Abstract
The chromosomal passenger complex (CPC) comprises at least four protein components and functions at various cellular localizations during different mitotic stages to ensure correct chromosome segregation and completion of cytokinesis. Borealin, the most recently identified member of the CPC, is an intrinsically unstructured protein of low solubility and stability. Recent reports have demonstrated the formation binary or ternary CPC sub-complexes incorporating short Borealin fragments in vitro. Using isothermal titration calorimetry, we show that full-length Borealin, instead of a Borealin fragment possessing the complete Survivin and INCENP-recognition sequence, is required for the composition of a Borealin-Survivin complex competent to interact with INCENP. In addition, we show evidence that full-length Borealin, which forms high-order oligomers in its isolated form, is a monomer in the Borealin-Survivin CPC sub-complex.
INTRODUCTION
The CPC functions in various key mitotic events, including chromatin modification (phosphorylation of histone H3), correction of kinetochore attachment errors, quality control of the spindle assembly checkpoint, the assembly of a stable bipolar spindle, chromosome segregation and completion of cytokinesis (1-4). During the progression of mitosis, the CPC moves to a series of locations in the cell, activating substrates in a coordinated fashion. Specifically, during prometaphase when chromosomes begin to bind to microtubules, the CPC localizes at the centromeres, which are the regions of the chromosomes to which the mitotic spindle fibers attach. At the onset of anaphase when chromosomes start to migrate to opposite poles of the cell, the CPC relocates to the central spindle. A fraction of the CPC then moves to the equatorial cortex just before cleavage furrow assembly and the ensuing cytokinesis (1-4). Despite the intriguing localization patterns of the CPC at mitosis, it is currently largely a mystery what the localization-specific CPC targets are at various mitotic stages, and the precise mechanistic basis of CPC function remains unclear.
There are four reported chromosomal passenger proteins within the CPC: the serine-threonine kinase Aurora B, the catalytic core of the CPC whose function is regulated by phosphorylation as well as by association with other CPC components (5-9); the inner centromere protein INCENP, an activating and targeting subunit of the CPC (10-13); Survivin, a protein that plays a clear role in cell mitosis (14, 15) and a plausible role in apoptosis which is based principally on the sequence composition of Survivin that includes a baculovirus inhibitor of apoptosis protein (IAP) repeat (BIR), and the observed over-expression of Survivin in a variety of cancer cells (16-18); and Borealin/Dasra B, the most recently reported CPC component that is important in kinetochore-microtubule attachment error correction (19-21). The Aurora B kinase appears to be at least partially activated at the onset of mitosis following association with INCENP and Survivin, after which further post-translational modification events (including phosphorylation of two serines in the C-terminus of INCENP) proceed and lead to full Aurora B activation (5-9). The remaining three CPC components (INCENP, Survivin and Borealin) are thought to act as adaptor proteins and direct the Aurora B kinase to specific substrates at various localizations during different stages of cell mitosis. For example, Borealin has been shown to be the only CPC component that demonstrates DNA binding and thus has been suggested to be responsible for recruiting the CPC to the inner centromere during prometaphase through direct interaction with centromeric DNA (22). Furthermore, a ternary CPC sub-complex comprising Survivin and a short N-terminal fragment each from Borealin and INCENP presents a composite molecular surface of conserved residues essential for central spindle and midbody localization (23).
Within the CPC, the four proteins interact extensively. Figure 1 summarizes the domain structures of the four CPC components and the mapping of their interaction regions. A general feature of most chromosomal passenger proteins is low solubility and stability. Borealin, an intrinsically unstructured protein, appears to be the least soluble CPC component since full-length and N/C-terminal fragments of Borealin have all exhibited high-order oligomerization characteristics (19, 22). Furthermore, our work has shown rapid degradation of recombinant full-length Borealin expressed in E. coli. So far structural and biophysical studies have only been reported for binary or ternary CPC sub-complexes involving short Borealin fragments (23, 24), but it is not known whether these complexes functionally behave in the same way as their corresponding native CPC sub-complexes incorporating full-length Borealin.
Figure 1.
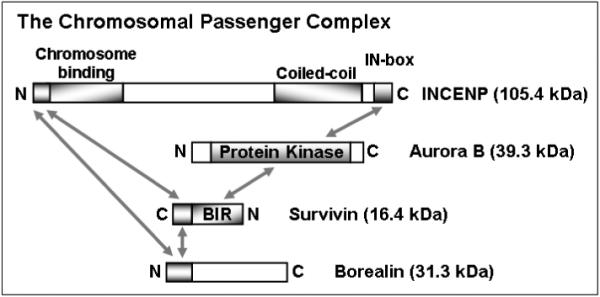
A summary of observed protein-protein interactions (illustrated by two-way arrows) within the CPC (1-4). Protein sequences of unknown structure and function are shown as blank boxes. The molecular weights of the four CPC components are those from Homo sapiens.
In this study, we have developed an approach to circumvent the unfavorable solubility and stability characteristics of full-length (f.l.) Borealin, and we produced sufficient amounts of a binary CPC sub-complex comprising f.l. Borealin and Survivin (f.l. Survivin has been used throughout this study) for protein-protein interaction studies using isothermal titration calorimetry. In parallel, we performed identical ITC characterization of a binary CPC sub-complex comprising an N-terminal fragment of Borealin (containing the entire Survivin-recognition sequence, a region that Borealin also uses to interact with INCENP) and Survivin. We observed striking differences in the protein-protein interaction behavior between the Survivin-Borealin fragment complex and the corresponding complex constituted by Survivin and f.l. Borealin. In addition, our study shows that the two Survivin-Borealin complexes adopt different stoichiometries providing insight into their respective three-dimensional (3D) structural assembly.
MATERIALS AND METHODS
DNA Cloning and Protein Sample Preparation
The Borealin constructs (the full-length protein and the fragment comprising residues 13-92) were each cloned into a pET21a vector derivative (Gev1, Addgene), which has a C-terminal His6 tag and an N-terminal GB1 (immunoglobulin G binding domain B1 of streptococcal protein G; 56 amino acid residues) tag. Survivin and INCENP1-69 were cloned into a pET21a vector (MerckBiosciences) as a non-tagged and a C-terminal His6-tagged protein. Proteins were expressed in E. coli strain DE3 T1R cells (Sigma-Aldrich) overnight at 18 °C for GB1Borealinf.l. and GB1Borealin13-92 or at 25 °C for Survivin and INCENP1-69. The Survivin-GB1Borealinf.l. and Survivin-GB1Borealin13-92 complexes were produced by co-purification of the overnight induction cell cultures containing separately expressed Borealin and non-tagged Survivin. The cell lysis buffer contains 50 mM Tris-HCl pH 8.0, 300 mM NaCl, 0.5 mM TCEP and Roche protease inhibitors (Roche Applied Sciences). The cleared cell lysate were purified using Ni-NTA affinity columns (MerckBiosciences) and the eluents were subjected to extensive dialysis in buffer containing 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1mM DTT. A final purification step was then carried out using size-exclusion chromatography (SEC) on a Superdex 200 prep grade column (GE Healthcare). The protein samples of His6-tagged INCENP1-69/Survivin/GB1 were purified using similar procedures.
Isothermal Titration Calorimetry (ITC)
ITC experiments were performed using a VP-ITC instrument (MicroCal Inc., USA). Samples of the Survivin-GB1Borealinf.l. and Survivin-GB1Borealin13-92 complexes and INCENP1-69 were dialyzed into buffer containing 50 mM Tris-HCl pH8.0, 150 mM NaCl, 1 mM DTT for 1 hour. Titrations were performed by injecting consecutive aliquots of INCENP1-69 (63 μM) into the ITC cell (volume 1.5 ml) containing the Survivin-GB1Borealinf.l. complex or the Survivin-GB1Borealin13-92 complex (both at 6.3 μM) at 10 °C. A repeat titration of INCENP1-69 (63 μM) into the Survivin-GB1Borealin13-92 complex (6.3 μM) was carried out at a different temperature of 20 °C in order to validate the near zero enthalpy change observed at 10 °C. ITC data were corrected for heats of dilution of the protein solution. Binding stoichiometry, enthalpy, entropy and binding constants were determined by fitting the corrected data to a one site binding model. The ITC data were fitted using Origin 7.0 (MicroCal Inc., USA). ITC experiments for the Survivin-GB1Borealinf.l. complex were carried out immediately after the 1 hour dialysis following the final step of purification using SEC. Each ITC experiment was completed within one hour.
Similar ITC procedures were carried out for the titrations of GB1 (100 μM) into Survivin (10 μM) at two temperatures (20 °C and 30 °C) in order to confirm that the GB1 tag used in the Borealin constructs does not interact with Survivin.
Analytical UltraCentrifugation (AUC)
Sedimentation velocity experiments were performed using a Beckman Optima XL-I analytical ultracentrifuge equipped with an interference optical system. The sample of the Survivin-GB1Borealin13-92 complex was prepared with dilution buffer containing 50mM Tris-HCl pH 8.0, 150mM NaCl, 0.5 mM TCEP and 1mM DTT. The aluminium double-sector centrepieces were filled with 400 μl of the protein complex and 420 μl of the dilution buffer, respectively. Samples were centrifuged at a speed of 40,000 rpm at 10°C using an An50-Ti rotor. Scans were acquired at a wavelength of 280 nm at time intervals of 600 seconds. The partial specific volume of 0.72 ml/g for a 2:2 stoichiometry of the Survivin-GB1Borealin13-92 complex was calculated based on amino acid composition (25). Sedimentation velocity data were analysed using SEDFIT (26).
Size-Exclusion Chromatography-Multi-Angle Laser Light Scattering (SEC-MALLS)
The molecular weight (M.W.) and M.W. distributions of the Survivin-GB1Borealin13-92 complex were determined by SEC conducted using a JASCO chromatography system composed of dual PU1580 pumps, a UV1575 detector, a Dawn HELOS Laser photometer and an Optilab rEX interferometric refractometer (Wyatt Technology, Santa Barbara, CA), with a Superdex 200 10/300 GL analytical column (Pharmacia) at a flow rate of 0.5 ml/min in buffer containing 50 mM Tris-HCl pH 8.0, 150 mM NaCl, and 0.5 mM TCEP. Molecular weight was analyzed using Astra (version 5.1.9.1) at a dn/dc value of 0.186 ml/g.
RESULTS
A Specific Protocol for the Preparation of a CPC Sub-Complex Comprising Full-Length Borealin and Survivin
The challenges in producing good quality protein samples of CPC sub-complexes incorporating f.l. Borealin include severe aggregation problems (most likely due to Borealin) and Borealin degradation. In order to alleviate these problems with f.l. Borealin, we introduced an N-terminal GB1 tag based on the highly soluble small immunoglobulin binding domain B1 (56 residues) of streptococcal protein G, a tag that has the potential to enhance the expression and solubility of target proteins (27). The addition of the GB1 tag to Borealin significantly improved the protein yield in the soluble fractions compared to Borealin constructs with no GB1 tag. In order to accurately assess whether GB1 interacts with Survivin, we carried out ITC experiments where aliquots of purified protein samples of GB1 (100μM) were titrated into 1.5 milliliters of purified Survivin sample (10 μM) at 20 °C and 30 °C. No heat (enthalpy) change was detected under both experimental conditions (Figure S1). These results are consistent with our initial observations that GB1 does not interact with Survivin using protein pull-down assays and analytical SEC analysis of a mixture of purified GB1 and Survivin where the two proteins elute separately (data not shown). We then devised a specific protocol to produce tractable protein samples of a binary CPC sub-complex comprising Survivin and f.l. Borealin (Survivin-GB1Borealinf.l.) for protein-protein interaction studies using ITC. The protocol which includes protein expression and purification using the nickel affinity column is described in the Materials and Methods section. The eluents from the nickel column require extensive dialysis (at least 48 hours) in buffer containing 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1mM DTT at 4 °C prior to the final purification of the Survivin-GB1Borealinf.l. complex using SEC. In addition, the Survivin-GB1Borealinf.l. complex is sensitive to high temperature (> 10 °C) and the concentration process using bench-top centrifuges where there are at least two adverse factors: the complex sticking to the concentrator membrane and the appreciable dissociation of the complex at a relative centrifugation force (RCF) of 3112 ×g (4000 RPM on a Heraeus Labofuge 400R bench top centrifuge with a swinging bucket rotor) as assessed using SEC (data not shown). Therefore the concentration process needs to be avoided or carried out at a lower RCF of 778 ×g.
ITC Reveals an Active Survivin-GB1Borealinf.l. Complex and an In-Active Survivin-GB1Borealin13-92 Complex
Previous studies have shown clear biochemical evidence for in vitro complex formation between Borealin and INCENP, and also between Survivin and INCENP, an interaction that is enhanced by the presence of Borealin (19, 22). Since the Survivin-GB1Borealinf.l. complex aggregates severely at a concentration above approximately 7 μM and shows signs of degradation within 2 hours after the final SEC purification step, we chose to use ITC to characterize the protein-protein interaction activity based on its speed and sensitivity. The following ITC experiments were carried out within 2 hours of passing the Survivin-GB1Borealinf.l. complex sample through the final SEC column. In the ITC experiments, 1.5 milliliters of the Survivin-GB1Borealinf.l. or the Survivin-GB1Borealin13-92 complex sample (both at 6.3 μM concentration) was titrated with aliquots of 63 μM purified protein samples of an N-terminal fragment of INCENP (INCENP1-69, containing the entire Borealin-Survivin recognition sequence). Strikingly, the ITC experiments suggest no detectable interaction between the Survivin-GB1Borealin13-92 complex and INCENP1-69 based on no observed heat (enthalpy) change at two different temperatures (10 °C and 20 °C; Figure 2). This observation is consistent with our protein pull-down assay results where non-tagged INCENP1-69 failed to bind to the His6-tagged Survivin-GB1 Borealin13-92 complex that was pre-bound to the nickel column (data not shown). On the other hand, the Survivin-GB1Borealinf.l complex demonstrated a strong interaction with INCENP1-69 (27 nM affinity; Figure 2). These data suggest that Survivin-GB1Borealinf.l., rather than Survivin-GB1Borealin13-92, is an active CPC sub-complex that is competent to interact strongly with INCENP. The stoichiometry of the interaction between INCENP1-69 and the Survivin-GB1Borealinf.l. complex is presumed to be 1:1 despite the fitted ITC data giving an N value of ~0.75. The lower value of N is likely due to a finite level of degradation of Borealin over the duration of the ITC experiment since our independent observations of freshly purified protein samples of the Survivin-GB1Borealinf.l. complex show a small percentage of degradation of Borealin within a 2 hour timescale (Figure S2).
Figure 2.
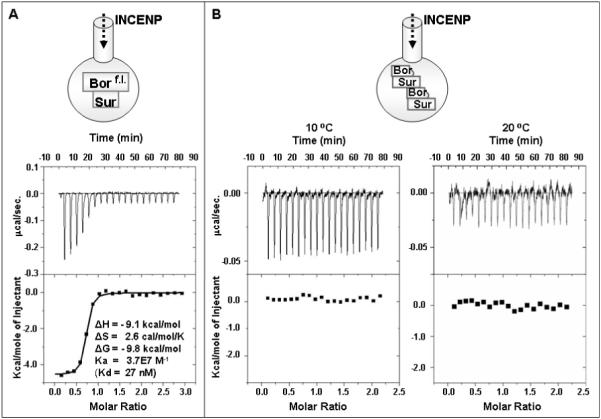
Isothermal titration calorimetry. (A) Titration of the Survivin-GB1Borealinf.l. complex with INCENP1-69 in buffer containing 50 mM Tris-HCl pH8.0, 150 mM NaCl, 1 mM DTT at 10 °C suggests strong binding interaction. (B) Titrations of the Survivin-GB1Borealin13-92 complex with INCENP1-69 at two temperatures of 10 °C (left) and 20 °C (right) in buffer containing 50 mM Tris-HCl pH8.0, 150 mM NaCl, 1 mM DTT suggest near zero enthalpy change implying no detectable interaction.
Different Stoichiometries Are Adopted by the Survivin-GB1Borealin13-92 and Survivin-GB1Borealinf.l. Complexes
Why do the two Survivin-Borealin complexes behave differently with respect to INCENP binding? We characterized their respective solubility, stability and stoichiometry. The complex of Survivin-GB1Borealin13-92 is highly soluble (up to 9 mg/ml without detectable aggregation (assessed using SEC, data not shown) and stable (< 2 months at -80 °C). Previous 3D structural studies show that Survivin is a homo-dimer in its isolated form and adopts an elongated shape due to the presence of a protruding C-terminal ~65 Å-long α-helix in each protomer (28-31). In our SEC analysis, purified protein samples of Survivin (predicted M.W. 35.6 kDa as a homo-dimer including a 14-residue sequence derived from the pET21a vector) elutes at a volume corresponding to a 58 kDa globular protein according to M.W. calibrations using 29-700 kDa protein M.W. standards (Sigma-Aldrich; data not shown). In order to accurately determine the M.W. and the stoichiometry of the Survivin-GB1Borealin13-92 complex, we applied AUC and SEC-MALLS, two techniques that allow estimation of the M.W. in a manner broadly independent of the hydrodynamic anisotropy of the molecules under investigation. The AUC and SEC-MALLS analyses of the Survivin-GB1Borealin13-92 complex suggested a M.W. of 73.9 ± 4.3 kDa and 80.9 ± 1.0 kDa, respectively (Figure 3). The data are consistent with a 2:2 stoichiometry between Survivin (predicted M.W. 17.8 kDa) and GB1Borealin13-92 (predicted M.W. 19.9 kDa) in the Survivin-GB1Borealin13-92 complex (predicted M.W. 75.4 kDa as a 2:2 complex).
Figure 3.
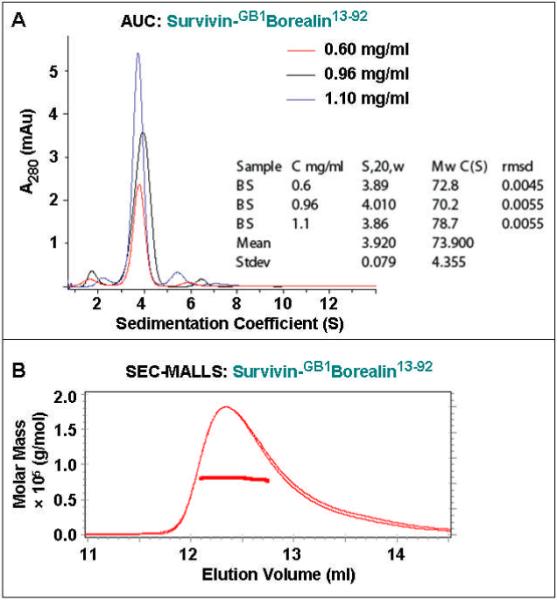
(A) Sedimentation velocity AUC analysis of the Survivin-GB1Borealin13-92 complex at three concentrations (0.6, 0.96, 1.1 mg/ml) suggests a mean M.W. of 73.9 ± 4.3 kDa. (B) SEC-MALLS analysis of the Survivin-GB1Borealin13-92 complex at 0.44 mg/ml suggests a M.W. of 80.9 ± 1.0 kDa.
In contrast, the Survivin-GB1Borealinf.l. complex has low solubility (severe aggregation at above approximately 7 μM concentration) and low stability (degradation starts within 2 hours after the final SEC purification) therefore precluding M.W. characterizations using AUC. We have attempted to use SEC-MALLS to determine the M.W. of the Survivin-GB1Borealinf.l. complex at a concentration of 6.3 μM at 4 °C using freshly-made samples. However, no reliable data could be obtained due to the low signal-to-noise level in the elution profile detected by the refractive index detector (whose sensitivity is 10-100 times lower than that of a UV detector).
According to analytical SEC, the Survivin-GB1Borealin13-92 2:2 complex elutes at 12.92 ml, whilst the Survivin-GB1Borealinf.l. complex elutes at a later volume of 13.27 ml (Figure 4). The difference in the SEC retention volumes of the two Survivin-Borealin complexes suggests that the M.W. of the Survivin-GB1Borealinf.l. complex is lower than that of the Survivin-GB1Borealin13-92 complex whose M.W. has been determined to be ~70-80 kDa using AUC and SEC-MALLS. Based on the computed molecular masses of the expressed polypeptides, the predicted M.W. of a Survivin-GB1Borealinf.l. heterodimer complex is 57.3 kDa. Therefore, the later retention volume of the Survivin-GB1Borealinf.l. complex compared to that of the Survivin-GB1Borealin13-92 complex suggests that the former is most likely to adopt a 1:1 stoichiometry in which f.l. Borealin is a monomer.
Figure 4.
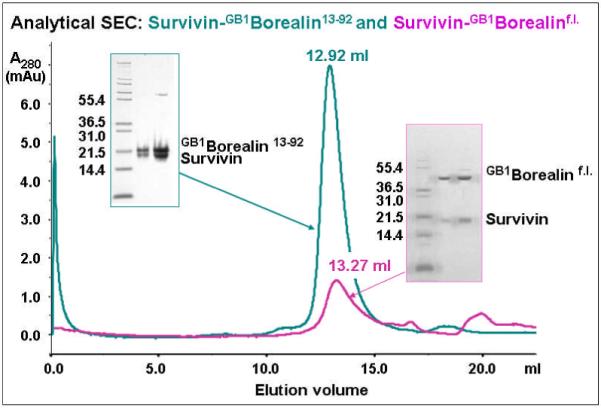
Analytical SEC reveals that the Survivin-GB1Borealinf.l. complex (magenta) elutes at a later volume than the Survivin-GB1Borealin13-92 complex (green). The SDS-PAGEs show that stable complexes are formed (the Survivin-GB1Borealin13-92 complex at 4.5 and 9.0 mg/ml, and the Survivin-GB1Borealinf.l. complex at 0.18 mg/ml and 0.36 mg/ml or 6.3 μM) and can be eluted by SEC.
DISCUSSION
The observed differences in stability, solubility, stoichiometry and protein-protein interaction activity between the two Survivin-Borealin complexes imply dramatic structural differences. According to the recent crystal structure of a core Survivin-Borealin10-109-INCENP1-58 1:1:1 complex, INCENP residues 1-47 and Borealin residues 15-62 form two long helices that bind to the hydrophobic C-terminal helix of Survivin thus forming a 3-helix bundle adjacent to the globular Survivin BIR domain ((23); Figure S3). Our data suggest that the Survivin-GB1Borealin13-92 2:2 complex forms a structure in which the INCENP binding surface is no longer presented, perhaps by forming a heterotetrameric 4-helix bundle. Our Survivin-GB1Borealin13-92 2:2 complex, as well as other recently reported Survivin-Borealin N-terminal fragment 2:2 complexes (23, 24), yielded only poorly diffracting crystals possibly implying conformational heterogeneity. Despite the observations that full-length Borealin as well as N- and C-terminal Borealin fragments form high-order oligomers in vitro (19, 22), our analysis suggest that full-length Borealin is likely to be a monomer when complexed with Survivin. Structurally the Survivin-GB1Borealinf.l. complex is predicted to adopt a similar assembly to that within the ternary Survivin-Borealin10-109-INCENP1-58 1:1:1 complex (23), in which the INCENP binding surface is clearly exposed. Overall, our study emphasizes the importance of the presence of the C-terminal region within f.l. Borealin for the composition of a fully active Survivin-Borealin complex, possibly by providing steric hindrance to the formation of an otherwise stable structure in which the INCENP recognition site is buried when a shorter Borealin fragment is used. In addition, our study provides an example where a protein complex composed of protein fragments that possess complete protein-protein interaction sequences demonstrates different stoichiometry and protein-protein interaction behavior from the corresponding protein complex of the full-length proteins.
So far, 3D structural studies of CPC sub-complexes involving full-length Survivin and fragments of Aurora B, Borealin and INCENP have all demonstrated monomeric protein-protein interactions (6, 23, 24). Our study provides evidence that full-length Borealin is likely to interact with Survivin as a monomer despite previous observations that Borealin and its N- and C-terminal fragments form high-order oligomers in vitro (19, 22). It is plausible that the quaternary CPC adopts a 1:1:1:1 stoichiometry. However, there are still extensive protein sequence regions in Borealin and INCENP (depicted as blank boxes in Figure 1) that await characterization of their 3D structure and analysis of their involvement in protein-protein interactions. In addition to the four reported CPC components, it cannot be ruled out that new members of the CPC assembly might still come to light. Understanding the structural geometry of the CPC represents an important step towards deciphering the functional mechanism of the CPC during cell mitosis, a question that also requires knowledge of the presently un-identified specific targets/substrates of the CPC at various localizations of the mitotic cell.
Supplementary Material
ACKNOWLEDGMENT
We thank Professor Paul Driscoll for critical reading and helpful discussions of the manuscript.
Abbreviations
- CPC
chromosomal passenger complex
- f.l.
full-length
- ITC
isothermal titration calorimetry
- 3D
three-dimensional
- GB1
immunoglobulin G binding domain B1 of streptococcal protein G
- SEC
size-exclusion chromatography
- AUC
analytical ultracentrifugation
- SEC-MALLS
size-exclusion chromatography-multi-angle laser light scattering
- M.W.
molecular weight
- BIR
baculovirus IAP (inhibitor of apoptosis proteins) repeat
- Bor
Borealin
- Sur
Survivin
- RCF
relative centrifugation force
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council [grant number BBC5208201]. JEL is a Wellcome Trust Senior Research Fellow. WCE is a Wellcome Trust Principal Research Fellow. XY is a BBSRC David Phillips Fellow.
REFERENCES
- 1.Vagnarelli P, Earnshaw WC. Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma. 2004;113:211–222. doi: 10.1007/s00412-004-0307-3. [DOI] [PubMed] [Google Scholar]
- 2.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 3.Carmena M. Cytokinesis: the final stop for the chromosomal passengers. Biochem Soc Trans. 2008;36:367–370. doi: 10.1042/BST0360367. [DOI] [PubMed] [Google Scholar]
- 4.Vader G, Maia AF, Lens SM. The chromosomal passenger complex and the spindle assembly checkpoint: kinetochore-microtubule error correction and beyond. Cell Div. 2008;3 doi: 10.1186/1747-1028-3-10. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sessa F, Mapelli M, Ciferri C, Tarricone C, Areces LB, Schneider TR, Stukenberg PT, Musacchio A. Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol Cell. 2005;18:379–391. doi: 10.1016/j.molcel.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Zappacosta F, Annan RS, Nurse K, Tummino PJ, Copeland RA, Lai Z. Characterization of INCENP-mediated Aurora B activation and the kinetic mechanism of Aurora B/INCENP. Biochem J. 2009;417:355–360. doi: 10.1042/BJ20081365. [DOI] [PubMed] [Google Scholar]
- 8.Vader G, Lens SM. The Aurora kinase family in cell division and cancer. Biochim Biophys Acta. 2008;1786:60–72. doi: 10.1016/j.bbcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Rosasco-Nitcher SE, Lan W, Khorasanizadeh S, Stukenberg PT. Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science. 2008;319:469–472. doi: 10.1126/science.1148980. [DOI] [PubMed] [Google Scholar]
- 10.Cooke CA, Heck MM, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987;105:2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheatley SP, Kandels-Lewis SE, Adams RR, Ainsztein AM, Earnshaw WC. INCENP binds directly to tubulin and requires dynamic microtubules to target to the cleavage furrow. Exp Cell Res. 2001;262:122–127. doi: 10.1006/excr.2000.5088. [DOI] [PubMed] [Google Scholar]
- 12.Wheatley SP, Carvalho A, Vagnarelli P, Earnshaw WC. INCENP is required for proper targeting of Survivin to the centromeres and the anaphase spindle during mitosis. Curr Biol. 2001;11:886–890. doi: 10.1016/s0960-9822(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Lakshmikanth GS, Spudich JA, De Lozanne A. The localization of inner centromeric protein (INCENP) at the cleavage furrow is dependent on Kif12 and involves interactions of the N terminus of INCENP with the actin cytoskeleton. Mol Biol Cell. 2007;18:3366–3374. doi: 10.1091/mbc.E06-10-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yue Z, Carvalho A, Xu Z, Yuan X, Cardinale S, Ribeiro S, Lai F, Gudmundsdottir E, Gassmann R, Morrison C, Ruchaud S, Earnshaw WC. Deconstructing Survivin: Comprehensive genetic analysis of Survivin function by conditional knockout in a verterbrate cell line. J Cell Biol. 2008;183:279–296. doi: 10.1083/jcb.200806118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lens SM, Vader G, Medema RH. The case for Survivin as mitotic regulator. Curr Opin Cell Biol. 2006;18:616–622. doi: 10.1016/j.ceb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Wheatley SP, McNeish IA. Survivin: a protein with dual roles in mitosis and apoptosis. Int Rev Cytol. 2005;247:35–88. doi: 10.1016/S0074-7696(05)47002-3. [DOI] [PubMed] [Google Scholar]
- 17.Knauer SK, Mann W, Stauber RH. Survivin’s dual role: an export’s view. Cell Cycle. 2007;6:518–521. doi: 10.4161/cc.6.5.3902. [DOI] [PubMed] [Google Scholar]
- 18.Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: molecular mechanism, prognostic, and therapeutic potential. Cancer Res. 2007;67:5999–6002. doi: 10.1158/0008-5472.CAN-07-0494. [DOI] [PubMed] [Google Scholar]
- 19.Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, Nigg EA, Gerloff DL, Earnshaw WC. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol. 2004;166:179–191. doi: 10.1083/jcb.200404001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Jelluma N, Brenkman AB, van den Broek NJ, Cruijsen CW, van Osch MH, Lens SM, Medema RH, Kops GJ. Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell. 2008;132:233–246. doi: 10.1016/j.cell.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 22.Klein UR, Nigg EA, Gruneberg U. Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol Biol Cell. 2006;17:2547–2558. doi: 10.1091/mbc.E05-12-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131:271–285. doi: 10.1016/j.cell.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 24.Bourhis E, Hymowitz SG, Cochran AG. The mitotic regulator Survivin binds as a monomer to its functional interactor Borealin. J Biol Chem. 2007;282:35018–35023. doi: 10.1074/jbc.M706233200. [DOI] [PubMed] [Google Scholar]
- 25.Laue TM, Shah BD, Ridgeway TM, Pelletier SL. Computer-aided interpretation of analytical sedimentation data for proteins. In: Harding SE, Rowe AJ, Horton JC, editors. Analytical Ultracentrifugation in Biochemistry and Polymer Science. Royal Society of Chemistry; Cambridge, UK: 1992. pp. 90–125. [Google Scholar]
- 26.Schuck P, Perugini MA, Gonzales NR, Howlett GJ, Schubert D. Size-distribution analysis of proteins by analytical ultracentrifugation: strategies and application to model systems. Biophys J. 2002;82:1096–1111. doi: 10.1016/S0006-3495(02)75469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou P, Lugovskoy AA, Wagner G. A solubility-enhancement tag (SET) for NMR studies of poorly behaving proteins. J Biomol NMR. 2001;20:11–14. doi: 10.1023/a:1011258906244. [DOI] [PubMed] [Google Scholar]
- 28.Verdecia MA, Huang H, Dutil E, Kaiser DA, Hunter T, Noel JP. Structure of the human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat Struct Biol. 2000;7:602–608. doi: 10.1038/76838. [DOI] [PubMed] [Google Scholar]
- 29.Muchmore SW, Chen J, Jakob C, Zakula D, Matayoshi ED, Wu W, Zhang H, Li F, Ng SC, Altieri DC. Crystal structure and mutagenic analysis of the inhibitor-of-apoptosis protein survivin. Mol Cell. 2000;6:173–182. [PubMed] [Google Scholar]
- 30.Chantalat L, Skoufias DA, Kleman JP, Jung B, Dideberg O, Margolis RL. Crystal structure of human survivin reveals a bow tie-shaped dimer with two unusual alpha-helical extensions. Mol Cell. 2000;6:183–189. [PubMed] [Google Scholar]
- 31.Sun C, Nettesheim D, Liu Z, Olejniczak ET. Solution structure of human survivin and its binding interface with Smac/Diablo. Biochemistry. 2005;44:11–17. doi: 10.1021/bi0485171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


