Abstract
Objective
Epidemiological studies suggest that consumption of ω-3 polyunsaturated fatty acids (ω-3 PUFA) decreases the risk of heart failure. We assessed the effects of dietary supplementation with ω-3 PUFA from fish oil on the response of the left ventricle (LV) to arterial pressure overload.
Methods
Male Wistar rats were fed a standard chow or a ω-3 PUFA-supplemented diet. After 1 week rats underwent abdominal aortic banding or sham surgery (n=9-12/group). LV function was assessed by echocardiography after 8 weeks. In addition, we studied the effect of ω-3 PUFA on the cardioprotective adipocyte-derived hormone adiponectin, which may alter the pro-growth serine-threonine kinase Akt.
Results
Banding increased LV mass to a greater extent with the standard chow (31%) than with ω-3 PUFA (18%). LV end diastolic and systolic volumes were increased by 19% and 105% with standard chow, respectively, but were unchanged with ω-3 PUFA. The expression of adiponectin was up-regulated in adipose tissue, and the plasma adiponectin concentration was significantly elevated. Treatment with ω-3 PUFA increased total Akt protein expression in the heart, but decreased the fraction of Akt in the active phosphorylated form, and thus did not alter the amount of active phospho-Akt.
Conclusion
Dietary supplementation with ω-3 PUFA attenuated pressure overload-induced LV dysfunction, which was associated with elevated plasma adiponectin.
1. Introduction
A diet rich in ω-3 polyunsaturated fatty acids (ω-3 PUFA) reduces plasma triglycerides [1], cardiac arrhythmias and sudden death [2], and the risk of ischemic heart disease and heart failure [3, 4], and thus is recommended for optimal cardiovascular health [5]. The most common source of ω-3 PUFA is fish oil, which is high in eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). While it has been suggested that ω-3 PUFA from fish oil may be effective in preventing heart failure and left ventricular hypertrophy (LVH) [3, 6], there is little direct evidence supporting this concept [7].
The mechanisms for a potential beneficial effect of fish oil in the prevention of LVH and heart failure are unclear. While fatty acids are classically viewed as an energy substrate, they are also endogenous ligands for peroxisome proliferator-activated receptors (PPARs) and regulate the expression of genes encoding key proteins controlling multiple aspects of metabolism [8, 9]. ω -3 PUFA from fish oil, specifically EPA and DHA, are ligands for PPARα and PPARγ [10]. PPARα regulates the expression of proteins involved in fatty acid metabolism in the heart [9] and mitochondria biogenesis [11], and is suppressed in severe LVH and heart failure [9, 12, 13]. ω-3 PUFA supplementation could preserve cardiac mitochondrial function by stimulating expression of proteins involved in cardiac lipid metabolism and mitochondrial function [12-16]. In adipocytes, ω-3 PUFA from fish oil activate expression and secretion of adiponectin [17, 18], a hormone that limits LVH, remodeling and contractile dysfunction in response to pressure overload [19-21]. The effects of adiponectin in the heart and vasculature are not well understood [21-23]. The heart expresses adiponectin receptors [24], suggesting there is a direct effect of adiponectin in the heart. The mechanisms for adiponectin-induced suppression of LVH and dysfunction have been linked to activation of AMP-activated protein kinase (AMPK) [20, 21], but could also be due to inhibition of the serine-threonine kinase Akt [25]. Activation of the Akt increases protein synthesis and suppresses protein breakdown, leading to LVH26. Treatment of tumor cells with ω-3 PUFA inactivates Akt[25], and a similar effect in the heart could blunt LVH in response to arterial pressure overload.
This study examined the effects of dietary ω-3 PUFA intake from fish oil on LVH, LV remodeling and contractile dysfunction, and adiponectin expression and concentration, in a rat model of chronic pressure overload induced by abdominal aortic banding. We hypothesize that ω-3 PUFA supplementation attenuates LVH and dysfunction, and that this effect is related to increased plasma concentration of adiponectin secondary to stimulation of adiponectin expression in adipose tissue. Furthermore, we postulated that these effects are related to activation of PPARα and AMPK in the heart, and reduced Akt activation.
2. Methods
2.1. Experimental Design
Measurements were performed with investigators blinded to treatment. The animal protocol was conducted according to the guideline for the care and use of laboratory animals (NIH publication No. 85-23) and was approved by the Institutional Animal Care and Use Committee of the Case Western Reserve University. Animals were maintained on a reverse 12-hour light-dark cycle. All procedures were performed in the fed state between 3 and 6 hours from initiation of the dark phase cycle.
Five-week old male Wistar rats were fed either a standard chow or a modified standard chow containing ω-3 PUFA from fish oil. After one week on the assigned diet, rats were randomly assigned to either sham surgery or abdominal aortic banding (AAB) (n=9-12/group), and dietary treatment was continued for 9 wks. Echocardiographic assessment of LV function was performed 1-2 days before and 8 weeks post-surgery. Nine weeks after surgery, rats were weighed and anesthetized with 1.5-2.0% isoflurane, and 3 mL blood was drawn from the inferior vena cava for metabolic measurements. The LV and adipose tissue were quickly removed, weighed, freeze clamped and stored at -80°C for biochemical analysis.
2.2. Diets
All chows were custom manufactured (Research Diets Inc. New Brunswick, NJ). The standard chow was similar to typical commercial rodent chows, with 70% of total energy from carbohydrate (75% from cornstarch, 15% maltodextrin and 10% from sucrose by energy), 10% of energy from fat (78% from cocoa butter and 22% from soybean oil) and 20% protein (casein supplemented with L-cystine). ω-3 PUFA diet also derived 10% of the total energy from fat, with 4% the total energy from fish oil (Ocean Nutrition, Dartmouth, Nova Scotia, Canada; comprised of 21% EPA and 49% DHA by mass, thus EPA and DHA comprised 0.8% and 2.0% of the total energy consumption, respectively), 3.8% from cocoa butter, and 2.2% from soybean oil. The protein and carbohydrate composition of the ω-3 PUFA diet matched the standard chow.
2.3. Abdominal Aortic Banding
The rats (180-200g) were anesthetized with 2.0-2.5% isoflurane by mask. A midline abdominal incision was used to expose the suprarenal abdominal aorta. The aorta was tied with a 3-0 silk suture against a blunt needle (19G). The needle was immediately removed, leaving the aortic lumen constricted to the diameter of the needle. Sham surgery animals were subjected to the same procedure without the aortic banding.
2.4. Echocardiography
LV function was evaluated using a Sequoia C256 system (Siemens Medica) with a 15-MHz linear array transducer as previously described [26]. Briefly, rats were anesthetized with 1.5-2.0% isoflurane by mask, the chest was shaved, the animal was placed supine on a warming pad, and ECG limb electrodes were placed. 2-D guided M-mode, 2-dimensional, and Doppler echocardiographic studies of aortic and transmitral flows were performed from parasternal and foreshortened apical windows. All data were analyzed offline with software resident on the ultrasound system at the end of the study as was described previously [26].
2.5. Metabolic measurements
Plasma free fatty acid and triglyceride concentrations were measured using enzymatic spectrophotometric assays (Wako and Sigma, respectively). Blood glucose concentration was also measured by enzymatic spectrophotometric assays from perchloric acid deproteinized whole blood samples (Stannbio laboratories). Serum levels of leptin and plasma concentration of adiponectin and insulin were measured by ELISA kit (ALPCO Diagnostics, Salem, NH). Myocardial activity of medium chain acyl-CoA dehydrogenase (MCAD) and citrate synthase was measured spectrophotometrically as previously described [27].
2.6. mRNA measurement
RNA isolation from rat heart and adipose tissue
Rat heart tissue was disrupted and homogenized by shaking with 5mm stainless-steel bead for 3min at 30sec-1 (Mixermill 300; Qiagen, Valencia, CA), followed by RNA isolation (RNeasy Mini Kit, Qiagen; Valencia, CA). On column DNase digestion was performed using RNase-free DNase set (Qiagen). RNA samples were eluted in 50μL of nuclease-free water and stored at -80°C.
cDNA preparation
RNA (1μg) was mixed with 2μL Oligo(dT)16 (Applied Biosystems; Foster City, CA) and 0.5μL random primers (Invitrogen), and brought to 15μL total volume. Sample/primer mix was heated to 70°C for 10min, then placed immediately on ice for 2min, followed by the addition of the RT reaction mix containing 5× buffer (5μL) & 0.1M DTT (2.5μL) (Superscript II RT, Invitrogen), 10mM dNTP (1.25μL; Invitrogen), and RNase inhibitor (0.25μL; Applied Biosystems). Reaction mix was incubated for 2min at 42°C, 1μL reverse transcriptase added, and incubation continued at 42°C for 1h. Reaction mix was then incubated at 70°C for 10min, and placed on ice for 2min. The resulting cDNA samples were stored at -20°C.
RT-PCR
Quantitative RT-PCR was performed using an ABI 7900 and the following protocol: 2 min at 50°C, 10 min at 95°C, 40 cycles at 95°C for 15 s, and 1 min at 60°C. Each reaction was 25μL, consisting of 1.0μL cDNA sample, 1.25μL TaqMan Gene Expression Assay, 12.5μL 2xTaqMan PCR master mix, and 10.25μL nuclease-free water. To control for sample-to-sample differences in RNA concentration, the mRNA level for cyclophilin A was quantitatively measured in each sample. PCR was performed for each of the following genes, using TaqMan Gene Expression Assays from Applied Biosystems: Adiponectin (Rn00595250_m1); Adiponectin R1 (Rn01483784_m1); Adiponectin R2 (Rn01463177_m1); PPPARa (Rn00566193_m1); CPT1β (Rn00566242_m1); UPC3 (Rn00565874_m1); Citrate Synthase (Rn00756225_m1); MCAD (Rn00566390_m1); PDK4 (Rn00585577_m1), cyclophilin A (Rn00690933_m1). mRNA was normalized to fold increase to the standard chow sham group.
2.7. Western blot analysis
Protein was extracted from frozen LV tissue [13], separated by electrophoresis in 10% SDS-PAGE gels, transferred onto a nitrocellulose membrane, and incubated with specific antibodies to either phospho-AMPK (Thr172 of α subunit) or phospho-Akt (Ser473) (all at 1:1000, from Cell Signaling Technology, Inc.), adiponectin receptors 1 and 2 (1:500, from Alpha Diagnositcs, Inc.). Fluorescence-conjugated secondary antibodies (IRDye 680/800, 1:5000; LI-COR Bioscience) were used for incubation before the membranes were scanned with Odyssey® infrared imaging system (LI-COR Bioscience). Digitized image was analyzed with Odyssey® software. Membranes were then stripped (Pierce Restore® stripping buffer) and re-probed for total-AMPK and Akt (1:1000; Cell Signaling Technology, Inc).
2.8. Statistical Analysis
Comparisons were made using two-way ANOVA, followed as appropriate by post hoc Bonferroni-corrected t tests. Mean values are presented ± S.E.M.
3. Results
3.1. LV Mass and Function
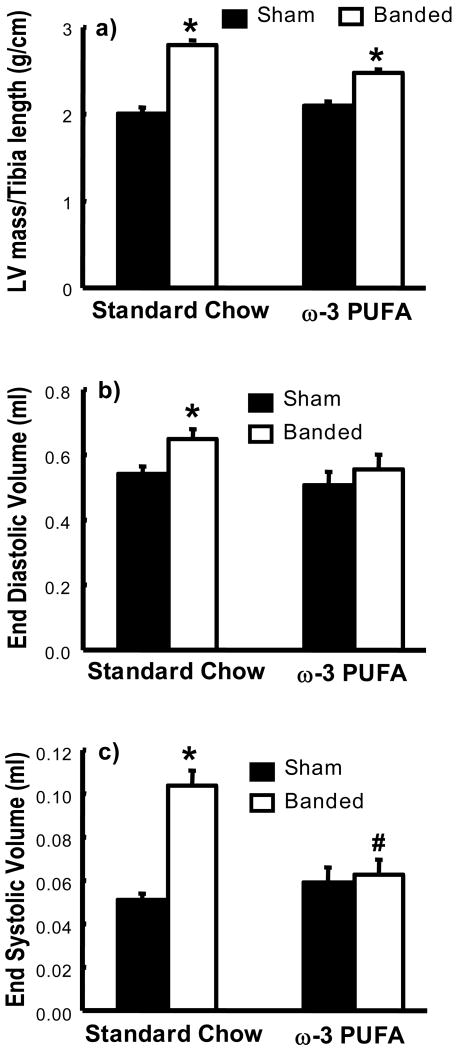
There was no significant a difference in the preliminary echocardiography measurements or body mass (data not shown), and body mass was not different among groups at the end of the study (Table 1). At 8 weeks abdominal aortic banding (AAB) increased LV mass/tibia length in both groups; however the increase was greater with the standard chow (31%) than with the ω-3 PUFA diet (18%) (Figure 1). With standard chow diet there was significant LV remodeling and systolic dysfunction with AAB compared to sham, as seen in the increase in end diastolic and systolic volumes and a reduction in ejection fraction., which were attenuated by the ω-3 PUFA diet (Figure 1 and Table 2).
Table 1.
Heart, body and adipose tissue masses.
| Standard Chow Sham (n=9) |
Standard Chow Banded (n=10) |
ω-3 PUFA Sham (n=11) |
ω-3 PUFA Banded (n=12) |
|
|---|---|---|---|---|
| Pre-surgery body mass (g) | 183±3 | 185±2 | 188±3 | 188±2 |
| Terminal body mass (g) | 476±16 | 500±11 | 511±11 | 524±13 |
| Tibia length (cm) | 4.43±0.04 | 4.51±0.05 | 4.44±0.04 | 4.52±0.07 |
| LV mass/body mass (g/cm) (P=0.068) | 2.14±0.02 | 2.80±0.01* | 2.26±0.06 | 2.67±0.06*# |
| RV mass/tibia length (g/cm) | 0.57±0.01 | 0.64±0.03 | 0.60±0.03 | 0.64±0.02 |
| Biventricular mass/tibia length (g/cm) (P=0.085) | 2.71±0.09 | 3.45±0.08* | 2.87±0.03 | 3.32±0.08* |
| Epididymal adipose mass (g) (PD=0.002) | 9.2±0.9 | 9.7±0.8 | 7.1±0.6# | 7.2±0.4# |
| Visceral adipose mass (g) | 16.6±1.3 | 17.1±1.3 | 17.0±1.2 | 18.6±1.1 |
| Epididymal adipose mass/body mass (g/kg) (PD<0.001) | 19.1±1.2 | 19.2±1.4 | 13.7±1.0# | 13.7±0.8# |
| Visceral adipose mass/body mass (g/kg) | 34.6±2.0 | 33.7±2.0 | 33.2±2.1 | 35.5±2.0 |
Data are the mean±SEM;
p< 0.05 vs. respective sham;
p<0.05 vs. standard chow diet.
P, interaction between surgery groups (sham vs. banded) and diet (standard chow vs. ω-3 PUFA)
PD, interaction between standard chow vs. ω-3 PUFA diet.
Figure 1.
LV mass/tibia length ratio (a) and echocardiograpgic assessment of LV end diastolic volume (b), and end systolic volume (c). * p< 0.05 vs. respective sham (n=9-12/group). Significant interactions between surgery group (sham vs. banded) and diet (standard chow vs. ω-3 PUFA) were observed for LV mass/tibia length ratio (P=0.045) and end systolic volume (P<0.001). Additionally, there were significant interactions between sham and banding for end diastolic volume (PG=0.032).
Table 2.
Echocardiography results.
| Standard Chow Sham (n=9) |
Standard Chow Banded (n=10) |
ω-3 PUFA Sham (n=11) |
ω-3 PUFA Banded (n=12) |
|
|---|---|---|---|---|
| Velocity of circumferential shortening (1/s) (P=0.007) | 9.09±0.17 | 7.31±0.29* | 8.53±0.26 | 8.37±0.36# |
| Ejection fraction (%) (P<0.001) | 90.6±0.5 | 83.6±1.3* | 88.2±0.9 | 88.8±0.8# |
| Anterior wall thickness (mm) (P<0.001) | 1.88±0.04 | 2.24±0.03* | 1.92±0.04 | 2.00±0.03# |
| Posterior wall thickness (mm) (P<0.001) | 1.90±0.02 | 3.34±0.05* | 1.93±0.03 | 2.05±0.04*# |
| Relative wall thickness (mm) (P=0.078) | 0.48±0.01 | 0.55±0.02* | 0.50±0.02 | 0.52±0.02 |
Data are the mean±SEM;
p< 0.05 vs. respective sham;
p<0.05 vs. standard chow diet.
P, interaction between surgery group (sham vs. banded) and diet (standard chow vs. ω-3 PUFA)
3.2. Metabolic and Endocrine Results
The ω-3 PUFA diet reduced plasma free fatty acid and triglyceride concentrations, but had no effect on glucose and insulin concentrations (Table 3).
Table 3.
Metabolic measurement and activities for enzymes.
| Metabolic Parameter | Standard Chow Sham (n=9) |
Standard Chow Banded (n=10) |
ω-3 PUFA Sham (n=11) |
ω-3 PUFA Banded (n=12) |
|---|---|---|---|---|
| Plasma Free Fatty Acids (mmol/L) (PD<0.001) | 0.31±0.03 | 0.30±0.02 | 0.23±0.02# | 0.20±0.01# |
| Plasma Trigliceryde (mg/mL) (PD<0.001) | 1.32±0.33 | 1.12±0.48 | 0.73±0.28# | 0.75±0.25# |
| Glucose (μmol/mL) | 4.73±0.15 | 4.29±0.27 | 4.25±0.24 | 4.19±0.15 |
| Plasma Insulin (ng/mL) | 1.27±0.19 | 1.65±0.07 | 1.48±0.31 | 1.57±0.14 |
| Enzyme activities | ||||
| MCAD activity (μmol min-1 g-1) (PD=0.03) | 5.59±0.23 | 5.59±0.28 | 6.72±0.41# | 5.85±0.25 |
| Citrate Synthase (μmol min-1 g-1) | 109.2±6.1 | 106.1±5.1 | 107.0±6.5 | 109.0±4.6 |
| MCAD/CS activity | 0.052±0.003 | 0.054±0.004 | 0.064±0.004 | 0.054±0.002 |
Data are the mean±SEM;
p<0.05 vs. standard chow diet.
PD, interaction between standard chow vs. ω-3 PUFA diet.
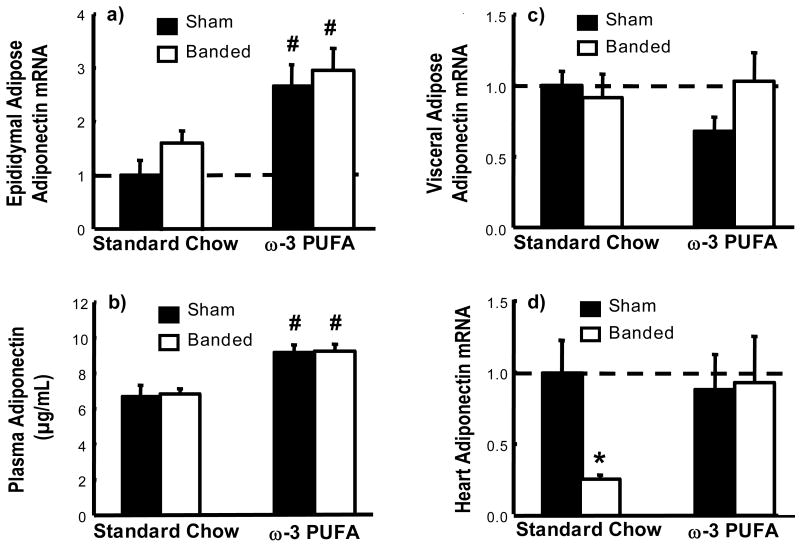
The mRNA for adiponectin was significantly increased in epididymal adipose in both sham and ABB groups with the ω-3 PUFA diet, which corresponded with a significant increase in plasma adiponectin concentration (Figure 2a-b). In contrast to epididymal adipose, there were no differences in visceral adipose for adiponectin mRNA (Figure 2c). LV adiponectin mRNA content was approximately 1/1500th of that found in fat when normalized to cyclophilin A, and was reduced by 80% by AAB in standard chow, which was attenuated in the ω-3 PUFA group (Figure 2d). Epididymal adipose mass was reduced by ∼25% in both sham and AAB groups on ω-3 PUFA compared to standard chow, but visceral adipose mass was unchanged (Table 1).
Figure 2.
Plasma adiponectin concentration (b) and adiponectin mRNA expression in epididymal adipose (a), visceral adipose (c) and heart (d) expressed as a fraction of the sham standard chow group; and. * p< 0.05 vs. respective sham; # p<0.05 vs. standard chow diet (n=9-12/group). Significant interactions between standard chow vs. ω-3 PUFA diet were observed for plasma adiponectin concentration (PD<0.001) and adiponectin mRNA expression in epididymal adipose (PD<0.001).
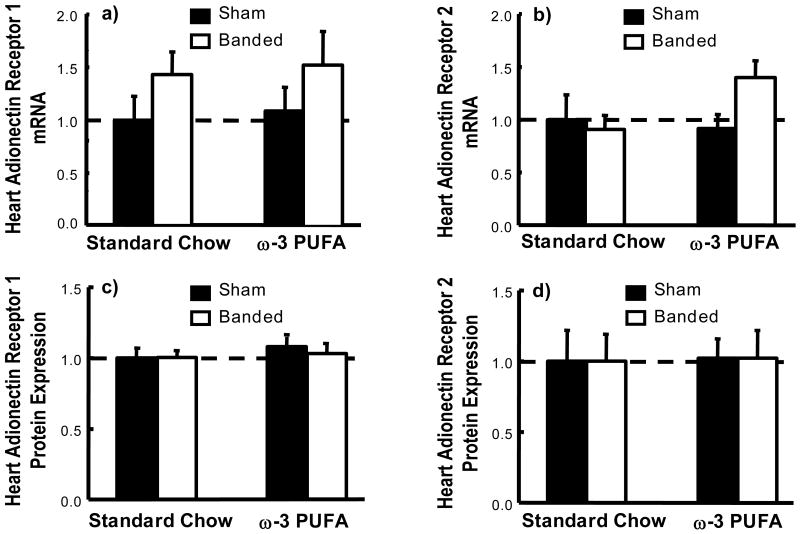
The mRNA and protein expression of adiponectin receptors R1 and R2 were unchanged in the heart (Figure 3).
Figure 3.
Cardiac mRNA (a-b) and protein (c-d) expression of adiponectin receptors R1 and R2 showed as a fraction of the sham standard chow group.
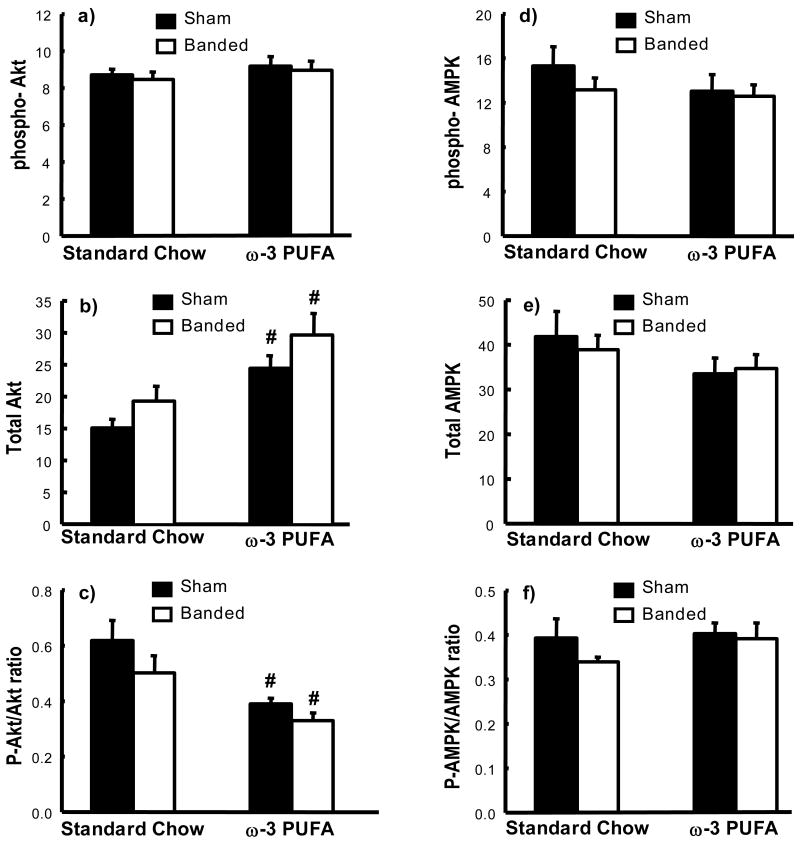
The amount of phospho-Akt in the heart, as assessed by western blot, was not different among groups, however treatment with ω-3 PUFA caused a significant increase in total Akt levels in the heart in both sham and AAB animals, resulting in a decrease in the faction of Akt in the active phosphorylated form (Figure 4). There were no differences in total or phosphorylated AMPK, or the ratio of phosphoryalated to total AMPK (Figure 4).
Figure 4.
Summary of western blot densitometry of total and phosphorylated Akt and AMPK (in arbitrary units). Phosphorylated Akt (a); total Akt (b); the ratio of phosphorylated to total Akt (c); phosphorylated AMPK (d); total AMPK (e); and the ratio of phosphorylated to total AMPK (f) # p<0.05 vs. standard chow diet (n=9-12/group). Significant interactions between standard chow vs. ω-3 PUFA diet were observed for total Akt (PD<0.001) and ratio of phosphorylated to total Akt (PD<0.001).
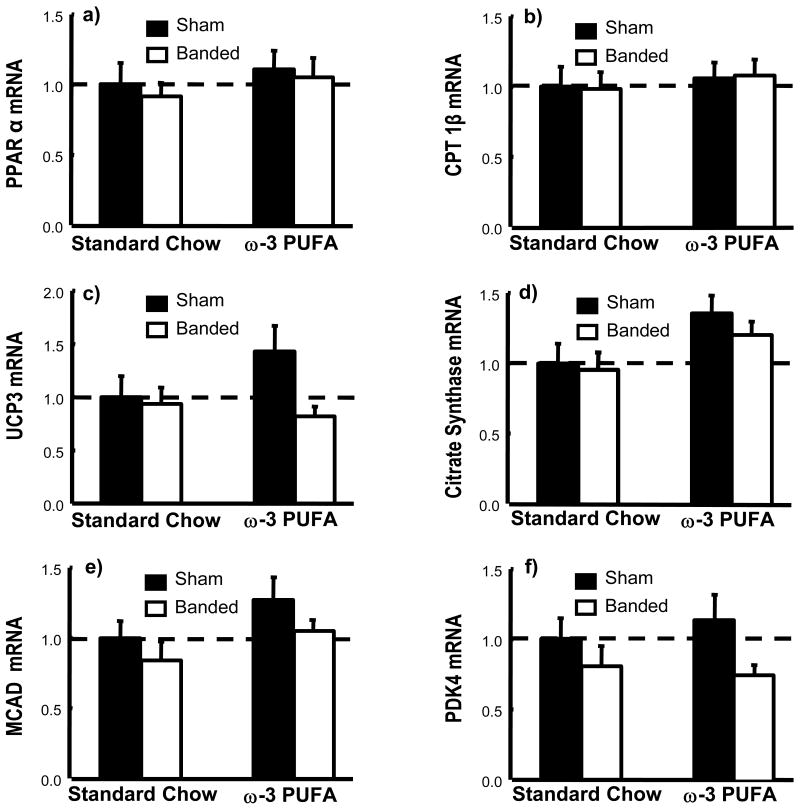
The mRNA for PPARα and PPARα-regulated genes were similar among groups (Figure 5). The activities of citrate synthase and MCAD were similar among groups except for significantly higher activity of MCAD in the sham ω-3 PUFA group (Table 3).
Figure 5.
mRNA for PPARα and PPARα-regulated genes in LV myocardium expressed as a fraction of the sham standard chow group. Data are the mean±SEM; n=9-12.
4. Discussion
We show that dietary supplementation with ω-3 PUFA attenuated pressure overload induced LVH, LV remodeling and contractile dysfunction. The beneficial effects of ω-3 PUFA were associated with increased expression of adiponectin in adipose tissue and elevated plasma adiponectin concentration. This suggests the novel concept that dietary supplementation with ω-3 PUFA attenuates LVH and cardiac dysfunction in hypertension, and that this effect is partially mediated through activation of adiponectin expression in adipose tissue.
The moderation of LVH and contractile dysfunction with ω-3 PUFA was not associated with activation of PPARα in heart; however the 2-fold increase in the mRNA for adiponectin in epididymal adipose is consistent with activation of PPARγ. Previously studies observed a similar response with a high fat diet of marine origin [17, 18]. However, in the present investigation we supplemented a low fat diet with purified fish oil containing a high concentration of EPA and DHA, which is similar to clinically-prescribed ω-3 PUFA supplements. Both EPA and DHA are potent activators of PPARα in cell culture [28]; however the mRNA for PPARα-regulated genes failed to increase in the present study. While these results clearly suggest that ω-3 PUFA supplementation does not increase expression of PPARα regulated genes in the normal heart or during moderate LVH, further studies are needed to determine if dietary ω-3 PUFA supplementation can prevent the de-activation of PPARα that occurs in advanced LVH and heart failure [9, 12].
Previous studies in adiponectin-/- mice found enhanced LVH and dysfunction following aortic banding compared to wild-type animals [19, 20], and that this can be rescued by adenovirus-mediated adiponectin supplementation [20]. In the present study we show that the increase in plasma adiponectin above normal levels induced by ω-3 PUFA is associated with attenuation of LVH and cardiac dysfunction without altered cardiac expression of adiponectin or adiponectin receptors. ω-3 PUFA did not affect the amount of phospho-Akt and AMPK, although the total Akt content was increased. Studies in adiponectin-/- mice did not assess Akt or AMPK activation in the heart [19, 20]; however, studies in isolated neonatal rat ventricular myocytes showed that adiponectin increased AMPK phosphorylation but not Akt [20]. On the other hand, adiponectin inhibited phosphorylation of Akt in breast carcinoma cells [25]. Additional work is needed to clarify the cardioprotective mechanism(s) provided by ω-3 PUFA.
Dietary supplementation with ω-3 PUFA has multiple effect on cardiac biochemistry and function that were not evaluated in the present study. Takahashi et al observed that supplementation with fish oil attenuated hypertrophic cardiomyopathy in mice with carnitine deficiency [29]. Fish oil alters the diacylglycerol composition in the heart and prevented activation of protein kinase C (PKC) isoforms α, β2, and ε by reducing their translocation from the cytosol to the plasma membrane. Since chronic PKC activation has been linked to LVH and heart failure [30], the consumption of ω-3 PUFA could reduce the risk for heart failure [29] by suppressing PKC activity. In addition, ω-3 PUFA supplementation affect membrane composition and ion permeability, and could potential improve Ca2+ uptake into the sarcoplasmic reticulum and optimize the ability of the mitochondria to generate ATP [31, 32]. Additional studies are needed to fully elucidate the mechanisms responsible for the improved cardiac response to pressure overload with ω-3 PUFA supplementation.
It is important to note that the results of the present investigation are limited by the absence of measurement of the cross sectional area and pressure gradient across the aortic stenosis. Future studies should include careful ultrasound measurement of the cross sectional area and flow velocity at the site of the aortic band. In addition, measurements of proximal aortic and peripheral blood pressure should be made. While there is no rationale for a ω-3 PUFA-induced reduction in aortic diameter or pressure in this experimental model, our findings would be strengthened by a thorough evaluation of these parameters.
In summary, the present study demonstrates that dietary supplementation with ω-3 PUFA attenuated pressure overload-induced LVH, remodeling, and contractile dysfunction. The protective effect of ω-3 PUFA was associated with up-regulation of adiponectin expression in adipose tissue and elevated plasma adiponectin.
Acknowledgments
This research was supported by NIH grant HL074237. The authors thank Drs Margaret Chandler and Isidore Okere, and Cody Rutledge and Jenny Cui for assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial disclosures.
Reference List
- 1.Weber P, Raederstorff D. Triglyceride-lowering effect of omega-3 LC-polyunsaturated fatty acids--a review. Nutr Metab Cardiovasc Dis. 2000;10:28–37. [PubMed] [Google Scholar]
- 2.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107:2646–52. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–21. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 4.Schacky C, Harris WS. Cardiovascular benefits of omega-3 fatty acids. Cardiovasc Res. 2007;73:310–5. doi: 10.1016/j.cardiores.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Gottdiener JS, Siscovick DS. Intake of tuna or other broiled or baked fish versus fried fish and cardiac structure, function, and hemodynamics. Am J Cardiol. 2006;97:216–22. doi: 10.1016/j.amjcard.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Stanley WC, Recchia FA, Okere IC. Metabolic therapies for heart disease: fish for prevention and treatment of cardiac failure? Cardiovasc Res. 2005;68:175–7. doi: 10.1016/j.cardiores.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–35. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 9.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–78. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- 10.Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 11.Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–17. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 13.Lei B, Lionetti V, Young ME, Chandler MP, D' Agostino C, Kang E, et al. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol. 2004;36:567–76. doi: 10.1016/j.yjmcc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, et al. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–12. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 15.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–42. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 16.Davila-Roman VG, Vedala G, Herrero P, de las FL, Rogers JG, Kelly DP, et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271–7. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 17.Neschen S, Morino K, Rossbacher JC, Pongratz RL, Cline GW, Sono S, et al. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes. 2006;55:924–8. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 18.Flachs P, Mohamed-Ali V, Horakova O, Rossmeisl M, Hosseinzadeh-Attar MJ, Hensler M, et al. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia. 2006;49:394–7. doi: 10.1007/s00125-005-0053-y. [DOI] [PubMed] [Google Scholar]
- 19.Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, et al. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67:705–13. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–9. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007;74:11–8. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han SH, Quon MJ, Kim JA, Koh KK. Adiponectin and cardiovascular disease: response to therapeutic interventions. J Am Coll Cardiol. 2007;49:531–8. doi: 10.1016/j.jacc.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 23.Hug C, Lodish HF. The role of the adipocyte hormone adiponectin in cardiovascular disease. Curr Opin Pharmacol. 2005;5:129–34. doi: 10.1016/j.coph.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, et al. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006;66:11462–70. doi: 10.1158/0008-5472.CAN-06-1969. [DOI] [PubMed] [Google Scholar]
- 26.Morgan EE, Faulx MD, McElfresh TA, Kung TA, Zawaneh MS, Stanley WC, et al. Validation of echocardiographic methods for assessing left ventricular dysfunction in rats with myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H2049–H2053. doi: 10.1152/ajpheart.00393.2004. [DOI] [PubMed] [Google Scholar]
- 27.Panchal AR, Stanley WC, Kerner J, Sabbah HN. Beta-receptor blockade decreases carnitine palmitoyl transferase I activity in dogs with heart failure. J Card Fail. 1998;4:121–6. doi: 10.1016/s1071-9164(98)90252-4. [DOI] [PubMed] [Google Scholar]
- 28.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997;94:4318–23. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi R, Okumura K, Asai T, Hirai T, Murakami H, Murakami R, et al. Dietary fish oil attenuates cardiac hypertrophy in lipotoxic cardiomyopathy due to systemic carnitine deficiency. Cardiovasc Res. 2005;68:213–23. doi: 10.1016/j.cardiores.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Bayer AL, Heidkamp MC, Patel N, Porter M, Engman S, Samarel AM. Alterations in protein kinase C isoenzyme expression and autophosphorylation during the progression of pressure overload-induced left ventricular hypertrophy. Mol Cell Biochem. 2003;242:145–52. [PubMed] [Google Scholar]
- 31.Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation. 2002;105:2303–8. doi: 10.1161/01.cir.0000015604.88808.74. [DOI] [PubMed] [Google Scholar]
- 32.Pepe S. Effect of dietary polyunsaturated fatty acids on age-related changes in cardiac mitochondrial membranes. Exp Gerontol. 2005;40:369–76. doi: 10.1016/j.exger.2005.03.005. [DOI] [PubMed] [Google Scholar]