Abstract
Goal
Adiponectin is an anti-inflammatory and insulin-sensitizing adipokine produced by adipose tissue. The purpose of this study was to determine the relationships between adiponectin and glucose metabolism in stroke survivors and to compare adiponectin levels between stroke subjects and non-stroke controls similar in age, gender, and body mass index (BMI).
Materials and Methods
Fifty-two stroke survivors (35 men, 17 women) and 33 (22 men, 11 women) non-stroke controls had plasma adiponectin levels measured by RIA, an oral glucose tolerance test, and a peak oxygen consumption (VO2peak) graded treadmill test. Insulin resistance and insulin sensitivity were assessed using the Homeostasis Model Assessment (HOMA-IR) and Insulin Sensitivity Index (ISIM).
Results
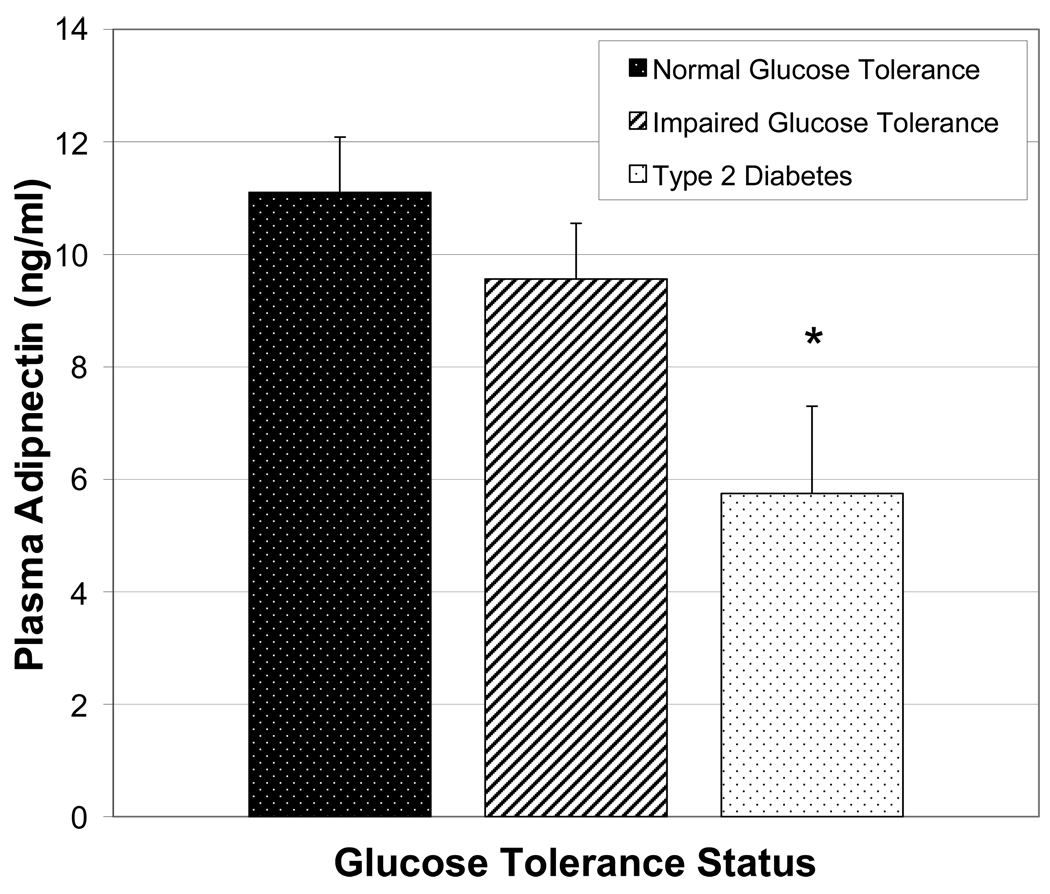
Adiponectin levels were positively associated with age (r=0.32, P<0.05) and negatively associated with glucose homeostasis (fasting glucose: r=−0.42; insulin: r=−0.36; G120min: r=−0.39; HOMA-IR: r=−0.45; and ISIM: r=0.44, all P<0.01) in stroke survivors. Adiponectin levels were significantly different between normal glucose tolerant (NGT), impaired glucose tolerant (IGT) and diabetic stroke subjects (11.1±0.99 vs. 9.56±0.99 vs. 5.75±1.55 ng/ml, P<0.05).
Adiponectin levels were 62% higher in stroke than controls (9.29±0.62 vs. 5.80±0.40 ng/ml, P<0.001) despite greater fasting insulin levels (81%) and 120min insulin (70%) in stroke survivors than controls (P<0.05). HOMA-IR was 78% higher and ISIM was 81% lower in stroke survivors than controls (P<0.05).
Conclusions
Plasma adiponectin levels are associated with age and insulin sensitivity but not adiposity in stroke survivors. The paradoxical finding that the more insulin resistant stroke survivors had higher adiponectin levels than more insulin sensitive controls suggests that perhaps, anti-inflammatory cytokines increase to counter an inflamed and insulin resistant state in stroke survivors.
Keywords: Stroke, Adiponectin, Inflammation, Insulin Sensitivity, Diabetes
INTRODUCTION
Inflammatory processes generally contribute to atherosclerotic plaque instability leading to ischemic vascular events and simultaneously contributing to disease risk progression for type 2 diabetes. Stroke survivors are at a heightened risk for type 2 diabetes [1] and the prevalence of abnormal glucose metabolism approaching 80% according to our recent studies [2]. As systemic inflammation plays a critical role in atherosclerotic diseases, vascular event risk, insulin resistance, and the development of type 2 diabetes [3][4][5][6], inflammatory processes may be upregulated in stroke survivors.
Adipose tissue produces a variety of relevant cytokines collectively known as “adipokines” including adiponectin [7]. Ironically, adiponectin is decreased in obesity [8], negatively associated with abdominal adiposity [9], and positively predicts increased glucose utilization and insulin sensitivity [9] [10]; findings which are replicated regardless of obesity status as determined by body mass index (BMI) [11]. Furthermore, adiponectin levels are reduced in patients with type 2 diabetes and coronary heart disease suggesting it may have some protective effect against atherosclerosis with anti-inflammatory effects in some conditions [12] [13]. To our knowledge, the role of adiponectin as a cytokine implicated in the insulin resistance of stroke is unknown.
We tested the hypothesis that adiponectin levels would be positively associated with measures of insulin sensitivity and lower in stroke survivors than healthy controls. Thus, the purpose of this study was to determine the relationships between adiponectin and glucose metabolism in stroke survivors and to compare adiponectin levels between stroke subjects and non-stroke controls.
MATERIALS AND METHODS
Subjects
Fifty-two individuals (35 men and 17 women) between the ages of 47 – 86 years and BMI between 19.8 – 49.7 kg/m2 with chronic residual hemiparetic deficits more than six months after index cerebral infarction were recruited by advertisement in local media (newspaper and television advertisements) and local area outpatient centers. All stroke survivors had mild to moderate hemiparetic gait deficits and completed conventional rehabilitation therapy. Evaluations included medical history and physical examination, fasting blood profile, and screening for dementia and depression [14][15]. Stroke participants were excluded if they had unstable angina, congestive heart failure (NYHA II), severe peripheral arterial disease, major post-stroke depression, dementia, impaired comprehension, orthopedic or chronic pain conditions.
Thirty-three men (n=22) and women (n=11) aged 51 –74 years and a BMI range of 24.6 – 35.3 kg/m2 participated in the study as controls. Control subjects were screened by medical history questionnaire, physical examination, fasting blood profile, and a graded exercise treadmill test to exclude those with cardiovascular disease (CVD). Stroke and control subjects had no evidence of cancer, liver, renal, or hematologic disease. Stroke participants and controls were sedentary (<20 min of aerobic exercise 2x/wk).
All methods and procedures for the study were approved by the Institutional Review Board of the University of Maryland and Baltimore VA Research & Development Committee. Each participant provided written informed consent to participate in the study.
Treadmill Exercise Tests
Maximal oxygen uptake (VO2max) was measured because of its positive association with insulin sensitivity [16]. A graded submaximal treadmill test with open circuit spirometry was conducted to measure VO2 peak [17], defined as the highest VO2 attained in the last minute of exercise. VO2max was measured using a continuous treadmill test protocol in non-stroke controls as previously described [18]. Validation for attainment of VO2max included meeting two of the following three criteria: 1) a plateau in oxygen uptake with an increased work load as evidenced by a difference in oxygen uptake of < 2 ml·kg−1·min−1; 2) a respiratory exchange ratio >1.10; and 3) a maximal heart rate within 10 beats/min of the age-predicted maximal value.
Body Composition
Height (cm) and weight (kg) were measured to calculate body mass index (BMI) as weight (kg)/height (m2). Fat mass, lean tissue mass, and % body fat were determined by dual-energy X-ray absorptiometry (DXA, Model Prodigy LUNAR GE version 7.53.002 analyses).
Glucose Metabolism
All subjects underwent a 75 g 2-hour oral glucose tolerance test (OGTT) [19] with blood samples drawn at baseline, 30, 60, 90 and 120 min for measurement of plasma glucose and insulin levels.
Analysis of Blood Samples
Blood samples were collected in heparinized syringes and placed in prechilled test tubes containing 1.5 mg EDTA/ml of blood. The blood samples were centrifuged at 4°C and a 1ml aliquot of plasma was rapidly frozen (−80°C) for subsequent hormone analysis. All determinations were performed in duplicate. Plasma glucose was measured with the glucose oxidase method (2300 STAT Plus, YSI, Yellow Springs, OH). Immunoreactive insulin and human adiponectin levels were determined by RIA (Linco Research Inc., St. Charles, MO).
Statistical Analyses
The insulin sensitivity index was calculated using the method of Matsuda and DeFronzon (ISIM) [20] [10,000/square root of (fasting plasma glucose X fasting plasma insulin) X (mean OGTT glucose concentration X mean OGTT insulin concentration). Homeostasis model assessment for insulin resistance, IR (HOMA-IR) was calculated as described by Matthews et al. [21] [(fasting insulin (µU/ml) X fasting glucose [mmol/l])/22.5]. All data were analyzed using SPSS 12.0. The data were analyzed for the total group, as well as by glucose tolerance status (normal, pre-diabetic, and diabetic) in stroke subjects. Standard methods were used to compute means, standard error of the means, and Pearson correlation coefficients. Multiple regression models were used to examine the effects of age, body fat, VO2 peak, basal glucose, and insulin levels on adiponectin levels. All standard tests were two-tailed. Data are means ± SEM and P values < 0.05 were regarded as statistically significant.
RESULTS
Stroke survivors and controls were well matched for gender with each group consisting of 67% men and 33% women. Physical characteristics for each group are presented in Table 1. As shown, there was no difference in age, body weight, BMI, and total body fat mass between stroke survivors and controls but lean body mass was 14% lower in stroke survivors than controls (P < 0.05). In addition, VO2peak was 57% lower in stroke survivors than controls (P < 0.001).
Table 1.
Physical characteristics of stroke survivors and controls.
| Stroke Survivors (n = 52) |
Controls (n = 33) |
|
|---|---|---|
| Age (years) | 65 ± 1.4 | 62 ± 1.3 |
| Weight (kg) | 84.3 ± 3.1 | 87.7 ± 2.3 |
| BMI (kg/m2) | 29.2 ± 0.9 | 29.7 ± 0.5 |
| Total body fat mass (kg) | 28.0 ± 1.6 | 30.9 ± 1.6 |
| Lean body mass (kg) | 47.9 ± 2.2* | 54.6 ± 1.8 |
| Percent body fat | 34.3 ± 1.5 | 34.8 ± 1.5 |
| VO2peak (ml·kg.min−1) | 13.1 0.6† | 23.3 1.0 |
| VO2peak (l/min) | 1.1 ± 0.1† | 2.1 ± 0.1 |
BMI = body mass index; VO2peak = peak oxygen consumption. Values are means ± SEM.
P < 0.05
P < 0.001.
The metabolic characteristics and adiponectin concentrations for both groups are presented in Table 2. Two stroke subjects had diabetes by elevated fasting glucose and six had G120> 200 mg/dl (n=8, 15%); however, none were treated with oral hypoglycemic agents or insulin. Nineteen (36%) stroke subjects had impaired glucose tolerance. Three of the controls (9%) had type 2 diabetes by G120 and 14 (42%) had impaired glucose tolerance. Thus, 51% of both groups had abnormal glucose metabolism. Both fasting plasma glucose and G120 were not different between stroke survivors and controls. However, fasting insulin levels and insulin120 levels were 81% and 70% higher in stroke survivors than controls (P < 0.05). Furthermore, HOMA-IR was 78% higher in stroke survivors and ISIM was 81% lower in stroke survivors than controls (P < 0.05), both indicating insulin resistance in stroke. Contrary to our hypothesis, adiponectin levels were 62% higher in stroke subjects than controls (P < 0.001).
Table 2.
Metabolic characteristics of stroke survivors and controls.
| Stroke Survivors (n = 52) |
Controls (n = 33) |
|
|---|---|---|
| Normal glucose tolerant (%) | 48 | 52 |
| Impaired glucose tolerant (%) | 37 | 42 |
| Type 2 diabetes (%) | 15 | 6 |
| Fasting plasma glucose (mmol/l) | 5.4 ± 0.1 | 5.4 ± 0.1 |
| Fasting plasma insulin (pmol/l) | 98 ± 7* | 79 ± 4 |
| Glucose120 (mmol/l) | 8.4 ± 0.4 | 7.6 ± 0.4 |
| Insulin120 (pmol/l) | 718 ± 55* | 502 ± 61 |
| ISI M | 2.62 ± 0.17* | 3.24 ± 0.26 |
| HOMA-IR | 3.45 ± 0.26* | 2.71 ± 0.16 |
| Adiponectin (ng/ml) | 9.29 ± 0.62† | 5.80 ± 0.40 |
BMI= body mass index; ISI = insulin sensitivity index; HOMA IR = homeostatic insulin resistance. Values are means ± SEM.
P < 0.05
P < 0.001 stroke vs. controls
Differences in adiponectin levels were observed as a function of glucose tolerance status in stroke subjects (Figure 1). Comparisons of adiponectin (ANOVA) between normal glucose tolerant (NGT) (n=25), impaired glucose tolerant (IGT) (n=19) and diabetic stroke subjects (n=8) were significant at the 0.05 level (NGT vs. IGT vs. diabetic: 11.1 ± 0.99 vs. 9.56 ± 0.99 vs. 5.75 ± 1.55 ng/ml). As expected, fasting glucose and G120 levels were higher and ISIM was lower in those with type 2 diabetes and IGT than normals (P < 0.01). The stroke group was also divided into those on (n = 31) and those not on (n = 21) statin medication in order to gain insight to the unexpectedly high adiponectin levels. These groups also did not differ in glucose or insulin levels, ISIM or HOMA-IR (data not shown) and there were no differences in adiponectin concentrations according to statin use (10.0 ± 1.0 vs. 8.8 ± 0.8 ng/ml, non-statin vs. statin).
Figure 1.
Plasma adiponectin concentrations in stroke survivors grouped by glucose tolerance status (X±SEM, *P<0.05 type 2 diabetes vs. impaired glucose tolerance vs. normal glucose tolerance).
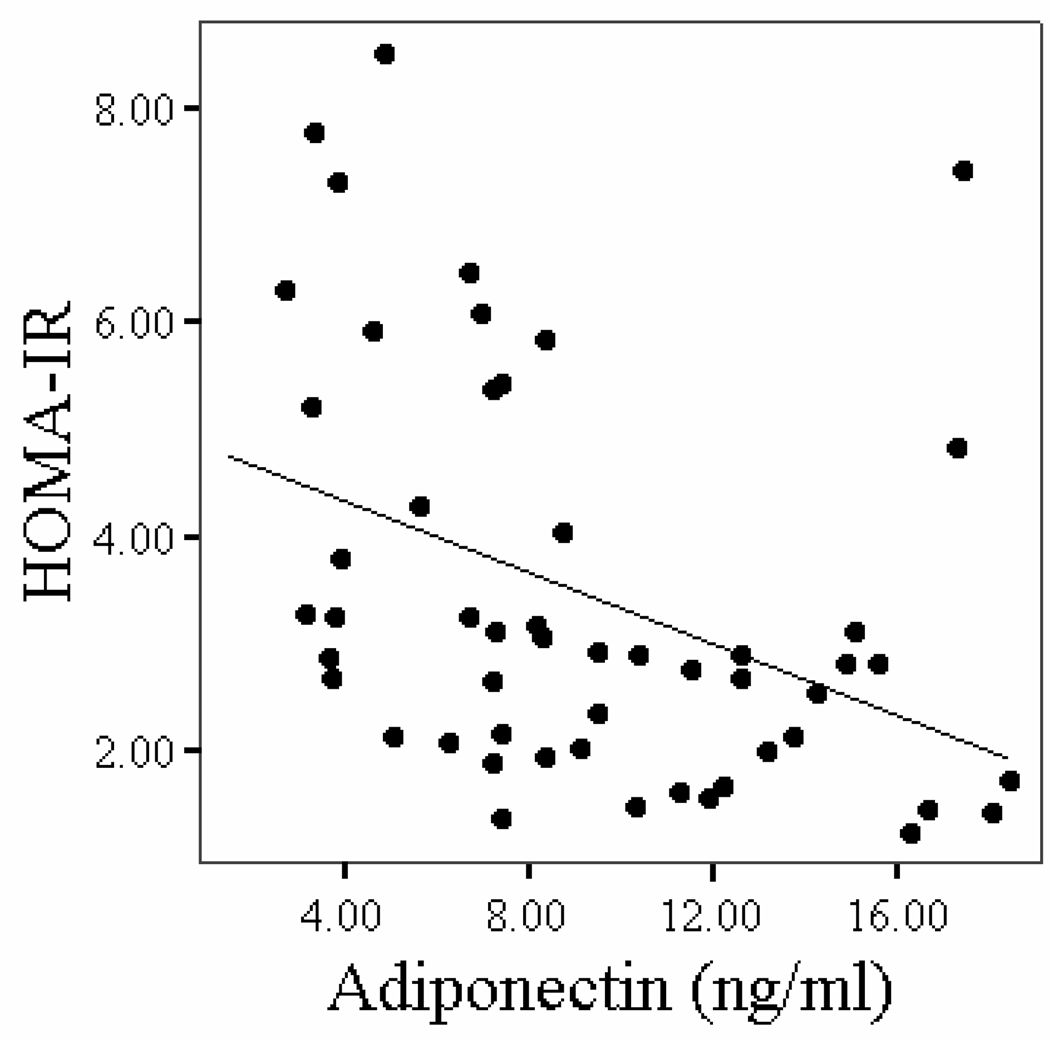
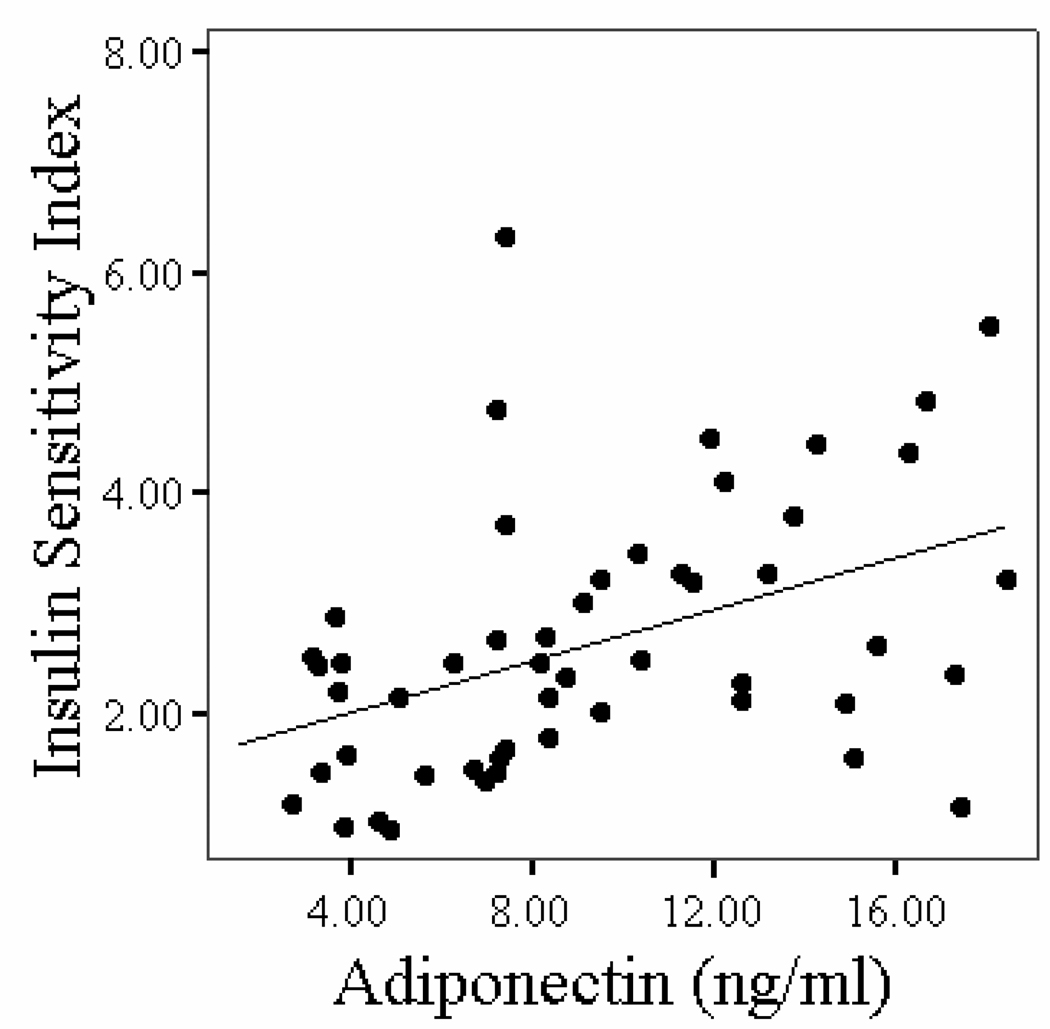
Correlations between plasma adiponectin concentrations and age, body composition, fitness, and glucose metabolism variables in stroke survivors are shown in Table 3. Older age was associated with higher plasma adiponectin in stroke (P < 0.05). Body weight, body fat mass, BMI, and VO2peak were not associated with adiponectin levels after stroke. Fasting and G120 levels and insulin concentrations (P < 0.05) and HOMA-IR (Figure 2, P < 0.01) were negatively associated with adiponectin levels. ISI index was positively correlated to adiponectin concentrations in stroke survivors (Figure 3, P < 0.01). In a multiple stepwise regression model to predict adiponectin levels, we put in the variables age, fasting glucose, G120, HOMA-IR and ISIM. Age and fasting glucose remained in the model at P < 0.005 (r=0.53).
Table 3.
The relationships between the plasma adiponectin concentrations and body composition and glucose metabolism variables in stroke survivors.
| Pearson Correlation Coefficients Plasma Adiponectin |
P-value | |
|---|---|---|
| Age | 0.32 | 0.02 |
| Weight | −0.24 | 0.09 |
| BMI | −0.26 | 0.84 |
| Body fat mass | −0.11 | 0.46 |
| VO2peak (ml/kg/min) | −0.18 | 0.23 |
| Fasting plasma glucose | −0.42 | 0.002 |
| Fasting plasma insulin | −0.36 | 0.008 |
| Glucose120 | −0.39 | 0.004 |
| Insulin120 | −0.18 | 0.22 |
| ISIM | 0.44 | 0.001 |
| HOMA-IR | −0.45 | 0.001 |
BMI = body mass index; ISIM = Insulin Sensitivity Index; HOMA-IR = Homeostasis model assessment for insulin resistance
Figure 2.
Figure 2a: Relationship of HOMA-IR with plasma adiponectin concentrations in stroke survivors (n=52, r = −0.45, P = 0.001).
Figure 2b: Relationship of insulin sensitivity index with plasma adiponectin concentrations in stroke survivors (n=52, r = 0.44, P = 0.001).
Figure 3.
DISCUSSION
Adiponectin levels were associated with age and insulin sensitivity in stroke subjects with the lowest concentrations found in those with type 2 diabetes. Our results also indicate that despite greater insulin resistance in stroke survivors, adiponectin concentrations are higher in stroke subjects than non-stroke controls. It is possible that aging and stroke trigger a counter-regulating response to increase adiponectin levels in this patient population, perhaps to offset an increase inflammatory state. Moreover, we would hypothesize that adiponectin levels would fall in response to worsening of glucose status in stroke survivors.
Stroke survivors have a 70% prevalence of type 2 diabetes and insulin resistance [22]. Several studies report an association of insulin resistance with risk for stroke [23][24][25]. Impaired ISIM [20] was highly prevalent among nondiabetic patients with a recent TIA or nondisabling ischemic stroke [26]. Thus, there is considerable epidemiological evidence that links insulin resistance and diabetes to stroke. To our knowledge, we are the first to report a relationship between adiponectin and insulin sensitivity in stroke survivors. Our results in stroke survivors are similar to observations in healthy individuals that plasma adiponectin concentrations predict increased glucose utilization and insulin sensitivity [9] [10]. The stroke patients in this study had progressively lower adiponectin levels according to worsening diabetes status, as had previously been reported in non-stroke volunteers [12][13]. In this group of stroke survivors, no subjects were treated with oral glucose agents and none were on insulin. Thus, we were able to examine adiponectin levels without the confounding effect of medication. The adiponectin levels were highest in the normal glucose tolerant stroke group and were lower with worsening of glucose metabolism which confirms the observed relationships between adiponectin levels and insulin resistance in stroke survivors.
The beneficial effects of adiponectin extends beyond insulin sensitivity. It also has an observed protective role against ischemic vascular events. Although inflammation may lead to the cardiovascular disease progression through accelerated local weakening of atherosclerotic plaques, leading to rupture and thrombus formation [27], adiponectin may exert an opposite effect. For example, low adiponectin levels are significantly and independently associated with the development of coronary artery disease [28] [29]. Low levels of adioponectin are predictive of cardiac and cerebrovascular events after percutaneous coronary intervention [30] and are a significant risk factor for cardiovascular events such as cardiovascular death, myocardial infarct and stroke in patients with type 2 diabetes [31]. Hypoadiponectinemia is reportedly a biomarker for ischemic stroke and increased mortality after stroke [32][33]. An adiponectin knock-out mouse model of middle cerebral artery occlusion supports the role of adiponectin in stroke. In this case, reperfusion led to increased infarct size, whereas injection of adiponectin reduced the infarct size, suggesting in this model that adiponectin protects the brain from acute ischemic injury [34]. By contrast, in one study, adiponectin measured greater than 5 years prior to stroke was not predictive of future stroke [35].
We unexpectedly observed 60% higher adiponectin concentrations in the stroke survivors than non-stroke controls, despite similar age and adiposity, and higher fitness and insulin sensitivity index in the controls. Although statin use has been described in one paper to increase adiponectin levels [36], adiponectin levels were not different in our stroke survivors based on statin use, nor did these sub-groups differ in any of the glucose or insulin variables including HOMA-IR and ISIM. Thus, statin use does not appear to explain the higher adiponectin levels observed in our group of stroke patients. We also excluded patients with liver disease, moderate alcohol intake and renal dysfunction which are known to increase adiponectin. We also observed an association between adiponectin and age in the stroke group; a relationship that has previously been demonstrated [37] [38] [39]. We hypothesize that the combination of aging and a history of stroke leads to compensatory increases in plasma adiponectin in an attempt to counteract the ensuing insulin resistance associated with stroke.
One mechanism by which adiponectin exerts its insulin-sensitizing effects is through a decrease in muscle and liver triglyceride content [40]. In rodent skeletal muscle, adiponectin increases fatty acid oxidation [40][41] by activating AMPK and inactivating aceytyl CoA carboxylase (ACC) [42], thereby regulating glucose metabolism. It remains to be investigated whether there are changes in fatty acid oxidation in skeletal muscle of stroke subjects. Our findings of increased intramuscular fat by CT in the paretic leg of stroke survivors [43] would tend to contradict the observed higher adiponectin levels in our stroke participants and yet, support a possible reduced fatty acid oxidation in stroke.
We cannot rule out the possibility that genetic variations in the adiponectin gene contribute to our findings. Several quantitative trait loci have been identified that have significant evidence of linkage for obesity-related phenotypes with serum adiponectin levels [44]. The polymorphisms in the adiponectin gene are also associated with adiponectin plasma concentrations, obesity and insulin resistance [45][46][47]. In a large prospective cohort of healthy men, adiponectin gene variations were associated with incident ischemic stroke [48] suggesting that certain genetic variants in the ADIPOQ gene have a potential protective effect.
We conclude that plasma adiponectin levels are associated with insulin sensitivity index after stroke and that concentrations are lowest in stroke survivors with type 2 diabetes. The finding of higher adiponectin in insulin resistant stroke survivors compared to non-stroke individuals was unexpected and suggests a possible counter-regulating protective response to the physiological stress of aging with a chronic neurological condition. Additional studies are needed to determine whether changes in adiponectin concentrations with treatments or interventions are associated with improvements in insulin sensitivity in stroke survivors and the mechanisms whereby this occurs.
AUTHOR ACKNOWLEDGEMENTS
Our appreciation is extended to those stroke survivors and men and women who participated in this study. We are grateful to Andrew P. Goldberg, M.D. for support, the nurses in the Geriatrics Services at the Baltimore VA Medical Center, for technical assistance. We also thank Carole St. Clair, Melissa Gray, and Agnes Kohler, for laboratory assistance.
FUNDING
This study was supported by funds from: VA Research Service, VA Merit Review, NIH grants 01-AG19310, KO1-AG019242, KO1-AG021457, R29 AG14487, Claude D. Pepper Older mericans Independence Center (P30AG028747), Baltimore Veterans Administration Medical enter, Geriatric Research, Education, and Clinical Center, Department of Veterans Affairs, VA R&D Exercise & Robotics Center of Excellence, VA Stroke REAP, and T32-AG 200219.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kernan WN, Viscoli CM, Inzucchi SE, et al. Prevalence of abnormal glucose tolerance following a transient ischemic attack or ischemic stroke. Arch Intern Med. 2005;165:227–233. doi: 10.1001/archinte.165.2.227. [DOI] [PubMed] [Google Scholar]
- 2.Ivey FM, Ryan AS, Hafer-Macko CE, Garrity B, Sorkin J, Goldberg AP, Macko RF. High prevalence of abnormal glucose metabolism and poor sensitivity of fasting plasma glucose in the chronic phase of stroke. J Gerontol.A Biol.Sci.Med.Sci. 2006 doi: 10.1159/000094853. Ref Type: In Press. [DOI] [PubMed] [Google Scholar]
- 3.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 4.Van Exel E, Gussekloo J, de Craen AJ, Bootsma-van der Wiel A, Frolich M, Westendorp RG. Inflammation and stroke: the Leiden 85-Plus Study. Stroke. 2002;33:1135–1138. doi: 10.1161/01.str.0000014206.05597.9e. [DOI] [PubMed] [Google Scholar]
- 5.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 6.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 9.Ryan AS, Berman DM, Nicklas BJ, et al. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26:2383–2388. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 10.Tschritter O, Fritsche A, Thamer C, et al. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003;52:239–243. doi: 10.2337/diabetes.52.2.239. [DOI] [PubMed] [Google Scholar]
- 11.Abbasi F, Chu JW, Lamendola C, et al. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes. 2004;53:585–590. doi: 10.2337/diabetes.53.3.585. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto Y, Arita Y, Nishida M, et al. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res. 2000;32:47–50. doi: 10.1055/s-2007-978586. [DOI] [PubMed] [Google Scholar]
- 13.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Radloff LS, Rae DS. Susceptibility and precipitating factors in depression: sex differences and similarities. J Abnorm Psychol. 1979;88:174–181. doi: 10.1037//0021-843x.88.2.174. [DOI] [PubMed] [Google Scholar]
- 16.Ryan AS, Muller DC, Elahi D. Sequential hyperglycemic-euglycemic clamp to assess beta-cell and peripheral tissue: studies in female athletes. J Appl Physiol. 2001;91:872–881. doi: 10.1152/jappl.2001.91.2.872. [DOI] [PubMed] [Google Scholar]
- 17.Macko RF, DeSouza CA, Tretter LD, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients. A preliminary report. Stroke. 1997;28:326–330. doi: 10.1161/01.str.28.2.326. [DOI] [PubMed] [Google Scholar]
- 18.Ryan AS, Pratley RE, Elahi D, Goldberg AP. Resistive training increases fat-free mass and maintains RMR despite weight loss in postmenopausal women. J Appl Physiol. 1995;79:818–823. doi: 10.1152/jappl.1995.79.3.818. [DOI] [PubMed] [Google Scholar]
- 19.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Kernan WN, Inzucchi SE. Type 2 Diabetes Mellitus and Insulin Resistance: Stroke Prevention and Management. Curr Treat Options Neurol. 2004;6:443–450. doi: 10.1007/s11940-004-0002-y. [DOI] [PubMed] [Google Scholar]
- 23.Shinozaki K, Naritomi H, Shimizu T, et al. Role of insulin resistance associated with compensatory hyperinsulinemia in ischemic stroke. Stroke. 1996;27:37–43. doi: 10.1161/01.str.27.1.37. [DOI] [PubMed] [Google Scholar]
- 24.Gertler MM, Leetma HE, Koutrouby RJ, Johnson ED. The assessment of insulin, glucose and lipids in ischemic thrombotic cerebrovascular disease. Stroke. 1975;6:77–84. doi: 10.1161/01.str.6.1.77. [DOI] [PubMed] [Google Scholar]
- 25.Folsom AR, Rasmussen ML, Chambless LE, et al. Prospective associations of fasting insulin, body fat distribution, and diabetes with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Diabetes Care. 1999;22:1077–1083. doi: 10.2337/diacare.22.7.1077. [DOI] [PubMed] [Google Scholar]
- 26.Kernan WN, Inzucchi SE, Viscoli CM, et al. Impaired insulin sensitivity among nondiabetic patients with a recent TIA or ischemic stroke. Neurology. 2003;60:1447–1451. doi: 10.1212/01.wnl.0000063318.66140.a3. [DOI] [PubMed] [Google Scholar]
- 27.Alexander RW. Inflammation and coronary artery disease. N Engl J Med. 1994;331:468–469. doi: 10.1056/NEJM199408183310709. [DOI] [PubMed] [Google Scholar]
- 28.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura Y, Shimada K, Fukuda D, et al. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528–533. doi: 10.1136/hrt.2003.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shioji K, Moriwaki S, Takeuchi Y, Uegaito T, Mutsuo S, Matsuda M. Relationship of serum adiponectin level to adverse cardiovascular events in patients who undergo percutaneous coronary intervention. Circ J. 2007;71:675–680. doi: 10.1253/circj.71.675. [DOI] [PubMed] [Google Scholar]
- 31.Lim S, Koo BK, Cho SW, et al. Association of adiponectin and resistin with cardiovascular events in Korean patients with type 2 diabetes: the Korean atherosclerosis study (KAS): a 42-month prospective study. Atherosclerosis. 2008;196:398–404. doi: 10.1016/j.atherosclerosis.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Efstathiou SP, Tsioulos DI, Tsiakou AG, Gratsias YE, Pefanis AV, Mountokalakis TD. Plasma adiponectin levels and five-year survival after first-ever ischemic stroke. Stroke. 2005;36:1915–1919. doi: 10.1161/01.STR.0000177874.29849.f0. [DOI] [PubMed] [Google Scholar]
- 33.Chen MP, Tsai JC, Chung FM, et al. Hypoadiponectinemia is associated with ischemic cerebrovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:821–826. doi: 10.1161/01.ATV.0000157784.25920.a7. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura M, Izumiya Y, Higuchi A, et al. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008;117:216–223. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- 35.Soderberg S, Stegmayr B, Stenlund H, et al. Leptin, but not adiponectin, predicts stroke in males. J Intern Med. 2004;256:128–136. doi: 10.1111/j.1365-2796.2004.01351.x. [DOI] [PubMed] [Google Scholar]
- 36.Koh KK, Son JW, Ahn JY, et al. Vascular effects of diet and statin in hypercholesterolemic patients. Int J Cardiol. 2004;95:185–191. doi: 10.1016/j.ijcard.2003.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 38.Isobe T, Saitoh S, Takagi S, et al. Influence of gender, age and renal function on plasma adiponectin level: the Tanno and Sobetsu study. Eur J Endocrinol. 2005;153:91–98. doi: 10.1530/eje.1.01930. [DOI] [PubMed] [Google Scholar]
- 39.Adamczak M, Rzepka E, Chudek J, Wiecek A. Ageing and plasma adiponectin concentration in apparently healthy males and females. Clin Endocrinol (Oxf) 2005;62:114–118. doi: 10.1111/j.1365-2265.2004.02182.x. [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 42.Tomas E, Tsao TS, Saha AK, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83:1703–1707. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- 44.Comuzzie AG, Funahashi T, Sonnenberg G, et al. The genetic basis of plasma variation in adiponectin, a global endophenotype for obesity and the metabolic syndrome. J Clin Endocrinol Metab. 2001;86:4321–4325. doi: 10.1210/jcem.86.9.7878. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Sanchez JL, Zabena CA, Martinez-Larrad MT, et al. An SNP in the adiponectin gene is associated with decreased serum adiponectin levels and risk for impaired glucose tolerance. Obes Res. 2005;13:807–812. doi: 10.1038/oby.2005.91. [DOI] [PubMed] [Google Scholar]
- 46.Jang Y, Lee JH, Kim OY, et al. The SNP276G>T polymorphism in the adiponectin (ACDC) gene is more strongly associated with insulin resistance and cardiovascular disease risk than SNP45T>G in nonobese/nondiabetic Korean men independent of abdominal adiposity and circulating plasma adiponectin. Metabolism. 2006;55:59–66. doi: 10.1016/j.metabol.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Stumvoll M, Tschritter O, Fritsche A, et al. Association of the T-G polymorphism in adiponectin (exon 2) with obesity and insulin sensitivity: interaction with family history of type 2 diabetes. Diabetes. 2002;51:37–41. doi: 10.2337/diabetes.51.1.37. [DOI] [PubMed] [Google Scholar]
- 48.Hegener HH, Lee IM, Cook NR, Ridker PM, Zee RY. Association of adiponectin gene variations with risk of incident myocardial infarction and ischemic stroke: a nested case-control study. Clin Chem. 2006;52:2021–2027. doi: 10.1373/clinchem.2006.074476. [DOI] [PubMed] [Google Scholar]