Abstract
In previous studies we described a “counter-immunosurveillance” mechanism initiated by tumor-activated, IL-13-producing NKT cells that signal Gr-1+ cells to produce TGF-β1, a cytokine that suppresses the activity of tumor-inhibiting cytolytic CD8+ T cells. Here we show that in two tumor models (the CT-26 metastatic colon cancer and the 15-12RM fibrosarcoma regressor models) this counter-surveillance mechanism requires the expression of a novel IL-13 receptor, IL-13Rα2, on Gr-1intermediate cells, since down-regulation of IL-13Rα2 expression or the AP-1 signal generated by the receptor via in vivo administration of specific siRNA or decoy oligonucleotides leads to loss of TGF-β1 production. Furthermore, acting on prior studies showing that IL-13Rα2 expression is induced (in part) by TNF-α, we show that receptor expression and TGF-β1 production is inhibited by administration of a TNF-α neutralizing substance, TNF-αR-Fc (etanercept). Taking advantage of this latter fact, we then demonstrate in the CT-26 model that counter-immunosurveillance could be inhibited, anti-CT-26-specific CD8+ cytolytic activity restored, and CT-26 metastatic tumor nodules greatly decreased by administration of TNF-αR-Fc. Corroborative data was obtained using the 15-12RM fibrosarcoma model. These studies point to the prevention of metastatic cancer with an available agent with already known clinically acceptable adverse effects and toxicity.
Introduction
Recent studies of the murine 15-12RM fibrosarcoma regressor and CT-26 colon carcinoma lung metastasis models have shown that the function of T cells engaged in the immune surveillance of tumors can be undermined by tumor-driven immune counter-surveillance mechanisms. In these studies it was shown that after a period of initial growth the fibrosarcoma undergoes regression due to the development of cytolytic CD8+ T cells; however, the tumor later recurs and persists due to the appearance of cells producing TGF-β1 that inhibits the cytolytic T cells. The chain of cellular events that leads to such reversal of immune surveillance involves first the tumor-induced expansion of NKT cells that produce IL-13, followed by IL-13 induction of TGF-β1 by a cell bearing a Gr-1 marker (most likely a monocytic cell) (1-3).
An important question that arose from the above studies relates to how IL-13 signaling activates cells to produce TGF-β1. One possibility comes from studies of TGF-β1 production during inflammation that showed that IL-13 induction of TGF-β1 is a two stage process whose first stage is the induction of an IL-13 receptor previously thought to be a decoy receptor without signaling function, IL-13Rα2; this event is then followed by a second stage involving IL-13 signaling through this receptor. The initial induction of IL-13Rα2 expression requires IL-4 or IL-13 signaling via the IL-13Rα1 receptor to generate activated Stat6 and TNF-α signaling to generate NF-κB, whereas the induction of TGF-β1 secretion requires IL-13 signaling via IL-13Rα2 to generate an AP-1 variant composed of c-jun and Fra-2 (4).
In the present study we determined whether TGF-β1 production arising from IL-13 signaling via IL-13Rα2 as described above applies to counter-immunosurveillance in the syngeneic CT-26 colon cancer and 15-12RM fibrosarcoma regressor experimental tumor models.
Material and Methods
TNF-αR-Fc and Control IgG1
TNF-αR-Fc (Enbrel, etanercept) was purchased from Amgen (Thousand Oaks, California). It consists of a dimeric fusion protein of the human (75KD) tumor necrosis factor receptor linked to the Fc portion of human IgG1. Control IgG1 consisted of human polyclonal IgG1 obtained from Invitrogen (Carlsbad, California) or HuMik-β1 clinical grade human monoclonal IgG1 antibody obtained from Dr. T.A. Waldmann, NCI, NIH, Bethesda, Maryland. The amount of etanercept used was selected based on previous experience using this human agent in a mouse system (4, 5). The amount of 100μg of etanercept administered every other day is slightly higher than the dose suggested for use in humans.
Mice
Female BALB/c mice (8–10 weeks old) were used in studies of tumor development in both the CT-26 colon cancer and 15-12RM fibrosarcoma models. All mice were obtained from Jackson Laboratory and were maintained in the National Institute of Allergy and Infectious Diseases (NIAID) animal holding facilities. Animal use adhered to NIH Laboratory Animal Care Guidelines and was approved by the NIAID and NCI Animal Care and Use Committee Review Boards.
Cell lines
The CT-26 cell line (a N-nitro-N-methylurethane–induced BALB/c murine colon carcinoma cell) was purchased from the American Type Culture Collection (ATCC, Manassas, Virginia) and maintained in RPMI-1640 complete medium supplemented with 10% FCS, L-glutamine, sodium pyruvate, streptomycin and penicillin. The 15-12RM fibrosarcoma cell line (BALB/c 3T3 fibroblasts transfected with HIV-1 IIIB gp160, Ras and Myc) originally developed in this laboratory was maintained in RPMI-1640 complete medium with 200 μg/ml of G418 (6).
Assessment of CT-26 tumor cell pulmonary nodules
The CT-26 tumor model was initiated by tail-vein injection of 0.5 × 106 tumor cells derived from the CT-26 cell line. Thereafter, mice were randomly separated into several groups depending on the experiment being conducted. Enumeration of pulmonary nodules was performed at the time control mice had sufficient numbers of pulmonary tumor nodules to allow reliable quantitation. In effect, this occurred at day 21 after CT-26 cell injection in studies wherein treatment was initiated at the time of initial tumor cell injection and at day 28 in studies wherein treatment was delayed to a later point in time. CT-26 cell pulmonary nodes were enumerated by counting the number of macroscopically apparent nodules in dissected lungs (7).
Assessment of subcutaneous 15-12RM fibrosarcoma growth
One million 15-12RM cells were injected subcutaneously at a site on the right flank of a mouse under study. The size of tumor nodules was measured periodically by caliper gauge.
Encapsulation of siRNA and oligonucleotides in HVJ Envelope (HVJ-E) Vector
Encapsulation of siRNA or decoy oligonucleotides for in vivo transfection was performed as previously described (8).
IL-13Rα2-specific siRNA
IL-13Rα2-specific siRNA and control (scrambled) siRNA for use in gene silencing studies was obtained from Dharmacon (Chicago, Illinois). The specific mRNA targeting sequence was: 5′-GGAATCTAATTTACAAGGA-3′. The specificity and efficiency of this siRNA has been previously demonstrated (4, 9). For in vivo transfection and gene silencing 100μg of siRNA encapsulated in HVJ-E was administered by intra-nasal instillation every-other day starting on day 0 after CT-26 injection. The amount of siRNA for in vivo studies was selected based on previous experience in several different animal models (bleomycin induced lung fibrosis, chronic TNBS-colitis) (4, 9).
Decoy ODN
Decoy ODN targeting AP-1 were prepared from complementary single-stranded ODN obtained from Qiagen (Germantown, Maryland) by melting at 95°C 3-5 min followed by incubation for 3h at ambient temperature. For in vivo transfection 100μg of AP-1 decoy ODN or scrambled oligonucleotides were administered via an intra-tracheal route following encapsulation in HVJ-E. The following sequences were used: AP-1 decoy ODN, CGCTTGATGACTCAGCCGGAA-3′ and 3′-GCGAACTACTGAGTCGGCCTT-5′; scrambled decoy ODN, 5-CATGTCGTCACTGCGCTCAT-3′ and 3′-GTACAGCAGTGACGCGAGTA-5′.
CTL assays
CTL assays were performed using cells obtained from single-cell suspensions of splenocytes isolated from CT-26 tumor-bearing mice. Splenocytes (2 × 105 cells) were re-stimulated in vitro with CT-26 cells (5 × 104 cells) treated with mitomycin C. After 2 days, cytolytic activity against CT-26 cells was determined by the CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison, Wisconsin).
Western Blot
The cells were lysed by RIPA buffer and the whole cell lysates thus obtained were subjected to SDS-PAGE. The separated proteins obtained were transferred to a nitrocellulose membrane and immunoblotted. IL-13Rα2 was detected by incubation with a monoclonal rat anti-mouse IL-13Rα2 (R&D Systems, Minneapolis, Minnesota) followed by incubation with horseradish peroxidase-conjugated anti-rat IgG (Invitrogen, Carlsbad, California). Membranes were developed with SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, Illinois) and exposed to X-ray film.
RT-PCR
Cells were stored in RNAlater solution (Ambion, Austin, Texas) and then subjected to RNA extraction using RNeasy tissue kit (Qiagen, Germantown, Maryland). A total of 1μg template RNA was reverse transcribed with Superscript III RT-PCR Kit (Invitrogen, Carlsbad, California). Primer sequences were as follows: IL-13Rα1: 5′-GCAGCCTGGAGAAAAGTCGTCAAT-3′ and 5′-ACAGCCTCGGCAAGAACACCA-3′, and glyceraldehyde phosphodehydrogenase (GAPDH), 5′-GGTGAAGGTCGGTGTGAACGGA-3′ and 5′-TGTTAGTGGGGTCTCGCTCCTG-3′. Annealing temperature and cycle number were as follows: IL-13Rα1 60°C and 27 cycles; GAPDH 60°C and 25 cycles.
ELISA
Cytokine protein concentrations in culture supernatants were measured by ELISA kits according to the manufacturer's instructions. Isolated splenocytes were cultured at 37°C. For IL-13 and TNF-α measurements we cultured 1×106 cells per 1ml medium for 48h. For TGF-β1 measurements we cultured 1×105 cells per 100μl medium for 24h. To determine cytokine concentrations, cells were stimulated during the culture period with plate-bound anti-CD3 Ab (10μg/ml) and soluble anti-CD28 Ab (1μg/ml) (BD Biosciences-Pharmingen, San Jose, California) for measurement of IL-13. Splenocytes were stimulated during the culture period with Staphylococcus aureus Cowan I (1:10000 dilution of Pansorbin) (EMD Biosciences, Darmstadt, Germany) and IFN-γ (1000U/ml) (R&D Systems, Minneapolis, Minnesota) for measurement of TNF-α. Splenocytes were stimulated during the culture period with recombinant murine IL-13 (20ng/ml) (R&D Systems, Minneapolis, Minnesota) for measurement of TGF-β1. ELISA kits for IL-13 were purchased from R&D Systems (Minneapolis, Minnesota), for TNF-α from BD Biosciences-Pharmingen (San Jose, California), and for TGF-β1 from Invitrogen (Carlsbad, California).
Statistical analyses
Student's t-test or Wilcoxon signed-rank test computed by GraphPad Prism 4 was used to evaluate the significance of the differences. A value of p < 0.05 was considered statistically significant (*), a value of p < 0.01 was considered statistically highly significant (**).
Results
Expression of IL-13 signaling components in the CT-26 tumor model
In previous studies it was shown that IL-13 induces TGF-β1 production via a two stage process involving first the induction of IL-13Rα2 expression and second IL-13 signaling via this receptor (4). To establish if this mechanism of IL-13 activity occurs in relation to tumor immunity, we determined whether it was operative in the syngeneic CT-26 colon cancer cell model and, as described below, in the 15-12RM fibrosarcoma regression model.
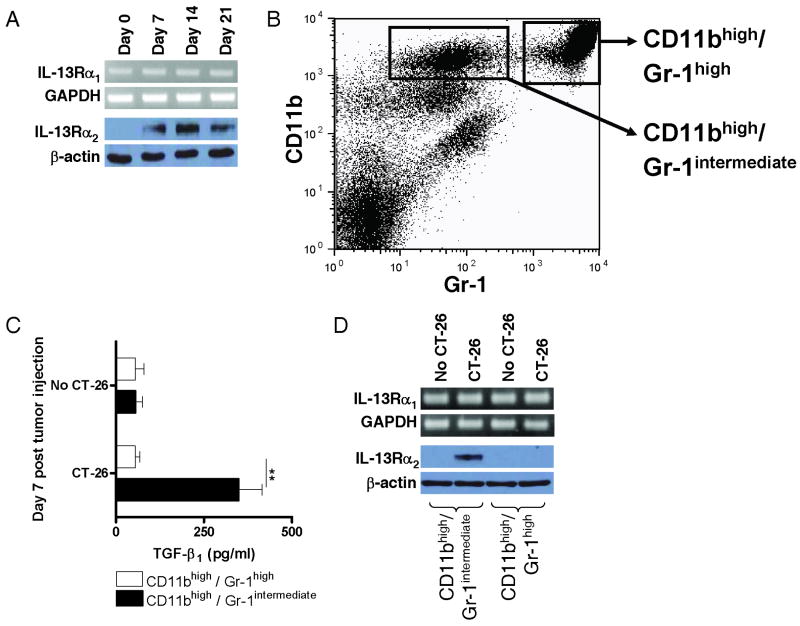
In Initial studies of the CT-26 model, we determined whether cells of mice injected with the CT-26 tumor cells activate the first stage of the IL-13 induction process, namely the induction of IL-13Rα2 expression. To this end, we first demonstrated that splenic CD4+ T cells and CD11b+ cells stimulated by anti-CD3/anti-CD28 and by SAC plus IFN-γ, respectively (see Methods), produced increased amounts of IL-13 and TNF-α when obtained from mice injected with tumor cells as compared to uninjected mice (data not shown). Thus, the cells of tumor-bearing mice were capable of actively synthesizing components that up-regulate expression of IL-13Rα2. Next, to determine whether IL-13Rα2 expression was indeed up-regulated in the target organ of the tumor, the lung, we performed Western blot studies on extracts of lung tissue. As shown in Figure 1A, naïve mice not bearing tumor (day 0 mice) express little or no IL-13Rα2 in the lung, whereas mice express this receptor beginning as early as day 7 after tumor injection. In contrast, IL-13Rα1 (measured by RT-PCR) is expressed constitutively. Similar findings were obtained with Western blot studies of spleen cells (Data not shown). Such differential expression of IL-13 receptors has been observed previously under in vivo conditions in which IL-13 is able to induce TGF-β1 (4, 9).
Figure 1.
IL-13 receptor expression and TGF-β1 production after CT-26 injection. (A) IL-13Rα1 and IL-13Rα2 expression during the CT-26 lung tumor model in BALB/c mice. IL-13Rα1 mRNA expression was determined by RT-PCR of RNA extracted from total lung and IL-13Rα2 protein expression was determined by Western blot analysis of total lung lysates obtained at indicated time points. (B) Representative FACS analysis of splenocytes for expression of CD11b and Gr-1 on day 7 after CT-26 injection. (C) TGF-β1 production by CD11bhigh/Gr-1intermediate cells isolated from the spleen on day 7 after CT-26 injection (or from control spleens from mice without CT-26 injection). Isolated splenocytes were separated by FACS sorting into CD11bhigh/Gr-1intermediate cells and CD11bhigh/Gr-1high cells and cultured for 24h in the presence of IL-13. Cytokine concentrations in the supernatants were determined by cytokine-specific ELISA. Data shown are mean ± SD of three independent experiments each containing pooled cells from at least 8 mice per group. **, p ≤ 0.01 (Student's t-test). (D) IL-13Rα1 and IL-13Rα2 expression of CD11bhigh/Gr-1intermediate cells isolated from the spleen on day 7 after CT-26 injection. Isolated splenocytes were separated by FACS-sorting into CD11bhigh/Gr-1intermediate cells and CD11bhigh/Gr-1high cells. IL-13Rα1 mRNA expression was determined by RT-PCR of RNA extracted from sorted cells and IL-13Rα2 protein expression was determined by Western blot analysis of lysates from sorted cells.
Characterization of cells responding to IL-13 and producing TGF-β1 in the CT-26 tumor model
Prior studies have disclosed that the TGF-β1-producing cell in a tumor-bearing mouse is a myeloid cell bearing surface CD11b and Gr-1 intermediate but that neither the number of these cells nor the ratio of Gr-1high cells (granulocytes) to Gr-1intermediate (monocytes and immature granulocytes) change as a result of tumor cell administration (2). To determine whether this is also the case in the CT-26 tumor model we subjected spleen cells of mice 7 days after CT-26 tumor injection to flow cytometric cell sorting to obtain purified populations of cells bearing these markers. These studies showed that once again the ratio of Gr-1high to Gr-1intermediate cells in the spleens of mice was the same in control mice and mice injected with tumor cells. In addition, as shown in Figure 1B, we found that the cells bearing high amounts of CD11b sorted into two distinct Gr-1 positive populations, one CD11bhigh/Gr-1high and the other CD11bhigh/Gr-1intermediate. To determine which of these cell populations is involved in TGF-β1 production we measured TGF-β1 production and IL-13R expression in the purified (sorted) cells. As shown in Figure 1C, after in vitro culture in the presence of IL-13, only the CD11bhigh/Gr-1intermediate cells from tumor-bearing mice produced substantial amounts of TGF-β1. In addition, as shown in Figure 1D, while both cell populations expressed constitutive levels of IL-13Rα1 regardless of tumor burden, only the CD11bhigh/Gr-1intermediate cells expressed IL-13Rα2, and such expression was noted only after tumor cell injection. While these studies were conducted with spleen cell populations, it is reasonable to assume that cells with similar properties are also present in the lung of the tumor-bearing mouse.
Inhibition of IL-13 induction of TGF-β1 in the CT-26 tumor model
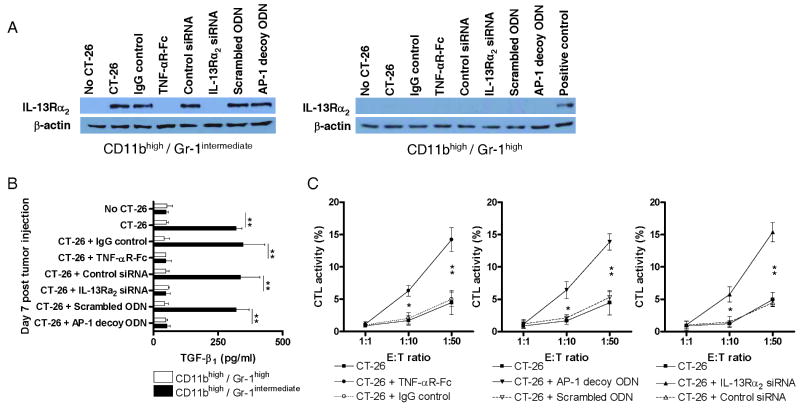
If indeed IL-13 induces TGF-β1 production via IL-13Rα2 signaling one might predict that such production should be inhibited by administration of various agents that block this pathway at various stages of its development. Recognizing that TNF-α (and IL-4 or IL-13) stimulation was necessary to induce surface expression of IL-13Rα2, in one approach to test this prediction we administered TNF-αR-Fc (etanercept; 100μg by intra-peritoneal injection every other day beginning on day 0 of CT-26 tumor cell administration) to block TNF-α signaling. As shown in the Western blot of cell extracts of purified CD11bhigh/Gr-1intermediate splenocytes prepared on day 7 after CT-26 administration depicted in Figure 2A, such treatment did prevent the expression of the IL-13Rα2 in these cells, whereas similar cells from mice treated with control IgG expressed this receptor. In contrast, expression of the IL-13Rα1 was constitutively present and unchanged in the face of TNF-αR-Fc treatment (data not shown). In concomitant studies, shown in Figure 2B, CD11bhigh/Gr-1intermediate cells obtained from the spleens of mice on day 7 after CT-26 administration and cultured with IL-13 produced increased amounts of TGF-β1 if obtained from mice subjected to control IgG treatment whereas those that were subjected to TNF-αR-Fc treatment did not produce increased amounts of this cytokine.
Figure 2.
Characteristics of CD11bhigh/Gr-1intermediate cells and CD11bhigh/Gr-1high cells appearing in mice subjected to treatments affecting tumor formation initiated on the day of CT-26 injection. Treatments included administration of TNF-R-Fc (administration every other day starting on day 0), IL-13Rα2-specific siRNA (administration every other day starting on day 0), and AP-1-specific decoy oligonucleotides (administration once every week starting on day 0). (A) IL-13Rα2 expression of CD11bhigh/Gr-1intermediate cells isolated from the spleen on day 7 after CT-26 injection. Isolated splenocytes were separated by FACS-sorting into CD11bhigh/Gr-1intermediate cells and CD11bhigh/Gr-1high cells. IL-13Rα2 protein expression was determined by Western blot analysis of lysates from sorted cells. Cell lysate of IL-13 and TNF-α stimulated J774 murine macrophage cell line was used as positive control. (B) TGF-β1 production of CD11bhigh/Gr-1intermediate cells isolated from the spleen on day 7 after CT-26 injection. Isolated splenocytes were separated by FACS-sorting into CD11bhigh/Gr-1intermediate cells and CD11bhigh/Gr-1high cells and cultured for 24h in the presence of IL-13. Cytokine concentrations in the supernatants were determined by cytokine-specific ELISA. Data shown are mean ± SD of three independent experiments each containing pooled cells from at least 8 mice per group. **, p ≤ 0.01 (Student's t-test). (C) Cytotoxicity of CD8+ T cells against CT-26 cells. Cells were isolated from the spleen on day 7 after CT-26 injection and start of treatment. Data shown are mean ± SD of three independent experiments each containing pooled cells from at least 8 mice per group. *, p ≤ 0.05; **, p ≤ 0.01 comparing treatment group with control treatment group (Student's t-test).
In a second approach along these lines we administered IL-13Rα2-specific siRNA or control siRNA to mice to directly inhibit IL-13Rα2 synthesis at the molecular level. In these studies the siRNA was administered (at 100μg siRNA) every other day by an intra-nasal route and the siRNA was encapsulated in a viral coat preparation to enhance cell entry in vivo (HVJ-E, see Methods). As also shown in Figure 2A and 2B, the result was similar to that obtained with TNF-αR-Fc, in that again, treatment prevents IL-13Rα2 expression and decreased the capacity of CD11bhigh/Gr-1intermediate cells to produce TGF-β1.
In a third and final approach to testing the prediction that blockade of IL-13Rα2 signaling would block TGF-β1 production we blocked such signaling by administration of a decoy oligonucleotide that competitively inhibits the binding of the receptor's down-stream signal, AP-1, to its consensus target sequence. As in the case of the siRNA, the decoy oligonucleotide (or a scrambled control oligonucleotide) was administered by an intra-nasal route (at 100μg ODN) following encapsulation in HVJ-E. As shown again in Figure 2A and 2B, while the specific AP-1 decoy oligonucleotide had no effect on IL-13Rα2 expression in CD11bhigh/Gr-1intermediate cells, it prevented IL-13-induced TGF-β1 production, whereas a control (scrambled) decoy had no such effect.
In summary, blockade of IL-13 signaling via IL-13Rα2 in the CT-26 tumor system using three distinct blocking strategies led to inhibition of IL-13-induced TGF-β1 production and it thus seems reasonable to conclude that IL-13 induces TGF-β1 in this system via this signaling pathway.
Anti-CT-26 cytotoxic activity of CD8+ T cells following inhibition of IL-13 induction of TGF-β1
In previous studies it was shown that the induction of TGF-β1 by IL-13 (produced by NKT cells) leads to diminished CD8+ cell-mediated anti-tumor cell cytotoxicity and resultant counter-immunosurveillance (2). We therefore examined the effect of TNF-αR-Fc, IL-13Rα2-specific siRNA, or AP-1 decoy oligonucleotide administration as indicated above on CD8+ T cell-mediated cytotoxic activity directed against CT-26 cells. As shown in Figure 2C, whereas CD8+ spleen T cells from untreated mice 7 days after CT-26 administration exhibited little or no toxicity for CT-26 cells, CD8+ T cells from mice administered all three inhibitors of IL-13 induction of TGF-β1 exhibited substantial cytotoxic activity against CT-26 tumor cell targets. Parallel studies of CD4+ spleen T cell showed that the latter population exhibited no cytotoxicity for CT-26. Finally, to establish the specificity of CD8+ T cell cytotoxic activity for CT-26 tumor cells we determined if these cells were cytotoxic for 4T1 cells (BALB/c mouse mammary tumor cells). We found that CD8+ spleen cells from mice inoculated with CT-26 cells did not exhibit cytotoxicity for 4T1 cells, indicating the specificity of the cytotoxicity against CT-26 cells (data not shown). Thus, the inhibition of IL-13 induction of TGF-β1 led to the acquisition of an immune function that could mediate immune surveillance.
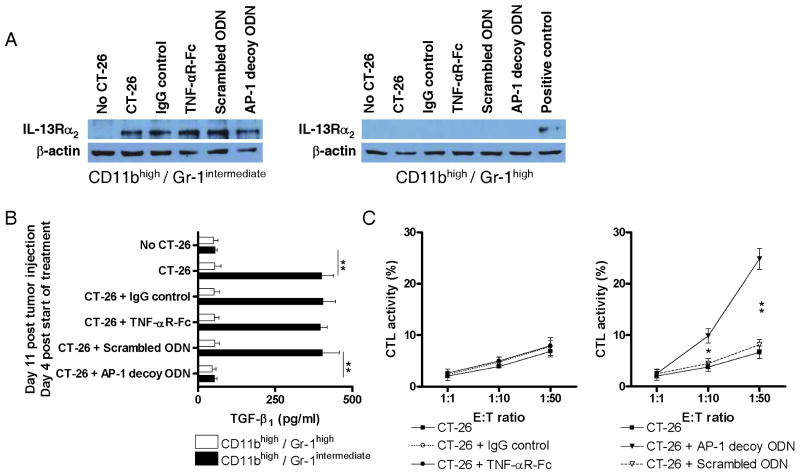
Effect of delayed administration of IL-13 signaling inhibitors on TGF-β1 production and CD8+ T cell cytotoxic activity
As a further test of the effect of inhibitors of IL-13 induction of TGF-β1 on immune counter-surveillance we determined the effect of delayed administration of TNF-αR-Fc (every other day, starting on day 7), and AP-1 decoy oligonucleotides (weekly, starting on day 7) after tumor cell injection and evaluation of effects on IL-13Rα2 expression, TGF-β1 production, and cytotoxicity of CD8+ cells on day 11 after tumor injection. As shown in the Western blot analysis of extracts of CD11bhigh/Gr-1intermediate cells isolated from the spleens of mice on day 11 after CT-26 cell injection depicted in Figure 3A, the cells continued to express IL-13Rα2 after delayed treatment with both inhibitors. However, as shown in Figure 3B, although CD11bhigh/Gr-1intermediate cells isolated on day 11 produced increased TGF-β1 in response to IL-13 after delayed treatment of mice with TNF-αR-Fc starting on day 7, they did not do so after delayed treatment with AP-1 decoy oligonucleotides. In addition, as shown in Figure 3C, a similar dichotomy was observed with respect to the cytotoxic activity of CD8+ cells for CT-26 tumor cells: delayed administration on day 7 of TNF-αR-Fc was associated with low level cytotoxicity of CD8+ T cells isolated on day 11, whereas administration of AP-1 decoy oligonucleotides was associated with robust cytotoxicity of CD8+ cells for tumor cells. Taken together, these studies show that delayed administration of TNF-αR-Fc had little or no effect on immune counter-surveillance in the time frame studied, probably because this inhibitor did not affect the function of cells already expressing IL-13Rα2. In contrast, the AP-1 decoy interfered with the downstream signaling of the receptor even in cells already bearing the receptor.
Figure 3.
Characteristics of CD11bhigh/Gr-1intermediate cells and CD11bhigh/Gr-1high cells appearing in mice subjected to treatments affecting tumor formation initiated on the day 7 after CT-26 injection. Treatments included administration of TNF-αR-Fc (administration every other day starting on day 7), and AP-1-specific decoy oligonucleotides (administration once every week starting on day 7). (A) IL-13Rα2 expression of CD11bhigh/Gr-1intermediate cells isolated from the spleen on day 11 after CT-26 injection (4 days post start of treatment). Isolated splenocytes were separated by FACS-sorting into CD11bhigh/Gr-1intermediate cells and CD11bhigh/Gr-1high cells. IL-13Rα2 protein expression was determined by Western blot analysis of lysates from sorted cells. Cell lysate of IL-13 and TNF-α stimulated J774 murine macrophage cell line was used as positive control. (B) TGF-β1 production of CD11bhigh/Gr-1intermediate cells isolated from the spleen on day 11 after CT-26 injection (4 days post start of treatment). Isolated splenocytes were separated by FACS-sorting into CD11bhigh/Gr-1intermediate cells and CD11bhigh/Gr-1high cells and cultured for 24h in the presence of IL-13. Cytokine concentrations in the supernatants were determined by cytokine-specific ELISA. Data shown are mean ± SD of three independent experiments each containing pooled cells from at least 8 mice per group. **, p ≤ 0.01 (Student's t-test). (C) Cytotoxicity of CD8+ T cells against CT-26 cells. Cells were isolated from the spleen on day 11 after CT-26 injection (4 days post start of treatment). Data shown are mean ± SD of three independent experiments each containing pooled cells from at least 8 mice per group. *, p ≤ 0.05; **, p ≤ 0.01 comparing treatment group with control treatment group (Student's t-test).
As in previous studies conducted at an earlier time point, CD11bhigh/Gr-1high cells isolated at day 11 did not express IL-13Rα2 and did not produce TGF-β1 in response to IL-13. Finally, it should be noted that treatment of mice with TNF-αR-Fc, IL-13Rα2-specific siRNA, or AP-1 decoy oligonucleotides did not influence the serum levels of soluble IL-13Rα2 throughout the entire time course the mice were monitored in this animal model (data not shown).
Clinical impact of inhibition of IL-13 induction of TGF-β1 by targeting IL-13Rα2 signaling
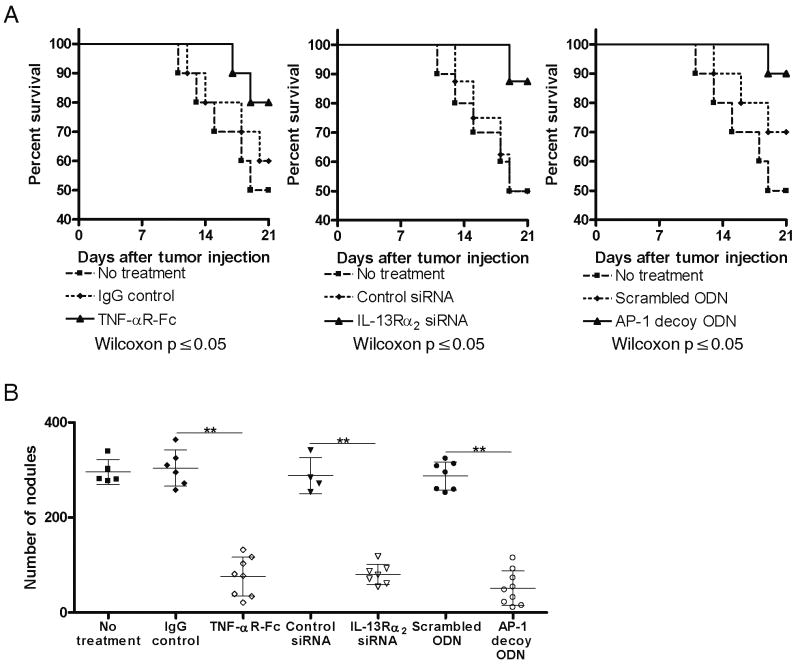
The clinical impact of inhibition of IL-13 induction of TGF-β1 by targeting IL-13Rα2 in the CT-26 tumor model was subsequently determined by mouse mortality and enumeration of macroscopic pulmonary nodules at 21 days after tumor cell injection. Treatment was performed by using: 100μg TNF-αR-Fc every other day starting on day 0, 100μg IL-13Rα2-specific siRNA every other day starting on day 0, or 100μg AP-1 decoy oligonucleotides once weekly starting on day 0. As shown in Figure 4A and 4B, mice administered tumor cells without any form of treatment or mice administered control materials (IgG control, siRNA control, or scrambled oligonucleotide control) displayed more than 250 macroscopic pulmonary nodules and suffered a mortality rate of 50% by day 21. In contrast, treatment groups with administration of either TNF-αR-Fc, IL-13Rα2-specific siRNA, or AP-1 decoy oligonucleotides at the time of tumor cell injection exhibited greatly reduced numbers (and smaller size) macroscopic tumor nodules and significantly lower mortality on day 21. It should be noted that since the number of pulmonary nodules in mice receiving control IgG did not differ from those not receiving the latter (siRNA control and scrambled oligonucleotie control mice) were comparable there was no evidence that the control IgG had any effect on tumor development.
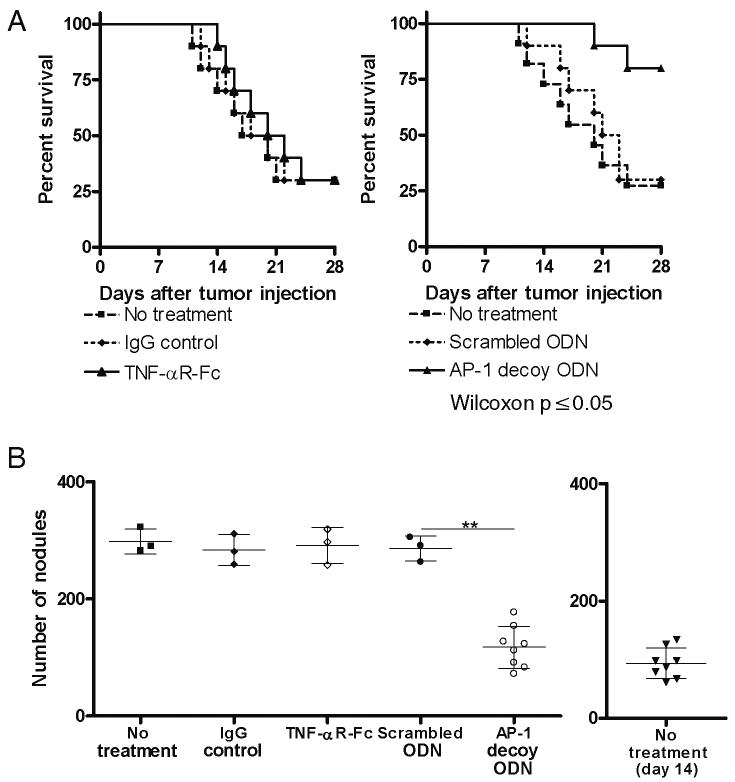
Figure 4.
CT-26 tumor development in mice subjected to treatment with TNF-αR-Fc, IL-13Rα2-specific siRNA, and AP-1-specific decoy oligonucleotides. Treatment started on the day of CT-26 injection. (A) Survival rates of mice until day 21 after CT-26 injection. Data shown are representative of three independent experiments containing 10 mice per group for TNF-αR-Fc treatment and AP-1 decoy ODN treatment, and 8 mice per group for IL-13Rα2 siRNA treatment. P ≤ 0.05 comparing treatment group with control treatment group (Wilcoxon test). (B) Number of pulmonary tumor nodules present on day 21 after CT-26 injection. Data shown are mean ± SD representative of three independent experiments each containing 10 mice per group. Each dot represents a living mouse at the termination of the study on day 21. **, p ≤ 0.01 comparing treatment group with control treatment group (Student's t-test).
In a more stringent test of the possible therapeutic benefit of targeting IL-13Rα2 signaling in the CT-26 tumor model we evaluated the effect of administration of inhibitors beginning at day 14 after initial tumor cell injection when pulmonary nodules already made their appearance. Treatment was performed by using: 100μg TNF-αR-Fc every other day starting on day 14, 100μg IL-13Rα2-specific siRNA every other day starting on day 14, or 100μg AP-1 decoy oligonucleotides once weekly starting on day 14. As shown in Figure 5A and 5B, untreated mice or mice treated with control materials beginning on day 14 again exhibited high numbers of pulmonary tumor nodules and high mortality rates on day 28. In addition, in this case administration of TNF-αR-Fc every other day beginning at day 14 did not reduce the number of pulmonary nodules at day 28 and had no effect on mortality, whereas administration of AP-1 decoy oligonucleotides on day 14 and day 21 led to a significant decrease in the number of pulmonary nodules at day 28 and greatly reduced mortality. In fact, mice administered AP-1 decoy oligonucleotides exhibited a similar number of pulmonary nodules at day 28 as control, untreated mice did at day 14, indicating that this treatment had almost completely suppressed the progression of tumor formation and also greatly improved survival of the mice even with delayed treatment.
Figure 5.
CT-26 tumor development in mice subjected to delayed treatment with TNF-αR-Fc, IL-13Rα2-specific siRNA, and AP-1-specific decoy oligonucleotides. Treatment started 14 days after CT-26 injection. (A) Survival rates of mice until day 28 after CT-26 injection. Data shown are representative of three independent experiments each containing 10 mice per group. P ≤ 0.05 comparing treatment group with control treatment group (Wilcoxon test). (B) Number of pulmonary tumor nodules on day 28 after CT-26 injection. Additional panel also shows baseline pulmonary tumor nodules of untreated mice on day 14 after CT-26 injection. Data shown are representative of three independent experiments each containing 10 mice per group. Each dot represents a living mouse at the termination of the study on day 28. **, p ≤ 0.01 comparing treatment group with control treatment group (Student's t-test).
These findings are compatible with the supposition that by day 14 after tumor cell injection IL-13Rα2 expression had already been induced and was therefore beyond the point it could be inhibited by TNF-αR-Fc, whereas at this time point AP-1 decoy ODN could still exert its negative effect on IL-13Rα2 down-stream signaling, hence the continued clinical effectiveness of the latter blocker.
Effects of targeting IL-13Rα2 signaling on the growth of 15-12RM fibrosarcoma
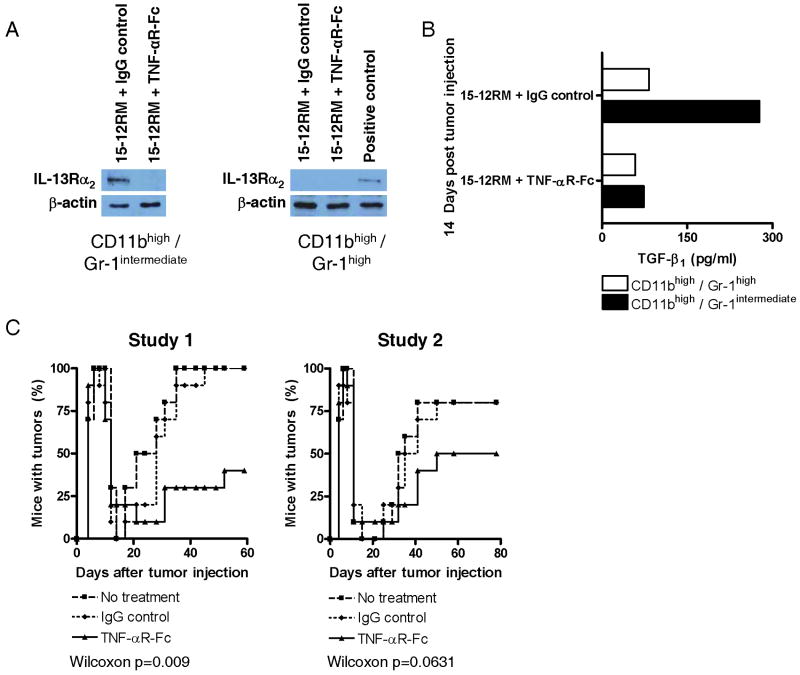
To determine if the IL-13 induction of TGF-β1 also requires signaling via IL-13Rα2 in a second tumor model exhibiting counter-immunosurveillance we turned to the 15-12RM fibrosarcoma tumor model. As noted above, this was in fact the first tumor model in which immune counter-surveillance via IL-13 secretion had been shown. As in the case of the CT-26 tumor cell model, tumor growth in this model was associated with the expression of IL-13, TNF-α, and TGF-β1 and, as shown in Figure 6A, CD11bhigh/Gr-1intermediate spleen cells but not CD11bhigh/Gr-1high spleen cells isolated on day 14 after tumor cell injection expressed IL-13Rα2. Repeated administration of TNF-αR-Fc initiated at the time of tumor cell injection inhibited such expression. In addition, as shown in Figure 6B, administration of TNF-αR-Fc greatly reduced production of TGF-β1 by CD11bhigh/Gr-1intermediate spleen cells. Finally, and most importantly, as shown in Figure 6C, treatment of mice initiated at the time of tumor cell inoculation with TNF-αR-Fc (every other day with 100μg or every day with 60μg TNF-αR-Fc) greatly reduced tumor recurrence in two separate studies: on day 60 or day 80 at the end of the observation period, tumor recurrence was reduced by about 50% in TNF-αR-Fc-treated mice as compared to control IgG treated mice. These studies therefore verified that the IL-13 induction via IL-13Rα2 is a central pathway in counter-immunosurveillance in a second tumor cell model.
Figure 6.
Key features of the 15-12RM fibrosarcoma model. (A) IL-13Rα1 and IL-13Rα2 expression of CD11bhigh/Gr-1intermediate cells isolated from the spleen on day 14 after 15-12RM injection. Isolated splenocytes were separated by FACS-sorting into CD11bhigh/Gr-1intermediate cells and CD11bhigh/Gr-1high cells. IL-13Rα1 mRNA expression was determined by RT-PCR of RNA extracted from sorted cells and IL-13Rα2 protein expression was determined by Western blot analysis of lysates from sorted cells. (B) TGF-β1 production of CD11bhigh/Gr-1intermediate cells isolated from the spleen on day 14 after 15-12RM injection. Isolated splenocytes were separated by FACS-sorting into CD11bhigh/Gr-1intermediate cells and CD11bhigh/Gr-1high cells and cultured for 24h in the presence of IL-13. Cytokine concentrations in the supernatants were determined by cytokine-specific ELISA. Data shown are representative of two independent experiments each containing pooled cells from at least 8 mice per group. (C) Recurrence of tumor-bearing mice over time in 15-12RM fibrosarcoma model of mice administered TNF-αR-Fc, control IgG or nothing every other day from day 0 to day 14. Data shown are two independent experiments in which each experimental group consisted of at least 10 mice. Statistical analysis is performed by using Wilcoxon test.
Discussion
In a series of previous studies it was shown that immunologic inhibition of tumor growth (immune surveillance) could be undermined in several different tumor models by tumor-induced secretion of TGF-β1 and the inhibitory effect of this cytokine on tumor antigen-specific cytolytic T cells (2, 10-13). Furthermore, it was shown that such counter-immunosurveillance was initiated by NKT cells that secrete IL-13 and thereby induce Gr-1 cells to produce TGF-β1 (1-3, 13). These studies led to attempts to treat tumors by disarming counter-immunosurveillance. While these included the use of antagonists that block the activity of either of the above cytokines, they did not include approaches that address key signaling mechanisms involved such as those mediating IL-13 induction of TGF-β1 since the latter were as yet poorly understood.
With regard to this latter point, previous studies showing that IL-13 signals via the type II IL-4R, a heterodimer of the IL-4Rα and IL-13Rα1 that responds to either IL-13 or IL-4, left unanswered the question of why stimulation of Gr-1 cells to produce TGF-β1 required IL-13 and was independent of IL-4 (14-16). The answer to this conundrum came from recent studies that showed that induction of TGF-β1 by IL-13 involves signaling via a second IL-13 receptor, IL-13Rα2, that does not recognize IL-4 (4) and that previously thought to be a non-signaling receptor that is secreted and acts as a decoy for IL-13 (17-21). The fact that the IL-13Rα2 was in fact a signaling receptor and one that signaled cells to produce TGF-β1 was proven on several grounds (4). First, it was shown that cells lacking cell surface IL-13Rα2 or expressing a mutant form of this receptor lacking the cytoplasmic tail could not be induced by IL-13 to produce TGF-β1. Second, it was shown that agents that blocked the induction of receptor expression also blocked IL-13 induction of TGF-β1. Third and finally, it was shown that signaling via this receptor resulted in AP-1 generation, and blockade of AP-1 with an AP-1-specific decoy oligonucleotide also blocked IL-13 induction of TGF-β1. In summary then, the evidence that IL-13Rα2 serves as a signaling receptor for IL-13 during TGF-β1 induction is quite strong and there appears to be little question that while this receptor can indeed function as a decoy under some circumstances, it also functions as a bona fide receptor for IL-13. This conclusion is consistent with a recent study showing that the soluble and membrane-bound forms of IL-13Rα2 are actually different splice variants of the IL-I3Rα2 gene, with the soluble form lacking the transmembrane domain of the molecule and therefore lacks signaling capability (22).
Further studies of the IL-13Rα2 receptor have shown that this receptor is not constitutively expressed on the cell surface (as is the IL-13Rα1) and for this reason the induction of TGF-β1 by IL-13, is, in reality, a two stage process characterized by an initial receptor induction step that is only then followed by the receptor signaling step described above (4, 9). In addition, such induction requires a dual signal consisting of IL-4 or IL-13 acting through IL-13Rα1 and TNF-α acting through its receptor to generate activated Stat6 and NF-κB respectively, the factors that transactivate the IL-13Rα2 promoter. With the elucidation of this induction step the major factors involved in IL-13 induction of TGF-β1 were now known and indeed, it could now be shown that such induction was inhibited in several models of inflammation by administration of an agent that blocked IL-13Rα2 receptor induction, TNF-αR-Fc, an agent that down-regulated the IL-13Rα2 itself, IL-13Rα2-specific siRNA and, finally, an agent that blocks the activity of IL-13Rα2 signaling, AP-1 decoy oligonucleotide.
In the present study, all of the major features of the model of IL-13 induction of TGF-β1 discussed above were noted in the context of tumor immunosurveillance. In particular, it was observed that inoculation of mice with CT26 tumor cells was promptly followed by increased IL-13 and TNF-α secretion as well as the appearance of the IL-13Rα2 on CD11bhigh/Gr-1intermediate cells and the secretion of TGF-β1 by such cells. Furthermore, in inhibition studies it was shown that as in the various inflammatory models mentioned above, TNF-αR-Fc, IL-13Rα2-specific siRNA and AP-1 decoy oligonucleotide had predicted effects on IL-13Rα2 expression on CD11bhigh/Gr-1intermediate cells and blocked TGF-β1 induction by IL-13; as a result, administration of these inhibitors restored the ability of CD8+ T cell to kill tumor cells.
In actual studies of control of CT-26 growth via immunosurveillance we in effect again verified the IL-13 signaling mechanism described above in an in vivo context by showing that the various inhibitors of the IL-13 signaling greatly reduced tumor burden in the lung and thereby reduced mortality from the tumor. Of interest, the response pattern seen was fully consistent with function of an inhibitor of the signal cascade in that delayed administration of TNF-αR-Fc rendered this agent ineffective in treatment, presumably because an agent that blocks induction of IL-13Rα2 expression could not work after the receptor was already being expressed (at least in a short term experiment). In contrast, AP-1 decoy oligonucleotide continued to exert a therapeutic effect in the face of delayed therapy, presumably because continued synthesis of TGF-β1 is necessary to maintain inhibition of cytotoxic CD8+ T cells. More limited studies of the 15-12RM fibrosarcoma were fully consistent with these results in that in this case as well administration of TNF-αR-Fc inhibited expression of IL-13Rα2 on CD11bhigh/Gr-1intermediate cells as well as negative regulation of immunosurveillance.
A major outcome of this study is the discovery that administration of TNF-αR-Fc, a relatively safe TNF-α inhibitor that is widely used in the treatment of inflammatory diseases, could have an anti-tumor effect by enhancing immune surveillance (23, 24). This finding, as well as others, support the idea that TNF-α can have pro-tumor activity and thus, the treatment of tumors via TNF-α blockade is a reasonable approach to the treatment of tumors (25). It should be noted, however, that there is also a considerable body of evidence supporting the idea that TNF-α can have anti-tumor activity especially when present at pharmacologic concentrations. This is buttressed by studies of the effect of TNF-α on tumor cell line growth in vitro and studies of the treatment of patients with tumors with TNF-α in vivo (26-28). Thus, assuming that endogenous TNF-α may have an inhibitory effect on tumor growth in some circumstances, it is possible that treating cancer patients with TNF-α blockers may enhance tumor development. On balance, then, it appears that the effect of anti-TNF-α therapy on any particular tumor is difficult to predict and the final outcome will depend on such factors as the inherent susceptibility of the tumor to immunosurveillance, its tendency to undergo TNF-α-induced apoptosis and, finally, the ability of TNF-α to elicit pro- or anti-tumor effects of immune cells in the milieu of the tumor. These issues are best sorted out by appropriate studies of patients with tumors.
References
- 1.Terabe M, Matsui S, Noben-Trauth N, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–20. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 2.Terabe M, Matsui S, Park JM, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terabe M, Park JM, Berzofsky JA. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunol Immunother. 2004;53:79–85. doi: 10.1007/s00262-003-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha(2) receptor is involved in induction of TGF-beta(1) production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 5.Wooley PH, Dutcher J, Widmer MB, Gillis S. Influence of a recombinant human soluble tumor necrosis factor receptor FC fusion protein on type II collagen-induced arthritis in mice. J Immunol. 1993;151:6602–7. [PubMed] [Google Scholar]
- 6.Matsui S, Ahlers JD, Vortmeyer AO, et al. A model for CD8+ CTL tumor immunosurveillance and regulation of tumor escape by CD4 T cells through an effect on quality of CTL. J Immunol. 1999;163:184–93. [PubMed] [Google Scholar]
- 7.Brutkiewicz RR, Sriram V. Natural killer T (NKT) cells and their role in antitumor immunity. Crit Rev Oncol Hematol. 2002;41:287–98. doi: 10.1016/s1040-8428(01)00198-6. [DOI] [PubMed] [Google Scholar]
- 8.Shimamura M, Morishita R, Endoh M, et al. HVJ-envelope vector for gene transfer into central nervous system. Biochem Biophys Res Commun. 2003;300:464–71. doi: 10.1016/s0006-291x(02)02807-3. [DOI] [PubMed] [Google Scholar]
- 9.Fichtner-Feigl S, Fuss IJ, Young CA, et al. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–70. doi: 10.4049/jimmunol.178.9.5859. [DOI] [PubMed] [Google Scholar]
- 10.Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–24. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–80. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–40. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 13.Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Curr Opin Immunol. 2004;16:157–62. doi: 10.1016/j.coi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–90. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol. 2000;105:1063–70. doi: 10.1067/mai.2000.107604. [DOI] [PubMed] [Google Scholar]
- 16.Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–8. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 17.Chiaramonte MG, Mentink-Kane M, Jacobson BA, et al. Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J Exp Med. 2003;197:687–701. doi: 10.1084/jem.20020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–56. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 19.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wynn TA, Hesse M, Sandler NG, et al. P-selectin suppresses hepatic inflammation and fibrosis in mice by regulating interferon gamma and the IL-13 decoy receptor. Hepatology. 2004;39:676–87. doi: 10.1002/hep.20102. [DOI] [PubMed] [Google Scholar]
- 21.Donaldson DD, Whitters MJ, Fitz LJ, et al. The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J Immunol. 1998;161:2317–24. [PubMed] [Google Scholar]
- 22.Tabata Y, Chen W, Warrier MR, Gibson AM, Daines MO, Hershey GK. Allergy-driven alternative splicing of IL-13 receptor alpha2 yields distinct membrane and soluble forms. J Immunol. 2006;177:7905–12. doi: 10.4049/jimmunol.177.11.7905. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Nebro A, Irigoyen MV, Urena I, et al. Effectiveness, predictive response factors, and safety of anti-tumor necrosis factor (TNF) therapies in anti-TNF-naive rheumatoid arthritis. J Rheumatol. 2007;34:2334–42. [PubMed] [Google Scholar]
- 24.Davis JC, van der Heijde DM, Braun J, et al. Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks. Ann Rheum Dis. 2005;64:1557–62. doi: 10.1136/ard.2004.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer. 2006;42:745–50. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Pastorakova A, Hlubinova K, Altaner C. Treatment of human tumor cells by combine gene therapy harnessing plasmids expressing human tumor necrosis factor alpha and bacterial cytosine deaminase suicide gene. Neoplasma. 2006;53:478–84. [PubMed] [Google Scholar]
- 27.Zins K, Abraham D, Sioud M, Aharinejad S. Colon cancer cell-derived tumor necrosis factor-alpha mediates the tumor growth-promoting response in macrophages by up-regulating the colony-stimulating factor-1 pathway. Cancer Res. 2007;67:1038–45. doi: 10.1158/0008-5472.CAN-06-2295. [DOI] [PubMed] [Google Scholar]
- 28.Bartlett DL, Libutti SK, Figg WD, Fraker DL, Alexander HR. Isolated hepatic perfusion for unresectable hepatic metastases from colorectal cancer. Surgery. 2001;129:176–87. doi: 10.1067/msy.2001.110365. [DOI] [PubMed] [Google Scholar]