Abstract
Myofibroblast apoptosis is critical for the normal resolution of wound repair responses, and impaired myofibroblast apoptosis is associated with tissue fibrosis. Lung expression of endothelin (ET)-1, a soluble peptide implicated in fibrogenesis, is increased in murine models of pulmonary fibrosis and in the lungs of humans with pulmonary fibrosis. Mechanistically, ET-1 has been shown to induce fibroblast proliferation, differentiation, contraction, and collagen synthesis. In this study, we examined the role ET-1 in the regulation of lung fibroblast survival and apoptosis. ET-1 rapidly activates the prosurvival phosphatidylinositol 3′-OH kinase (PI3K)/AKT signaling pathway in normal and fibrotic human lung fibroblasts. ET-1–induced activation of PI3K/AKT is dependent on p38 mitogen-activated protein kinase (MAPK), but not extracellular signal-regulated kinase (ERK) 1/2, JNK, or transforming growth factor (TGF)-β1. Activation of the PI3K/AKT pathway by ET-1 inhibits fibroblast apoptosis, and this inhibition is reversed by blockade of p38 MAPK or PI3K. TGF-β1 has been shown to attenuate myofibroblast apoptosis through the p38 MAPK–dependent secretion of a soluble factor, which activates PI3K/AKT. In this study, we show that, although TGF-β1 induces fibroblast synthesis and secretion of ET-1, TGF-β1 activation of PI3K/AKT is not dependent on ET-1. We conclude that ET-1 and TGF-β1 independently promote fibroblast resistance to apoptosis through signaling pathways involving p38 MAPK and PI3K/AKT. These findings suggest the potential for novel therapies targeting the convergence of prosurvival signaling pathways activated by these two profibrotic mediators.
Keywords: myofibroblast, fibrosis, Fas, p38 mitogen-activated protein kinase, mesenchymal cells
CLINICAL RELEVANCE
The primary finding of this study is that two fibrogenic growth factors, endothelin (ET)-1 and transforming growth factor (TGF)-β1 independently activate antiapoptotic signaling mechanisms in human lung fibroblasts by p38 mitogen-activated protein kinase–dependent activation of phosphatidylinositol 3′-OH kinase/AKT. These results provide important insights into the mechanisms regulating fibroblast survival and apoptosis, with broad implications in the regulation of lung injury and repair processes. The convergence antiapoptotic signaling pathways mediated by ET-1 and TGF-β1 may represent novel therapeutic targets for the treatment of pulmonary fibrosis.
Endothelin (ET)-1 is a soluble fibrogenic peptide that has been implicated in the pathogenesis of pulmonary fibrosis. Secreted as a preproenzyme, active ET-1 is generated through cleavage by ET-converting enzyme. The two Gαq/11-coupled ET receptors (ET-A and ET-B) activate phospholipase (PL) C to initiate intracellular signaling events (1). Although ET-1 has been extensively studied in the pathobiology of pulmonary arterial hypertension, accumulating evidence supports a role for ET-1 in the pathogenesis of pulmonary fibrosis (2). Animal models show that increased ET-1 levels precede collagen deposition in the lungs after administration of intratracheal bleomycin, and that transgenic overexpression of ET-1 is sufficient to induce progressive pulmonary fibrosis (3, 4). Moreover, ET-1 levels are elevated in patients with pulmonary fibrosis from several underlying etiologies (5). In vitro, ET-1 has profibrotic effects on lung fibroblasts, promoting proliferation (6), differentiation (7), and extracellular matrix (ECM) synthesis (8, 9).
A key feature of pulmonary fibrosis is the accumulation of myofibroblasts, contractile mesenchymal cells with a phenotype that is intermediate between fibroblasts and smooth muscle cells, which synthesize, secrete, organize, and remodel the ECM (10). Myofibroblasts are critical effectors of wound-repair responses, and normal repair requires the ultimate clearance of myofibroblasts by apoptosis (10, 11). Insufficient myofibroblast apoptosis is associated with progressive ECM deposition and contraction, leading to tissue fibrosis (12).
We have previously shown that transforming growth factor (TGF)-β1, a cytokine strongly associated with fibrosis in the lung and other organs/tissues, protects myofibroblasts from apoptosis by p38 mitogen-activated protein kinase (MAPK)–dependent secretion of a soluble factor that activates phosphatidylinositol 3′-OH kinase (PI3K)/AKT signaling (13). The PI3K/AKT signaling pathway is activated after ligation of receptor tyrosine kinases and seven transmembrane G protein–coupled receptors, or through integrin-associated adhesion–mediated signaling cascades (14). Activated AKT may support cell survival through a number of potential mechanisms, including regulation of BCL-2 family proteins, NF-κB, caspase-9, and forkhead family transcription factors (14). TGF-β1 has been shown to induce secretion of ET-1 by fibroblasts (15). This study was undertaken to investigate the role of ET-1 in the regulation of fibroblast fate and to determine if ET-1 mediates TGF-β1 activation of PI3K/AKT and apoptosis resistance in fibroblasts. Portions of this work have been previously published in abstract form (16).
MATERIALS AND METHODS
Cells and Cell Culture
Normal primary human fetal lung fibroblasts (IMR-90; Institute for Medical Research, Camden, NJ) between passages 7 and 12 were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 100 U/ml penicillin/streptomycin (Sigma, St. Louis, MO), fungizone (Invitrogen, Carlsbad, CA), and 5% FBS (Sigma). IMR-90 fibroblasts were incubated in 5% CO2 at 37°C to 60–80% confluence, and growth arrested for 24 hours in DMEM with 0.01% FBS before treatment.
Primary fibroblast cultures were generated using methods previously described (17). Normal lung fibroblasts were cultured from histologically normal portions of lung from patients undergoing surgical resection of biopsy-proven non–small cell lung cancer. Idiopathic pulmonary fibrosis (IPF) fibroblasts were obtained via surgical lung biopsy or from lung explants from patients undergoing lung transplantation. For experiments using normal adult and IPF fibroblasts, three different normal and three different IPF fibroblast lines were used. All studies were approved by the Institutional Review Board at the University of Michigan.
Reagents
ET-1 (human, porcine) and cycloheximide were from Sigma. Porcine-derived TGF-β1 and goat polyclonal antibodies to uncleaved and cleaved caspase-3 were from R&D Systems (Minneapolis, MN). SB203580, SP600125, U73122, Wortmannin, LY294002, and SB431542, along with antibodies to serine-473 phospho AKT, total AKT, phospho-p44/p42 extracellular signal-regulated kinase (ERK) MAPK, phospho p38 MAPK, phospho JNK MAPK, and the corresponding total MAPK antibodies were from Cell Signaling Technology (Beverly, MA). Rabbit polyclonal antibodies to glyceraldehyde-3-phosphate dehydrogenase were from Abcam (Cambridge, MA). Secondary horseradish peroxidase–conjugated anti-mouse, anti-goat, and anti-rabbit antibodies were obtained from Pierce (Rockford, IL). Anti-Fas (activating) antibody clone CH11 and mouse monoclonal antibody to single-stranded DNA (ssDNA) were from Millipore (Billerica, MA).
Western Immunoblotting
Whole-cell lysates were collected in ice-cold RIPA buffer (1% nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, 0.01 M NaH2PO4, 2 mM EDTA, 0.5 mM NaF) containing 2 mM sodium orthovanadate and 1:100 dilution of protease inhibitor mixture III (Calbiochem, San Diego, CA). Protein estimation was done with the Bio-Rad DC protein assay (Bio-Rad, Hercules, CA). Whole-cell lysates were reduced by mixing with a 1:5 vol/vol ratio of 6× electrophoresis sample buffer (0.2 M EDTA, 40 mM dithiothreitol, 6% SDS, 0.06 mg/ml pyronin, pH 6.8) and boiling for 7 minutes. Equal amounts of protein were subjected to SDS-PAGE electrophoresis, as previously described (18).
Real-Time PCR
Total RNA was isolated using the TRIzol reagent, according to the manufacturer's instructions (Invitrogen). A total of 1.0 μg RNA was reverse transcribed into cDNA using M-MLV reverse transcriptase (Invitrogen). The cDNA was then amplified by real-time quantitative TaqMan PCR using an ABI Prism 7,700 sequence detection system (Applied Biosystems, Foster City, CA); β-actin was used as an internal control. SYBR Green Master PCR mix (Applied Biosystems) was used to amplify ET-1, ET-A receptor (ET-A), and ET-B receptor (ET-B). Primers were as follows: (ET-1) forward, 5′-GCTCGTCCCTGATGGATAAA-3′, reverse, 5′-CTGTTGCCTTTGTGGGAAGT-3′; (ETAR) forward, 5′-GCTTCCTGGTTACCACTCATCAA-3′, reverse, 5′-TAGTCTGCTGTGGGCAATAGTTG-3′; (ETBR) forward, 5′-GCCAAGGACCCATCGAGAT-3′, reverse, 5′-GAAGTGTGGAGTTCCCGATGAT-3′; (β-actin) forward, 5′-GCCAC GGCTGCTTCCA-3′, reverse, 5′-GAACCGCTCATTGCCATTG-3′. Gene expression is shown as a fold increase in transcript expression in treated fibroblasts compared with untreated fibroblasts using the ΔCt method, per manufacturer's instructions (Applied Biosystems).
Small Interfering RNA Transfections
RNA interference was accomplished by transfecting cells with ON-TARGET plus SMART pool small interfering RNA (siRNA) for human ET-1 (EDN1;NM_001955), ET-A (EDNRA;NM_001957), and ET-B (EDNRB;NM_003991) from Dharmacon Inc.(Lafayette, CO). Transfections were performed using oligofectamine reagent (Invitrogen), according to the manufacturer's instructions. Briefly, for a 35-mm cell culture dish, 5 μl of oligofectamine and 100 nm of siRNA were mixed for 20 minutes at room temperature. A 200-μl aliquot of the oligofectamine/siRNA mixture was then combined with 800 μl of Opti–Eagle's minimum essential medium for treatment of the cells. After 24 hours, the media were changed to DMEM with 5% FBS. Cells were incubated for 48 hours, and growth arrested in serum-free DMEM for 24 hours before treatment. Nontransfected cells and cells transfected with a nontargeting pooled siRNA (siControl) (Dharmacon Inc.) were used as controls.
ET-1 ELISA
Cell culture supernatants collected after treatments were used for quantitative determination of ET-1 levels by EIA kit from Assay Designs (Ann Arbor, MI), according to the manufacturer's instructions.
Assessments of Apoptosis
Apoptosis was assessed by Western immunoblotting for cleaved caspase-3 and ELISA for ssDNA, as previously described (19).
RESULTS
ET-1 Activates PI3K/AKT in Normal Lung Fibroblasts
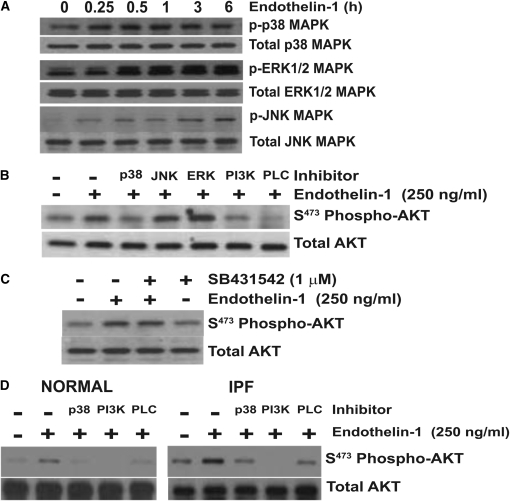
Activation of PI3K/AKT by TGF-β1 promotes myofibroblast resistance to apoptosis (13, 19, 20). To investigate the role of ET-1 in the regulation of fibroblast apoptosis, we first determined if ET-1 activated PI3K/AKT in normal human fetal lung fibroblasts. IMR-90 fibroblasts were treated with ET-1 at concentrations from 0 to 2,500 ng/ml for times ranging from 15 minutes to 24 hours, and AKT activity was assessed by Western immunoblotting with a specific antibody targeting S473 phosphorylation of AKT (Figure 1). A single treatment of ET-1 induced AKT phosphorylation within 1 hour. Maximal activation of AKT was seen within 3 hours, and AKT activation was maintained for 24 hours (Figure 1A). ET-1 induced AKT activation in normal lung fibroblasts at concentrations ranging from 25 to 2,500 ng/ml (10–1,000 nM), with optimal activation occurring at a concentration of 250 ng/ml (Figure 1B). As expected, AKT phosphorylation by ET-1 was blocked by inhibition of either PI3K or PLC (Figure 1B). Similar robust phosphorylation of AKT by 250 ng/ml ET-1 was seen in normal adult and IPF lung fibroblasts (Figure 2D).
Figure 1.
Endothelin (ET)-1 activation of phosphatidylinositol 3′-OH kinase (PI3K)/AKT. Normal human fetal lung fibroblasts (IMR-90) were cultured on tissue culture plates to 80% confluence in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% FBS before growth arrest for 24 hours in DMEM with 0.01% FBS. (A) IMR-90 fibroblasts were treated with ET-1 (250 ng/ml) for the times indicated. (B) IMR-90 fibroblasts were treated for 3 hours with the concentration of ET-1 shown, or with 250 ng/ml ET-1 in the presence/absence of inhibitors of PI3K (Wortmannin, 50 nM) or phospholipase (PL) C (U73122, 5 μM). At the conclusion of treatment, whole-cell lysates were collected and AKT phosphorylation was assessed by SDS-PAGE immunoelectrophoresis and Western immunoblotting. Membranes were stripped and probed for total AKT.
Figure 2.
AKT phosphorylation by ET-1 requires p38 mitogen-activated protein kinase (MAPK). (A) Quiescent IMR-90 fibroblasts were treated with ET-1 (250 ng/ml) for the time periods indicated. Whole-cell lysates were assessed by SDS-PAGE immunoelectrophoresis and Western immunoblotting for: phospho p38 MAPK, phospho extracellular signal-regulated kinase (ERK) 1/2 MAPK, and phospho JNK MAPK. Membranes were stripped and probed for the corresponding total MAPK. (B) IMR-90 fibroblasts were treated with ET-1 (250 ng/ml) for 3 hours in the presence/absence of inhibitors of p38 MAPK (SB203580, 6 μM), JNK MAPK (SP600125, 100 nM), ERK1/2 (PD98059, 20 μM), PI3K (Wortmannin 50 nM), or PLC (U73122, 5 μM). Whole-cell lysates were assessed for AKT phosphorylation by Western immunoblotting, and the membrane was stripped and probed for total AKT. (C) IMR-90 fibroblasts were treated with ET-1 (250 ng/ml) in the presence/absence of SB431542 (1 μM), an inhibitor of the type-1 TGF-β receptor (ALK5). Cell lysates obtained at 3 hours were assessed for AKT phosphorylation by Western immunoblotting, and the membrane was stripped and probed for total AKT. (D) Primary normal adult lung fibroblasts (left panel) and idiopathic pulmonary fibrosis (IPF) fibroblasts (right panel) were treated with ET-1 (250 ng/ml) for 3 hours in the presence/absence of inhibitors of p38 MAPK (SB203580, 6 μM), PI3K (Wortmannin 50 nM), or PLC (U73122, 5 μM). AKT phosphorylation was assessed by Western immunoblotting, and membranes were stripped and probed for total AKT. Data represent three normal and three IPF fibroblast cell lines.
ET-1 Activation of PI3K/AKT Is Mediated by p38 MAPK
MAPKs, including p38, ERK1/2, and JNK, have been shown to mediate the downstream signaling by ET-1 (21). To investigate the mechanism of PI3K/AKT activation by ET-1, IMR-90 fibroblasts were treated with ET-1 (250 ng/ml), and phosphorylation of these MAPKs was assessed at time points from 15 minutes to 6 hours (Figure 2A). Significant activation of p38 MAPK, ERK1/2, and JNK were observed within 15 to 30 minutes after treatment with ET-1. However, p38 MAPK phosphorylation plateaued after 15 to 30 minutes, whereas ERK1/2 and JNK phosphorylation increased gradually over the 6-hour time course studied (Figure 2A). To determine if these MAPKs had a role in ET-1 activation of PI3K/AKT, IMR-90 fibroblasts were treated for 3 hours with ET-1 in the presence/absence of specific inhibitors of these MAPK pathways, PI3K, or PLC, and AKT phosphorylation was determined. Inhibition of p38 MAPK, PI3K and PLC completely blocked ET-1 activation of AKT (Figure 2B). In contrast, inhibition of ERK1/2 or JNK did not inhibit, and appeared to increase, ET-1 phosphorylation of AKT. In separate experiments, blockade of the type-1 TGF-β receptor (ALK5) had no significant effect on AKT activation by ET-1 (Figure 2C). Similarly, inhibition of p38 MAPK blocked ET-1 activation of AKT in primary adult normal and IPF lung fibroblasts (Figure 2D).
ET-1 Attenuates Fibroblast Apoptosis
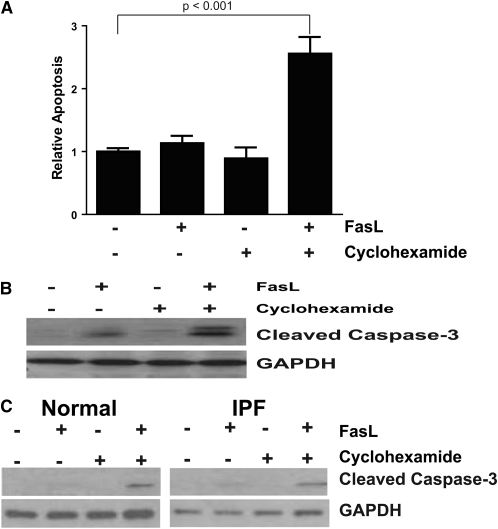
We next sought to determine if ET-1 activation of PI3K/AKT would confer an apoptosis-resistant phenotype to lung fibroblasts. Previous reports have shown fibroblast resistance to extrinsic apoptosis triggered by ligation of the Fas/CD95 receptor (22–24). However, fibroblast susceptibility to Fas-mediated apoptosis can be restored by sensitization with TNF-α, or by the addition of cycloheximide, an inhibitor of protein translation (22, 24). To determine the susceptibility of IMR-90 lung fibroblasts to apoptosis induced by Fas-activation, IMR-90 fibroblasts were treated with CH11, a Fas-activating antibody (FasL), in the presence/absence of cycloheximide, and apoptosis was measured by ELISA for ssDNA (Figure 3A) and by Western immunoblotting for cleaved caspase 3 (Figure 3B). Consistent with the previous reports, we found that IMR-90 fibroblasts were resistant to Fas-mediated apoptosis, and that susceptibility to apoptosis was restored in the presence of cycloheximide. As prior studies have reported variable susceptibility of normal and IPF lung fibroblasts after stimulation with FasL (22, 23), we additionally assessed apoptosis in normal adult and IPF lung fibroblasts (Figure 3C). Similar to the results in IMR-90 fibroblasts, apoptosis in the primary adult lung and IPF fibroblasts was only induced by the combination of Fas-activating ligand and cycloheximide.
Figure 3.
Fibroblast apoptosis requires combined treatment with FasL and cycloheximide. (A) IMR-90 fibroblasts were cultured in a 96-well plate to 80% confluence, and growth arrested for 24 hours before treatment with/without Fas-activating antibody (FasL; CH11, 250 ng/ml), cycloheximide (500 ng/ml), or the combination of FasL and cycloheximide for 16 hours. Apoptosis was assessed by ELISA for single-stranded DNA (ssDNA), and is normalized to the level of untreated controls. P < 0.001 for the combination of FasL plus cycloheximide compared with untreated controls. (B) IMR-90 fibroblasts in 35-mm cell culture dishes were similarly treated, and apoptosis was assessed by Western immunoblotting of whole-cell lysates for cleaved caspase-3. The membranes were stripped and reprobed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The data shown are representative of three separate experiments. (C) Primary normal adult fibroblasts (left panel) and IPF fibroblasts (right panel) were similarly treated, and apoptosis assessed by Western immunoblotting for cleaved caspase-3. Membranes were stripped and probed for GAPDH. The data shown are representative of experiments done with three normal and three IPF fibroblast cell lines.
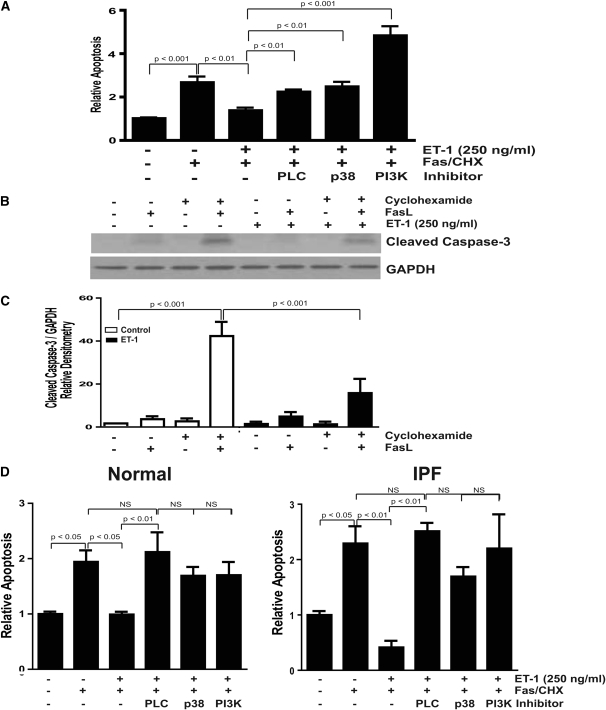
To determine if ET-1 modified apoptosis susceptibility, and to assess the role of PI3K/AKT by ET-1 in fibroblast survival, cells were treated with the combination of FasL and cycloheximide in the presence/absence of ET-1 with/without inhibitors of PLC, p38 MAPK, or PI3K (Figure 4). IMR-90 fibroblasts (Figures 4A–4C), normal primary adult lung fibroblasts (Figure 4D, left panel), and IPF fibroblasts (Figure 4D, right panel) all demonstrated significantly decreased apoptosis after treatment with ET-1. In each fibroblast cell line, the antiapoptotic effects conferred by ET-1 were lost after blockade of PLC, p38 MAPK, or PI3K. Interestingly, inhibition of PI3K enhanced the apoptotic response to Fas and cycloheximide in IMR-90 fibroblasts (Figure 4A), but not in adult (normal or IPF) fibroblasts (Figure 4D). These findings support a novel prosurvival mechanism of ET-1 mediated by p38 MAPK and PI3K/AKT in lung fibroblasts.
Figure 4.
ET-1–mediated protection from apoptosis is dependent on p38 MAPK and PI3K/AKT. (A–C) IMR-90 fibroblasts in a 96-well plate were treated with the combination of FasL (250 ng/ml) and cycloheximide (500 ng/ml) in the presence/absence of ET-1 (250 ng/ml) with/without inhibitors of PLC (U73122, 5 mM), p38 MAPK (SB203580, 6 μM), or PI3K (Wortmannin, 50 nM) for 16 hours. Apoptosis was assessed by (A) ELISA for ssDNA (four replicates per condition and three separate experiments) and (B) Western immunoblotting for cleaved caspase-3. The membrane in (B) was stripped and probed for GAPDH. (C) Densitometric analysis of cleaved caspase-3/GAPDH ratios from three independent experiments. (D) Similarly treated primary normal lung fibroblasts (left panel) and IPF fibroblasts (right panel) were assessed for apoptosis by ELISA for ssDNA (n = 3 normal lung fibroblast lines and 3 IPF fibroblast lines).
TGF-β1 Activation of PI3K/AKT Protects Fibroblasts from Apoptosis Induced by Fas Activation
We have shown that p38 MAPK is necessary for TGF-β1 activation of PI3K/AKT in IMR-90 fibroblasts and alveolar mesenchymal cells from the bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome (ARDS) (13). To determine if a similar signaling pathway is activated in normal adult lung fibroblasts and IPF fibroblasts, these primary cells were treated with TGF-β1 in the presence or absence of an inhibitor of p38 MAPK or an ALK5 inhibitor, and AKT phosphorylation was assessed after 16 hours. Consistent with IMR-90 and ARDS fibroblasts, inhibition of p38 MAPK blocked TGF-β1 activation of PI3K/AKT (Figure 5A). We next showed that TGF-β1 activation of AKT-attenuated apoptosis induced by FasL and cycloheximide in IMR-90 fibroblasts (Figure 5B). Consistent with the previous experiment (Figure 4A), apoptosis of IMR-90 fibroblasts was enhanced by inhibition of PI3K along with the combination of Fas and cycloheximide compared with the combination of Fas and cycloheximide alone. As we found that TGF-β1 and ET-1 confer similar degrees of protection from apoptosis via activation of PI3K/AKT, we next sought to determine the relationship between fibrogenic TGF-β1 and ET-1 in the regulation of fibroblast apoptosis.
Figure 5.
TGF-β1 activation of PI3K/AKT is dependent on p38 MAPK and protects fibroblasts from apoptosis induced by FasL and cycloheximide. (A) Primary normal adult lung fibroblasts (left panel) and IPF fibroblasts (right panel) were treated with TGF-β1 (2 ng/ml) in the presence/absence of inhibitors of p38 MAPK (SB203580, 6 μM) or ALK5 (SB431542, 1 μM) for 16 hours. AKT phosphorylation was assessed by Western immunoblotting. Membranes were stripped and probed for total AKT. Data shown represent three normal and three IPF fibroblast cell lines. (B) IMR-90 fibroblasts were treated with the combination of FasL (250 ng/ml) and cycloheximide (500 ng/ml) with/without TGF-β1 (2 ng/ml) alone or with an inhibitor of PI3K (LY294002, 10 μM) for 16 hours. Apoptosis was assessed by ELISA for ssDNA, and is normalized to the untreated control.
TGF-β1 Induces ET-1 Expression in Lung Fibroblasts
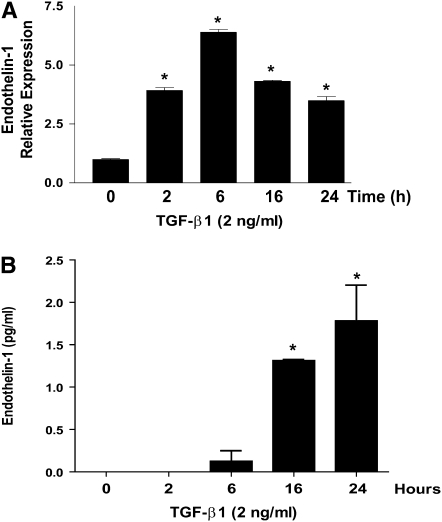
ET-1 is a soluble factor that activates PI3K/AKT, and protects fibroblasts from apoptosis. To determine if autocrine activation of PI3K/AKT by TGF-β1 is mediated by TGF-β1–induced secretion of ET-1, we examined the regulation of ET-1 by TGF-β1 in lung fibroblasts. RNA and cell culture supernatants were collected from IMR-90 fibroblasts between 0 and 24 hours after treatment with TGF-β1. Quantitative real-time PCR showed that TGF-β1 strongly up-regulates ET-1 transcription, with maximal mRNA expression noted between 6 and 16 hours (Figure 6A). Basal levels of active ET-1 in cell culture supernatants were undetectable, and stimulation with TGF-β1 led to a small, but significant, increase in active ET-1 (Figure 6B). Similar induction of ET-1 by TGF-β1 was found in normal adult and IPF lung fibroblasts (Figure 7C).
Figure 6.
ET-1 expression is increased by TGF-β1 treatment. Quiescent IMR-90 fibroblasts were treated with TGF-β1 (2 ng/ml) for the times indicated. (A) RNA was isolated and ET-1 expression was assessed by quantitative real-time PCR (n = 3). (B) Active ET-1 in the cell culture supernatants was assessed by ELISA (n = 3). *P < 0.001 compared with untreated controls.
Figure 7.
ET-1 induction by TGF-β1 is not dependent on p38 MAPK. (A and B) IMR-90 fibroblasts were treated with TGF-β1 (2 ng/ml) in the presence/absence of a p38 MAPK inhibitor (SB203580, 6 μM). (A) RNA was collected at 6 hours, and ET-1 expression was assessed by quantitative real-time PCR. (B) Cell culture supernatants were collected at 16 hours and ET-1 levels assessed by ELISA (n = 3) for (A) and (B). *P < 0.001 compared with untreated controls. (C) Primary normal adult fibroblasts (open bars) and IPF fibroblasts (closed bars) were treated with TGF-β1 (2 ng/ml) with/without a p38 MAPK inhibitor (SB203580, 6 μM) for 16 hours and soluble ET-1 was assessed by ELISA. n = 3 normal and 3 IPF fibroblast lines. *P < 0.001 compared with untreated controls).
TGF-β1 Activation of PI3K/AKT Is Not Mediated by ET-1
TGF-β1 activation of PI3K/AKT requires the p38 MAPK–dependent secretion of a soluble factor (13). Thus, if ET-1 mediates TGF-β1 activation of PI3K/AKT, it should be regulated by TGF-β1 in a p38 MAPK–dependent manner. To define the role of ET-1 in TGF-β1 activation of PI3K/AKT, we treated fibroblasts with TGF-β1 in the presence or absence of a p38 MAPK inhibitor (Figure 7). Surprisingly, inhibition of p38 MAPK had no significant effect on TGF-β1 induction of ET-1 transcription at 6 hours (Figure 7A), or on the secretion of active ET-1 at 16 hours (Figure 7B). Moreover, inhibition of p38 MAPK did not impact TGF-β1 induction of ET-1 in normal adult or IPF-lung fibroblasts (Figure 7C). As inhibition of p38 MAPK failed to block TGF-β1–induced synthesis or secretion of ET-1, these data suggest that ET-1 was not the soluble factor directly mediating TGF-β1 activation of PI3K/AKT.
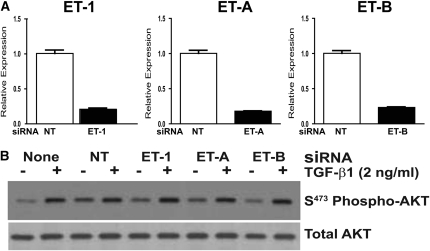
To confirm that TGF-β1 activation of PI3K/AKT was independent of ET-1, we employed siRNA to knock down ET-1 or its receptors, ET-A and ET-B, and examined the impact on TGF-β1 activation of AKT. Knockdown of ET-1 or its receptors was confirmed by real-time PCR 72 hours after siRNA transfection of IMR-90 fibroblasts compared with the expression of cells transfected with a nontargeting (control) siRNA (Figure 8A). Transfected fibroblasts were then treated with/without TGF-β1 for 16 hours, and AKT activation was assessed. As seen in Figure 8B, AKT phosphorylation by TGF-β1 was not affected by siRNA knockdown of ET-1, ET-A, or ET-B, confirming that neither ET-1 nor its receptors are required for TGF-β1 activation of PI3K/AKT.
Figure 8.
TGF-β1 activation of PI3K/AKT is independent of ET-1. IMR-90 fibroblasts were transfected with pooled small interfering RNA (siRNA) targeting ET-1, the ET-A receptor, the ET-B receptor, or with a nontargeting (NT) siRNA. (A) At 72 hours after transfection, RNA was isolated from the transfected cells for quantitative real-time PCR for ET-1, ET-A, or ET-B to demonstrate that there was significant knockdown of the targeted mRNA. (B) Similarly transfected IMR-90 fibroblasts, or nontransfected fibroblasts, were treated with/without TGF-β1 (2 ng/ml) for 16 hours, and AKT phosphorylation was assessed by Western immunoblotting. Membranes were stripped and probed for total AKT. Results are representative of three separate experiments.
DISCUSSION
TGF-β1 and ET-1 have been implicated in the pathogenesis of pulmonary fibrosis. Both cytokines have been shown to modulate pulmonary fibrosis in murine models, and both have been shown to be highly expressed in the lungs of patients with pulmonary fibrosis (4, 25–29). Moreover, both of these mediators induce fibroblast differentiation, ECM synthesis, and collagen gel contraction (7, 10). TGF-β1 has been shown to promote myofibroblast resistance to apoptosis (13, 19, 20, 30, 31), but few studies have addressed the effects of ET-1 on this profibrotic fibroblast phenotype. This study was undertaken to examine the effects of ET-1 on fibroblast survival signaling and apoptosis.
First, we examined the mechanism of ET-1 activation of PI3K/AKT in normal and IPF lung fibroblasts. Next, we investigated the role of PI3K/AKT activation by ET-1 in the regulation of fibroblast survival/apoptosis. Finally, we sought to determine if ET-1 functioned as a downstream mediator of TGF-β1 in the regulation of fibroblast survival, or if ET-1 functioned in a parallel, independent manner. Our primary findings are that ET-1 robustly activates PI3K/AKT in a p38 MAPK–dependent manner, and that ET-1 activation of PI3K/AKT confers apoptosis resistance to fibroblasts. In addition, although TGF-β1 induces ET-1 expression in fibroblasts, ET-1 is not required for TGF-β1 activation of PI3K/AKT. Collectively, these studies establish that TGF-β1 and ET-1 independently promote fibroblast survival through p38 MAPK–dependent activation of PI3K/AKT.
Prevailing hypotheses pose that pulmonary fibrosis results from dysfunctional repair after lung injury from known causes, as in scleroderma and ARDS, or unknown causes, as in IPF (32). Regardless of etiology, pulmonary fibrosis is characterized by myofibroblast accumulation and excessive deposition of ECM. After injury, recruited fibroblasts differentiate into myofibroblasts, which synthesize, secrete, organize, and contract the ECM (10, 33). The fate of recruited fibroblasts may be a critical determinant of the outcome of wound repair, as normal resolution requires the apoptotic clearance of myofibroblasts, whereas fibrosis is associated with insufficient myofibroblast apoptosis (11, 12, 33, 34). Although the current study was not designed to evaluate differences in the apoptosis susceptibility of normal and fibrotic fibroblasts, several studies have found that fibroblasts isolated from the patients with pulmonary fibrosis of different etiologies have increased resistance to apoptosis compared with normal lung fibroblasts (20, 22, 23, 35).
The physiologic stimuli of myofibroblast apoptosis during the resolution phase of wound repair have not been clearly defined. Consistent with several prior studies, we found that lung fibroblasts demonstrate resistance to extrinsic apoptosis induced by ligation of the membrane-bound death receptor, Fas/CD95 (22, 24, 35). In contrast, one study demonstrated fibroblast apoptosis susceptibility to Fas activation, but used a significantly higher concentration of Fas-activating ligand (23). Our study demonstrates fibroblast resistance to apoptosis induced by Fas, which is overcome by cotreatment with cycloheximide, an inhibitor of protein translation. The mechanism of fibroblast resistance to Fas-mediated apoptosis remains poorly defined, and several mechanisms have been proposed. Tanaka and colleagues (24) found that resistance to Fas-mediated apoptosis was due to increased constitutive expression of inhibitors of apoptosis, and that cycloheximide blocked that constitutive expression, allowing apoptosis to proceed. Frankel and colleagues (22) showed that TNF-α facilitates recruitment of the Fas-associated death domain to the cytoplasmic domain of Fas, thereby promoting apoptosis.
The regulation of fibroblast survival and apoptosis in lung homeostasis and disease remains poorly understood, but evidence supports a significant role for PI3K/AKT signaling. TGF-β1 activation of PI3K/AKT, and subsequent myofibroblast resistance to apoptosis, is mediated by the p38 MAPK–dependent, SMAD-independent secretion of a putative soluble factor (13, 19). Focal adhesion kinase, a nonreceptor tyrosine kinase that is activated by TGF-β1, has also been shown to induce apoptosis resistance in fibroblasts through activation of PI3K/AKT (18, 36). Murine models show that phosphorylated AKT is strongly expressed in areas of pulmonary fibrosis after intratracheal administration of bleomycin, and that blockade of PI3K/AKT signaling attenuates pulmonary fibrosis induced by intratracheal bleomycin or TGF-β1 overexpression (37, 38). Finally, increased AKT activation in primary lung mesenchymal cells is associated with resistance to apoptosis and a clinical course of persistent ARDS (20).
In this study, we show that ET-1 inhibits fibroblast apoptosis through activation of PI3K/AKT, suggesting a novel fibrogenic mechanism of ET-1. ET-1 has been shown to inhibit cancer cell apoptosis by activation of PI3K/AKT (39). However, no prior studies have examined the role of ET-1 activation of PI3K/AKT in lung fibroblast survival. One study reported that PI3K/AKT regulates ET-1–induced fibroblast differentiation and contraction (7). Another study recently showed that fibroblasts lacking Thy-1 have decreased apoptosis in contractile collagen gels after treatment with ET-1 when compared with ET-1–treated fibroblasts expressing Thy-1 (40). Our studies show that ET-1 protects normal fetal, normal adult, and IPF lung fibroblasts from apoptosis through activation of PI3K/AKT. Interestingly, the current study suggests that blockade of PI3K enhances Fas-mediated apoptosis in fetal, but not adult, lung fibroblasts (Figures 4A, 4D, and 5B). The mechanism of enhanced apoptosis is unclear, but these data suggest that constitutive expression of PI3K-mediated prosurvival signals contributes to apoptosis resistance in fetal lung fibroblasts.
In concordance with previous reports, we found that ET-1 activates p38, ERK1/2, and JNK MAPKs (21, 41). Early activation of p38 MAPK is required for ET-1 activation of PI3K/AKT, whereas blockade of ERK1/2 or JNK fails to inhibit ET-1 activation of PI3K/AKT. Consistently, blockade of p38 MAPK reversed the antiapoptotic effects of ET-1. This study is the first to demonstrate that p38 MAPK is required for ET-1 activation of PI3K/AKT and acquisition of apoptosis resistance in mesenchymal cells. Collectively, these findings support a novel mechanism by which ET-1 contributes to the pathobiology of pulmonary fibrosis.
TGF-β1 is associated with fibrosis of most organ systems studied, including the lung (42). Among its fibrogenic actions, TGF-β1 induces fibroblast resistance to apoptosis through p38 MAPK–dependent secretion of a soluble factor that activates PI3K/AKT (13). In normal and scleroderma lung fibroblasts, autocrine secretion of ET-1 by TGF-β1 has been found to promote fibroblast differentiation, collagen gel contraction, and ECM synthesis (15, 43). In the current study, we show that ET-1 confers protection from apoptosis in fibroblasts through activation of PI3K/AKT, and we questioned if autocrine induction of ET-1 mediates TGF-β1 activation of PI3K/AKT. Contrary to our original hypothesis, our studies show that TGF-β1 activation of PI3K/AKT is not mediated by ET-1. Although we found that TGF-β1 does induce expression of ET-1 at the gene and protein levels, the absolute increase in soluble, active ET-1 is several orders of magnitude lower than the concentrations required for in vitro activation of AKT in fibroblasts. The observed concentrations of active ET-1 are consistent with those reported in scleroderma fibroblasts exposed to TGF-β (7, 15). Similar concentrations of ET-1 were found in the epithelial lining fluid of patients with ARDS and bronchoalveolar lavage fluid of patients with interstitial lung disease (44–46). Although the concentration of ET-1 secreted by mesenchymal cells stimulated with TGF-β1 in vitro approximates these levels reported in vivo, the effective concentration of ET-1 within fibrotic tissue remains unclear, and may be markedly higher within the pericellular microenvironment.
Our studies indicate that TGF-β1 induction of ET-1 is not inhibited by blockade of p38 MAPK, and that siRNA-mediated knockdown of ET-1 or its receptors (ET-A or ET-B) has no significant effect on TGF-β1 activation of PI3K/AKT. Thus, although ET-1 and TGF-β1 use the same signaling intermediates—p38 MAPK and PI3K/AKT—they independently promote fibroblast survival. Additionally, although the biologic source of ET-1 in patients with IPF has not been defined, our studies suggest that ET-1 secreted by epithelial, endothelial, or inflammatory cells may activate fibroblast survival signaling in a paracrine manner (27, 47).
Effective pharmacologic therapies for pulmonary fibrosis are lacking, and current concepts favor therapies targeting fibrogenic mechanisms, including inhibition of TGF-β1 and ET-1 (32, 48). Studies with ET receptor antagonists in murine models of pulmonary fibrosis have generated conflicting results (49, 50). In one study, a dual ET receptor antagonist reduced the accumulation of connective tissue in mouse lungs 28 days after intratracheal bleomycin (50). A different dual ET receptor antagonist, however, had no significant effect on lung collagen content in rats at 14 or 21 days after bleomycin administration (49). Recently, a clinical trial of a dual ET receptor antagonist in IPF failed to meet the primary efficacy endpoints; however, patients in the treatment group demonstrated a trend toward delayed death and disease progression (51). Further clinical trials of ET receptor antagonists for IPF are ongoing (clinicaltrials.gov: NCT00768300 and NCT00391443). Additionally, ongoing clinical trials are investigating anti-TGF-β1 monoclonal antibodies in IPF (clinicaltrials.gov: NCT00125385). The current study shows that these two fibrogenic mediators—ET-1 and TGF-β1—use the same intracellular signaling intermediates to independently induce an apoptosis-resistant fibroblast phenotype. These findings support the hypothesis that the acquisition of a profibrotic mesenchymal cell phenotype, in the context of pulmonary fibrosis, may be the result of several mediators acting alone or in combination (52). It remains to be seen if individuals with pulmonary fibrosis have disease states dominated by a single mediator (i.e., TGF-β1 or ET-1), or if several mediators function in an additive or synergistic manner to induce fibrosis. Our data suggest that therapy targeting the convergence of downstream signaling pathways common to fibrogenic mediators, rather than targeting a single mediator, may provide improved clinical efficacy for the treatment of pulmonary fibrosis.
This work was supported by National Institutes of Health grants K08 HL081059 (J.C.H.) and R01 HL67967 (V.J.T.), the American Lung Association Dalsemer Award (J.C.H.), the Entelligence Young Investigator Award (J.C.H.), and the ACCP/LUNGevity Foundation (D.A.A.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0447OC on February 2, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annu Rev Physiol 1999;61:391–415. [DOI] [PubMed] [Google Scholar]
- 2.Denton CP, Black CM, Abraham DJ. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat Clin Pract Rheumatol 2006;2:134–144. [DOI] [PubMed] [Google Scholar]
- 3.Hocher B, Schwarz A, Fagan KA, Thone-Reineke C, El-Hag K, Kusserow H, Elitok S, Bauer C, Neumayer HH, Rodman DM, et al. Pulmonary fibrosis and chronic lung inflammation in ET-1 transgenic mice. Am J Respir Cell Mol Biol 2000;23:19–26. [DOI] [PubMed] [Google Scholar]
- 4.Mutsaers SE, Foster ML, Chambers RC, Laurent GJ, McAnulty RJ. Increased endothelin-1 and its localization during the development of bleomycin-induced pulmonary fibrosis in rats. Am J Respir Cell Mol Biol 1998;18:611–619. [DOI] [PubMed] [Google Scholar]
- 5.Antoniu SA. Targeting the endothelin pathway in the idiopathic pulmonary fibrosis: the role of bosentan. Expert Opin Ther Targets 2008;12:1077–1084. [DOI] [PubMed] [Google Scholar]
- 6.Shahar I, Fireman E, Topilsky M, Grief J, Schwarz Y, Kivity S, Ben-Efraim S, Spirer Z. Effect of endothelin-1 on alpha-smooth muscle actin expression and on alveolar fibroblasts proliferation in interstitial lung diseases. Int J Immunopharmacol 1999;21:759–775. [DOI] [PubMed] [Google Scholar]
- 7.Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, et al. Endothelin-1 Promotes myofibroblast induction through the ETA receptor via a Rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell 2004;15:2707–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi-Wen X, Denton CP, Dashwood MR, Holmes AM, Bou-Gharios G, Pearson JD, Black CM, Abraham DJ. Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. J Invest Dermatol 2001;116:417–425. [DOI] [PubMed] [Google Scholar]
- 9.Xu SW, Howat SL, Renzoni EA, Holmes A, Pearson JD, Dashwood MR, Bou-Gharios G, Denton CP, du Bois RM, Black CM, et al. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J Biol Chem 2004;279:23098–23103. [DOI] [PubMed] [Google Scholar]
- 10.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 2007;170:1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 12.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc 2006;3:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horowitz JC, Lee DY, Waghray M, Keshamouni VG, Thomas PE, Zhang H, Cui Z, Thannickal VJ. Activation of the pro-survival phosphatidylinositol 3-kinase/Akt pathway by transforming growth factor-beta1 in mesenchymal cells is mediated by p38 MAPK–dependent induction of an autocrine growth factor. J Biol Chem 2004;279:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 1999;13:2905–2927. [DOI] [PubMed] [Google Scholar]
- 15.Shi-Wen X, Rodriguez-Pascual F, Lamas S, Holmes A, Howat S, Pearson JD, Dashwood MR, du Bois RM, Denton CP, Black CM, et al. Constitutive Alk5-independent C-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol Cell Biol 2006;26:5518–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horowitz JC, Kulasekaran P, Rogers DS, Thannickal VJ. Endothelin-1 mediates TGF-B1 activation of Akt and resistance to apoptosis in lung fibroblasts [abstract]. Proc Am Thorac Soc 2008;177:A730. [Google Scholar]
- 17.Keane MP, Arenberg DA, Lynch JP III, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGiovine B, et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol 1997;159:1437–1443. [PubMed] [Google Scholar]
- 18.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem 2003;278:12384–12389. [DOI] [PubMed] [Google Scholar]
- 19.Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, Thannickal VJ. Combinatorial activation of Fak and Akt by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal 2007;19:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horowitz JC, Cui Z, Moore TA, Meier TR, Reddy RC, Toews GB, Standiford TJ, Thannickal VJ. Constitutive activation of prosurvival signaling in alveolar mesenchymal cells isolated from patients with nonresolving acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 2006;290:L415–L425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choukroun G, Hajjar R, Kyriakis JM, Bonventre JV, Rosenzweig A, Force T. Role of the stress-activated protein kinases in endothelin-induced cardiomyocyte hypertrophy. J Clin Invest 1998;102:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frankel SK, Cosgrove GP, Cha SI, Cool CD, Wynes MW, Edelman BL, Brown KK, Riches DW. TNF-α sensitizes normal and fibrotic human lung fibroblasts to Fas-induced apoptosis. Am J Respir Cell Mol Biol 2006;34:293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moodley YP, Caterina P, Scaffidi AK, Misso NL, Papadimitriou JM, McAnulty RJ, Laurent GJ, Thompson PJ, Knight DA. Comparison of the morphological and biochemical changes in normal human lung fibroblasts and fibroblasts derived from lungs of patients with idiopathic pulmonary fibrosis during Fasl-induced apoptosis. J Pathol 2004;202:486–495. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka T, Yoshimi M, Maeyama T, Hagimoto N, Kuwano K, Hara N. Resistance to Fas-mediated apoptosis in human lung fibroblast. Eur Respir J 2002;20:359–368. [DOI] [PubMed] [Google Scholar]
- 25.Wendel M, Petzold A, Koslowski R, Kasper M, Augstein A, Knels L, Bleyl JU, Koch T. Localization of endothelin receptors in bleomycin-induced pulmonary fibrosis in the rat. Histochem Cell Biol 2004;122:507–517. [DOI] [PubMed] [Google Scholar]
- 26.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 1997;100:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abraham DJ, Vancheeswaran R, Dashwood MR, Rajkumar VS, Pantelides P, Xu SW, du Bois RM, Black CM. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am J Pathol 1997;151:831–841. [PMC free article] [PubMed] [Google Scholar]
- 28.Cambrey AD, Harrison NK, Dawes KE, Southcott AM, Black CM, du Bois RM, Laurent GJ, McAnulty RJ. Increased levels of endothelin-1 in bronchoalveolar lavage fluid from patients with systemic sclerosis contribute to fibroblast mitogenic activity in vitro. Am J Respir Cell Mol Biol 1994;11:439–445. [DOI] [PubMed] [Google Scholar]
- 29.Khalil N, O'Connor RN, Flanders KC, Unruh H. TGF-β1, but not TGF-β2 or TGF-β3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol 1996;14:131–138. [DOI] [PubMed] [Google Scholar]
- 30.Horowitz JC, Rogers DS, Simon RH, Sisson TH, Thannickal VJ. Plasminogen activation–induced pericellular fibronectin proteolysis promotes fibroblast apoptosis. Am J Respir Cell Mol Biol 2008;38:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor β1. Am J Respir Cell Mol Biol 1999;21:658–665. [DOI] [PubMed] [Google Scholar]
- 32.Horowitz JC, Thannickal VJ. Idiopathic pulmonary fibrosis: new concepts in pathogenesis and implications for drug therapy. Treat Respir Med 2006;5:325–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349–363. [DOI] [PubMed] [Google Scholar]
- 34.Laurent GJ, McAnulty RJ, Hill M, Chambers R. Escape from the matrix: multiple mechanisms for fibroblast activation in pulmonary fibrosis. Proc Am Thorac Soc 2008;5:311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buhling F, Wille A, Rocken C, Wiesner O, Baier A, Meinecke I, Welte T, Pap T. Altered expression of membrane-bound and soluble CD95/Fas contributes to the resistance of fibrotic lung fibroblasts to Fasl induced apoptosis. Respir Res 2005;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem 2004;279:33024–33034. [DOI] [PubMed] [Google Scholar]
- 37.Kang HR, Lee CG, Homer RJ, Elias JA. Semaphorin 7A plays a critical role in TGF-beta1–induced pulmonary fibrosis. J Exp Med 2007;204:1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, Standiford TJ, Thannickal VJ. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol 2005;166:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Del Bufalo D, Di Castro V, Biroccio A, Varmi M, Salani D, Rosano L, Trisciuoglio D, Spinella F, Bagnato A. Endothelin-1 protects ovarian carcinoma cells against paclitaxel-induced apoptosis: requirement for Akt activation. Mol Pharmacol 2002;61:524–532. [DOI] [PubMed] [Google Scholar]
- 40.Sanders YY, Kumbla P, Hagood JS. Enhanced myofibroblastic differentiation and survival in Thy-1(−) lung fibroblasts. Am J Respir Cell Mol Biol 2007;36:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aquilla E, Whelchel A, Knot HJ, Nelson M, Posada J. Activation of multiple mitogen-activated protein kinase signal transduction pathways by the endothelin B receptor requires the cytoplasmic tail. J Biol Chem 1996;271:31572–31579. [DOI] [PubMed] [Google Scholar]
- 42.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 2007;117:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi-wen X, Kennedy L, Renzoni EA, Bou-Gharios G, du Bois RM, Black CM, Denton CP, Abraham DJ, Leask A. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor beta in human lung fibroblasts. Arthritis Rheum 2007;56:4189–4194. [DOI] [PubMed] [Google Scholar]
- 44.Nakano Y, Tasaka S, Saito F, Yamada W, Shiraishi Y, Ogawa Y, Koh H, Hasegawa N, Fujishima S, Hashimoto S, et al. Endothelin-1 level in epithelial lining fluid of patients with acute respiratory distress syndrome. Respirology 2007;12:740–743. [DOI] [PubMed] [Google Scholar]
- 45.Sofia M, Mormile M, Faraone S, Alifano M, Zofra S, Romano L, Carratu L. Increased endothelin-like immunoreactive material on bronchoalveolar lavage fluid from patients with bronchial asthma and patients with interstitial lung disease. Respiration 1993;60:89–95. [DOI] [PubMed] [Google Scholar]
- 46.Reichenberger F, Schauer J, Kellner K, Sack U, Stiehl P, Winkler J. Different expression of endothelin in the bronchoalveolar lavage in patients with pulmonary diseases. Lung 2001;179:163–174. [DOI] [PubMed] [Google Scholar]
- 47.Giaid A, Michel RP, Stewart DJ, Sheppard M, Corrin B, Hamid Q. Expression of endothelin-1 in lungs of patients with cryptogenic fibrosing alveolitis. Lancet 1993;341:1550–1554. [DOI] [PubMed] [Google Scholar]
- 48.Walter N, Collard HR, King TE Jr. Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc Am Thorac Soc 2006;3:330–338. [DOI] [PubMed] [Google Scholar]
- 49.Mutsaers SE, Marshall RP, Goldsack NR, Laurent GJ, McAnulty RJ. Effect of endothelin receptor antagonists (BQ-485, RO 47-0203) on collagen deposition during the development of bleomycin-induced pulmonary fibrosis in rats. Pulm Pharmacol Ther 1998;11:221–225. [DOI] [PubMed] [Google Scholar]
- 50.Park SH, Saleh D, Giaid A, Michel RP. Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med 1997;156:600–608. [DOI] [PubMed] [Google Scholar]
- 51.King TE Jr, Behr J, Brown KK, du Bois RM, Lancaster L, de Andrade JA, Stahler G, Leconte I, Roux S, Raghu G. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2008;177:75–81. [DOI] [PubMed] [Google Scholar]
- 52.Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J 2007;30:835–839. [DOI] [PubMed] [Google Scholar]