the BAL from wild-type and APN−/− mice after 4 weeks of OVA challenges (P = 0.011 for CCL11 and P = 0.071 for CCL24 wild-type versus APN−/− mice; n = 10 mice per group from two experiments). (E) CCL11 and CCL24 RNA expression in bone marrow–derived macrophages treated with IL-4 (50 ng/ml) and TNF-α (100 ng/ml) with or without APN pretreatment (10 μg/ml) (P = 0.048 for CCL11 and P = 0.18 for CCL24, comparing IL-4/TNF-α with and without APN pretreatment; n = 3 samples per group, repeated twice).
APN Suppresses Macrophage Production of CCL11
Lung macrophages are a critical source of CCL11 and CCL24 in allergic airway inflammation (41–47), suggesting that APN could influence eosinophil recruitment via effects on lung macrophages. To test this hypothesis, we measured CCL11 and CCL24 expression in bone marrow–derived murine macrophages stimulated with IL-4 and TNF-α (which has been shown to maximally stimulate CCL11 and CCL24 production) (48, 49). Some cells were pretreated with APN at a level within the range of serum and airway concentrations (10 μg/ml) that has been used to investigate the effect of APN in vitro (29, 50). APN pretreatment decreased the expression of CCL11 by nearly eightfold, and led to a trend toward lower expression of CCL24 in these cells after stimulation with IL-4 and TNF-α (Figure 3E).
APN Deficiency Does Not Affect Apoptosis in the Lung
There is evidence to suggest that APN inhibits macrophage phagocytic activity, including the ability to take up apoptotic bodies (51). Therefore, we evaluated the number of apoptotic cells using terminal desoxy nucleotidyl TUNEL analysis of the lung. As seen in other studies (28), there was no significant difference in the number of apoptotic bodies identified in APN−/− and wild-type mice after OVA immunization and challenge (data not shown), suggesting that APN was not effecting apoptosis or the clearance of apoptotic cells in this model.
APN Deficiency Does Not Affect Airway Fibrosis
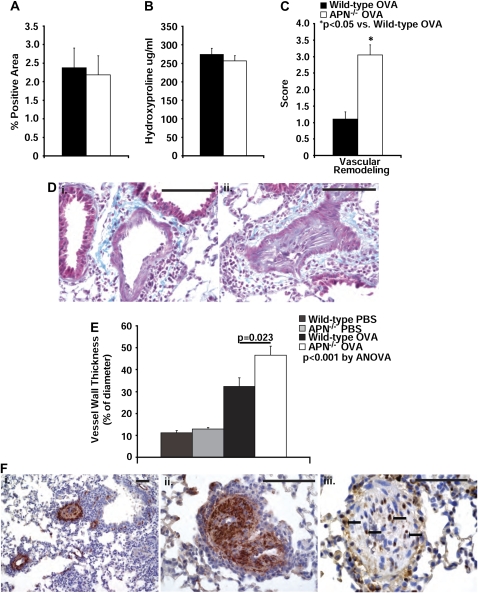
Many patients with asthma develop subepithelial fibrosis in the airways, a component of airway remodeling (52). Murine models of chronic asthma mimic many of the structural changes seen in the airways of humans with asthma, including airway fibrosis (53–56). We therefore evaluated airway fibrosis in our model of chronic asthma by Masson trichrome staining and Picrosirius red staining. This analysis revealed similar amounts of collagen deposition around the airways in lung sections from wild-type and APN−/− mice after OVA immunization and challenge (Figure 4D, panels i and ii, and Figure 4A). Quantification of the total collagen content in the lungs with the hydroxyproline assay revealed a nonsignificant trend toward higher baseline values of hydroxyproline in PBS-challenged APN−/− mice compared with wild-type mice (194.7 ± 9.7 versus 158.2 ± 10.6 μg/ml, respectively; P = 0.07; n = 3 lungs per group), but similar amounts of total lung hydroxyproline in both wild-type and APN−/− mice after OVA challenges (Figure 4B).
Figure 4.
Changes in fibrosis and pulmonary arteries in APN−/− mice by proliferating smooth muscle cells (SMCs). (A) The percentage area of lung sections that stained positive for Picrosirius red is not different in wild-type and APN−/− mice after OVA immunization and challenge (n = 9 mice per group from two experiments). (B) The concentration of hydroxyproline in the right lung is not different in wild-type and APN−/− mice after OVA immunization and challenge (n = 10 mice per group from two experiments). (C) Blinded histologic scoring of vascular obliteration in lung sections (scale of 0–4 [0 = normal, 4 = severe]) from wild-type and APN−/− mice harvested after 4 weeks of OVA challenges (*P < 0.001 compared with wild-type mice; n = 10 mice per group from two experiments). (D) Trichrome staining of lung sections from wild-type (i, 400×) and APN−/− mice (ii, 400×) (scale bars, 100 μm; pictures are representative from n = 10 mice per group from two experiments). (E) Vascular wall thickening in preacinar blood vessels in lung sections from wild-type and APN−/− mice (P < 0.001 by ANOVA between all four groups; P = 0.023 between wild-type and APN−/− mice after OVA challenge; n = 9 mice per OVA group; n = 3 mice per naive group; from two experiments). (F) Representative staining for α-SMC actin in lung sections from APN−/− mice (i, 100× magnification; ii, 400× magnification), demonstrating positivity within the obliterated pulmonary arteries. Representative staining for proliferating cell nuclear antigen in a lung section from APN−/− mice (iii, 400× magnification), demonstrating positivity in nuclei in cells (closed arrows) surrounding and within the obliterated pulmonary artery. Scale bars, 100 μm.
APN Deficiency Increases Pulmonary Vascular Remodeling and Pulmonary Hypertension
Although there was no difference in airway fibrosis between the groups of mice in our chronic asthma model, we observed greater pulmonary arterial remodeling (defined as thickening of the arterial wall) in APN−/− mice compared with wild-type mice (Figure 4C). In the OVA-challenged APN−/− mice, there was greater thickening of the vessel walls (Figure 4E), with near obliteration of the lumen by spindle-shaped cells (Figure 4D, panels i and ii). The cells filling the pulmonary vessels stained positively for α-SMC actin (Figure 4F, panels i and ii), and many of them expressed proliferating cell nuclear antigen (Figure 4F, panel iii). These data suggest that proliferating SMCs narrow the lumen of pulmonary vessels in APN−/− mice in this model. Consistent with the observed histologic changes, we measured increased RVSP in both the APN−/− and wild-type mice after OVA immunization and challenge compared with PBS-challenged mice with a greater increase in APN−/− mice than in wild-type mice (Figure 5A). Of note, arterial oxygen levels were normal in both wild-type and APN−/− mice while animals were breathing room air (data not shown).
Figure 5.
Changes in right ventricular systolic pressure (RVSP) and growth factor expression in APN−/− mice. (A) RVSP in wild-type and APN−/− mice measured after 4 weeks of OVA or PBS challenges (P < 0.001 by ANOVA; P = 0.0007 between wild-type and APN−/− mice after OVA challenge; n = 8 mice per group from two experiments). RVSP in wild-type and APN−/− mice measured after 3 weeks of hypoxia (n = 6 mice per group from one experiment). (B) Growth factor RNA expression in the lungs from APN−/− mice and wild-type mice harvested after 4 weeks of OVA or PBS challenges (n = 5 mice per group from two experiments).
We also measured lung RNA levels of several mediators important for fibrosis and SMC proliferation. In this analysis, we found an increase in the lung RNA levels of transforming growth factor-β, platelet-derived growth factor (PDGF)-α, and PDGF-β in OVA-challenged mice from both genotypes compared with PBS-challenged mice, but we did not detect any differences in the levels of these growth factors in OVA-challenged wild-type mice compared with APN−/− mice (Figure 5B). The levels of plasminogen activator inhibitor-1 were elevated nearly twofold in the APN−/− mice compared with wild-type mice; however, this difference was not significant when corrected for multiple comparisons.
APN Deficiency Does Not Affect Pulmonary Vascular Remodeling in Response to Hypoxia
To determine if the effects of APN deficiency on pulmonary artery SMC (PASMC) proliferation seen after OVA challenge were specific to chronic allergic inflammation, we analyzed the effects of chronic hypoxia on pulmonary vascular remodeling in the APN−/− mice. Although chronic hypoxia induced pulmonary vascular remodeling and pulmonary hypertension in both APN−/− and wild-type mice, there was no difference in the degree of remodeling, as assessed by lung histology and α-SMC staining (data not shown). Furthermore, prolonged hypoxia increased RVSP in both wild-type and APN−/− mice, and there was no difference in the degree of pulmonary hypertension between these two genotypes (Figure 5A).
DISCUSSION
There are multiple pathways linking metabolism to immunity (6), suggesting that obesity may influence immune function and affect the course of inflammatory diseases, such as asthma. Studies in humans and animals have supported a link between obesity and asthma (3, 21), demonstrating potential effects of obesity on airway inflammation (21, 57), AHR (7, 58), and remodeling (20). However, the exact molecular mechanisms that explain this association are not fully known. Our data demonstrate that APN-deficiency, which mimics one component of the obese state, enhances allergic airway inflammation in a murine model of chronic asthma. Although APN deficiency did not seem to modulate airway fibrosis in this model, surprisingly, we observed a dramatic increase in pulmonary arterial muscularization associated with pulmonary hypertension. Obesity has been linked to pulmonary hypertension and pulmonary vascular remodeling, and it is now recognized that lung inflammation is an important component of the pathogenesis of some forms of pulmonary hypertension (34, 59). Thus, our data suggest that APN deficiency in obesity could also contribute to the development of pulmonary hypertension in the setting of inflammatory lung disease.
In normal individuals, APN circulates at very high levels (3–30 μg/ml), and is the most abundant adipose tissue–specific protein produced in humans (9). However, individuals with obesity have low plasma APN levels, presumably due to feedback mechanisms. APN has a wide range of metabolic, antiinflammatory and antiproliferative activities (9), suggesting that decreased APN levels may contribute to the increased inflammatory state in obesity. Consistent with this, APN−/− mice are predisposed to diabetes, vascular disease, and ischemia–reperfusion injury (9–11). Furthermore, APN seems to affect aspects of organ tissue remodeling and vascular SMC proliferation in disease (9–11, 29). It has been proposed that APN may be a “starvation signal” that works to limit energy expenditure. Thus, secretion of APN in times of limited nutrient reserve would suppress processes, such as protein production and cellular proliferation, to preserve cellular energy for other processes. It follows that, in APN-deficient states, such as obesity, inflammation and remodeling may be more robust due to a lack of this suppression.
Links between APN and lung disease are not well defined; however, a few recent studies have suggested that APN can influence the development of airway inflammation (21, 28, 60). APN is normally found at high levels in airway lining fluid, and lung macrophages from APN−/− mice have been shown to be more activated at baseline compared with wild-type mice (28). In addition, all of the four known receptors for APN, AdipoR1, AdipoR2, T-cadherin, and calreticulin (51, 61, 62), are expressed on multiple cell types in the lung, including macrophages, endothelial cells, and SMCs. Thus, the molecular machinery necessary for APN signaling exists in the lung and airways in multiple cell types.
We initially examined the effects of APN deficiency in a model of acute asthma, and found no differences in measures of airway inflammation or AHR between wild-type and APN−/− mice. We next examined the effects of APN deficiency in a model of mild chronic allergic airway inflammation, reasoning that APN deficiency may have more of an impact over a long period of time, and that mild inflammation would be less likely to reduce APN levels in wild-type mice, as seen in the more intense acute model of asthma. In addition, a model of chronic asthma may be more relevant to obese patients with asthma. In this model, we did not see a decrease in APN levels in wild-type mice, and APN−/− mice had an increase in airway inflammation associated with increased accumulation of eosinophils and monocyte/macrophages in the airways. Consistent with the increased cellular recruitment, there were increased levels of the chemokines, CCL11, CCL2, CCL7, and CCL12, in the lung, providing a possible mechanism for the increased airway inflammation observed in APN−/− mice. Our data are consistent with recent human data, which revealed an inverse correlation between serum APN levels and CCL11 levels in patients with obesity (60). Alveolar macrophages from APN−/− mice have recently been shown to spontaneously produce more TNF-α than wild-type cells (28), which may contribute to increased CCL11 production in the lung. However, we did not see increased spontaneous levels of CCL11 or CCL24 in PBS-challenged mice, though, the eotaxins require IL-4/-13 either alone or in combination with TNF-α to be up-regulated (42, 48, 49).
CCL11 is produced by multiple cell types in the lung during allergic inflammation, including macrophages (41–47). Previous data indicate that APN can influence immune responses through actions on NF-κB signaling in macrophages (63), a cellular pathway that has been linked to the development of allergic inflammation and CCL11 production (48, 49, 64). Furthermore, in vitro studies on macrophages have demonstrated that APN inhibits NF-κB activation and the production of IFN-γ, CXCL10/IFN-γ–inducible 10-kD protein, and TNF-α (50, 51). Similar to this, we demonstrate a significant reduction in IL-4/TNF-α–induced CCL11 expression in bone marrow–derived macrophages after APN pretreatment. Overall, these data suggest that APN may modulate allergic inflammation in part via effects on CCL11 and reduced eosinophil recruitment.
AHR develops in response to airway inflammation in this model of chronic asthma (37). We also saw a modest increase in AHR in both wild-type and APN−/− mice, as measured by changes in airways resistance and dynamic compliance in response to methacholine. OVA-challenged APN−/− mice had a greater change in compliance in response to methacholine than OVA-challenged wild-type mice, whereas airways resistance increased in response to methacholine to a similar degree in both genotypes. Prior studies have demonstrated that C57BL/6 mice are more likely to manifest AHR with changes in dynamic compliance rather than airways resistance, reflecting a propensity for small airway inflammation in this strain in asthma models (65). Thus, our data might suggest that the greater change in dynamic compliance in APN−/− mice could reflect an increase in small airway inflammation. However, PBS-challenged APN−/− mice also have a greater decrease in compliance compared with PBS-challenged wild-type mice in response to methacholine. This suggests that there is a baseline decrease in dynamic compliance in mice with APN deficiency, which may also explain the differences between wild-type and APN−/− mice after OVA challenge. Recent data suggest that naive APN−/− mice develop spontaneous emphysema with macrophage activation (28, 66); thus, the decrease in dynamic lung compliance may be due to an increase in small airway closure from reduced lung elastic recoil and/or an increase in baseline airway reactivity. We did not see significant airspace enlargement in our APN−/− mice; however, quantitative measurements of mean airspace chord length were not performed in our studies.
The most unexpected finding in our study was the dramatic increase in pulmonary arterial muscularization and pulmonary hypertension seen in the APN−/− mice relative to wild-type mice in the model. In models of atherosclerosis, APN−/− mice develop increased vascular SMC proliferation in systemic arteries (35), but our study is the first to report similar changes in the pulmonary vasculature. Although asthma is not usually associated with the development of pulmonary vascular disease, inflammation is now recognized as an important stimulus for the development of pulmonary hypertension (30, 32–34). Interestingly, lung vascular remodeling has been reported in humans with asthma (in small vessels associated with inflamed airways) (67–71), and more recently in murine models of allergic airway inflammation (30–33). Male apolipoprotein E–deficient mice on a high-fat diet develop pulmonary hypertension associated with lower APN levels compared with wild-type mice (29). Treatment of these mice with the peroxisome proliferator–activated receptor-γ activator, rosiglitazone, resulted in higher plasma APN levels and complete regression of pulmonary hypertension and pulmonary artery remodeling. Thus, these data suggest that APN may modulate PASMC proliferation and/or migration in response to inflammation or injury.
Eosinophils and macrophages are an important source of growth factors in allergic inflammation, and are essential for airway remodeling in asthma (53, 54, 72–74). Thus, it is possible that the increased accumulation of these cells in APN−/− lungs may contribute to the more intense pulmonary vascular remodeling found in APN−/− mice via enhanced growth factor production. We did see an increase in the RNA levels of several growth factors important in remodeling and SMC proliferation (e.g., transforming growth factor-β, PDGF-α, PDGF-β, and plasminogen activator inhibitor-1) after OVA challenge in both wild-type and APN−/− mice; however, there was no difference in the levels of these factors between the two genotypes. These differences do not exclude differences in protein levels, growth factor activation (or activity), or microanatomical differences (i.e., around blood vessels) of these factors. In addition, recent in vitro studies have demonstrated that APN inhibits growth factor–mediated proliferation of murine PASMCs (29). Growth factors stimulate SMC proliferation in part through effects on the mammalian target of rapamycin–S6 kinase pathway (75–77), and APN has been shown to inhibit growth factor–mediated stimulation of mammalian target of rapamycin via AMPK activation (11, 78–83). Thus, the loss of direct suppressive effects of APN on PASMC proliferation could lead to the increase in pulmonary arterial muscularization found in APN−/− mice, independent of differences in inflammation or growth factor activity.
If the increase in PASMC proliferation seen after OVA challenge resulted largely from direct effects on PASMC, we might expect to see similar changes in the vessels with other models of pulmonary vascular remodeling. However, in the hypoxia model of pulmonary hypertension, APN−/− mice developed an amount of remodeling and pulmonary hypertension similar to that of wild-type mice. Although these data might suggest that the effects of APN deficiency on pulmonary vascular remodeling may be specific to allergic inflammation, recent studies have demonstrated that hypoxia suppresses APN secretion in wild-type mice (84–86); thus, any differences between wild-type and APN−/− mice may be attenuated.
In conclusion, our data suggest that low APN levels may increase asthmatic inflammation in obese individuals. We also observed the unexpected and novel finding of an increase in pulmonary arterial muscularization and pulmonary hypertension with APN deficiency in allergic inflammation, suggesting that coexisting inflammatory lung disease and obesity may predispose individuals to pulmonary hypertension due, in part, to reduced plasma APN levels.
Acknowledgments
The authors thank Dr. Galina Sukhova, Ms. Eugenia Shvartz, Mr. Frank Zincone, and Mr. Ryan Jackobek for skillful technical assistance.
This work was supported by National Institutes of Health grants HL072775 and HL088297 (B.D.M.), AI40618 (A.D.L.), HL34636 (P.L.), and HL074352 (K.D.B.), the Donald W. Reynolds Foundation (P.L.), and the American Heart Association and Eli Lilly (International Fellowship) (Y.O.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0415OC on January 23, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among us adults, 1999–2000. JAMA 2002;288:1723–1727. [DOI] [PubMed] [Google Scholar]
- 2.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007;175:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med 2006;174:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J 2006;13:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sztrymf B, Ioos V, Sitbon O, Parent F, Simonneau G, Humbert M. Pulmonary hypertension and obesity. Rev Pneumol Clin 2002;58:104–110. [PubMed] [Google Scholar]
- 6.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772–783. [DOI] [PubMed] [Google Scholar]
- 7.Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol 2005;115:925–927. [DOI] [PubMed] [Google Scholar]
- 8.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev 2007;18:313–325. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 2006;110:267–278. [DOI] [PubMed] [Google Scholar]
- 10.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 2002;8:731–737. [DOI] [PubMed] [Google Scholar]
- 11.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia–reperfusion injury through AMPK- and COX-2–dependent mechanisms. Nat Med 2005;11:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busse WW, Lemanske RF Jr. Asthma. N Engl J Med 2001;344:350–362. [DOI] [PubMed] [Google Scholar]
- 13.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol 2003;111:215–225. (quiz 226). [DOI] [PubMed] [Google Scholar]
- 14.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest 1999;104:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camargo CA Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med 1999;159:2582–2588. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Dales R, Tang M, Krewski D. Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol 2002;155:191–197. [DOI] [PubMed] [Google Scholar]
- 17.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol 2005;115:897–909. [DOI] [PubMed] [Google Scholar]
- 18.Mosen DM, Schatz M, Magid DJ, Camargo CA, Jr. The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol 2008;122:507–511. [DOI] [PubMed] [Google Scholar]
- 19.Thomson CC, Clark S, Camargo CA Jr. Body mass index and asthma severity among adults presenting to the emergency department. Chest 2003;124:795–802. [DOI] [PubMed] [Google Scholar]
- 20.Aaron SD, Fergusson D, Dent R, Chen Y, Vandemheen KL, Dales RE. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest 2004;125:2046–2052. [DOI] [PubMed] [Google Scholar]
- 21.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 2006;118:389–395. [DOI] [PubMed] [Google Scholar]
- 22.Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol 2003;95:938–945. [DOI] [PubMed] [Google Scholar]
- 23.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001;104:2797–2802. [DOI] [PubMed] [Google Scholar]
- 24.Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc 2008;5:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mokhlesi B, Tulaimat A. Recent advances in obesity hypoventilation syndrome. Chest 2007;132:1322–1336. [DOI] [PubMed] [Google Scholar]
- 26.Haque AK, Gadre S, Taylor J, Haque SA, Freeman D, Duarte A. Pulmonary and cardiovascular complications of obesity: an autopsy study of 76 obese subjects. Arch Pathol Lab Med 2008;132:1397–1404. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed Q, Chung-Park M, Tomashefski JF Jr. Cardiopulmonary pathology in patients with sleep apnea/obesity hypoventilation syndrome. Hum Pathol 1997;28:264–269. [DOI] [PubMed] [Google Scholar]
- 28.Summer R, Little FF, Ouchi N, Takemura Y, Aprahamian T, Dwyer D, Fitzsimmons K, Suki B, Parameswaran H, Fine A, et al. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol 2008;294:L1035–L1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator–activated receptor-gamma activation. Circulation 2007;115:1275–1284. [DOI] [PubMed] [Google Scholar]
- 30.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med 2008;205:361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tormanen KR, Uller L, Persson CG, Erjefalt JS. Allergen exposure of mouse airways evokes remodeling of both bronchi and large pulmonary vessels. Am J Respir Crit Care Med 2005;171:19–25. [DOI] [PubMed] [Google Scholar]
- 32.Rydell-Tormanen K, Uller L, Erjefalt JS. Remodeling of extra-bronchial lung vasculature following allergic airway inflammation. Respir Res 2008;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rydell-Tormanen K, Johnson JR, Fattouh R, Jordana M, Erjefalt JS. Induction of vascular remodeling in the lung by chronic house dust mite exposure. Am J Respir Cell Mol Biol 2008;39:61–67. [DOI] [PubMed] [Google Scholar]
- 34.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 2004;351:1655–1665. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, et al. Role of adiponectin in preventing vascular stenosis: the missing link of adipo-vascular axis. J Biol Chem 2002;277:37487–37491. [DOI] [PubMed] [Google Scholar]
- 36.Medoff BD, Seed B, Jackobek R, Zora J, Yang Y, Luster AD, Xavier R. CARMA1 is critical for the development of allergic airway inflammation in a murine model of asthma. J Immunol 2006;176:7272–7277. [DOI] [PubMed] [Google Scholar]
- 37.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest 2006;116:1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol 2004;287:L1241–L1247. [DOI] [PubMed] [Google Scholar]
- 39.Warren MK, Vogel SN. Bone marrow–derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J Immunol 1985;134:982–989. [PubMed] [Google Scholar]
- 40.Medoff BD, Wain JC, Seung E, Jackobek R, Means TK, Ginns LC, Farber JM, Luster AD. CXCR3 and its ligands in a murine model of obliterative bronchiolitis: regulation and function. J Immunol 2006;176:7087–7095. [DOI] [PubMed] [Google Scholar]
- 41.Lamkhioued B, Renzi PM, Abi-Younes S, Garcia-Zepada EA, Allakhverdi Z, Ghaffar O, Rothenberg MD, Luster AD, Hamid Q. Increased expression of eotaxin in bronchoalveolar lavage and airways of asthmatics contributes to the chemotaxis of eosinophils to the site of inflammation. J Immunol 1997;159:4593–4601. [PubMed] [Google Scholar]
- 42.Li L, Xia Y, Nguyen A, Lai YH, Feng L, Mosmann TR, Lo D. Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J Immunol 1999;162:2477–2487. [PubMed] [Google Scholar]
- 43.Lilly CM, Nakamura H, Belostotsky OI, Haley KJ, Garcia-Zepeda EA, Luster AD, Israel E. Eotaxin expression after segmental allergen challenge in subjects with atopic asthma. Am J Respir Crit Care Med 2001;163:1669–1675. [DOI] [PubMed] [Google Scholar]
- 44.Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo JA, Newman W, et al. Cloning of the human eosinophil chemoattractant, eotaxin: expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest 1996;97:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pope SM, Fulkerson PC, Blanchard C, Akei HS, Nikolaidis NM, Zimmermann N, Molkentin JD, Rothenberg ME. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem 2005;280:13952–13961. [DOI] [PubMed] [Google Scholar]
- 46.Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The eotaxin chemokines and ccr3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol 2005;175:5341–5350. [DOI] [PubMed] [Google Scholar]
- 47.Ying S, Meng Q, Zeibecoglou K, Robinson DS, Macfarlane A, Humbert M, Kay AB. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, rantes, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and c-c chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (intrinsic) asthmatics. J Immunol 1999;163:6321–6329. [PubMed] [Google Scholar]
- 48.Matsukura S, Stellato C, Plitt JR, Bickel C, Miura K, Georas SN, Casolaro V, Schleimer RP. Activation of eotaxin gene transcription by NF-kappa B and STAT6 in human airway epithelial cells. J Immunol 1999;163:6876–6883. [PubMed] [Google Scholar]
- 49.Lilly CM, Nakamura H, Kesselman H, Nagler-Anderson C, Asano K, Garcia-Zepeda EA, Rothenberg ME, Drazen JM, Luster AD. Expression of eotaxin by human lung epithelial cells: induction by cytokines and inhibition by glucocorticoids. J Clin Invest 1997;99:1767–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, et al. Adiponectin inhibits the production of cxc receptor chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res 2008;102:140–142. [DOI] [PubMed] [Google Scholar]
- 51.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor–dependent clearance of early apoptotic bodies. J Clin Invest 2007;117:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Homer RJ, Elias JA. Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology (Bethesda) 2005;20:28–35. [DOI] [PubMed] [Google Scholar]
- 53.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5–deficient mice. J Clin Invest 2004;113:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, et al. A critical role for eosinophils in allergic airways remodeling. Science 2004;305:1776–1779. [DOI] [PubMed] [Google Scholar]
- 55.Kelly MM, Leigh R, Bonniaud P, Ellis R, Wattie J, Smith MJ, Martin G, Panju M, Inman MD, Gauldie J. Epithelial expression of profibrotic mediators in a model of allergen-induced airway remodeling. Am J Respir Cell Mol Biol 2005;32:99–107. [DOI] [PubMed] [Google Scholar]
- 56.Leigh R, Ellis R, Wattie J, Southam DS, De Hoogh M, Gauldie J, O'Byrne PM, Inman MD. Dysfunction and remodeling of the mouse airway persist after resolution of acute allergen-induced airway inflammation. Am J Respir Cell Mol Biol 2002;27:526–535. [DOI] [PubMed] [Google Scholar]
- 57.Misso NL, Petrovic N, Grove C, Celenza A, Brooks-Wildhaber J, Thompson PJ. Plasma phospholipase A2 activity in patients with asthma: association with body mass index and cholesterol concentration. Thorax 2008;63:21–26. [DOI] [PubMed] [Google Scholar]
- 58.Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, Shore SA. Allergic airway responses in obese mice. Am J Respir Crit Care Med 2007;176:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 2008;118:2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herder C, Hauner H, Haastert B, Rohrig K, Koenig W, Kolb H, Muller-Scholze S, Thorand B, Holle R, Rathmann W. Hypoadiponectinemia and proinflammatory state: two sides of the same coin?: results from the Cooperative Health Research in the Region of Augsburg Survey 4 (KORA S4). Diabetes Care 2006;29:1626–1631. [DOI] [PubMed] [Google Scholar]
- 61.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high–molecular-weight forms of acrp30/adiponectin. Proc Natl Acad Sci USA 2004;101:10308–10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 2007;13:332–339. [DOI] [PubMed] [Google Scholar]
- 63.Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, Hanazawa S, Yamashita Y. Adiponectin inhibits Toll-like receptor family–induced signaling. FEBS Lett 2005;579:6821–6826. [DOI] [PubMed] [Google Scholar]
- 64.Desmet C, Gosset P, Pajak B, Cataldo D, Bentires-Alj M, Lekeux P, Bureau F. Selective blockade of NF-kappa B activity in airway immune cells inhibits the effector phase of experimental asthma. J Immunol 2004;173:5766–5775. [DOI] [PubMed] [Google Scholar]
- 65.Takeda K, Haczku A, Lee JJ, Irvin CG, Gelfand EW. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol 2001;281:L394–L402. [DOI] [PubMed] [Google Scholar]
- 66.Wert SE. Does adiponectin play a role in pulmonary emphysema? Am J Physiol Lung Cell Mol Physiol 2008;294:L1032–L1034. [DOI] [PubMed] [Google Scholar]
- 67.Green FH, Butt JC, James AL, Carroll NG. Abnormalities of the bronchial arteries in asthma. Chest 2006;130:1025–1033. [DOI] [PubMed] [Google Scholar]
- 68.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med 1997;156:229–233. [DOI] [PubMed] [Google Scholar]
- 69.Saetta M, Di Stefano A, Rosina C, Thiene G, Fabbri LM. Quantitative structural analysis of peripheral airways and arteries in sudden fatal asthma. Am Rev Respir Dis 1991;143:138–143. [DOI] [PubMed] [Google Scholar]
- 70.Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax 2001;56:902–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanaka H, Yamada G, Saikai T, Hashimoto M, Tanaka S, Suzuki K, Fujii M, Takahashi H, Abe S. Increased airway vascularity in newly diagnosed asthma using a high-magnification bronchovideoscope. Am J Respir Crit Care Med 2003;168:1495–1499. [DOI] [PubMed] [Google Scholar]
- 72.Masu K, Ohno I, Suzuki K, Okada S, Hattori T, Shirato K. Proliferative effects of eosinophil lysates on cultured human airway smooth muscle cells. Clin Exp Allergy 2002;32:595–601. [DOI] [PubMed] [Google Scholar]
- 73.Phan SH, McGarry BM, Loeffler KM, Kunkel SL. Regulation of macrophage-derived fibroblast growth factor release by arachidonate metabolites. J Leukoc Biol 1987;42:106–113. [DOI] [PubMed] [Google Scholar]
- 74.Shore SA. Airway smooth muscle in asthma—not just more of the same. N Engl J Med 2004;351:531–532. [DOI] [PubMed] [Google Scholar]
- 75.Krymskaya VP. Targeting the phosphatidylinositol 3-kinase pathway in airway smooth muscle: rationale and promise. BioDrugs 2007;21:85–95. [DOI] [PubMed] [Google Scholar]
- 76.Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 2006;574:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell 2000;103:253–262. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem 2005;280:18341–18347. [DOI] [PubMed] [Google Scholar]
- 79.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 2004;279:1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of AMP-activated protein kinase signaling. J Biol Chem 2004;279:28670–28674. [DOI] [PubMed] [Google Scholar]
- 81.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation 2007;116:2809–2817. [DOI] [PubMed] [Google Scholar]
- 82.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 2002;99:16309–16313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002;8:1288–1295. [DOI] [PubMed] [Google Scholar]
- 84.Magalang UJ, Cruff JP, Rajappan R, Hunter MG, Patel T, Marsh CB, Raman SV, Parinandi NL. Intermittent hypoxia suppresses adiponectin secretion by adipocytes. Exp Clin Endocrinol Diabetes 2009;117:129–134. [DOI] [PubMed] [Google Scholar]
- 85.Nakagawa Y, Kishida K, Kihara S, Sonoda M, Hirata A, Yasui A, Nishizawa H, Nakamura T, Yoshida R, Shimomura I, et al. Nocturnal reduction in circulating adiponectin concentrations related to hypoxic stress in severe obstructive sleep apnea–hypopnea syndrome. Am J Physiol Endocrinol Metab 2008;294:E778–E784. [DOI] [PubMed] [Google Scholar]
- 86.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 2007;293:E1118–E1128. [DOI] [PubMed] [Google Scholar]