Abstract
Pancreatic cancer is highly aggressive and refractory to existing therapies. Connective tissue growth factor (CTGF/CCN2) is a fibrosis-related gene that is thought to play a role in pancreatic tumor progression. However, CCN2 can be expressed in a variety of cell types, and the contribution of CCN2 derived from either tumor cells or stromal cells as it affects the growth of pancreatic tumors is unknown. Using genetic inhibition of CCN2, we have discovered that CCN2 derived from tumor cells is a critical regulator of pancreatic tumor growth. Pancreatic tumor cells derived from CCN2 shRNA-expressing clones showed dramatically reduced growth in soft agar and when implanted subcutaneously. We also observed a role for CCN2 in the growth of pancreatic tumors implanted orthotopically, with tumor volume measurements obtained by PET imaging. Mechanistically, CCN2 protects cells from hypoxia-mediated apoptosis, providing an in vivo selection for tumor cells that express high levels of CCN2. We found that CCN2 expression and secretion was increased in hypoxic pancreatic tumor cells in vitro, and we observed co-localization of CCN2 and hypoxia in pancreatic tumor xenografts and clinical pancreatic adenocarcinomas. Furthermore, we found increased CCN2 staining in clinical pancreatic tumor tissue relative to stromal cells surrounding the tumor, supporting our assertion that tumor cell-derived CCN2 is important for pancreatic tumor growth. Taken together, these data improve our understanding of the mechanisms responsible for pancreatic tumor growth and progression, and also indicate that CCN2 produced by tumor cells represents a viable therapeutic target for the treatment of pancreatic cancer.
Keywords: CCN2, CTGF, hypoxiz, pancreatic cancer, orthotopic tumors
Introduction
Pancreatic cancer is the tenth most common cancer type diagnosed in the United States each year, and is the fourth most common cause of cancer deaths. The 5-year survival rate for patients with pancreatic ductal adenocarcinoma is approximately 5%, and has not substantially improved over the past 25 years (1). There is a clear need for increased understanding of the mechanisms driving pancreatic cancer growth and progression so that novel therapeutic strategies can be devised to improve treatment. As with most solid tumors, pancreatic ductal adenocarcinomas contain tumor cells that are at low oxygen tensions (hypoxic), and tumor hypoxia is known to induce the expression of a variety of genes associated with tumor progression and aggressiveness. Pancreatic tumors also contain extensive desmoplasia, and this fibrotic tissue can store a variety of secreted factors that facilitate tumor progression (2). Genes such as connective tissue growth factor are thought to play a role in the formation of desmoplastic tissue and also in tumor progression, and therefore represent an attractive therapeutic target for the treatment of pancreatic cancer.
Connective tissue growth factor (CTGF/CCN2) is a member of the CCN family of proteins and is thought to be involved in extracellular matrix production, desmoplasia, tumor cell proliferation, adhesion, migration, angiogenesis, and metastasis (3, 4). The myriad of functions assigned to CCN2 may be partly explained by the modular domain structure of the protein. CCN2 has four structural domains, each of which is thought to have a distinct biological function (5), and cleaved versions of CCN2 have been detected in body fluids (6). CCN2 is known to interact with transforming growth factor-β (TGF-β) and bone morphogenetic protein-4 (BMP4) to modulate their activity (7), and also binds a variety of integrins involved in cell adhesion and migration (8-10). Thus CCN2 function in different cell types is likely dictated by a number of factors including CCN2 expression levels, structure of CCN2 molecules, and interaction with other proteins.
While the role of CCN2 in normal tissue fibrosis has been well-studied (11), the function of CCN2 in cancer is not as well understood. Interestingly, CCN2 has been identified as an oncogene in a variety of cancer types, but is considered a tumor-suppressor gene in other forms of cancer. Over-expression of CCN2 correlates with decreased survival in patients with esophageal adenocarcinoma (12), glioblastoma (13), breast cancer (14), gastric cancer (15), and adult acute lymphoblastic leukemia (16). Increased CCN2 expression has been associated with progression of cervical tumors (17), esophageal squamous cell carcinoma (18), and Wilm's tumor (19). Conversely, CCN2 expression levels correlated with increased survival in chondrosarcoma patients (20) and in patients with lung cancer (21). Whether these disparities in the literature are due to diversity in CCN2 structure, expression levels, and binding partners in different tumor types is an open question. Notably, CCN2 expression has been observed in tumor cells, tumor-associated fibroblasts, and endothelial cells, raising the question that the effects of CCN2 on tumor progression may be influenced by the source of CCN2 production.
Previous studies have shown that immunological inhibition of CCN2 delays the growth and metastasis of xenografted human pancreatic tumors (22, 23). However, the CCN2 antibody used in these studies cross-reacts with both human CCN2 (tumor cell-derived) and mouse CCN2 (stromal cell-derived), not permitting insight into the impact of tumor cell or stromal cell sources of CCN2 on pancreatic tumor growth. Determining the relative importance of tumor cell-derived CCN2 or stromal cell-derived CCN2 in pancreatic tumor growth would improve our understanding of pancreatic cancer progression, and would also provide a means to select patients that are more likely to benefit from immunological CCN2 inhibition.
We show herein that pancreatic tumor growth in both subcutaneous and orthotopic sites is critically dependent on CCN2 expressed by tumor cells, and that there is a robust in vivo selection for tumor cells that express high levels of CCN2. We observed elevated levels of CCN2 in clinical pancreatic adenocarcinomas when compared to either normal pancreatic tissue or to stromal cells surrounding the tumor. We also found that CCN2 co-localized with tumor hypoxia in clinical pancreatic adenocarcinomas and in human tumor xenografts, and that CCN2 secretion was increased in hypoxic pancreatic tumor cells in vitro. CCN2 increased the growth of pancreatic tumor cells in soft agar and decreased apoptosis of pancreatic tumor cells in response to hypoxic stress in vitro, potentially explaining the in vivo selection for tumor cells that express high levels of CCN2. Taken together, these data indicate the importance of tumor cell-derived CCN2 in pancreatic tumor growth and support the inhibition of CCN2 in clinical pancreatic cancer therapy.
Methods
Patient samples
Human tissue was obtained from pancreatic cancer patients undergoing pancreaticoduodenectomies at Stanford Hospital; informed consent was obtained prior to the procedure under the approval of the Stanford Institutional Review Board. Paraffinembedded samples were stained as previously described for CCN2 (22) or carbonic anhydrase-IX (24). CCN2 staining intensity was scored on a scale from 0+ to 3+ (where 0+ = no staining and 3+ = strong staining), and the fraction of tissue with a given level of CCN2 staining intensity was estimated. The overall CCN2 staining scores were based on the most intense staining found in a given section, such that a staining intensity of 3+ in >50% of tumor tissue = 3, a staining intensity of 2+ in >50% of tumor tissue = 2, and a staining intensity of 1+ in >50% of tumor tissue = 1. In sections that contained lesser amounts of stained tissue, the overall CCN2 staining scores were based on a staining intensity of 3+ in <50% of tumor tissue = 2, a staining intensity of 2+ in <50% of tumor tissue = 1, and a staining intensity of 0-1+ in <50% of tumor tissue = 0.
Carbonic anhydrase-IX (CAIX) staining intensities were typically classified as either 3+ or 0+, and the overall CAIX staining scores were derived from the relative tissue area stained with 3+ CAIX intensity: 3+ staining in >50% of tumor tissue = 3, 3+ staining in <50% and >15% of tumor tissue = 2, 3+ staining in <15% of tumor tissue = 1, and no observable 3+ staining = 0.
Tumor cells
Human pancreatic Panc-1 and Su86.86 tumor cells were obtained from the American type culture collection (ATCC) and used within 10 passages. Panc-1 cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS), while Su86.86 cells were maintained in RPMI 1640 medium supplemented with 10mM HEPES, 1mM sodium pyruvate, and 10% FBS. CCN2 shRNAs were designed using the Dharmacon shRNA design algorithm: A=5'-CTATGATTAGAGCCAACTG-3', B=5'-GCTGACCTGGAAGAGAACA-3', C=5'-GAGAACATTAAGAAGGGCA-3', D=5'-TGACATCTTTGAATCGCTG-3', E=5'-TCGCTGTACTACAGGAAGA-3', and F=5'-GATGTACGGAGACATGGCA-3'. Oligonucleotides were inserted into pSiren vector for retroviral transfection and CCN2 shRNA-expressing cells were puromycin-selected. Surviving cells were either pooled or single clones were picked and expanded to make clonal cell populations. CCN2 in the pBabe Puro vector was used to induce CCN2 expression.
For monolayer growth curves, 105 cells were plated in 60mm plates and grown for 3-5 days. Cells were trypsinized, counted using a Coulter Z1 particle counter (Beckman Coulter Inc., Fullerton, CA), and 105 cells were replated and allowed to grow for another 3-5 days. For soft agar growth, 0.5% Noble agar (BD Biosciences, San Diego, CA) was allowed to solidify in a 12-well plate, and 5×103 cells were plated in 0.3% Noble agar on top. Tumor cell colonies were stained with 0.02% Geimsa stain in PBS after 14-21 days.
Subcutaneous and orthotopic tumor implants
All animal studies were performed in accordance with the Stanford University Animal Care and Use Committee. For subcutaneous tumor growth, male 8-10 week old Nu/Nu mice were anesthetized with 2% isoflurane in O2 and 107 Panc-1 or Su86.86 tumor cells were implanted in 100μl of phosphate buffered saline (PBS). Tumor volumes were calculated as the volume of an ellipsoid based on three orthogonal caliper measurements (L × W × H × π/6).
For orthotopic tumor growth, male 8-10 week old Nu/Nu mice were anesthetized with 100mg/kg ketamine and 20mg/kg xylazine administered intraperitoneally prior to a transverse abdominal incision. The stomach, spleen, and pancreas were exposed and 106 tumor cells in 50μl PBS were injected into the tail of the pancreas. In order to prevent leakage of the cell suspension from the injection site, a cotton swab was held in contact with the injection site for 30 seconds post-injection and the area was then monitored for evidence of leakage for an additional 30 seconds (25). A successful injection was determined by the presence of a fluid-filled bleb in the pancreas without intraperitoneal leakage. The incision was then sutured closed. We have found this method to form reproducible orthotopic pancreatic tumors (Ham and Giaccia, unpublished data).
Positron emission tomography
Relative volumes of orthotopic pancreatic tumors were estimated by measuring uptake of 18F-2-deoxyglucose (FDG) using positron emission tomography (PET). 18F was produced through bombardment of 18O-enriched H2O on a PETTrace cyclotron (GE Medical Systems, Milwaukee, WI) and FDG was produced on an FX-FN synthesis unit (GE Medical Systems, Milwaukee, WI) through nucleophilic substitution of a precursor with 18F. Mice were anesthetized with 2% isoflurane in O2 and 200-250μCi FDG was administered in ~100μl via the lateral tail vein. Mice remained anesthetized for one hour following radiotracer injection in order to minimize FDG uptake by skeletal muscle and brown fat tissue. Mice were transferred to the bed of a Vista microPET scanner (GE Medical Systems, Milwaukee, WI), and microPET data were collected for 10 minutes over a 4cm longitudinal field of view. The coincidence events measured were then reconstructed into a three-dimensional image of FDG concentration using an ordered subsets expectation maximization (OSEM) algorithm (26).
Immunofluorescence
Tumors were frozen in OCT and 8μm sections were cut and stained with the appropriate antibodies: polyclonal goat anti-CCN2 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), monoclonal anti-pimonidazole (NPI Inc., Burlington, MA), and Alexa 488 or 594 secondary antibodies (Invitrogen, Carlsbad, CA). Slides were mounted in Vectashield mounting medium containing 4',6-diamidino-2-phenylindole (DAPI; Vector Laboratories Inc., Burlingame, CA). Images were captured at 20× magnification using a Qimaging Retica EXi camera mounted on a Leica DM6000B microscope (JH Technologies, San Jose, CA).
Hypoxia treatment and apoptosis assay
Cells were placed in a humidified Invivo2 400 hypoxia workstation (Ruskinn Inc., Cincinnati, OH) at 5% CO2 and the indicated oxygen tensions. For the apoptosis assay, cells were incubated in 0.5% O2 prior to cell harvest and staining with annexin-V-conjugated FITC antibody according to manufacturer's instructions. Apoptotic cells were quantified using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) with subsequent data analysis using CellQuest software.
CCN2 Northern blots and Western blots
For Northern blot analysis, cells were harvested in Trizol reagent (Invitrogen, Carlsbad, CA), and 5-10μg of glyoxal-treated total RNA was loaded on a sodium phosphate agarose gel using standard methods. Northern blots were probed with 32P-labeled CCN2 and visualized by exposure to a phosphorscreen with subsequent scanning on a Storm 860 scanner (Molecular Dynamics, Sunnyvale, CA).
To analyze CCN2 levels in vivo, small pieces of excised tumors were stored in RNAlater stabilization reagent (Qiagen Inc., Valencia, CA) at -20°C according to manufacturer's instructions. Tissues were removed from the RNAlater reagent and homogenized in 1ml Trizol for RNA extraction and subsequent Northern blot.
For analyzing secreted CCN2 protein, equal numbers of cells were plated 24 hours prior to the experiment and the media was changed to DMEM containing 0.25% bovine serum albumin (BSA) at time 0. Plates were kept under either 21% or 0.5% O2 and conditioned media was collected (and cell lysates harvested) at the indicated time points. The conditioned media was cleared by centrifugation and the media volume was normalized to 10ml with water. 180μl of heparin sepharose CL-6B beads (Amersham Biosciences, Piscataway, NJ) were added to 4.5ml of conditioned media and rocked for 24hr at 4°C. Beads were washed three times with cold PBS and CCN2 protein was collected by incubating the beads with 200μl of RIPA buffer at 95°C for 10min. 20μl of each sample was loaded for Western blot analysis.
Protein lysates were harvested at the indicated time points using a 9M Urea, 0.075M Tris buffer (pH 7.6). Protein lysates were quantified using the Bradford assay, and subjected to reducing SDS-PAGE using standard methods. Western blots were probed with the following antibodies: HIF-1α (610959; BD Biosciences, San Diego, CA), actin (AC-40 A3853; Sigma-Aldrich, St. Louis, MO), CCN2 (SC-14939; Santa Cruz Biotechnology Inc., Santa Cruz, CA).
Statistical analysis
Soft agar colony numbers or apoptotic cell numbers were assessed by Student's t-test, and survival of orthotopic tumor-bearing mice were assessed by log-rank test. Areas under the curves (AUCs) were calculated for each tumor growth curve and assessed by ANOVA or Student's t-test as appropriate. Chi-squared analyses were performed on the clinical data. GraphPad Prism was used for statistical analyses.
Results
To investigate the role of tumor cell-derived CCN2 in pancreatic cancer, we generated a number of stable CCN2 shRNA-expressing Panc-1 cell lines. Six CCN2 shRNA sequences were tested, with three shRNAs producing varying degrees of CCN2 knockdown (Figure 1A, upper panel). As controls against potential off-target effects of the CCN2 shRNA, we used a scrambled shRNA sequence and also added CCN2 back into cells stably expressing shRNA D, which had produced the most potent CCN2 knockdown (Figure 1A, lower panel).
Figure 1. CCN2 knockdown decreases soft agar growth of pancreatic tumor cells.
(A) Upper, Northern blot of total RNA from wild-type Panc-1 cells and Panc-1 cells stably expressing one of six shRNA sequences targeting CCN2 (labeled A-F). Scr = scrambled control shRNA. Lower, Northern blot of Panc-1 cells stably expressing shRNA D and subsequently transfected with CCN2.
(B) 105 Panc-1 cells stably expressing the indicated shRNA constructs were plated as monolayers and counted every 4 days. 105 cells were replated after each count and the cumulative cell numbers are plotted. Data are mean ± SEM.
(C) 5×103 Panc-1 cells stably expressing the indicated shRNA constructs were plated in soft agar and allowed to grow for 2-3 weeks. Colonies were stained with Giemsa stain for visualization and representative images are shown.
(D) Quantification of the soft agar colonies from (C). Data are mean ± SEM; *p<0.05 relative to control.
CCN2 levels did not affect the proliferation of Panc-1 tumor cells (Figure 1B) or Su86.86 tumor cells (Supplemental Figure 1) in monolayer culture in air (or at 2% oxygen; data not shown). However, CCN2 knockdown induced significant decreases in the ability of Panc-1 cells to grow in soft agar (Figure 1C) with the greatest decrease in soft agar growth induced by shRNA “D” (Figure 1D). Soft agar growth was not decreased in cells expressing either a scrambled control shRNA sequence or shRNA constructs that did not effectively knockdown CCN2. Furthermore, adding CCN2 expression back to cells expressing CCN2 shRNA D significantly increased the soft agar growth of these cells. These data indicate that the level of CCN2 knockdown influences the ability of Panc-1 cells to grow in soft agar.
We then subcutaneously implanted Nude mice with wild-type Panc-1 cells or a pooled population of Panc-1 cells expressing the most potent CCN2 shRNA construct D. We observed a modest growth delay in tumors derived from the pooled population of CCN2 shRNA-expressing cells (Figure 2A), but the difference was not statistically significant. Interestingly, the take-rate of shRNA-expressing tumors was only 80%, and cell lines generated from these tumors at the end of the experiment indicated a marked increase in CCN2 expression relative to the input shRNA-expressing cells (Figure 2B). These data indicate a heterogeneous loss of stable shRNA expression in vivo and/or an outgrowth of tumor cells that had incomplete knockdown of CCN2. Similarly, cell lines generated from wild-type Panc-1 tumors excised at the end of the experiment had increased CCN2 expression relative to the initial Panc-1 input cells. These data suggest that cells expressing high levels of CCN2 have a growth advantage in solid tumors.
Figure 2. Partial knockdown of CCN2 induces modest delays in subcutaneous pancreatic tumor growth.
(A) 107 wild-type or CCN2 shRNA-expressing Panc-1 cells were implanted subcutaneously in nu/nu mice and tumor volumes were monitored over time. Data are mean ± SEM with at least 7 mice per group; no significant difference by Student's t-test of AUCs.
(B) Northern blots of wild-type or CCN2 shRNA-expressing Panc-1 cells used for tumor implants compared to cell lines generated from representative tumors excised 15-18 weeks after tumor implant.
(C) 107 wild-type or CCN2 shRNA-expressing Su86.86 cells were implanted subcutaneously in nu/nu mice and tumor volumes were monitored over time. Data are mean ± SEM with at least 5 mice per group; *p<0.05 by Student's t-test of AUCs. Inset, Northern blot of total RNA from wild-type and CCN2 shRNA-expressing Su86.86 cells.
(D) Upper, Northern blot of cell lines generated from representative subcutaneous CCN2 shRNA-expressing Su86.86 tumors harvested 8 weeks after tumor implant. Input cells represent the original CCN2 shRNA-expressing Su86.86 cells used for the tumor implants. Lower, Northern blot of total RNA extracted directly from excised subcutaneous wild-type or CCN2 shRNA-expressing Su86.86 tumors.
In agreement with these data, pooled populations of Su86.86 cells expressing CCN2 shRNA-D exhibited a modest statistically significant tumor growth delay (Figure 2C) relative to wild-type Su86.86 tumors and had a 75% take-rate. An increase in CCN2 expression was again observed when cell lines generated from CCN2 shRNA-expressing tumors at the end of the experiment were compared to the input shRNA-expressing cells (Figure 2D, upper panel). Furthermore, when excised tumor tissue was directly assayed for CCN2, we observed CCN2 expression levels in shRNA-expressing tumors that were comparable to wild-type Su86.86 tumors (Figure 2D, lower panel). Taken with Figure 2A-B, these data indicate that the microenvironment in pancreatic tumors promotes the outgrowth of tumor cells that express high levels of CCN2.
To determine if clonal populations of cells with more effective knockdown of CCN2 would result in greater suppression of tumor growth, we screened 40 clones from the heterogeneous Panc-1 CCN2 shRNA D-expressing population. We observed a wide range of CCN2 knockdown efficiency (Supplemental Figure 2) despite puromycin selection of the cells; all clones demonstrated at least some CCN2 knockdown with several clones showing >90% reductions in CCN2 levels. We were unable to derive clonal populations of Su86.86 shRNA-expressing cells because Su86.86 cells do not grow from single cells in our hands.
We also tested the efficacy of CCN2 knockdown in selected clones after exogenous stimulation of CCN2 over-expression. CCN2 is considered an immediate-early response gene, and we have found that serum stimulation of Panc-1 cells induces a transient increase in CCN2 expression as reported in other cell types (27, 28). Wild-type Panc-1 cells exposed to a change of fresh DMEM containing 10% FBS showed remarkable increases in CCN2 RNA and protein expression from 30 minutes up to 8 hours, at which time CCN2 expression returned to control levels (Figure 3A). Changing the media to DMEM without serum did not induce changes in CCN2 RNA or protein expression (data not shown). We chose two clones (#1 and #5) for further study based on sustained knockdown of CCN2 by >90% despite stimulation by 10% serum (Figure 3B).
Figure 3. Clonal populations of CCN2 shRNA-expressing Panc-1 cells show decreased growth in soft agar.
(A) Kinetics of CCN2 RNA and protein induction after changing media on the cells to fresh (10% FBS-containing) DMEM.
(B) CCN2 expression in the indicated cell types was stimulated by changing the media. Clonal populations with high levels of CCN2 knockdown after serum-stimulation were selected for further study.
(C) 5×103 wild-type, CCN2 over-expressing, and CCN2 shRNA-expressing Panc-1 clones were plated in soft agar and allowed to grow for 2 weeks. Colonies were stained with Geimsa stain for visualization, and photographs were taken using a dissecting microscope (2×).
(D) Quantification of soft agar colonies from (C). Data are mean ± SEM; *p<0.05 relative to control.
CCN2 shRNA-expressing Panc-1 clones displayed dramatically decreased growth in soft agar, while Panc-1 cells that over-expressed CCN2 demonstrated significantly increased soft agar growth relative to wild-type Panc-1 cells (Figure 3C-D). We also found that neither complete knockdown of CCN2 in clonal cell populations nor over-expression of CCN2 affected monolayer growth of Panc-1 tumor cells (Supplemental Figure 3). These data suggest that CCN2 increases the growth and/or survival of pancreatic tumor cells under non-ideal growth conditions as are found in soft agar matrices, and also illustrate the importance of tumor cell-derived CCN2 in an in vitro system devoid of stromal cells.
We then tested our hypothesis that CCN2 shRNA-expressing clones with more efficient CCN2 knockdown than the pooled CCN2 shRNA-expressing cells would provide greater effects on tumor growth. Indeed, the CCN2 shRNA-expressing Panc-1 clones with the greatest knockdown of CCN2 did not form tumors when implanted subcutaneously (Figure 4A). Furthermore, subcutaneous tumors derived from CCN2 over-expressing Panc-1 cells grew significantly faster than wild-type Panc-1 tumors. These data indicate an essential role for tumor cell-derived CCN2 relative to stromal cell-derived CCN2 in the growth of subcutaneous pancreatic tumor xenografts.
Figure 4. CCN2 knockdown decreases subcutaneous and orthotopic tumor growth, and increases the survival of orthotopic tumor-bearing mice.
(A) 107 wild-type, CCN2 over-expressing, and CCN2 shRNA-expressing Panc-1 clones were subcutaneously implanted in nu/nu mice. Tumor volumes were monitored weekly using calipers. Panc-1 + CCN2 shRNA (pool) curve is included from Figure 2A for visual comparison purposes only. Data are mean ± SEM with at least 5 mice per group; *p<0.05 by ANOVA of AUCs.
(B) 107 wild-type, CCN2 over-expressing, or CCN2 shRNA-expressing Panc-1 cells were orthotopically implanted in nu/nu mice. Tumor volume was monitored by uptake of intravenously administered 18F-deoxyglucose (FDG) using positron emission tomography (PET). Representative maximum intensity projections are shown, with regions of high FDG uptake highlighted. G = Harderian glands, H = heart, K = kidney, B = bladder, T = tumor.
(C) Survival plot of mice bearing orthotopic pancreatic tumors. Data from 3-9 mice per group; p-values indicate comparison with mice bearing wild-type tumors by log-rank test.
We wanted to determine the role of tumor cell-derived CCN2 in the growth of tumors implanted in tissue that is more representative of clinical pancreatic tumors. Orthotopically implanted pancreatic tumors (29) model the local development and distal metastasis of clinical pancreatic adenocarcinomas, and are responsive to stimulation by TGF-β (30) which is an upstream inducer of CCN2. Furthermore, transcription factors such as hypoxia-inducible factor-1 (HIF-1) can have opposite effects on the growth of tumors implanted in relatively poorly-vascularized subcutaneous sites compared to the often better-perfused tissues of origin (31). Thus the tissue of origin represents an optimal site for tumor xenograft implantation, and we therefore followed up our subcutaneous studies by testing the effect of CCN2 on the growth of pancreatic tumor xenografts implanted orthotopically.
Mice bearing orthotopically-implanted tumors were imaged by positron emission tomography (PET) for 18F-2-deoxyglucose (FDG) uptake (Figure 4B) and monitored for overall survival (Figure 4C). CCN2 over-expression enhanced the growth and metastasis of orthotopic pancreatic tumors as detected by FDG, leading to significantly decreased survival times when compared to wild-type Panc-1 tumors. Mice implanted with CCN2 shRNA-expressing Panc-1 clones exhibited smaller orthotopic tumors by PET imaging with a concomitant statistically significant increase in survival (Figure 4C). Taken together, these data indicate that tumor cell-derived CCN2 is important for the growth of both subcutaneous and orthotopic pancreatic tumor xenografts.
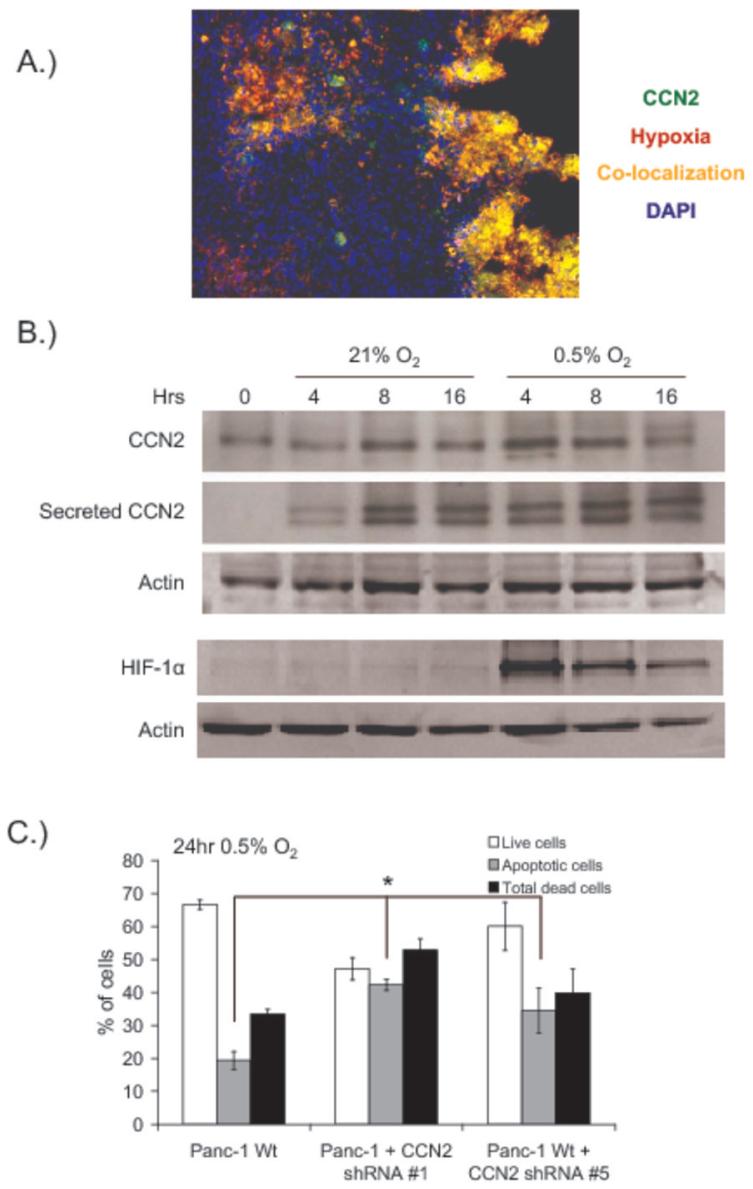
CCN2 expression can be induced in some cell types by exposure to low levels of oxygen (32-35), and pancreatic tumors contain relatively large numbers of hypoxic cells. We found that CCN2 co-localized with the hypoxia marker pimonidazole (Hypoxyprobe-1) in orthotopic Panc-1 tumors (Figure 5A), suggesting that CCN2 may be regulated by hypoxia in pancreatic tumors. However, CCN2 is a secreted protein that is capable of binding to the extracellular matrix (36) and the presence of CCN2 protein in hypoxic tumor regions is not necessarily indicative of CCN2 production by the hypoxic cells.
Figure 5. CCN2 expression and secretion is increased by hypoxia and protects pancreatic tumor cells from hypoxia-induced apoptosis.
(A) Pimonidazole (Hypoxyprobe-1) was administered 90 minutes prior to orthotopic Panc-1 tumor excision, and frozen tumor sections were analyzed for pimonidazole (red) and CCN2 (green). Areas of co-localization are indicated (yellow), and nuclei are stained with DAPI (blue).
(B) Western blots of CCN2 and HIF-1α in Panc-1 cells incubated at 21% or 0.5% oxygen for the indicated periods of time before collection of cell lysate and conditioned media. Secreted CCN2 was obtained by contacting conditioned media with heparin sepharosecoated beads prior to loading. Actin in the cell lysate was used as a loading control.
(C) Flow cytometric quantification of wild-type Panc-1 cells or Panc-1 shRNA-expressing clones exposed to 0.5% O2 for 24hr. Apoptotic cells were measured by annexin-V staining and quantified by flow cytometry. *p<0.05 relative to control cells.
We therefore studied the hypoxia-inducibility of CCN2 in Panc-1 cells in vitro. CCN2 protein levels in cell lysates and secreted into the surrounding media were elevated after exposure of cells to 0.5% O2 (Figure 5B). When cell lysates were assayed for HIF-1α, we found that the increase in CCN2 production and secretion in response to hypoxia occured when HIF-1α levels were maximally induced (at 4 hours). These data are consistent with previously published data suggesting a role for HIF-1 in CCN2 regulation in some cell types (32, 33). The levels of HIF-1 and intracellular CCN2 decreased by 16 hours of 0.5% O2 in relation to death of significant numbers of tumor cells. Increases in secreted CCN2 have been observed without significant changes in intracellular CCN2 levels (37), indicative of rapid and quantitative secretion of CCN2 upon up-regulation (38). Thus the secreted CCN2 in Figure 5B is a cumulative indication of CCN2 levels secreted from the cells over time while the intracellular CCN2 levels are more representative of CCN2 production at the time of cell harvest. CCN2 expression and secretion is therefore induced by hypoxia in Panc-1 tumor cells in vitro, and CCN2 protein co-localizes with hypoxic cells in Panc-1 tumors.
Our data indicate that CCN2 derived from tumor cells is required for efficient pancreatic tumor growth, and in vivo growth provides a selective pressure for the outgrowth of tumor cells that express high levels of CCN2. CCN2 also enhances the growth and survival of pancreatic tumor cells in soft agar, but had no effect on cells grown as monolayers. These observations led us to postulate that CCN2 may provide a growth or survival advantage to tumor cells under non-ideal growth conditions. Solid tumor hypoxia can represent a significant microenvironmental stress for cells, and we hypothesized that CCN2 affected tumor growth partly by modulating the survival of tumor cells found in hypoxic regions of tumors. We therefore treated Panc-1 cells with 0.5% O2 for 24 hours and monitored cell viability and apoptosis by annexin-V staining. Interestingly, we found that wild-type Panc-1 cells did not survive well in 0.5% O2 in vitro, and that CCN2 inhibition produced statistically significant increases in apoptotic cell death in response to hypoxia (Figure 5C). Importantly, CCN2 inhibition did not affect the survival of Panc-1 cells in normoxia (data not shown). These data indicate that CCN2 protects Panc-1 cells against hypoxia-induced apoptosis and, when taken with our observations that CCN2 increases the growth of tumor cells in soft agar, provide an explanation for the observed in vivo selection for the enhanced survival and outgrowth of tumor cells that express high levels of CCN2.
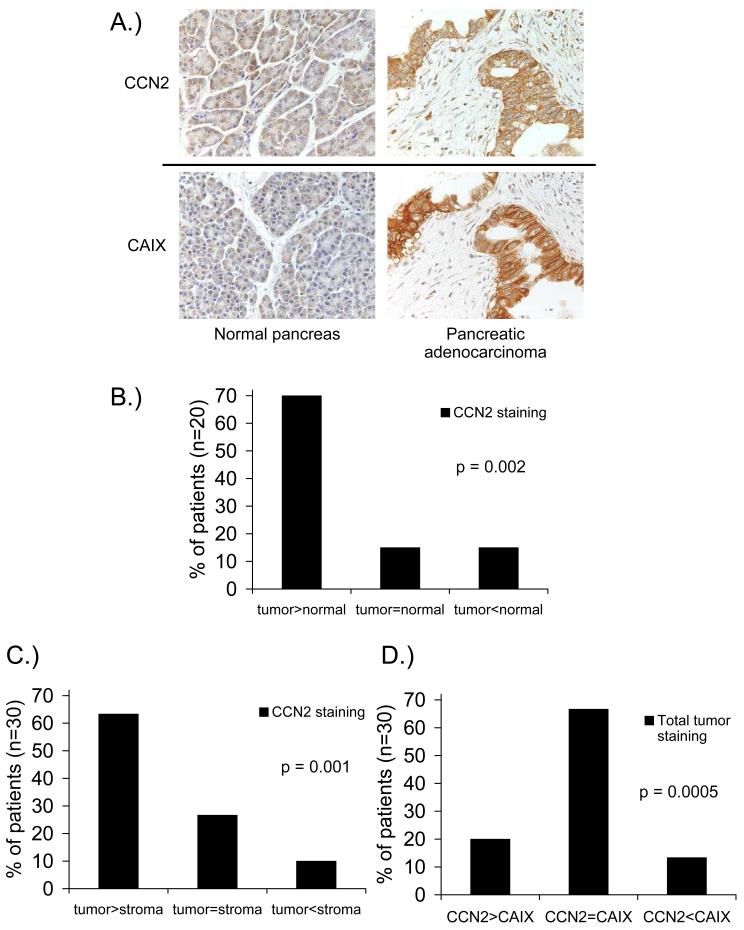
In order to put our data in context with clinical pancreatic cancer, we obtained pancreatic adenocarcinoma samples and stained them for CCN2. Pancreatic adenocarcinoma and normal pancreas tissue samples were collected from the same patient where possible (20/30 patients). Both the intensity and the degree of CCN2 staining (Figure 6A) were scored and an overall rating of CCN2 staining was determined. We found that 14/20 patients (70%) had higher CCN2 staining in pancreatic adenocarcinoma tissue compared to normal pancreatic tissue (Figure 6B), and that 19/30 patients (63%) had more intense CCN2 staining in tumor tissue compared to the stromal tissue surrounding the tumor (Figure 6C). We also compared CCN2 staining with levels of the classical endogenous hypoxia marker carbonic anhydrase-IX (CAIX) in step-sections. We found that 20/30 patients (67%) demonstrated similar overall levels of staining for both CAIX and CCN2 (Figure 6D), and there was a considerable degree of overlap between CCN2 and CAIX in individual tumor samples (Figure 6A). Taken together, these data indicate that CCN2 expression is elevated in primary pancreatic adenocarcinomas and that CCN2 is found preferentially associated with hypoxic pancreatic tumor cells rather than the surrounding stroma.
Figure 6. CCN2 is associated with tumor tissue and hypoxia in clinical pancreatic adenocarcinoma samples.
(A) Immunohistochemical staining for CCN2 and carbonic anhydrase-IX (CAIX) in paraffin-embedded samples from clinical pancreatic cancer patients.
(B) CCN2 staining in pancreatic adenocarcinoma vs normal pancreatic tissue from the same patient (n=20).
(C) CCN2 staining in pancreatic adenocarcinoma tissue vs stroma surrounding the tumor in the same section (n=30).
(D) Overall CCN2 staining and CAIX staining in step-sections of clinical pancreatic adenocarcinoma samples (n=30).
Discussion
We have identified a critical role for tumor cell-derived CCN2 in the growth of pancreatic tumors. A robust selective pressure is present in pancreatic tumors for cells that express high levels of CCN2 and this selection is driven, at least in part, by tumor hypoxia. CCN2 protects tumor cells against microenvironmental stresses such as hypoxia by inhibiting apoptosis and enhancing tumor growth. Greater than 90% knockdown of CCN2 by shRNA in clonal populations of cells was required to prevent CCN2-expressing cells from populating the tumors. It is important to note that wild-type Panc-1 cells and CCN2 shRNA-expressing clones exhibited differences in tumor growth despite being implanted in the same tissue-type, suggesting that any CCN2 derived from the surrounding stromal cells was not sufficient to affect the differential growth rates of the tumors. Similarly, immunological inhibition of CCN2 is the most robust in suppressing the growth of tumors that contain CCN2-expressing tumor cells (22, 23), indicating the role of stromal cell-derived CCN2 in pancreatic tumor growth is minimal.
CCN2 is downstream of the ras/MEK/ERK pathway (39), and activation of the ras pathway mediates basal over-expression of CCN2 in pancreatic cancer cells in a Smad4-independent manner (40). Thus activating ras mutations, which are known to occur in the vast majority of pancreatic adenocarcinomas, would induce CCN2 over-expression in the early stages of pancreatic tumor development. When combined with our data illustrating a role for CCN2 in enhancing the growth of pancreatic tumor cells in soft agar (Figure 1C-D, Figure 3C-D), the combination of an activating ras mutation with the resultant over-expression of CCN2 would lead to enhanced proliferation of pancreatic tumor cells. Interestingly, secreted CCN2 is known to directly interact with TGF-β and enhance binding to the TGF-B receptor (7), while CCN2 expression is also induced by TGF-β. These observations suggest a feedforward loop mediated by TGF-β and CCN2 resulting in further increases in CCN2 production and tumor growth. There are therefore multiple mechanisms that can increase CCN2 expression levels in pancreatic tumor cells throughout the development and growth of a solid tumor.
As tumors grow, increasing numbers of tumor cells become hypoxic and induction of CCN2 expression by hypoxia has been observed in a number of normal and neoplastic cell types. Mechanistically, hypoxic induction of CCN2 is mediated by hypoxia-inducible factor-1 (HIF-1) transcriptional activity in murine primary renal tubular epithelial cells (32), and in dermal fibroblasts from systemic sclerosis patients (33). Our data are consistent with these reports in that we observed increased CCN2 expression and secretion with HIF-1α stabilization in response to hypoxia (Figure 5B). Interestingly, HIF-2 mediated CCN2 expression has been observed in human 786-0 renal cell carcinoma cells that lack HIF-1 (34), indicating that both HIF-1 and HIF-2 can influence induction of CCN2 by hypoxia in different cell types. Hypoxia has also been shown to increase CCN2 expression in human chondrosarcoma cells by 3'-untranslated region (UTR) mediated increases in CCN2 mRNA stability during hypoxia (35). We observed modest increases in intracellular CCN2 protein levels with greater increases in secreted CCN2 protein from hypoxic tumor cells, consistent with reports of hypoxia-induced increases in CCN2 secretion from human trophoblasts with lesser increases observed in intracellular CCN2 levels (37). CCN2 is known to be efficiently secreted through the Golgi from rat hepatic stellate cells and TGF-β-stimulated human dermal fibroblasts (38), and we have observed localization of CCN2 to the Golgi in hypoxic pancreatic tumor cells (data not shown). Taken together, these data indicate that hypoxia induces increased expression and rapid secretion of CCN2 from pancreatic tumor cells, providing a further mechanism for increased CCN2 production with tumor growth.
Hypoxia also represents a microenvironmental stressor for tumor cells, and we observed increased apoptosis of pancreatic tumor cells in response to hypoxia with genetic inhibition of CCN2 (Figure 5C). These data are consistent with reports of CCN2 inhibition increasing apoptosis of human rhabdomyosarcoma cells (41), and with observations that the lungs of perinatally lethal CCN2 knockout mice contain increased levels of apoptosis compared to wild-type mice (42). CCN2 also enhances TGF-β-mediated apoptosis in human breast cancer cells (43), human aortic smooth muscle cells (44), and rat peritoneal endothelial cells (45), while CCN2 has been reported to protect murine endothelial cells against growth factor deprivation-induced apoptosis (10), and impair apoptosis of chondrocytes (46). Whether these conflicting reports are due to cell type-specific differences in CCN2 action and/or differences in CCN2 structure in these systems is an open question, and is the subject of active investigation.
Healthy human pancreatic tissue does not typically express high levels of CCN2. Significant over-expression of CCN2 mRNA has been observed in acute necrotizing pancreatitis (47) and in pancreatic cancer tissue relative to normal pancreas (48, 49), although one group observed only a modest (non-statistically significant) increase in CCN2 expression in pancreatic cancer tissues (50). CCN2 mRNA over-expression has been shown in fibroblasts, scattered acinar cells, and pancreatic tumor cells, while CCN2 protein is associated with tumor cells and fibroblasts surrounding pancreatic tumor tissue (49). We have found that scattered acinar cells and subsets of islet cells in normal pancreatic tissue can stain intensely for CCN2 (data not shown), but that overall CCN2 staining is typically low in the healthy pancreas. We observed more intense and more abundant CCN2 staining in the majority of pancreatic tumor samples when compared to normal pancreatic tissue from the same patient (Figure 6B). CCN2 also co-localizes with CAIX staining in human pancreatic tumor tissue (Figure 6A, D) and in fibroblasts adjacent to necrosis. Taken together, these data highlight the relationship between hypoxia and CCN2 expression in clinical pancreatic cancer, and support our pre-clinical findings that CCN2 derived from tumor cells is important for pancreatic tumor growth and progression.
Taken together, our data indicate that CCN2 derived from tumor cells is up-regulated by hypoxia, enhances the survival of hypoxic tumor cells, and increases tumor growth. CCN2 therefore acts as a protective factor for pancreatic tumor cells, enhancing the growth and progression of pancreatic tumors despite relatively high fractions of pancreatic tumor cells residing in hypoxic tumor regions. The selective pressure exerted by the tumor microenvironment produces tumor cells that express high levels of CCN2 which ultimately leads to enhanced tumor growth. Our data indicate a critical role for CCN2 derived from pancreatic tumor cells in the growth of pancreatic tumors and identify CCN2 as a therapeutic target for the clinical treatment of pancreatic cancer.
Supplementary Material
Acknowledgements
The authors thank Drs. Scott Welford, Tom Johnson, and Sandra Turcotte for expert technical assistance and helpful discussion. The authors also thank Drs. Barbara Bedogni, Nadja Dornhoefer, and Derek Bingham for helpful discussion.
Grant support: Supported by a grant from the National Institute of Health (AJG), the Blue Dot Fund (AK), and a post-doctoral fellowship from the Canadian Institutes of Health Research (KLB).
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Korc M. Pancreatic cancer-associated stroma production. Am J Surg. 2007;194:S84–6. doi: 10.1016/j.amjsurg.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- 4.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–10. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 5.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–4. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 6.Brigstock DR, Steffen CL, Kim GY, Vegunta RK, Diehl JR, Harding PA. Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids. Identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J Biol Chem. 1997;272:20275–82. doi: 10.1074/jbc.272.32.20275. [DOI] [PubMed] [Google Scholar]
- 7.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heng EC, Huang Y, Black SA, Jr, Trackman PC. CCN2, connective tissue growth factor, stimulates collagen deposition by gingival fibroblasts via module 3 and alpha6- and beta1 integrins. J Cell Biochem. 2006;98:409–20. doi: 10.1002/jcb.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao R, Brigstock DR. A novel integrin alpha5beta1 binding domain in module 4 of connective tissue growth factor (CCN2/CTGF) promotes adhesion and migration of activated pancreatic stellate cells. Gut. 2006;55:856–62. doi: 10.1136/gut.2005.079178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–66. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leask A, Denton CP, Abraham DJ. Insights into the molecular mechanism of chronic fibrosis: the role of connective tissue growth factor in scleroderma. J Invest Dermatol. 2004;122:1–6. doi: 10.1046/j.0022-202X.2003.22133.x. [DOI] [PubMed] [Google Scholar]
- 12.Koliopanos A, Friess H, di Mola FF, et al. Connective tissue growth factor gene expression alters tumor progression in esophageal cancer. World J Surg. 2002;26:420–7. doi: 10.1007/s00268-001-0242-x. [DOI] [PubMed] [Google Scholar]
- 13.Xie D, Yin D, Wang HJ, et al. Levels of expression of CYR61 and CTGF are prognostic for tumor progression and survival of individuals with gliomas. Clin Cancer Res. 2004;10:2072–81. doi: 10.1158/1078-0432.ccr-0659-03. [DOI] [PubMed] [Google Scholar]
- 14.Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001;61:8917–23. [PubMed] [Google Scholar]
- 15.Liu L, Li Z, Feng G, You W, Li J. Expression of connective tissue growth factor is in agreement with the expression of VEGF, VEGF-C, -D and associated with shorter survival in gastric cancer. Pathol Int. 2007;57:712–8. doi: 10.1111/j.1440-1827.2007.02162.x. [DOI] [PubMed] [Google Scholar]
- 16.Sala-Torra O, Gundacker HM, Stirewalt DL, et al. Connective tissue growth factor (CTGF) expression and outcome in adult patients with acute lymphoblastic leukemia. Blood. 2007;109:3080–3. doi: 10.1182/blood-2006-06-031096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong YF, Cheung TH, Tsao GS, et al. Genome-wide gene expression profiling of cervical cancer in Hong Kong women by oligonucleotide microarray. Int J Cancer. 2006;118:2461–9. doi: 10.1002/ijc.21660. [DOI] [PubMed] [Google Scholar]
- 18.Deng Y-Z, Chen P-P, Wang Y, et al. Connective Tissue Growth Factor Is Overexpressed in Esophageal Squamous Cell Carcinoma and Promotes Tumorigenicity through -Catenin-T-cell Factor/Lef Signaling. J Biol Chem. 2007;282:36571–81. doi: 10.1074/jbc.M704141200. [DOI] [PubMed] [Google Scholar]
- 19.Zirn B, Hartmann O, Samans B, et al. Expression profiling of Wilms tumors reveals new candidate genes for different clinical parameters. Int J Cancer. 2006;118:1954–62. doi: 10.1002/ijc.21564. [DOI] [PubMed] [Google Scholar]
- 20.Shakunaga T, Ozaki T, Ohara N, et al. Expression of connective tissue growth factor in cartilaginous tumors. Cancer. 2000;89:1466–73. [PubMed] [Google Scholar]
- 21.Chen PP, Li WJ, Wang Y, et al. Expression of Cyr61, CTGF, and WISP-1 correlates with clinical features of lung cancer. PLoS ONE. 2007;2:e534. doi: 10.1371/journal.pone.0000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dornhofer N, Spong S, Bennewith K, et al. Connective tissue growth factorspecific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2006;66:5816–27. doi: 10.1158/0008-5472.CAN-06-0081. [DOI] [PubMed] [Google Scholar]
- 23.Aikawa T, Gunn J, Spong SM, Klaus SJ, Korc M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol Cancer Ther. 2006;5:1108–16. doi: 10.1158/1535-7163.MCT-05-0516. [DOI] [PubMed] [Google Scholar]
- 24.Le QT, Kong C, Lavori PW, et al. Expression and prognostic significance of a panel of tissue hypoxia markers in head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2007;69:167–75. doi: 10.1016/j.ijrobp.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 25.Bruns CJ, Harbison MT, Kuniyasu H, Eue I, Fidler IJ. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia. 1999;1:50–62. doi: 10.1038/sj.neo.7900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graves EE, Quon A, Loo BW., Jr RT_Image: an open-source tool for investigating PET in radiation oncology. Technol Cancer Res Treat. 2007;6:111–21. doi: 10.1177/153303460700600207. [DOI] [PubMed] [Google Scholar]
- 27.Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol. 2001;54:57–79. doi: 10.1136/mp.54.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryseck RP, Macdonald-Bravo H, Mattei MG, Bravo R. Structure, mapping, and expression of fisp-12, a growth factor-inducible gene encoding a secreted cysteine-rich protein. Cell Growth Differ. 1991;2:225–33. [PubMed] [Google Scholar]
- 29.Vezeridis MP, Doremus CM, Tibbetts LM, Tzanakakis G, Jackson BT. Invasion and metastasis following orthotopic transplantation of human pancreatic cancer in the nude mouse. J Surg Oncol. 1989;40:261–5. doi: 10.1002/jso.2930400412. [DOI] [PubMed] [Google Scholar]
- 30.Rowland-Goldsmith MA, Maruyama H, Matsuda K, et al. Soluble type II transforming growth factor-beta receptor attenuates expression of metastasis-associated genes and suppresses pancreatic cancer cell metastasis. Mol Cancer Ther. 2002;1:161–7. [PubMed] [Google Scholar]
- 31.Blouw B, Song H, Tihan T, et al. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell. 2003;4:133–46. doi: 10.1016/s1535-6108(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 32.Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. Hypoxic induction of Ctgf is directly mediated by Hif-1. Am J Physiol Renal Physiol. 2004;287:F1223–32. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- 33.Hong KH, Yoo SA, Kang SS, Choi JJ, Kim WU, Cho CS. Hypoxia induces expression of connective tissue growth factor in scleroderma skin fibroblasts. Clin Exp Immunol. 2006;146:362–70. doi: 10.1111/j.1365-2249.2006.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chintalapudi MR, Markiewicz M, Kose N, et al. Cyr61/CCN1 and CTGF/CCN2 mediate the pro-angiogenic activity of VHL mutant renal carcinoma cells. Carcinogenesis. 2008;29:696–703. doi: 10.1093/carcin/bgn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondo S, Kubota S, Mukudai Y, et al. Hypoxic regulation of stability of connective tissue growth factor/CCN2 mRNA by 3′-untranslated region interacting with a cellular protein in human chondrosarcoma cells. Oncogene. 2006;25:1099–110. doi: 10.1038/sj.onc.1209129. [DOI] [PubMed] [Google Scholar]
- 36.Kireeva ML, Latinkic BV, Kolesnikova TV, et al. Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp Cell Res. 1997;233:63–77. doi: 10.1006/excr.1997.3548. [DOI] [PubMed] [Google Scholar]
- 37.Rimon E, Chen B, Shanks AL, Nelson DM, Sadovsky Y. Hypoxia in human trophoblasts stimulates the expression and secretion of connective tissue growth factor. Endocrinology. 2008;149:2952–8. doi: 10.1210/en.2007-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Segarini P, Raoufi F, Bradham D, Leask A. Connective tissue growth factor is secreted through the Golgi and is degraded in the endosome. Exp Cell Res. 2001;271:109–17. doi: 10.1006/excr.2001.5364. [DOI] [PubMed] [Google Scholar]
- 39.Pickles M, Leask A. Analysis of CCN2 promoter activity in PANC-1 cells: regulation by ras/MEK/ERK. J Cell Commun Signal. 2007;1:85–90. doi: 10.1007/s12079-007-0008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwon S, Munroe X, Crawley SC, et al. Expression of connective tissue growth factor in pancreatic cancer cell lines. Int J Oncol. 2007;31:693–703. [PubMed] [Google Scholar]
- 41.Croci S, Landuzzi L, Astolfi A, et al. Inhibition of connective tissue growth factor (CTGF/CCN2) expression decreases the survival and myogenic differentiation of human rhabdomyosarcoma cells. Cancer Res. 2004;64:1730–6. doi: 10.1158/0008-5472.can-3502-02. [DOI] [PubMed] [Google Scholar]
- 42.Baguma-Nibasheka M, Kablar B. Pulmonary hypoplasia in the connective tissue growth factor (Ctgf) null mouse. Dev Dyn. 2008;237:485–93. doi: 10.1002/dvdy.21433. [DOI] [PubMed] [Google Scholar]
- 43.Hishikawa K, Oemar BS, Tanner FC, Nakaki T, Luscher TF, Fujii T. Connective tissue growth factor induces apoptosis in human breast cancer cell line MCF-7. J Biol Chem. 1999;274:37461–6. doi: 10.1074/jbc.274.52.37461. [DOI] [PubMed] [Google Scholar]
- 44.Hishikawa K, Nakaki T, Fujii T. Connective tissue growth factor induces apoptosis via caspase 3 in cultured human aortic smooth muscle cells. Eur J Pharmacol. 2000;392:19–22. doi: 10.1016/s0014-2999(00)00115-1. [DOI] [PubMed] [Google Scholar]
- 45.Szeto CC, Chow KM, Lai KB, Szeto CY, Kwan BC, Li PK. Connective tissue growth factor is responsible for transforming growth factor-beta-induced peritoneal mesothelial cell apoptosis. Nephron Exp Nephrol. 2006;103:e166–74. doi: 10.1159/000092907. [DOI] [PubMed] [Google Scholar]
- 46.Fujisawa T, Hattori T, Ono M, et al. CCN family 2/connective tissue growth factor (CCN2/CTGF) stimulates proliferation and differentiation of auricular chondrocytes. Osteoarthritis Cartilage. 2008;16:787–95. doi: 10.1016/j.joca.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 47.di Mola FF, Friess H, Riesle E, et al. Connective tissue growth factor is involved in pancreatic repair and tissue remodeling in human and rat acute necrotizing pancreatitis. Ann Surg. 2002;235:60–7. doi: 10.1097/00000658-200201000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wenger C, Ellenrieder V, Alber B, et al. Expression and differential regulation of connective tissue growth factor in pancreatic cancer cells. Oncogene. 1999;18:1073–80. doi: 10.1038/sj.onc.1202395. [DOI] [PubMed] [Google Scholar]
- 49.Hartel M, di Mola FF, Gardini A, et al. Desmoplastic reaction influences pancreatic cancer growth behavior. World J Surg. 2004;28:818–25. doi: 10.1007/s00268-004-7147-4. [DOI] [PubMed] [Google Scholar]
- 50.Aoyagi Y, Oda T, Kinoshita T, et al. Overexpression of TGF-beta by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer. Br J Cancer. 2004;91:1316–26. doi: 10.1038/sj.bjc.6602141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.