Abstract
Most, perhaps all cells in epithelial sheets are polarised in the plane of the sheet. This type of polarity, referred to as planar cell polarity (PCP), can be expressed in the orientation of cilia, stereocilia, in oriented outgrowths such as hairs, in the plane of cell division, in directed cell movement and possibly in the orientation of axon extension1, 2. Researchers are also studying growth: there is an attempt to find systems that determine the shape and size of organs. Although both polarity and growth are subject to overall control by morphogen gradients3 the mechanisms of this control are almost completely unknown. Here we discuss recent evidence for a “steepness hypothesis” that links these two apparently disconnected features of animal development.
The history of the steepness hypothesis
Grafting experiments by Wigglesworth, Piepho, Bohn, Locke, Lawrence and Stumpf in the 1940s, 50s and 60s first linked morphogenetic gradients, growth and polarity (reviewed in REF. 4). When pieces of cockroach limb taken from different regions of one leg segment were grafted together, growth was elicited from cells on either side of the junction to fill in the missing region. Bohn argued that each leg segment is patterned by a morphogenetic gradient in the proximo-distal axis and that the polarity and length of the new tissue depends on the net difference between the scalar values of the gradient that were juxtaposed by the graft. This idea was then refined further from work on other insects (Hemiptera): if, in the wildtype, the direction of slope, or vector of the gradient normally determines polarity5 then the steepness could measure dimension and regulate growth4 (see BOX 1).
BOX 1. The steepness hypothesis.
The steepness hypothesis has been evolving over many years but it remains speculative, unclear and incomplete. We make 5 propositions:
First, the decision as to whether to divide or not to divide, to live or to die, to differentiate or not is made by single cells in a population. These cell-by-cell decisions, summed over the whole, regulate the growth of an organ and fix its size and shape.
Second, in each axis, there is a mechanism that senses the dimension of the organ and this feeds back to regulate these die/divide decisions.
Third, this dimension sensing depends on a linear gradient of some signal set up between the boundaries of a defined and growing population of cells whose maximum and minimum is constant; consequently, as the organ grows, the gradient becomes less steep. Thus the steepness of the gradient is effectively a measure of dimension in one axis that could be conveyed to every cell (BOX. 1 figure).
Fourth, the morphogens responsible for overall pattern of an organ (such as Dpp, Hedgehog and Wingless) set up and orient the Dachsous/Fat (Ds/Ft) system which then provides a linear gradient. The Ds/Ft system regulates both growth and PCP.
Fifth, in the Ds/Ft system, the direction of a gradient (the vector) determines cell polarity while the steepness of the same gradient feeds into the ‘die or divide’ decisions via the “Hippo pathway”, a pathway linked to growth. Note that the Ds/Ft system may be responsible for one axis of growth (for example, anteroposterior in the abdomen) but there may still be other inputs into that axis; moreover different systems may be responsible for the other axis (dorsoventral in the abdomen).
In the 1960s and 70s, we thought that the gradient would prove to be a morphogen. However, morphogens responsible for patterning, such as Hedgehog in the Drosophila abdomen, Decapentaplegic (Dpp) and Wingless in the Drosophila wing, were later shown to operate upstream of the systems controlling PCP6, 7. Even later, evidence was gathered from the fly abdomen that morphogens act through two independent systems to determine PCP, one depending on Frizzled, Van Gogh and Flamingo and another on the protocadherins Ds and Ft8. Mutations in the latter system affect growth as well as PCP, suggesting that the Ds/Ft system might provide some measure of dimension to the cells9-11. Recently it was also found that if cells with widely different levels of Dpp signalling are apposed, cell division is elicited locally12, suggesting that disparity of Dpp signalling itself is mitogenic, or that it regulates a subordinate system for controlling growth. As we explain below, and as proposed in Rogulja and Irvine12, it now appears that Dpp controls growth indirectly through the Ds/Ft system.
The Ds/Ft system and the Hippo pathway
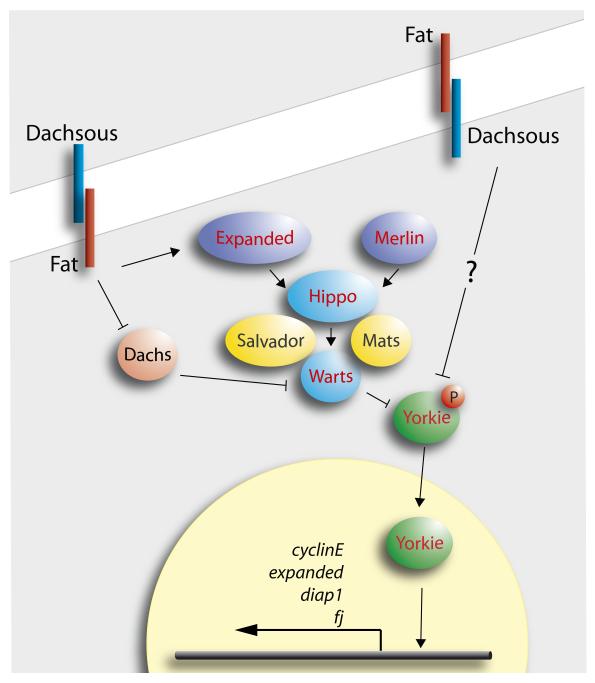
In the last few years the concept of a Hippo “pathway”, or “kinase cassette” (including Merlin, Expanded, Hippo, Salvador, Mats, Warts and Yorkie) has been developed (FIG. 1, reviewed in REF. 13). A flurry of papers have proposed that the Hippo pathway acts to suppress tumours and is involved in size control. However, these conclusions were based on mutant phenotypes and on experiments that upregulate Yorkie to cause excess growth, and thus leave open what the Hippo pathway really does during normal development. Nevertheless, it was recently found that ft− cells, long known to grow excessively14, upregulate target genes of the Hippo pathway that promote growth and inhibit apoptosis, such as Cyclin E and DIAP1 — suggesting that the wildtype function of Ft is to regulate cell proliferation via the Hippo pathway15-18. Ft has even been called a “tumour suppressor”19, 20 but it is not clear how it might Ft act in size control.
Figure 1.
The Hippo pathway, see REF. 35. Note it is not clear how Ft and Ds feed into the pathway, but we imagine the input within one cell to be from both types of Ft/Ds heterodimers in the membrane. Warts is thought to regulate the phosphorylation of the transcription factor Yorkie; unphosphorylated Yorkie enters the nucleus and drives transcription of target genes.
The essentials of the Ds/Ft system for PCP, “the Ds/Ft model”
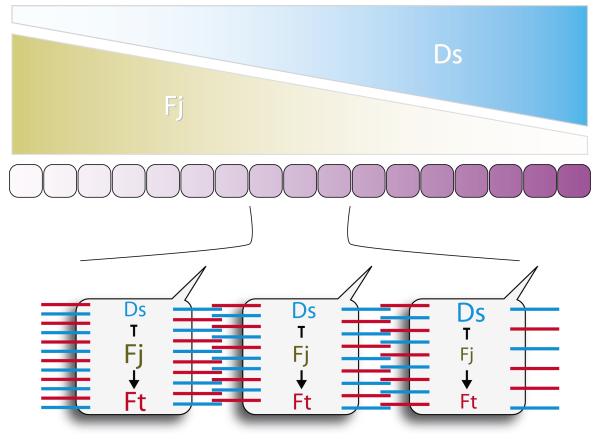
Here follows a simplified summary of our current model of how the Ds/Ft system generates PCP, based on the abdominal epidermis of Drosophila8; we refer to this as “the Ds/Ft model” (FIG. 2). Ds and Four-jointed (Fj), a Golgi kinase21, are expressed in opposing gradients in the anteroposterior axis (set up by the morphogens, Hedgehog and Wingless). The heterodimeric bridges formed by Ds and Ft from cell to cell ensure that the amounts of Ft to Ds on the surface of one cell can affect the distribution of Ds and Ft on neighbouring cells22, 23. According to the Ds/Ft model, when clones of cells are made that express, for example, a large amount of extra Ds, Ft and Ds molecules are redistributed on abutting cells on both sides of the clone/host interface for a few rows of cells. These redistributions of Ds and Ft cause local changes in steepness and/or direction of the Ds/Ft slopes. Of course there can only be a visible reversal of polarity where the effects of the clone oppose, rather than reinforce, the background polarity. For example, in the abdomen, changes in hair orientation are found either anterior or posterior to the clone but not both. Note also and importantly for what follows, the experimental results8 show that cells having either Ds or Ft can repolarise their neighbours. However these neighbours need both Ds and Ft to be repolarised8. These observations argue against a simple ligand-receptor relationship between Ds and Ft7, 16; instead both Ds and Ft act as “ligands” and “receptors” for each other8.
Figure 2.
A sketch of the Ds/Ft model. There is evidence that the Ds and Fj gradients are set up by the primary morphogens; they make a Ds/Ft gradient that is responsible for both PCP7, 8 and for the activation of Hippo targets that drive growth15-18. In the model, Ds and Fj concentration gradients span the organ and interact with uniformly expressed Ft molecules to build together, in one axis, a linear gradient of Ds/Ft heterodimers. Putative distributions of Ds and Ft heterodimers are indicated below. In the model, Ds and Ft function as trans-heterodimers acting, in effect, as ligands and receptors for each other. This model explains, for example, why ds− or ft− cells do not show PCP or growth responses — ds− or ft− cells could not compare numbers of Ds/Ft heterodimers on their two faces.
New evidence for the steepness hypothesis
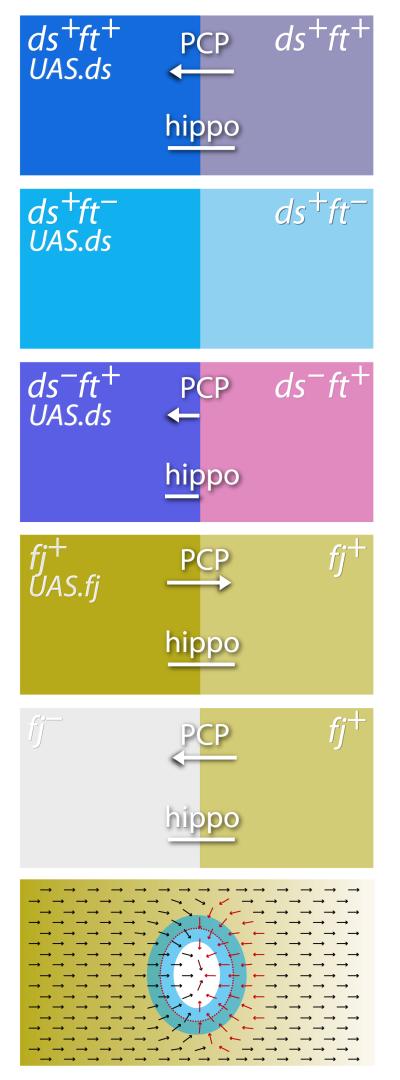
Recently the steepness hypothesis and the Ds/Ft model have been supported by two exciting papers24, 25. As with studies of PCP8 the method used was to make clones of cells that set up sharp disparities in the Ds/Ft system but, instead of assessing hair polarity, Rogulja et al and Willecke et al looked at expression of gene targets of the Hippo pathway. What is exciting is that their results demonstrate that the effects of the Ds/Ft system on Hippo targets are coextensive with the effects shown earlier on PCP8, suggesting that growth and PCP are both outputs of the same signalling mechanism. To illustrate, we choose a few of their experiments from many (FIG. 3).
Figure 3.
The effects of juxtaposing cells with different levels or states of the Ds/Ft system. The left column shows the genotypes of clones and the right column the background genotypes — the interfaces between cells of these two genotypes drive the effects which produce coextensive outputs onto PCP and Hippo targets. The arrows show formally the sign as well as the extent of polarity effects that reverse the background polarity on the appropriate sides of the clones8. The bars indicate the extent of upregulation of Hippo targets above background levels — these extend a few cell rows on one or both sides of the interfaces as shown24, 25. Ft is indicated in pink, Ds in blue and Fj in cadmium green. Below we show a single example of an ellipsoidal fj− clone. The clone is outlined with a dotted red line, the arrows indicate the PCP of oriented structures such as cuticular hairs, red arrows showing where polarity has been changed by the clone-induced Ds/Ft slopes. Blue marks the zone, including both the periphery of the clone and its surround, in which Hippo targets are upregulated as a result of local steepening of those slopes.
Clones overexpressing Ds
Clones were made in the wing disc that overexpress the ds gene, creating new Ds/Ft differentials all around the perimeter of the clone. Given that Ds and Ft are present in all cells, the Ds/Ft model predicts that, when Ds is expressed strongly in a clone, all around its perimeter, inside and outside, there will be sharp local increases in steepness and, in some places, changes in slope direction (the latter seen in the earlier PCP8 experiments). Both new papers24, 25 report that, when the Ds/Ft model predicts changes in steepness, a Hippo target gene is upregulated. The ranges of effects on PCP and on Hippo targets are similar, extending to about 2-4 cells from the interface, leaving the centre of the clone relatively unaffected.
If clones containing extra Ds are made in a ft− fly, the Ds/Ft model predicts there should be no effects on the Hippo pathway, as all cells in the animal lack Ft, both inside the clone and outside, and thus should be refractory to any incoming Ds/Ft signal. Indeed, there is no effect on the Hippo pathway24, 25. If clones expressing Ds are made in a ds− fly, cells outside the clone lack Ds and therefore cannot respond. But, just inside the clone, where both Ds and Ft are present, an abrupt, local increase in steepness is predicted by the Ds/Ft model, and indeed an increase in Hippo target expression, as well as growth, is observed25.
Loss and gain of Fj
Fj is a protein kinase present in the Golgi lumen21, 26 that modulates Ds/Ft interactions, enhancing Ft activity and reducing Ds activity8, 26. Removing or adding Fj to clones of cells have opposite effects on hair orientation: in the abdomen, removing Fj reverses hairs behind the clone while adding Fj reverses hairs in front27. Even though the polarity is changed on only one face of the clones, the Ds/Ft model predicts that, in both experiments, steepness of the gradient will be increased all around the clone borders. Accordingly, in the wing and eye, Hippo target genes are upregulated in both experiments on both sides of the interface between mutant and wildtype cells25, even though polarity changes are found on one face27, 28. Further, in PCP it was observed that effects in fj− territory have a longer range8 and, suggestively, the range of effects on Hippo targets may also be increased where the Fj concentration is lower25.
This spatial fit between the PCP results and the upregulation of Hippo targets in these experiments (and others described in the two recent papers24, 25) argues that PCP and the Hippo pathway are both outputs of the same Ds/Ft landscape: the orientation of hairs depending on the vectors of local gradients and the activation of Hippo targets being correlated with the steepness of these slopes.
Hippo pathway and cell division
There is good evidence that upregulating Hippo targets leads to mitosis. Both new articles24, 25 describe the effects of clones expressing Ds and Fj and they find stimulation of mitosis in the vicinity of the clone borders, both outside, and in some cases, inside the clone. They report a greater stimulation when the Ds and Fj expressing clones are located near the nadirs of the Ds and Fj gradients, respectively, as is also the case for the activation of Hippo targets24, 25 and PCP8, 27, 28. Again these results argue that it is the degree of difference across the interface in Ds/Ft activity that drives Hippo targets and cell division as well as changes in PCP. The effects on cell division were blocked in dachs− flies24, a gene needed downstream of fat for both growth and, possibly PCP29. These experiments all support the steepness hypothesis — the steepness of the Ds/Ft gradient regulating Hippo target expression and cell proliferation and its direction providing information used to polarise the cells.
Problems with the main conclusions and some unanswered questions
There are some results that do not fit.
First, according to the Ds/Ft model, ft− clones should show a uniform level of expression of Hippo targets inside the clone and should induce non-autonomous upregulation in the surrounding wildtype cells. Even though ft− clones do cause non-autonomous effects on PCP8, 27, the same non-autonomous effect has not (yet) been observed on Hippo targets or growth18. If this latter result proves to be correct, there will be a problem with the straightforward case we have presented.
Second, the Ds/Ft model predicts that the effect of Ft on neighbouring cells, the key element of PCP, depends on its extracellular domain interacting with Ds in the next cell. It follows that the intracellular domain should be ineffective on its own, and we found that, when this domain was over-expressed locally, it had no detectable PCP activity8. However uniform overexpression of the intracellular domain can partially rescue the ft− overgrowth phenotype30. These divergent results between PCP and growth appear to argue in different directions and are currently unresolved.
Third, some results suggest that the steepness hypothesis is insufficient. As expected, substituting uniform expression of both Ds and Fj in place of the normal, opposing gradients of Fj and Ds (that is, flattening the slope of the Ds/Ft system) does reduce growth, but the effects, as monitored by BrdU incorporation, are only transient24, and the resulting wings are only modestly reduced to about half their normal size24, 25, 31. Removing either Ds or Ft (and activating the Hippo pathway) results in enhanced growth, but again, the effects are weak, as the rare surviving adult flies make wings that are only moderately enlarged30. If the Ds/Ft model were as central as we like to believe, then perhaps flattening or removing the Ds/Ft gradient should have more catastrophic effects.
Fourth, the precise nature of the Ft/Ds gradient is unknown; even though our Ds/Ft hypothesis posits that the numbers of Ds/Ft trans-heterodimers are the key variable, this is not proven. In the model8 a difference in the number of heterodimers between the two faces of a cell might be the cue for planar polarity and the amount of that difference could represent the steepness, but there is no direct evidence. Finally, it seems important for the steepness hypothesis that the gradient be more or less linear, as only a linear gradient could convey consistent information of dimension to all the cells. But it is not known if the gradient is linear and if so how this might be achieved. Nor is it known how the upstream morphogen gradients help establish the Ft/Ds gradient landscape.
The steepness hypothesis has other implications, as yet unresolved. For example it might seem that growth should be related to organ size — faster, early in development, when primordia are small and the slope steep, and slower later on, as the cells increase in number and the linear gradient becomes shallow (BOX 1). Yet, the rate of growth, for example of the Drosophila wing, is relatively constant. But here we begin to enter a continent beyond the scope of this commentary — there it may be found that the controls of size and shape apply to animals of all sizes and probably depend on many inputs and feedback mechanisms4, 32, 33 operating in two or even three axes.
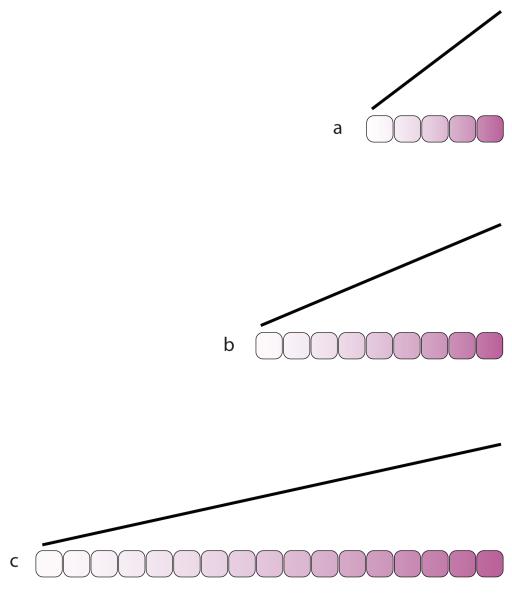
BOX 1 Figure.
The gradient is assumed to be linear. As the organ grows, the maximum and minimum limits are conserved while recently divided cells take up intermediate scalar values from their neighbours (some evidence for this can be found in REF. 34). The steepness of the gradient at each point, measured perhaps as a differential across each cell, correlates with the dimension of the organ. A measure that, in principle, could be delivered locally to each cell. During normal growth and development, the identity of cells changes, so that the “target steepness” will vary from stage to stage. This process could be responsible for the observed and precise logarithmic increase in the length of organs, such as limbs, from instar to instar in hemimetabolic insects10.
Acknowledgements
We thank the Wellcome Trust and the Medical Research Council, UK for support. GS is a Howard Hughes Medical Institute Investigator.
Footnotes
Competing interests statement: The authors declare they have no competing financial interests.
References
- 1.Wang Y, Nathans J. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 2.Zallen JA. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 3.Zecca M, Basler K, Struhl G. Development. 1995;121:2265–2278. doi: 10.1242/dev.121.8.2265. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence PA. Adv. Insect Physiol. 1970;7:197–266. [Google Scholar]
- 5.Lawrence PA. J. Exp. Biol. 1966;44:607–620. doi: 10.1242/jeb.44.3.507. [DOI] [PubMed] [Google Scholar]
- 6.Struhl G, Barbash DA, Lawrence PA. Development. 1997;124:2155–2165. doi: 10.1242/dev.124.11.2155. [DOI] [PubMed] [Google Scholar]
- 7.Yang C, Axelrod JD, Simon MA. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 8.Casal J, Lawrence PA, Struhl G. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day SJ, Lawrence PA. Development. 2000;127:2977–2987. doi: 10.1242/dev.127.14.2977. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence PA. Nature. 2004;429:247. doi: 10.1038/429247a. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence PA, Struhl G, Casal J. Nat Rev Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogulja D, Irvine KD. Cell. 2005;123:449–461. doi: 10.1016/j.cell.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Reddy BV, Irvine KD. Development. 2008;135:2827–2838. doi: 10.1242/dev.020974. [DOI] [PubMed] [Google Scholar]
- 14.Bryant PJ, Huettner B, Held LI, Jr., Ryerse J, Szidonya J. Dev Biol. 1988;129:541–554. doi: 10.1016/0012-1606(88)90399-5. [DOI] [PubMed] [Google Scholar]
- 15.Bennett FC, Harvey KF. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 16.Cho E, et al. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 17.Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. Curr Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Willecke M, et al. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Hariharan IK, Bilder D. Annu Rev Genet. 2006;40:335–361. doi: 10.1146/annurev.genet.39.073003.100738. [DOI] [PubMed] [Google Scholar]
- 20.Mahoney PA, et al. Cell. 1991;67:853–868. doi: 10.1016/0092-8674(91)90359-7. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Science. 2008;321:401–404. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Nature. 2003 doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 23.Strutt H, Strutt D. Dev Cell. 2002;3:851–863. doi: 10.1016/s1534-5807(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 24.Rogulja D, Rauskolb C, Irvine KD. Dev Cell. 2008;15:309–321. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willecke M, Hamaratoglu F, Sansores-Garcia L, Tao C, Halder G. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0805201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strutt H, Mundy J, Hofstra K, Strutt D. Development. 2004;131:881–890. doi: 10.1242/dev.00996. [DOI] [PubMed] [Google Scholar]
- 27.Casal J, Struhl G, Lawrence PA. Curr Biol. 2002;12:1189–1198. doi: 10.1016/s0960-9822(02)00974-0. [DOI] [PubMed] [Google Scholar]
- 28.Zeidler MP, Perrimon N, Strutt DI. Dev Biol. 2000;228:181–196. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- 29.Mao Y, et al. Development. 2006;133:2539–2551. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- 30.Matakatsu H, Blair SS. Development. 2006 doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- 31.Simon MA. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- 32.Zecca M, Struhl G. Development. 2007;134:3001–3010. doi: 10.1242/dev.006411. [DOI] [PubMed] [Google Scholar]
- 33.Zecca M, Struhl G. Development. 2007;134:3011–3020. doi: 10.1242/dev.006445. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence PA, Crick FHC, Munro M. J Cell Sci. 1972;11:815–853. doi: 10.1242/jcs.11.3.815. [DOI] [PubMed] [Google Scholar]
- 35.Saucedo LJ, Edgar BA. Nat Rev Mol Cell Biol. 2007;8:613–621. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]