Abstract
OBJECTIVE
A direct comparison of the incidence and risk factors of major thrombosis in essential thrombocythemia (ET) and polycythemia vera (PV) according to their respective JAK2V617F allele burden is the object of this study.
METHODS
We compared the rate (%/pts/yr) of major thrombosis in 867 ET patients (57% JAK2V617F) with that of 415 PV patients (all JAK2V617F) and examined risk factors.
RESULTS
Patients with ET wild type, ET V617F and PV showed a rate of thrombosis of 1.4, 2.1, and 2.7 %/pt/yr respectively. This latter was found to progressively increase according to the time of diagnosis. The actuarial probability of arterial and venous thrombosis in the first 5 years of diagnosis was roughly similar in the three groups. While, in the subsequent periods, the curves of mutated ET patients diverged from wild type and after 10–15 years, ET mutated arm approached PV.
CONCLUSION
These findings support the concept of a continuum between ET JAK2 mutated and PV not only in reference to the hematological phenotype but also in terms of vascular events
Keywords: JAK2V617F allele burden, rate of thrombosis, essenthial thrombocythemia, polycythemia vera
INTRODUCTION
Patients with essential thrombocythemia (ET) can be categorized as either JAK2V617F mutated (V617F+) or wild type (V617F−)1, 2. Mutated patients display multiple features resembling polycythemia vera (PV), with significantly higher hemoglobin level and neutrophil counts, lower platelet count, more pronounced bone marrow erythropoiesis and granulopoiesis and higher tendency to transform to PV3, 4. This has suggested that mutated ET patients and PV are phenotypic expression of the same pathological process in which the effects of the V617F mutation on erythropoiesis are constrained by physiological mechanisms, including erythropoietin suppression and depleted iron stores, or by genetic modifiers, either acquired or constitutional5.
Remarkably, the presence of leukocytosis6 and V617F mutation and/or the burden of mutated allele have been related to the rate of thrombosis both in ET and PV7 but no data are available on direct comparability of the two diseases in terms of vascular complications.
Aim of this paper was to see whether ET patients harboring JAK2V617F mutation had a rate of major thrombosis comparable to PV patients depending on V617F allele burden. A positive result would support the hypothesis that ET and PV may be viewed as a continuum not only for the hematological phenotype but also in terms of vascular complications and may strength the prognostic role of allele burden mutation.
MATERIALS AND METHODS
A total of 1282 patients with ET (n=867) and PV (n=415) followed in two Italian Institutions (Ospedali Riuniti Bergamo, Italy and Policlinico Careggi, Florence, Italy) constituted the study sample. ET patients were selected from the previously reported cohort of 1063 patients8 on the basis of JAK2V617F allele burden that was tested in patients from Bergamo and Florence centers. Permission was obtained from local IRBs to review the medical records.
All patients were diagnosed by the PVSG, and from 2002 by WHO criteria. Patients were at low- or high-risk for thrombosis according to standard risk factors (age ≥ 60 years and/or a previous major thrombotic event). Low risk patients were not given cytoreductive therapy whereas hydroxyurea (HU) was prescribed in the great majority of high-risk patients. Low dose aspirin was prescribed according to the judgement of physicians in charge. A small group of elderly patients (n=11, 1%) was given busulfan. Cytotoreductive therapy was aimed to reduce platelet number to < 600 ×109/L and in the majority of PV patients, these drugs were associated with phlebotomies, to maintain hematocrit level to less than 45%.
Vascular events included ischemic stroke, cerebral transient ischemic attack (TIA), acute myocardial infarction (AMI), peripheral arterial thrombosis (PAT) and venous thromboembolism (VTE). Diagnostic procedures were previously reported9. Follow-up of patients was updated until the occurrence of vascular event or at the last visit and patients developing disease transformation were truncated at the time of evolution.
JAK2V617F mutation was tested in all 1282 ET and PV patients while JAK2V617F allele burden was quantitated, at different times from diagnosis (see result section), in 719 cases representing 79,4% of mutated patients. Quantitative Real Time PCR (QRT-PCR) assay was performed as previously published9.
Statistical methods
Continuous variables were compared using the extension of the Wilcoxon rank-sum non-parametric test for trend across ordered groups and categorical variables were analysed using the Pearson extended chi-square and Fisher exact tests to see differences across the three groups in Table 1.
TABLE 1.
Features of 1,282 patients
| ET JAK2 wild type |
ET JAK2V617F |
PV JAK2V617F |
p | ||
|---|---|---|---|---|---|
| Patients number | 376 | 491 | 415 | ||
| Laboratory features | |||||
| Age (years, median, range) | 49 (8 – 88) | 56 (12 – 93) | 61 (17 – 91) | <.0001† | |
| Gender, F/M (number, %) | 249/125 (67/33) | 338/155 (69/31) | 191/222 (46/54) | <.0001† | |
| Hemoglobin (g/dL, median, range) | 13.6 (9.7 –15) | 14.5 (8.9 – 15) | 17.6 (13 – 24) | <.0001† | |
| Hematocrit (%, median, range) | 41 (30 – 51.5) | 43 (30 – 54) | 53.8 (40 – 71.5) | <.0001† | |
| White Blood Cells (×109/L, median, range) |
8.3 (3.3 – 26) | 9.1 (4 – 35) | 10.5 (4.9 – 49.7) | <.0001† | |
| Platelets (×109/L, median, range) | 817 (407 – 2269) | 733 (304 – 2149) | 487 (112 – 1639) | <.0001† | |
| Quantitative JAK2V617F (%)* | |||||
| 0 | 376 (100) | - | - | ||
| 1–25 | 190 (49) | 64 (19) | |||
| 26–50 | 177 (45) | 118 (36) | |||
| >50 | 23 (6) | 147 (45) | <.0001‡ | ||
| Years from diagnosis to assay (median, range) |
- | 2.26 (0 – 24.8) | 1.32 (0 – 24.5) | 0.060† | |
| Evolutions (number, %) | 26 (7) | 51 (10.5) | 28 (7) | ||
| PV | 1 (0.5) § | 23 (5) | - | 0.004 ¶ | |
| MF | 24 (6) | 25 (5) | 25 (6) | 0.842‡ | |
| AML | 1 (0.5) | 3 (0.5) | 3 (1) | 0.568‡ | |
| Clinical features | |||||
| High-risk patients (number, %) | 136 (36) | 285 (58) | 248 (60) | <.0001‡ | |
| Previous Thrombosis (number, %) | 61 (16) | 132 (27) | 70 (17) | 0.003‡ | |
| IMA | 12 (3) | 40 (8) | 10 (2) | ||
| Ictus/TIA | 20 (5) | 36 (7) | 26 (7) | ||
| PAT | 8 (2) | 15 (3) | 1 (0) | ||
| VTE | 21 (6) | 41 (9) | 33 (8) | ||
Percentages calculated respectively on 390 and 329 ET and PV JAK2V617F patients, for whom quantitative determination was carried out
Wilcoxon rank-sum non-parametric test for trend
Pearson extended chi-square test
Fisher exact test
JAK2 persistently negative
Thrombotic episodes occurring in follow-up were calculated as rates % patient/year (pt/yr). Thrombosis-free survival curves adjusted for patients’ age at diagnosis were calculated by the Kaplan-Meier method and the test for trend of the survivor function across ordered groups was used to compare JAK2 wild type ET, JAK2 mutated ET and PV patients. Patients were divided in groups according to the time from diagnosis to JAK2 mutation and allele burden assay.
JAK2V617F allele burden and other meaningful covariates (leukocytosis) were examined as risk factors for thrombosis by using multivariable Cox proportional hazard model performed at diagnosis and after selecting patients who remained asymptomatic up to 5 years from diagnosis. The cut-off values chosen for WBC count were the quartiles of the whole distribution at diagnosis. Estimates were adjusted for disease (PV versus ET) and presence of standard risk factors in both models.
RESULTS
Patients were divided in three groups: ET wild type, ET JAK2V617F and PV (all JAK2V617F). Table 1 reports their main clinical and laboratory findings. The groups were significantly different for age and gender distribution. As expected, hemoglobin, hematocrit and leukocyte levels progressively increased across groups, whereas platelet number were lower. JAK2V617F allele burden greater than 50% was found in 45% of PV and 6% of ET patients, respectively. The values between 26 and 50% were almost equally distributed in ET and PV patients (45% versus 36%). Note that only 298 (45%) of patients were tested for JAK2V617F allele burden assay at presentation or within two years from diagnosis
Vascular events occurred during follow-up are shown in Table 2. The cumulative incidence of total major arterial and venous thrombosis was 146 out of 1282 patients (11%). Annual rates of thrombosis were 1.4% patients/year in ET JAK2 wild type, 2.1% in ET mutated and 2.7% in PV (p=0.001).
TABLE 2.
Vascular events in follow-up in the whole series of 1,282 patients
| ET JAK2 wild type |
ET JAK2V617F |
PV JAK2V617F |
||
|---|---|---|---|---|
| Patients number | 376 | 491 | 415 | |
| Follow-up (years, median, range) | 5.0 (0 – 27) | 3.7 (0 – 25) | 3.8 (0 – 26) | |
| Thrombosis in the follow-up (number, %) | 34 (9) | 54 (11) | 58 (14) | |
| IMA | 9 (2) | 9 (2) | 13 (3) | |
| Ictus/TIA | 13 (4) | 15 (3) | 17 (4) | |
| PAT | 3 (1) | 6 (1) | 3 (1) | |
| VTE | 9 (2) | 24 (5) | 25 (6) | |
| Rate of thrombosis (%/patients/year) | 1.4 | 2.1 | 2.7 | |
| Relative Risk (95% CI) | 1 | 1.5 (1.00–2.31) | 2.3 (1.50–3.49) | |
Thrombosis-free survival is presented in Figure 1. In the first 5 years from diagnosis, there was no significant difference among the three groups; subsequently, the curves of mutated patients started to diverge from ET wild type and, in a long-term prevision, the ET mutated curve approached PV (test for trend p=0.05).
FIGURE 1. Thrombosis-Free Survival by disease and JAK2V617F status.
Curves plotted using the Kaplan Meier method, adjusting for age at diagnosis. P-value associated to the test for trend across ordered groups is quoted.
To see whether this pattern could be associated with changes of JAK2V617F allele burden, we divided patients in groups according to the time from diagnosis to the date of quantitative JAK2 assay (Figure 2). In the majority of ET cases (57%), the allele burden at presentation or within two years from diagnosis was between 1–25% and this percentage lowered progressively according to the duration of disease in favour of groups between 26–50% and over 50%. This progressive change was also seen in PV patients in whom the initial allele burden values between 1–25% were found in only 24% of cases, while the categories 25–50% and over 50% were more represented .
FIGURE 2. JAK2V617F allele burden distribution by the time of the test.
ET patients (n=390) and PV patients (n=329)
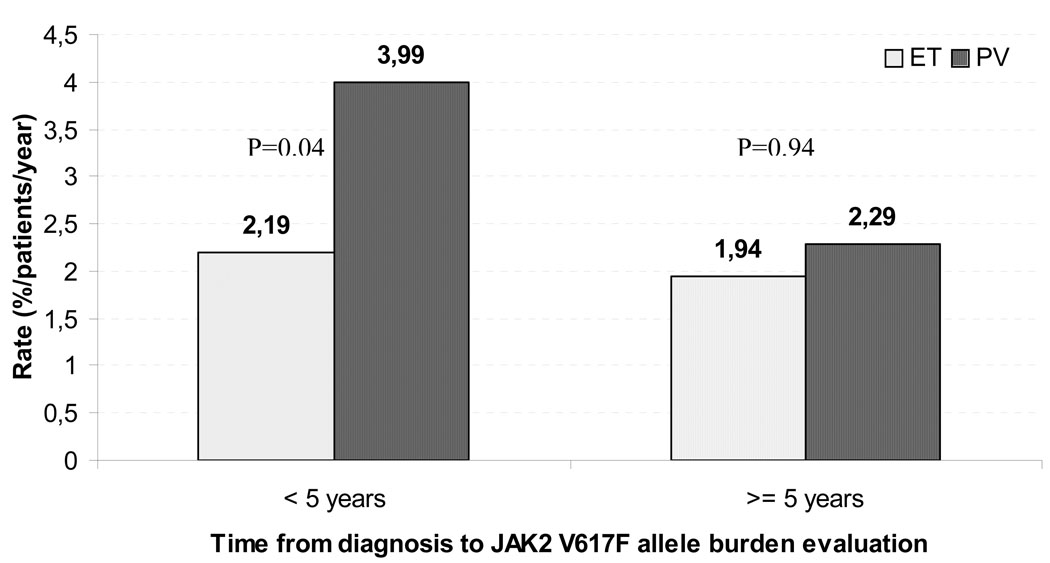
The impact of JAK2V617F allele values on the incidence of thrombosis was analyzed in two multivariable Cox models (Table 3). The first included whole population at diagnosis and the second only patients who remained thrombosis-free for 5 years from diagnosis (N=605, 80 major vascular events). The two groups showed different prognostic factors for thrombosis. At diagnosis, PV (versus ET), standard risk factors (age above 60 years and/or previous thrombosis) and leukocytosis were independently associated with an increased risk of events while after 5 years from diagnosis JAK2 allele burden became the only independent factor for predicting subsequent thrombotic events (HR=1.74, 95% CI 1.00–3.50, p=.01; and HR=2.89, 95% CI 1.47–5.61, p=.002, respectively). It is worth noting that after 5 years of diagnosis the median leukocyte counts lowered from 9.1 to 7.6 ×109/L due to the effect of HU therapy. This analysis was extended by comparing the rate of thrombosis in patients with the same allele burden (26–50%) but different disease (ET vs. PV), at different times from diagnosis. Results shown in Figure 3 stress that in the first 5 years from diagnosis the rate of thrombosis is influenced by the hematological phenotype, despite similar allele burden, whereas this is not true in the later period.
TABLE 3.
Multivariable analyses for the prediction of thrombosis in follow-up
| At diagnosis | HR | 95% (CI) | p |
|---|---|---|---|
| Disease (PV vs ET) | 1.55 | 1.02 – 2.36 | 0.04 |
| Standard risk factors* | 1.89 | 1.34 – 2.66 | 0.001 |
| JAK2V617F (1–25%) | 0.98 | 0.46 – 1.63 | 0.73 |
| JAK2V617F (26–50%) | 1.12 | 0.79 – 1.87 | 0.56 |
| JAK2V617F (>50%) | 1.19 | 0.81 – 2.01 | 0.52 |
| WBC 7.5 – 9.1 (× 109/L) † | 1.52 | 0.88 – 2.61 | 0.13 |
| WBC 9.1 – 11.3 (× 109/L) † | 1.67 | 0.92 – 2.70 | 0.09 |
| WBC ≥ 11.3 (× 109/L) † | 1.93 | 1.01 – 2.77 | 0.04 |
| At 5 years from diagnosis | |||
| Disease (PV vs ET) | 1.54 | 0.80 – 2.97 | 0.19 |
| Standard risk factors* | 1.19 | 0.71 – 2.01 | 0.51 |
| JAK2V617F (1–25%) | 1.61 | 0.75 – 3.44 | 0.22 |
| JAK2V617F (26–50%) | 1.74 | 1.00 – 3.50 | 0.01 |
| JAK2V617F (>50%) | 2.89 | 1.47 – 5.61 | 0.002 |
Aged 60 years or older and/or previous thrombosis
Reference categories: ET, age below 60 years and asymptomatic, JAK2 wild type, WBC less than 7.5 (× 109/L).
Quartiles of the WBC count distribution at diagnosis used as cut-off; multivariable model using the PVSG cut-off for leukocytosis at 10 ×10^9/L produced an HR of 1.826 with 95% confidence interval 1.065–3.133 (p=0.0287)
FIGURE 3.
Rate of thrombosis in patients with 26–50% of JAK2 V617F allele burden according to time.
DISCUSSION
This is a retrospective analysis of a large cohort of 1282 unselected patients with ET and PV diagnosed from 1981 to 2008. In JAK2 mutated ET patients, a higher rate of thrombosis, compared to wild type, was documented. This finding is in keeping with other experiences10,11 and three recent metanalyses11,12,13 where the odds ratio of thrombosis was almost double in mutated vs. non mutated ET. We previously reported that the thrombosis rate in mutated ET patients was similar to that observed in PV.14 However, that study was restricted to the mutational status and did not include JAK2 allele burden measurements. Moreover, the number of patients was limited and the analysis considered the sum of thrombotic episodes occurring at presentation and during follow-up. We confirm here in a larger patient population that the rate of thrombotic complications in mutated ET patients is intermediate between ET wild-type and PV in the first 15 years of diagnosis.
Considering JAK2V617F allele burden, recent reports have indicated that in PV and ET, considered separately, the rate of vascular events progressively increased according to the amount of mutated alleles 3,15,16 but a direct comparison of the two diseases was not done. Thus, whether the same allele burden values could produce comparable rates of events in ET and PV remained undetected. In the present analysis, we have found that the frequency of thrombosis progressively increased according to the amount of allele burden both in ET and PV. The highest rate of vascular complications was seen in groups with allele burden over 50% found in the majority of PV and in a small proportion of ET.
Time distribution of vascular events in our three groups showed an actuarial probability of arterial and venous thrombosis in the first 5 years of diagnosis roughly similar. In the subsequent periods, the curves diverged from wild type and after 10–15 years ET mutated curve approached PV. Based on multivariable analysis, our interpretation is that this pattern is due to the burden of JAK2 mutant clones that, in analogy to BCR/ABL clones in chronic myeloid leukemia, can manifest a survival advantage enabling them to expand after many years of diagnosis.
This process appears slower in ET since around 5% of mutated alleles were acquired over 5 years period. In the first 2 years of diagnosis, 38% of ET patients had JAK2 allele burden between 26–50% whereas the percentage was 55% after 10 years (p=0.02). In PV, this change seems more pronounced, as shown by the percentage of patients with allele burden more than 50% that moved from 34% to 66% after 10 years of diagnosis (p<.001). From these data, one could assume an allele accumulation over time, but we are aware that this interpretation is based on findings not collected from the same patients tested at multiple time points. The only way to support the hypothesis of allele burden accumulation would be to collect prospective data over many years. In primary myelofibrosis an even more significant progress over time of JAK2V617F allele burden has been reported by the GIMEMA group.17 These investigators showed in a prospective evaluation that heterozygous cases progressed to a homozygous V617F status at the calculated rate of 10% patients-year in the period from diagnosis to 8 or more years of disease duration.
Three previous papers faced the question of clonal evolution in ET and PV. The first was published by Gale et al.18, who in a long-term serial analysis of X chromosome inactivation patterns and JAK2V617F mutant levels in patients with ET showed that minor mutant-positive clones can remain stable for many years. The second by Tefferi et al.19 who observed a time dependent increase in JAK2V617F allele burden in PV whereas a similar phenomenon was not shown in ET. The third by Theocharides et al.20 who demonstrated in a 5 year longitudinal study little change in the allelic ratios of JAK2 mutations of PV and ET. Several variables may account for these discrepancies, including therapy, study design, length of observational period and number of patients.
We have explored, in two multivariable models, the clinical relevance of allele burden in terms of vascular complications. Considering patients at diagnosis, we confirmed that standard risk factors (age above 60 years and/or previous thrombosis) and leukocytosis were significantly associated with an increased risk of events in follow-up.8,9,21 PV versus ET at diagnosis was also an independent predictor of thrombosis and the reason could be ascribed to a more pronounced myeloproliferation including also an expansion of red cell mass. On the contrary, after 5 years from diagnosis, multivariable model showed that these factors lost their prognostic value very likely in relation to a good control of myeloproliferation by cytoreductive therapy. At this time, the strongest and unique risk factor for subsequent thrombosis was JAK2V617F allele burden so that PV or ET phenotype did not influence the rate of subsequent events. Values between 26 and 50% and above 51% were predictors of subsequent events independently on standard risk factors and diagnosis of PV versus ET.. Interestingly enough is that ET genotype progressively approached PV even though hematological phenotype remained that of ET entity in the great majority of patients. In fact, in this series, only 5% of ET cases transformed and met the criteria of PV diagnosis.
In conclusion, these findings support the concept of a continuum between ET JAK2 mutated and PV not only in reference to the hematological phenotype but also in terms of vascular events. In addition, they draw attention on the accumulation of allele burden over long-time and its possible role in predicting thrombotic complications.
Acknowledgments
This work was supported by fundings from FIRC - Fondazione Italiana per la Ricerca sul Cancro (AC fellowship), European LeukemiaNet Sixth Framework Programme LSH-2002-2.2.0-3, Grant NIH – Clinical Trails Consortium Myeloproliferative Disease Research Consortium, MIUR - Cofin 2006067001_003 (AMV), Associazione Paolo Belli and AIL - Associazione Italiana Lotta alla Leucemia AIL, sezione Paolo Belli.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
No financial interest/relationships with financial interest relating to the topic of this article have been declared.
REFERENCES
- 1.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 2.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 3.Kittur J, Knudson RA, Lasho TL, et al. Clinical Correlates of JAK2 V617F Allele Burden in Essential Thrombocythemia. Cancer. 2007;109:2279–2284. doi: 10.1002/cncr.22663. [DOI] [PubMed] [Google Scholar]
- 4.Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366:1945–1953. doi: 10.1016/S0140-6736(05)67785-9. [DOI] [PubMed] [Google Scholar]
- 5.Pardanani A, Fridley BL, Lasho TL, Gilliland DG, Tefferi A. Host genetic variation contributes to phenotypic diversity in myeloproliferative disorders. Blood. 2008;111:2785–2789. doi: 10.1182/blood-2007-06-095703. [DOI] [PubMed] [Google Scholar]
- 6.Barbui T, Carobbio A, Rambaldi A, Finazzi G. Perspectives on thrombosis in essential thrombocythemia and polycythemia vera: is leukocytosis a causative factor? Blood. doi: 10.1182/blood-2009-02-206797. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vannucchi AM, Antonioli E, Guglielmelli P, Pardanani A, Tefferi A. Clinical correlates of JAK2V617F presence or allele burden in myeloproliferative neoplasms: a critical reappraisal. Leukemia. 2008;22:1299–1307. doi: 10.1038/leu.2008.113. [DOI] [PubMed] [Google Scholar]
- 8.Carobbio A, Finazzi G, Antonioli E, et al. Thrombocytosis and leukocytosis interaction in vascular complications of essential thrombocythemia. Blood. 2008;112:3135–3137. doi: 10.1182/blood-2008-04-153783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carobbio A, Antonioli E, Guglielmelli P, et al. Leukocytosis and risk stratification assessment in essential thrombocythemia. J Clin Oncol. 2008;26:2732–2736. doi: 10.1200/JCO.2007.15.3569. [DOI] [PubMed] [Google Scholar]
- 10.Larsen TS, Pallisgaard N, Moller MB, Hasselbalch HC. High prevalence of arterial thrombosis in JAK2 mutated essential thrombocythaemia: independence of the V617F allele burden. Hematology. 2008;13:71–76. doi: 10.1179/102453308X315960. [DOI] [PubMed] [Google Scholar]
- 11.Dahabreh IJ, Zoi K, Giannouli S, Zoi C, Loukopoulos D, Vougarelis M. Is JAK2 V617F mutation more than a diagnostic index? A meta-analysis of clinical outcomes in essential thrombocythemia. Leuk Res. 2009;33:67–73. doi: 10.1016/j.leukres.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Ziakas PD. Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica. 2008;93:1412–1414. doi: 10.3324/haematol.12970. [DOI] [PubMed] [Google Scholar]
- 13.Lussana F, Caberlon S, Pagani C, Kamphuisen PW, Büller HR, Cattaneo M. Association of V617F Jak2 mutation with the risk of thrombosis among patients with essential thrombocythaemia or idiopathic myelofibrosis: A systematic review. Thromb Res. doi: 10.1016/j.thromres.2009.02.004. In press. [DOI] [PubMed] [Google Scholar]
- 14.Finazzi G, Rambaldi A, Guerini V, Carobbo A, Barbui T. Risk of thrombosis in patients with essential thrombocythemia and polycythemia vera according to JAK2 V617F mutation status. Haematologica. 2007;92:135–136. doi: 10.3324/haematol.10634. [DOI] [PubMed] [Google Scholar]
- 15.Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110:840–846. doi: 10.1182/blood-2006-12-064287. [DOI] [PubMed] [Google Scholar]
- 16.Vannucchi AM, Antonioli E, Guglielmelli P, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952–1959. doi: 10.1038/sj.leu.2404854. [DOI] [PubMed] [Google Scholar]
- 17.Barosi G, Bergamaschi G, Marchetti M, et al. JAK2 V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis. Blood. 2007;110:4030–4036. doi: 10.1182/blood-2007-07-099184. [DOI] [PubMed] [Google Scholar]
- 18.Gale RE, Allen AJ, Nash MJ, Linch DC. Long-term serial analysis of X-chromosome inactivation patterns and JAK2 V617F mutant levels in patients with essential thrombocythemia show that minor mutant-positive clones can remain stable for many years. Blood. 2007;109:1241–1243. doi: 10.1182/blood-2006-06-029769. [DOI] [PubMed] [Google Scholar]
- 19.Tefferi A, Strand JJ, Lasho TL, et al. Bone marrow JAK2V617F allele burden and clinical correlates in polycythemia vera. Leukemia. 2007;21:2074–2075. doi: 10.1038/sj.leu.2404724. [DOI] [PubMed] [Google Scholar]
- 20.Theocharides A, Passweg JR, Medinger M, et al. The allele burden of JAK2 mutations remains stable over several years in patients with myeloproliferative disorders. Haematologica. 2008;93:1890–1893. doi: 10.3324/haematol.13074. [DOI] [PubMed] [Google Scholar]
- 21.Carobbio A, Finazzi G, Guerini V, et al. Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: interaction with treatment, standard risk factors, and Jak2 mutation status. Blood. 2008;109:2310–2313. doi: 10.1182/blood-2006-09-046342. [DOI] [PubMed] [Google Scholar]