Abstract
Objective
We have previously demonstrated that the transcription factor, HIF-1, promoted the onset of autophagy in chondrocytes. The overall goal of this study was to test the hypothesis that another HIF family transcription factor, HIF-2, modulated the induction of autophagy by chondrocytes.
Methods
Expression of HIF-1, HIF-2 and LC-3 were visualized by immunohistochemistry. Suppression of HIF-2 was achieved using siRNA technology. Autophagic flux, lysosomal activity and ultrastructural analysis was assessed in chondrocytes in cell culture.
Results
HIF-2 was expressed abundantly by cells in mouse and human articular cartilage and the cartilage of mineralizing vertebrae. Protein levels were reduced in articular cartilage of older animals, the end plate cartilage and in human osteoarthritic chondrocytes. HIF-2 was robustly expressed in the pre-hypertrophic cells of the mouse growth cartilage. When HIF-2α was silenced, ROS generation was elevated with a concomitant decrease in catalase and superoxide dismutase activity. Suppression of HIF-2 was associated with decreased Akt-1 and mTOR activities, reduced Bcl-XL expression and a robust autophagic response even under nutrient replete conditions; in these silenced chondrocytes, HIF-1 expression was elevated. Decreased HIF-2 expression was associated with autophagy in osteoarthritic tissue and aging cartilages. Finally, we examined the autophagic response of chondrocytes in HIF-2α knockout mouse growth plate. There was elevated autophagic response throughout the plate.
Conclusions
Based on the above mentioned observations, it is concluded that HIF-2 is a potent regulator of autophagy in maturing chondrocytes. Our data suggests that this protein acts as a brake to the autophagy accelerator function of HIF-1.
Keywords: HIF-2, autophagy, chondrocyte, ROS, HIF-1
INTRODUCTION
Among the skeletal elements of the body, the cartilages comprise a group of highly diverse tissues that perform a wide array of functions including support, growth and locomotion. Articular cartilage is present at the ends of long bones where it serves as a load bearing surface. Although cell distribution within the tissue is sparse, the highly glycosylated proteoglycan molecules of the extracellular matrix bind water, and by rapidly changing the cartilage hydration state, accommodate biomechanical forces. The network of orientated collagen fibers form a network that endows the tissue with much of its mechanical strength. During aging and progression of the osteoarthritic disease, there is a net loss of matrix macromolecules (in particular, the proteoglycan aggrecan), disruption of tissue architecture and a concomitant limitation in joint function (1).
In the axial and appendicular skeleton, much of bone growth is regulated by the activity of chondrocytes residing in a specialized transient cartilage, the epiphyseal growth plate. The maturing cells in this cartilage undergo a series of phenotypic changes which include secretion of a unique sets of proteins into the avascular extracellular matrix, up-regulation of alkaline phosphatase and the release and subsequent mineralization of matrix vesicles (2). Moreover, we have recently shown that prior to deletion from the plate, the mature hypertrophic chondrocyte becomes glycolytic and undergoes functional and immunohistochemical changes that are characteristic of autophagy (3). We have recently demonstrated that HIF-1, a transcription factor that responds to the tissue oxemic state, promotes chondrocyte autophagy (4). We have suggested that autophagy is an intermediate step in the chondrocyte life cycle that permits the cells to assume a mature phenotype, prior to elimination by apoptosis (4, 5). A second HIF isoform, HIF-2, has also been shown to be present in cartilage (6). In contrast to HIF-1, which serves to metabolically adapt chondrocytes to their microenvironment and sensitizes them to apoptogens, HIF-2 is cytoprotective. Thus, upregulation of HIF-2 lowers intracellular ROS levels by promoting the activities of the dismutating proteins, catalase and superoxide dismutase (7). In addition, the observation that HIF-2 knockout animals are small suggests that there may be an increased rate of chondrocyte apoptosis that serves to impede normal long bone growth.
The overall goal of this study was to test the hypothesis that HIF-2 modulated the induction of autophagy by chondrocytes. We show that HIF-2α was expressed by cells in a number of cartilages. Furthermore, its expression is decreased with maturation, aging and onset of disease. Since in cartilage, a decrease in HIF-2 expression is accompanied by an increase in HIF-1 expression, it lends support to the view that the activities of these two isoforms can be visualized as opposing arms of a rheostat that functions to modulate autophagic activity, thereby allowing chondrocytes to complete their life cycle.
MATERIALS AND METHODS
Reagents
The following antibodies were used in this study: HIF-2α (cat #NB 100–122) and Beclin-1 were from Novus Biologicals (Littleton, CO); LC-3 was from Abgent (San Diego, CA); Tubulin, Lamin A/C, Bcl-XL and Bcl-2 were from Santa Cruz Biotechnology (Santa Cruz, CA); HIF-1α was from R&D labs (Minneapolis, MN); Akt-1, p-Akt-1, mTOR, p-mTOR, S-6-K and p-S-6-K were from Cell Signaling (Danvers, MA). Cell culture reagents were obtained from Fisher Scientific (Malvern, PA). Transfection reagents were bought from Invitrogen (Carlsbad, CA). Mammalian Protein Extraction Reagent (M-PER), protein A/G sepharose beads and HRP secondary antibody was purchased from Pierce (Rockford, IL). Alexafluor 594-labeled and fluorescein-labeled secondary antibodies (Southern Biotechnology, Birmingham, AL) were used in the immunohistochemical studies. Reagents for Western blotting were from Bio-Rad, (Hercules, CA). All other reagents were from Sigma-Aldrich (St. Louis, MO).
Cell Culture
N1511 mouse chondrocytes and derived cell lines were maintained in culture at 37°C, in 5%, CO2, 95% air in α-MEM medium. Details of the cell culture system have been described in an earlier report (7). For hypoxia studies, chondrocytes were maintained at 2% O2 in an inVIVO2 Hypoxia Workstation (Ruskinn, Cincinnati, OH). In some studies, cells were treated with bafilomycin (LC laboratories, Woburn, MA) at 200 nM for 4 h.
In vivo immunolocalization of HIF-2α, LC-3 and HIF-1α in cartilage
Expression of HIF-2α, LC-3 or HIF-1α was assessed in normal and osteoarthritic human proximal femoral cartilage obtained from the NDRI tissue bank; in the distal tibial and proximal femoral cartilages of new born and 18 and 24 month mice; in mouse neonatal mineralizing vertebral cartilage and intervertebral end plate cartilage; and the new born mouse femoral epiphyseal growth plate. Mice were sacrificed in accordance with ethical guidelines approved by the IACUC of Thomas Jefferson University. Articular, end plate and epiphyseal cartilage was demineralized with EDTA, paraffin-embedded, serially sectioned (5 μm), permeabilized with proteinase K (10 mg/mL), and fixed in 4.0% (w/v) paraformaldehyde. Next, sections were treated with antibodies to HIF-2α, LC-3 or HIF-1α at a dilution of 1:50 v/v. Following treatment with the primary antibody, sections were treated with Alexafluor 594 labeled secondary antibodies, counterstained with DAPI, and visualized by fluorescent microscopy.
Immunohistochemical localization of LC-3 in chondrocytes in culture
LC-3 expression in N1511 chondrocytes was assessed by immunohistochemistry. After washing in PBS, cells were fixed with 4% (v/v) paraformaldehyde [pH 8.0] for 10 min. Following permeabilization with 0.5% (v/v) Triton-X 100, antigenic sites were blocked in 10% (v/v) calf serum. The cells were incubated with LC-3 antibody overnight at 4° C. Subsequently, the cells were incubated with a fluorescein labeled secondary antibody. Proteins were visualized by confocal microscopy (Olympus Fluoview, Japan).
Electron microscopic evaluation of autophagy
Isolated cells were fixed in 2% (v/v) paraformaldehyde, 0.1% (v/v) glutaraldehyde in 0.1 M sodium cacodylate for 2 h, postfixed with 1% (w/v) OsO4 for 2 h and finally stained for 1 h in 2% (w/v) aqueous uranyl acetate. The samples were then washed, dehydrated with graded alcohol, and embedded in Epon-Araldite resin. Ultrathin sections were cut on a Leica ultramicrotome, counterstained with 0.3% (w/v) lead citrate and examined at 60 kV using a JEOL 1230 electron microscope.
Detection of lysosomal activity
Lysosomal activity was assessed using the Lysotracker assay. Cells were serum-starved for 4 h and then incubated with LysoTracker Red for 1 h at 30° C. Lysosomal activity was assessed using confocal microscopy.
Western blot analysis
Cells were lysed with MPER. 100 μg of protein was then loaded onto 4–20% Tris gradient gels. Following transfer to PVDF membranes and blocking, blots were treated overnight with the appropriate primary antibody. Membranes were then washed and treated with HRP labeled secondary antibody. Blots were visualized with Lumigen TMA-6 (Amersham Biosciences, Piscataway, NJ).
siRNA mediated suppression of HIF-2α
An siRNA construction kit (pSilencer, Ambion, Austin, TX) was utilized for silencing. The following phosphorylated oligonucleotides were used (Forward [F], Reverse [R]):
F: gatccggagacggaggtcttctatttcaagagaatagaagacctccgtctccttttttggaaa
R: agcttttccaaaaaaggacacggaggtcttctattctcttgaaatagaagacctccgtctccg
Permanent cell lines were generated by clonal selection using 800 μg/mL of hygromycin B. Cell line with backbone vector containing scrambled sequences served as controls (pSHH).
Measurement of chondrocyte ROS levels
Following culture, cells were treated with dihydroethidium (50μM) for 30 min. The cells were lysed with 0.1% (v/v) Triton-X100 in dH2O, and fluorescence was determined at 485 nm and 595 nm.
Suppression of ROS
Cellular ROS generation was blocked using 2-,3-tert-butyl-4-hydroxy-anisole (BHA) (Supelco, Bellefonte, PA) at a concentration of 100 μM for 1 h.
Measurement of chondrocyte catalase and SOD activities
Catalase activity was measured using the Fluoro Catalase kit (Cell Technologies, Mountain View, CA). Samples were treated with 20 μM H2O2 for 1 h and then treated with the detection reagent. Fluorescence was determined at 540 and 590 nm. SOD activity was measured using a kit from Cell Technologies (Mountain View, CA) according to the manufacturer’s instructions.
Immunoprecipitation of Bcl-2 - Beclin-1 complex
To detect Bcl-2 - Beclin complexes cells were serum-starved for 4 h and whole cell extracts were prepared as described previously (4, 5). Cell lysates (150 μg) were pre-cleared using 20 μL of protein A/G Sepharose beads and 10 μL of rabbit antiserum for 1 h at 4°C. The supernatant was then incubated with Beclin-1 antibody and protein A/G Sepharose beads overnight at 4°C. The beads were then washed in lysis buffer. Proteins were then resolved by SDS-PAGE followed by Western blot analysis for Bcl-2 and Beclin-1.
Immunohistochemical analysis of HIF-2α knockout mouse growth plate
Immunohistochemistry for HIF-1α, HIF-2α and LC-3 expression were performed on sections of the epiphyseal growth plate of a HIF-2α knockout mice that was generated as described earlier (7).
Statistical analysis
All measurements were performed in triplicate; data is presented as mean ± standard deviation. Differences between groups were analyzed by the Student’s t-test; *P < 0.05.
RESULTS
HIF-2 expression in cartilage
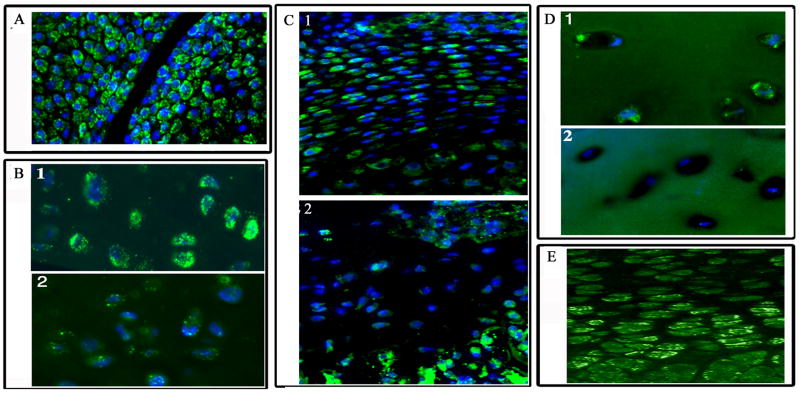
Cartilage tissue sections were immunostained for HIF-2α. Figure 1A shows that chondrocytes of new born mouse distal tibial and proximal femoral articular cartilage express HIF-2a; Noteworthy, there is a robust expression of the HIF-2a and the highest level of expression is seen in the chondrocytes in the superficial cells of the cartilage. We next examined HIF-2 expression in articular chondrocytes from the proximal femurs of mice of two different age groups. While chondrocytes of mature mice (18 months) show abundant HIF-2a expression (Fig 1B), there is a dramatic reduction in HIF-2α expression in the chondrocytes of older animals (24 months) (Fig 1 B2). When we compared neonatal cartilage in the mineralizing vertebrae with end plate cartilage of older mice (Fig 1 C2) a dramatic decrease in HIF-2α expression is seen. Similarly, there appears to be a reduction in HIF-2α expression in chondrocytes from osteoarthritic cartilage (Fig 1D) when compared to normal cartilage (Fig 1D). To evaluate HIF-2α expression in the growth cartilage, sections of mouse neonatal femoral growth plate were immunostained for HIF-2α. Noteworthy, while HIF-2α staining was observed in the proliferating chondrocytes, the most densely stained cells are localized in the early-hypertrophic region (Fig 1E). We and others have demonstrated that cells in this region have elevated alkaline phosphatase activity and express collagen type X (7,8). HIF-2α expression decreases as chondrocytes become terminally differentiated. While in most cases there is very limited staining of the chondrocyte nuclei, this is not unexpected as there is good evidence to indicate that HIF-2 is preferentially retained or trapped in the cytosol, with a minimum levels of the protein present the nucleus (16). Specificity of HIF-2α staining was ascertained by using sections of the growth plate obtained from the HIF-2α knockout mice (not shown).
Figure 1. Expression of HIF-2α in cartilage.
Sections through demineralized cartilage were stained with an antibody to HIF-2α. Subsequently the sections were treated with Alexafluor 594 labeled secondary antibodies and nuclei counterstained blue with DAPI. Rabbit IgG was used as a negative control. HIF-2 expression in: (A) new born mouse distal tibial and proximal femoral articular cartilage; note, the intense HIF-2α staining in the cells at the articular surface of the joint. Mag × 200. (B1) articular chondrocytes from mouse proximal femur (18 mos) compared with (B2) older mouse (30 mos); with age a dramatic decrease inHIF-2α expression is evident. Mag × 200 (C1) neonatal cartilage in the mineralizing vertebrae compared with end plate cartilage in older mice (C2); again a dramatic decrease in HIF-2α expression is seen with age. (D1) chondrocytes of healthy articular cartilage compared with cells in osteoarthritic cartilage (D2). HIF-2α expression is significantly reduced in cells of the osteoarthritic cartilage. Mag × 200 (E) pre-hypertrophic cells of the mouse growth plate. A robust signal was evident in cells of the pre-hypertrophic zone (PHZ) compared with the proliferating (PZ) (Mag × 200).
HIF-2 regulates ROS dismutation in chondrocytes
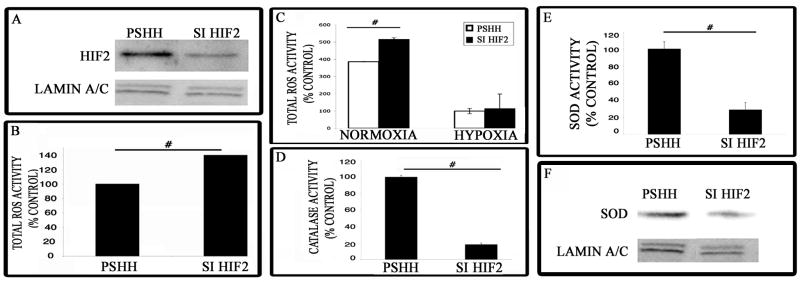
In order to examine the role of HIF-2 in chondrocyte biology in greater detail, we examined the expression of HIF-2α in N1511 chondrocytes. In culture these cells express aggrecan and type II collagen and generate an abundant extracellular matrix. We silenced HIF-2α gene expression using siRNA technology (Fig 2A) and measured ROS generation. Noteworthy, compared with control (pSHH) chondrocytes, the total ROS levels are significantly greater in the silenced chondrocytes (Fig 2B). Under hypoxic conditions however, while ROS levels decrease there is no difference between control and HIF-2α silenced chondrocytes (Fig 2C). Since the levels of ROS are regulated by the activities of catalase and superoxide dismutase (SOD), we next examined the activity of these dismutating enzymes in chondrocytes. Figure 2D and E show that the activity of both dismutating agents is significantly suppressed in HIF-2α silenced cells. We also examined the levels of SOD by Western blot analysis. Figure 2F shows that the protein expression level is lowered in silenced chondrocytes.
Figure 2. HIF-2α silencing impairs ROS dismutation and suppresses SOD and catalase activity.
(A) Suppression of HIF-2α by siRNA technology. Protein was extracted from the chondrocyte cell line and Western blot analysis was used to measure HIF-2α expression in the silenced cells and cells transfected with the control construct (pSHH). A marked decrease in HIF-2α protein was observed in the silenced cells. (B) Analysis of total ROS levels in maturing chondrocytes. Cells were treated with dihydroethidium and the change in fluorescence was measured. The increase in dihydroethidium fluorescence in HIF-2α silenced cells was due to decreased ROS dismutation. [# = significantly different from control. p < 0.05]. (C) Assessment of ROS generation in chondrocytes grown under normoxic and hypoxic conditions. Cells were cultured either under normoxic (20%) or hypoxic (2%) oxygen tensions. Subsequently, cells were treated with dihydroethidium and the change in fluorescence was measured. The increase in dihydroethidium fluorescence in HIF-2α silenced cells was due to decreased ROS dismutation. [# = significantly different from control. p < 0.05]. There was no difference in ROS dismutation under hypoxic conditions. (D) Determination of catalase activity in chondrocytes. There was a significant decrease in catalase activity in siHIF-2α chondrocytes compared to the control (pSHH) cells. [# = significantly different from control. p < 0.05] (E) Assessment of superoxide dismutase (SOD) activity in chondrocytes. SOD activity was determined in both control (pSHH) and silenced chondrocytes. SOD activity was significantly reduced in siHIF-2α chondrocytes [# = significantly different from control. p < 0.05]. (F) Expression of SOD protein in HIF-2 silenced chondrocytes by Western blot analysis. Total SOD protein levels were reduced when HIF-2α is silenced. Lamin A/C was used as a loading control.
LC-3 expression in cartilage
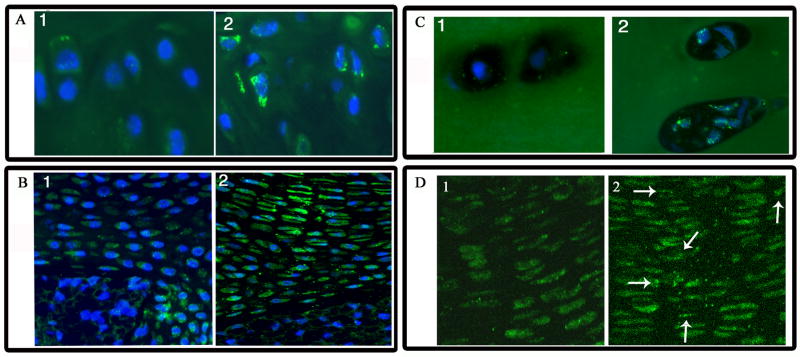
Since HIF-2 has been shown to regulate ROS levels, and ROS have been shown to induce autophagy, we next examined cartilaginous tissues for the expression of the autophagy marker, LC-3. Figure 3A shows that LC-3 is expressed in chondrocytes from both young and older mice. However, while younger mice exhibit diffuse staining (Fig 3A), distinct punctate staining is commonly seen in the older mice, indicative of autophagy (Fig 3A). Figure 3B shows LC3 staining of neonatal and endplate cartilage of mice vertebrae. Compared with end plate cartilage of older animals, end plate cartilage cells of the intervertebral disc from young mice displayed diffuse LC3 staining (Fig. 3 B1). Chondrocytes in the end plate showed distinct puncta characteristic of autophagic cells (Fig. 3 B2). LC3 expression was also evaluated in chondrocytes of human healthy and osteoarthritic articular cartilage (Fig 3C). Chondrocytes in osteoarthritic tissue display numerous autophagic LC3 puncta (Fig. 3 C2). In contrast, cells from healthy tissue do not show an elevation in punctate LC3 distribution (Fig. 3 C1). Finally, we examined LC-3 expression in the HIF-2 knockout growth plate cartilage. Distinct punctate LC-3 staining is observed throughout the growth plate, including the proliferating zone cells (Fig 3D), indicating that suppression of HIF-2 results in the induction of autophagy. In the wild type control, LC3 staining is seen mainly in the pre-hypertrophic zone (Fig. 3D1)
Figure 3. LC3 expression in cartilage.
Cartilage sections were stained with an antibody to the microtubule associated protein, LC3. Subsequently the sections were treated with Alexafluor 594 labeled secondary antibodies. Rabbit IgG was used as a negative control. Mag × 200 (A) Cells of the articular cartilage of the mouse proximal femur (18 mos). There is diffuse staining for the microtubule associated protein, LC3 (Fig 3A1). In contrast, LC3 was distributed in a distinct punctate manner in the chondrocytes of older mice (30 mos) (Fig 3A2). Mag × 200 (B) Neonatal and endplate cartilage of the mineralizing mice vertebrae. Compared with end plate cartilage of older animals, cartilage cells of the intervertebral disc from young mice also diplayed diffuse LC3 staining (Fig 3B1) Mag × 200. Chondrocytes in the end plate of older animals showed distinct puncta charecteristic of autophagic cells (Fig 3B2). (C) Chondrocytes of healthy and osteoarthritic human articular cartilage. Chondrocytes in osteoarthritic tissue display numerous autophagic LC3 puncta (Fig 3C2). In contrast, cells from healthy tissue do not show an elevation in punctate LC3 distribution (Fig 3C1). (D) Proliferative cells of the HIF-2α knockout mouse growth plate. In the HIF-2α knockout growth plate (Fig 3D2) there is LC3 punctate staining even in the proliferative zone of the growth plate. In the wildtype growth plate, there is diffuse LC3 staining in the proliferative zone (Fig 3D1).
Induction of autophagy in HIF-2 suppressed chondrocytes
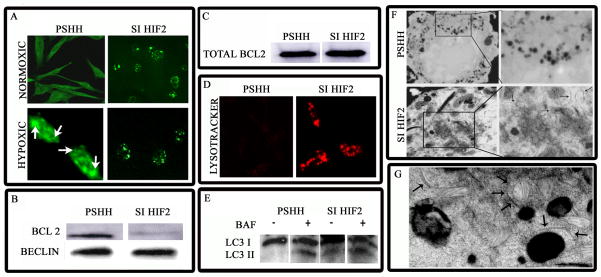
To learn if the change in HIF-2 levels promoted autophagy, cells were probed with an anti-LC3 antibody. Figure 4A shows that when HIF-2 is silenced there is induction of autophagy; thus, there is redistribution of the microtubule associated protein, LC-3, to form punctate structures in the HIF-2 silenced cells. Significantly, induction is evident in serum-rich conditions. Under hypoxic conditions, while some LC-3 reorganization is seen in control cells, once again, in HIF-2 silenced cells there is significant reorganization of LC3 into punctate structures (Fig 4A). The association of the autophagic protein Beclin-1 with Bcl-2 was also evaluated in chondrocytes under serum-replete conditions. We noted that in control cells, Bcl-2 is co-immunoprecipitated with Beclin-1. In contrast, immunoprecipitates of the silenced cells indicate a reduced affinity of Bcl-2 for Beclin-1 (Fig 4B). However, there is no difference in total Bcl-2 levels between the two cell lines (Fig. 4C). We also used LysoTracker Red to probe for autophagic activity. The increase in LysoTracker fluorescence (Fig 4D), due to an elevation in lysosomal activity, confirms that the silenced cells in serum-replete medium are autophagic. Similarly bafilomycin, an agent that inhibits the fusion of autophagosomes with lysosomes causes an accumulation of LC-3 II isoform (Fig 4E). Finally, we confirmed the presence of autophagosomes by TEM. Figure 4F shows that autophagosomes are formed in HIF-2 silenced cells in serum-rich conditions. Double membrane lined vesicles as well as autophagic isolation membranes are visible in these silenced cells. The presence of mitochondria in the vesicles (Fig 4G) suggests that HIF-2 silencing results in the onset of mitophagy in these cells.
Figure 4. Autophagic response of HIF-2 suppressed cells.
(A) LC-3 expression in HIF-2 suppressed cells examined by immunofluorescence analysis. Cells were cultured either under normoxic (20% O2) or hypoxic (2% O2) conditions. Under normoxic conditions, LC-3 was expressed in speckled structures (autophagosomes) in HIF-2 suppressed cells, even in serum-replete conditions. In contrast, LC-3 fluorescence was diffuse in control (pSHH) cells. Under hypoxic conditions, both HIF-2α silenced cells and control cells show a speckled distribution of LC-3 (mag. × 200). (B) Beclin-1 – Bcl-2 association in HIF-2 suppressed cells probed by immunoprecipitation and Western blot analysis. Control and HIF-2 suppressed cells that were grown under serum-rich conditions were lysed, immunoprecipitated with an anti-Beclin-1 antibody and subjected to Western blot analysis. Note, Bcl-2 was associated with Beclin-1 in control cells. In contrast, in HIF-2 silenced cells this association was blocked, even in serum-rich conditions. (C) Analysis of total Bcl-2 expression. Total Bcl-2 expression was assessed by Western blot analysis of whole cell lysates. Note that there is no significant difference in total Bcl-2 expression between HIF-2 silenced and control cells. (D) Lysosomal activity of HIF-2 suppressed chondrocytes. Cells were incubated with LysoTracker Red (70 nm) for 1 h at 37°C in serum-replete media and viewed by confocal microscopy. Note the near complete suppression of lysosomal activity in control cells. In contrast, there was extensive lysosomal fluorescence in HIF-2α silenced cells. (E) Increase in LC-3 II expression upon bafilomycin A1 treatment. Cells were grown either in the absence or presence of bafilomycin A1. Subsequently, whole cell lysates were assessed for LC-3 expression by Western blot analysis. Note the presence of LC-3 II in HIF-2α silenced cells grown in the presence of serum. Also note the accumulation of this isoform in cells treated with bafilomycin A1. (F and G) TEM analysis of control and HIF-2α silenced chondrocytes. Note the presence of autophagosomes and isolation membranes in HIF-2α silenced cells cultured under serum-rich conditions (arrows; Fig 3F; Mag × 8000). Also note the presence of a mitochondria surrounded by a double layered membrane (3G; three arrows) and isolation membranes (single arrows) in HIF-2α silenced cells viewed at higher magnification (Mag × 10,000).
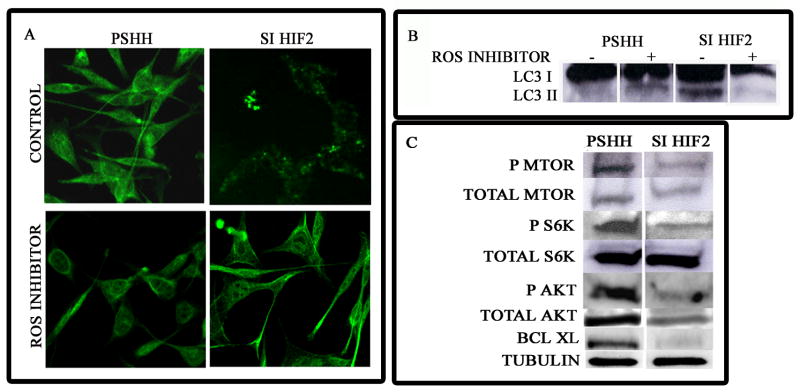
ROS inhibition suppresses autophagy
Since silencing of HIF-2 results in the loss of dismutating activity, it was necessary to determine if the autophagic activity was due to elevated ROS generation. To test this hypothesis, we inhibited ROS generation in these cells using BHA. Figure 5A shows that while HIF-2 silenced cells display a punctate pattern of LC-3 distribution, this pattern is absent from cells treated with BHA. Thus, ROS inhibition results in the diffuse redistribution of LC-3. Similarly, while distinct LC-3 I and LC-3 II isoforms are seen in HIF-2 silenced cells, ROS suppression results in the disappearance of the LC-3 II isoform (Fig 5B).
Figure 5. Effect of HIF-2 silencing on ROS generation, autophagy induction, mTOR activity and expression of Akt-1 and Bcl-XL.
Cellular ROS generation was blocked using 2-,3-tert-butyl-4-hydroxy-anisole (BHA) at a concentration of 100 μM for 1 h. Subsequently LC-3 expression was assessed either by (A) immunofluorescence analysis or (B) by Western blot analysis. In A, compared with control cells, there was disappearance of the punctate pattern of LC-3 expression in HIF-2α silenced cells treated with the inhibitor. Note the presence of the LC-3 II isoform. Again, note the disappearance of the LC-3 II isoform in HIF-2α silenced cells treated with BHA. (C) Total protein isolated from cells cultured under serum-replete conditions was examined by Western blot analysis for the expression of mTOR, S6K, Akt-1 and Bcl-XL. Note the reduction in the activity of mTOR and its target gene S6K (reduced levels of p-mTOR and p-S6K). In addition, note the reduction in expression of total Akt-1 and Bcl-XL.
Expression of mTOR, Akt-1 and Bcl-XL in HIF-2 silenced cells
Since HIF-2 silencing resulted in the induction of autophagy, we next examined the expression and activity of the autophagic repressor, mTOR. As shown in Figure 5C, while there was a minimal decrease in mTOR expression, there was a significant reduction in activated mTOR. Thus, while control cells showed robust expression of phosphomTOR, HIF-2 silenced cells showed minimal expression of the activated form of mTOR. In addition, there was a reduction of the mTOR target, phospho-S6K. We also examined the expression of another mTOR activated protein, the survival pathway kinase, Akt. Figure 5C shows that not only was Akt activation (pAkt) suppressed in HIF-2 silenced cells, but overall expression was also reduced. We next examined the expression of the anti-apoptotic protein Bcl-XL. While total Bcl-2 expression was not effected in HIF-2 silenced cells (Fig 4C), expression of Bcl-XL was significantly reduced (Fig 5C).
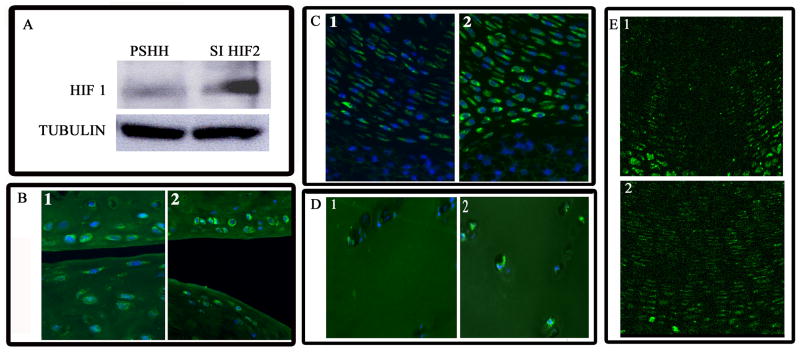
HIF-1 expression in HIF-2 silenced cells and cartilage
To explore the possible interaction between HIF-1 and HIF-2 we examined HIF-1 expression in the HIF-2 silenced cells. Figure 6A shows that there is robust expression of HIF-1α in the HIF-2 silenced cells. We next examined HIF-1 expression in various cartilaginous tissues. While moderate levels of HIF-1 are seen in the superficial chondrocytes of the articular surface of the tibia and femur (Fig 6B), there is a significant increase in expression in the cartilage of older mice (Fig 6B). Similarly, while cells of the intervertebral cartilage from young mice display low levels of HIF-1 (Fig 6C), in the end plate of mature mice, there is elevated HIF-1 expression (Fig 6C). Furthermore, there are high levels of HIF-1 expression in osteoarthritic chondrocytes (Fig 6D) when compared to normal articular chondrocytes (Fig 6D). We next examined the expression of HIF-1 in the growth plate. Previous studies have demonstrated high levels HIF-1 expression in hypertrophic chondrocytes and moderate to low expression in proliferative cells. However, HIF-1 expression pattern in the growth plate of a HIF-2 knockout mouse was different. Thus, high levels of HIF-1 expression are seen in the proliferating zone cells (Fig 6E) unlike the wildtype mouse wherein very low HIF-1 expression is seen in the proliferative zone cells (Fig 6E).
Figure 6. Increased HIF-1α expression in HIF-2α suppressed cells and cells of aging and osteoarthritic cartilage.
(A) Control and HIF-2α silenced cells were assessed for the expression of HIF-1α by Western blot analysis. Note the robust expression of HIF-1α in HIF-2α silenced cells. Tubulin served as a loading control. (B) HIF-1α expression in cells of the articular cartilage of the mouse proximal femur and distal femur at 18 and 30 mo. Note the low levels of HIF-1α staining in the superficial cells of the articular cartilage (Fig 6B1). In the older mice, there was elevated staining of the superficial chondrocytes (Fig 6B2). (C) Neonatal and endplate cartilage of the mineralizing mice vertebrae. The cartilage cells in the younger mouse showed low levels of HIF-1α expression (Fig 6C1), while cells from the older end plate showed significantly higher levels of HIF-1α (Fig 6C2). (D) Chondrocytes of human healthy and osteoarthritic articular cartilage. Osteoarthritic chondrocytes displayed high levels of HIF-1α (Fig 6D2) expression when compared to cells from healthy tissue (Fig 6D1). (E) Chondrocytes of the HIF-2α knockout growth plate. Cells of the proliferative zone of the growth plate were probed for HIF-1α. Note the low level expression in proliferative cells of wildtype growth plate (Fig 6E1). In contrast, the proliferative cells of the growth plate of HIF-2α knockout animals displayed an increase in HIF-1α expression (Fig 6E2).
DISCUSSION
A number of recent studies have shown that the local oxygen tension provides a microenvironmental signal that influences skeletal cell function. (9, 10) While it is clear that the oxemic response is transduced by HIF-1, there is some evidence to indicate that HIF-2 expression may be a requirement for cartilage function. For example, a recent study of HIF-2α knockout mice indicates that deletion of this gene (EPAS−/−) resulted in small animals, suggesting an abnormality of chondrocyte function (7). Herein, we showed that the HIF-2 homologue was expressed by chondrocytes of a number of different cartilages. If HIF-2α has a protective function and serves to promote chondrocyte survival and function, then loss or deletion of this homolog is likely to be associated with development of disease or possibly aging. Lending support to this notion, we noted that while HIF-2 was expressed abundantly by cells in articular cartilage and the cartilage of mineralizing vertebrae, expression was reduced in cartilage of older animals and was low in the mature end plate. Similarly, there was a significant drop in expression in osteoarthritic chondrocytes compared to healthy cells. Based on these findings, it is plausible to consider that this HIF homologue, together with HIF-1, may play a role in maintaining chondrocyte functional activity and tissue health.
We also examined the expression of HIF-2α in the epiphyseal growth plate and the N1511 chondrocytic cell line. This HIF homolog was robustly expressed in vivo; in hypoxic culture, there was expression of this transcription factor. When we evaluated ROS generation in the HIF-2α silenced cells, we noted that there was a small, but significant (20–30%) increase in total ROS values when compared to the pSHH controls. Thus, we confirmed earlier findings that indicated that HIF-2 regulates dismutation of ROS. Next, we evaluated the activity of catalase and superoxide dismutase in HIF-2 silenced cells. These two genes encode proteins that serve to decrease levels of intracellular ROS. Predictably, when HIF-2α was suppressed, there was a marked decrease in the activities of both enzymes, and the SOD protein levels were also low. Noteworthy, since these two dismutating enzymes are HIF-2 target genes, it is likely that their expression serves to minimize ROS generation in the chondrocytes. Minimization is of considerable importance as ROS accumulation can influence structural as well as signaling activities of a number of cell types. The recent observation by Scherz-Shouval et al (11) that H2O2 modifies a conserved cysteine residue in the autophagy protein ATG4, couples HIF-2α activity with the induction of autophagy. We explored this possible relationship, by evaluating autophagy and HIF-2 expression in a number of cartilages. We noted that low levels or suppression of HIF-2 correlated with a robust autophagic response in osteoarthritic tissue and also in aging cartilage; in the growth plate cartilage of HIF-2α knockout mouse there was elevated autophagy. From this perspective, HIF-1 and HIF-2α have separate and opposing effects on the induction of autophagy.
To further examine the relationship between HIF-2 and autophagy, we probed for LC-3 protein expression in the suppressed chondrocytes. Thus, there was redistribution of the microtubule associated protein to form punctate structures that are characteristic of the autophagic state. Since LC-3 redistribution was evident in serum-rich conditions, we infer that autophagy is directly controlled by HIF-2, and is not an artifact induced by the culture system. We also used LysoTracker Red to probe cells for autophagic activity. The increase in LysoTracker fluorescence, due to an elevation in lysosomal activity, confirmed that the silenced cells in serum-replete medium displayed the functional characteristics of an autophagic chondrocyte. In addition, ultramicroscopic analysis indicated the presence of double membrane vacuoles and isolation membranes, morphological characteristics of an autophagic cell. Based on these observations, it is concluded that HIF-2 is a potent regulator of autophagy in maturing chondrocytes. Thus, in contrast to HIF-1, which serves to promote autophagy (4, 12), HIF-2 regulates the extent of the autophagic response and can be viewed as acting as a brake to the accelerator function of HIF-1.
Our earlier studies (4), together with those discussed above, suggest that both HIF-1 and HIF-2 regulate chondrocyte maturation and the development of autophagy. Moreover, since the impact of each of these transcription factors is maximal at different stages of the chondrocyte maturation and pathology, (HIF-2α is maximal in the pre-hypertrophic cells, while HIF-1α expression is highest in the hypertrophic zone of the growth plate), the following question was raised: Does HIF-2 expression regulate HIF-1 activity? While the details of this relationship are fragmentary, one possible indication may come from the contrasting suppressive effects of HIF-1 and HIF-2 on each other (13) Thus, suppression or loss of HIF-2 results in a higher expression of HIF-1, a potent inducer of autophagy (4, 12). In young healthy chondrocytes, HIF-2 dependent expression of SOD and catalase would serve to down regulate ROS generation. Furthermore, HIF-1 is expressed at a minimal level. With aging and the onset of disease, the subsequent lowered expression of HIF-2 would lead to loss of dismutating activity, an increase in ROS generation and promotion of HIF-1 expression. In addition, there was a decrease in mTOR activity and a reduction in the expression of both Akt-1 and the anti-apoptotic protein, Bcl-XL. These changes would cause an increase in chondrocyte autophagy as is evident in cells of osteoarthritic and aging cartilage.
Finally, from the perspective of the oxemic microenvironment, there were no significant differences in total ROS levels between hypoxic control cells and HIF-2 silenced cells. However, under normoxic conditions, there was a significant increase in ROS levels in HIF-2 silenced cells. This observation may explain the refractory nature of hypoxic chondrocytes to an apoptogen challenge. (8, 14) From a physiological perspective, the hypoxic environment would allow the cells to complete their developmental and maturation process in the presence of potential apoptogens generated by matrix degradation (15). We hypothesize that at the chondro-osseous junction, the increase in oxygen tension as a result of vascular invasion would lead to an increase in ROS generation in a manner similar to that reported for ischemia-reperfusion injury. In addition to the elevation in ROS generation, the decrease in HIF-2 levels and stabilization of HIF-1 would further elevate chondrocytes autophagic activity, resulting in sensitization to apoptogen challenges and final removal by apoptosis.
Acknowledgments
Supported by grants RO3 DE 015694 and RO1 DE 016383 (to VS) and RO1 DE 010875 and RO1 DE 013319 (to IMS) from the National Institutes of Health. SPT was supported by NIH grant F31 DE 015753.
References
- 1.Shapiro IM, Adams CS, Srinivas V, Freeman TA. Chondrocyte hypertrophy and apoptosis at the cartilage-bone interface. In: Bronner F, Farach-Carson MC, editors. Bone and Osteoarthritis (Topics in Bone Biology Vol 4) Springer; 2006. pp. 109–130. [Google Scholar]
- 2.Anderson HC, Garimella R, Tague SE. The role of matrix vesicles in growth plate development and biomineralization. Front Biosci. 2005;10:822–37. doi: 10.2741/1576. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro IM, Srinivas V. Metabolic consideration of epiphyseal growth: Survival responses in a taxing environment. Bone. 2007;40:561–567. doi: 10.1016/j.bone.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohensky J, Shapiro IM, Leshinsky S, Terkhorn SP, Adams CS, Srinivas V. HIF-1 regulation of chondrocyte apoptosis: induction of the autophagic pathway. Autophagy. 2007;3:207–14. doi: 10.4161/auto.3708. [DOI] [PubMed] [Google Scholar]
- 5.Bohensky J, Shapiro IM, Leshinsky S, Watanabe H, Srinivas V. PIM-2 is an independent regulator of chondrocyte survival and autophagy in the epiphyseal growth plate. J Cell Physiol. 2007;213:246–51. doi: 10.1002/jcp.21117. [DOI] [PubMed] [Google Scholar]
- 6.Stewart AJ, Houston B, Farquharson C. Elevated expression of hypoxia inducible factor-2alpha in terminally differentiating growth plate chondrocytes. J Cell Physiol. 2006;206:435–40. doi: 10.1002/jcp.20481. [DOI] [PubMed] [Google Scholar]
- 7.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet. 2003;35:331–40. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 8.Terkhorn SP, Bohensky J, Shapiro IM, Koyama E, Srinivas V. Expression of HIF prolyl hydroxylase isozymes in growth plate chondrocytes: relationship between maturation and apoptotic sensitivity. J Cell Physiol. 2007 Jan;210(1):257–65. doi: 10.1002/jcp.20873. [DOI] [PubMed] [Google Scholar]
- 9.Reginato AM, Shapiro IM, Lash JW, Jimenez SA. Type X collagen alterations in rachitic chick epiphyseal growth cartilage. J Biol Chem. 1988 Jul 15;263:9938–45. [PubMed] [Google Scholar]
- 10.Provot S, Zinyk D, Gunes Y, Kathri R, Le Q, Kronenberg HM, Johnson RS, Longaker MT, Giaccia AJ, Schipani E. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol. 2007;177:451–64. doi: 10.1083/jcb.200612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scherz-Shouval R, Shvets E, Elazar Z. Oxidation as a post-translational modification that regulates autophagy. Autophagy. 2007;3:371–373. doi: 10.4161/auto.4214. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–86. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe H, Bohensky J, Freeman T, Srinivas V, Shapiro IM. Hypoxic induction of UCP3 in the growth plate: UCP3 suppresses chondrocyte autophagy. J Cell Physiol. 2008;216:419–25. doi: 10.1002/jcp.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro IM, Adams CS, Freeman T, Srinivas V. Fate of the hypertrophic chondrocyte: microenvironmental perspectives on apoptosis and survival in the epiphyseal growth plate. Birth Defects Res C Embryo Today. 2005;75:330–9. doi: 10.1002/bdrc.20057. [DOI] [PubMed] [Google Scholar]
- 16.Park S, Dadak AM, Haase VH, Fontana L, Giaccia AJ, Johnson RS. Hypoxia-Induced Gene Expression Occurs Solely through the Action of Hypoxia-Inducible Factor 1 (HIF-1): Role of Cytoplasmic Trapping of HIF-2. Molecular and Cellular Biology. 2003;23:4959–71. doi: 10.1128/MCB.23.14.4959-4971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]