Abstract
The survival rate of infected vectors represents one of the fundamental components that influence the transmission dynamics of mosquito-borne diseases. Despite the occurrence of a number of studies investigating mosquito survival after infection with filarial worms, there remains conflicting evidence from both laboratory and field experiments as to the existence and mechanism for parasite-induced mortality among filarial mosquitoes. Here, we used a mixed effects meta-analytical framework to combine the data from all available vector–human host blood feeding experiments to evaluate the evidence for the impact of parasite load on the mortality rates of the three major lymphatic filariasis transmitting mosquito genera, Culex, Aedes, and Anopheles mosquitoes, over the extrinsic incubation period of parasitic infection. The results show that, despite the application of this approach, or in the case of Anopheles using a convention fixed effects logistic regression analysis supplemented with additional survival analysis of longitudinal data, no strong association between mortality rate and microfilariae (mf) uptake for either of the three mosquito genera is apparent in the combined data. Instead, a key finding is that study effects played a more crucial role in determining the levels of mortality observed in these experimental studies. This was most revealing in the case of Culex, given that the largest single study in terms of both the number of data points and range of mf intensities, in contrast to smaller studies, showed a significant positive association between mf intensity and mortality, indicating that in this genus at least, the detrimental effect of infection may be manifested only at the highest mf intakes. Although no density dependence in vector mortality was also observed for Aedes, possibly because of the use of restricted human mf intensity range in previous studies, an intriguing finding was that a significantly higher overall mortality was observed for this genus over mf intake ranges that produced much less corresponding mortality in Culex and Anopheles. The results also indicate that currently very little can be said about the survival rate of Anopheles mosquitoes infected with filarial worms because of the striking paucity of data for this genus. Further studies, using standardized methods and covering an appropriate range of mf uptake intensities and using study frameworks that allow the design and comparison of data from both experimental and field experiments, are clearly indicated if we are to reliably quantify the likely effect of filarial infection on vector survival.
Keywords: Wuchereria bancrofti, lymphatic filariasis, vector survival, ecological meta-analysis
The ability of the vector to survive infection is one of the major factors that determine the transmission of mosquito-borne diseases, such as lymphatic filariasis. Because the extrinsic incubation periods of parasites are usually relatively long compared with the vector mean life expectancy, estimates of vectorial capacity and hence the parasite basic reproductive number or ratio, R0, are particularly susceptible to changes in vector survival rates (Dye 1986, 1992). In addition to the natural daily mortality experienced by mosquitoes in the field (Laurence 1963), it is has been suggested that there may be additional mortality experienced by infected mosquitoes as a result of carrying filarial infection (Rosen 1955, Wharton 1957a, Jordan and Goatly 1962, Lindsay and Denham 1986). Such infection-induced mortality is of particular interest when trying to understand the population dynamics of lymphatic filariasis transmission for a number of reasons. At its simplest, it may act to regulate disease transmission in endemic areas by reducing the number of infected vectors. Morever, if mortality is density-dependent, i.e., higher mortality is associated with increased infection intensity within the mosquito, then mass drug administration aimed at reducing human microfilarial (mf) levels (and hence mosquito infection intensity) may lead to an increase in survival of the mosquito population and hence to an increase in transmission in the long-term (Pichon 2002). The study of this question has become even more pressing given the growing realization that, in endemic areas where filariasis and malaria co-occur and are transmitted by the same anopheline mosquitoes, any intervention that may increase vector survival could clearly result in an increase in the incidence of malaria in treated communities (Manga 2002, Burkot et al. 2006).
The difficulties in measuring mosquito survival in the field, and particularly in separating out the effects of natural mortality, infection related mortality, and parasite mortality within the mosquito host, mean that there exist few studies that has allowed conclusive analysis of this topic under natural field conditions (Laurence 1963, Krafsur and Garrett-Jones 1977, Das et al. 1995). For this reason, the study of the detrimental effects that filarial worms may induce on their vectors has been largely restricted to laboratory studies, where vector mortality can be investigated under more controlled conditions. However, although a number of such studies have indicated a detrimental impact of high intensity infection on vector survival, manifested either at specific points in the extrinsic incubation period or as a steady reduction in survival over this period (Rosen 1955; Wharton 1957a, b; Jordan and Goatly 1962; Crans 1973; Lindsay and Denham 1986; Ellrot 1987; Failloux et al. 1995), others by contrast have found no association (Samarawickrema et al. 1985b, Bryan and Southgate 1988). Furthermore, it may be expected that different species and genera of mosquito will vary in their susceptibility to the adverse affects of heavy infection because of regulatory mechanisms limiting the numbers of parasites within the mosquitoes (Bryan et al. 1976). We also suggest that differences between studies in experimental factors such as variations in mosquito collection and maintenance, sample sizes used, host infection density, and methods used to measure infection intensity mean that making simple comparisons based on these data are likely to be problematic.
This paper forms the third in a series of ecological meta-analyses aiming to use published data from mosquito–human feeding experiments to elucidate the occurrence of density-dependent infection processes in the vectors of lymphatic filariasis (Snow and Michael 2002, Snow et al. 2006). We have previously shown, using this approach, significant evidence for the occurrence and operation of density-dependent mechanisms governing the uptake and development of W. bancrofti larvae within vector mosquitoes. The primary aim of this study was to apply a similar amalgamated analysis of available experimental data to inspect the evidence for any additional density-dependent effect of parasite load on the mortality of the three main lymphatic filariasis transmitting vector genera: Culex, Aede, and Anopheles mosquitoes.
Materials and Methods
An extensive survey of the literature (using both electronic searches and manual tracing of references from publications obtained from the electronic search) showed seven experimental vector–human host blood feeding studies that fitted the criteria for inclusion to our meta-analysis that they must report raw data on (1) the microfilarial (mf) density of human hosts on which mosquitoes were fed and (2) the subsequent survival of mosquitoes at the end of the extrinsic incubation period for each of the three main filariasis transmitting vectors (Table 1). Because of a lack of information regarding the intrinsic differences in the nature of infection caused by Wuchereria and Brugia worms in the mosquito, the analysis was restricted to studies in which only W. bancrofti infected human volunteers were used. One additional unpublished study, carried out in Papua New Guinea (PNG) using Anopheles punctulatus s.l. by one of the authors (Bockarie), was also included in the analysis because it contained data not only on the survival of mosquitoes after the completion of the incubation period but also of survival over time during this period, which enabled a more robust longitudinal analysis of parasite-induced mortality for this genus in addition to that outlined above.
Table 1. Details of studies used in this analysis.
| Author | Vector species | na | Mean human mf (range) | No. denistiesb | Durationc |
|---|---|---|---|---|---|
| Brito et al. 1997 | Cx. quinquefasciatusd | 44.70 (33–106) | 389.0 mf/ml (17–700) | 6 | 20 |
| Jordan and Goatly 1962 | Cx. quinquefasciatus | Not given | 179.4 mf/20 μl (10–471) | 21 | 14.5 |
| McGreevy et al. 1982 | Cx. quinquefasciatusd | 107.50 (35–200) | 620.7 mf/ml (1.3–2705) | 14 | 10–13 |
| Rosen 1955 | Ae. polynesiensis | 128.00 (46–207) | 88.0 mf/20 μl (0.4–555.1) | 23 | 12.5 |
| Samarawickrema et al. 1985b | Ae. polynesiensisd | 216.93 (59–490) | 413.3 mf/ml (1–5,290) | 29 | 12–14 |
| Ae. samoanus | 199.17 (93–296) | 396.0 mf/ml (1–1,449) | 18 | 12–14 | |
| Hicks 1932 | An. costalis | 22.25 (13–40) | 6.10 mf/μl (0.8–13.75) | 4 | 14 |
| An. funestus | 10 (10) | 15 mf/μl (15) | 1 | 14 | |
| Bockarie (unpublished) | An. punctualtus | 112.00 (72–152) | 4,018 mf/ml (2–80,32) | 2 | 15 |
Mean numbers plus ranges (in parentheses) of mosquitoes fed per mf density.
No. of human mf densities studied.
Approximate time from feeding to end of study.
These mosquitoes were from established laboratory colonies.
Variation in the methods used to estimate human mf density can cause significant problems when attempting to compare filarial infection data from different studies (Snow and Michael 2002). To allow the use of the data from different sources in this study, it was thus necessary to carry out a standardization, first to numerically standardize all measures of human mf density into mf per 20 μl of peripheral blood and then to convert all human mf densities into expected values for the mean number of mf ingested by the mosquitoes. The latter also enabled estimates of mortality based on mf intensity in the mosquito rather than human mf density, a more reliable estimate of mosquito infection levels because of nonlinearitites in the relationship between the two (Snow and Michael 2002).
We used a logistic regression framework to study the relationship between the mean mf uptake and the proportion of mosquitoes alive at the end of the extrinsic incubation period. To effectively use data from all the available studies and to generalize the results beyond these studies, binomial logistic regression analyses were carried out by fitting separate generalized linear mixed-effects models (GLMMs) to the data from each of the three vector genera (Pinheiro and Bates 2000, Zuur et al. 2007). This method enables the estimation of both the overall genera-specific relationship in addition to study-specific survival relationships within each genus. The GLMMs were fitted by either defining the intercept, or both the slope and intercept, of a linear logistic regression model for the relationship between mean mf host density and vector survival, as random effects (and so allowing analysis of not only how the overall level of survival varies between studies but also how the relationship with mf uptake intensity varies between studies within each genera [Pinheiro and Bates 2000]). Determining which of these terms required to be specified as a random parameter was done by inspecting the variation observed in the parameter values (intercepts and slopes) obtained by first fitting conventional linear logistic regressions to the data from each study (Pinheiro and Bates 2000). Thus, for each genera, after the fits of conventional linear logistic regression models relating vector survival to mf uptake, the study-specific 95% confidence intervals (CLs) of the intercepts and slopes of these fitted models were inspected to determine which of these parameters varied significantly between studies and thus required to be incorporated into the GLMM as a random effect. Both the conventional linear logistic regression and the mixed-effects logistic regression GLMM models were fitted using the Splus statistical software. The linear logistic regression model was fit to the data using the generalized linear model (GLM) function in Splus, whereas the GLMM models were fitted using the glmmPQL routine in the MASS library under a binomial family argument: this method relies on penalized quasi likelihood, an approximation to maximum likelihood, to fit mixed effects regression models (Schall 1991, Breslow and Clayton 1993). Because of the scarcity of data on mosquito survival for anopheline mosquitoes, however, only the linear logistic regression model was applied to examine the relationship between infection intensity and survival for this genus with the data from all studies pooled. In the additional study of M.J.B. (unpublished data), two groups of An. punctulatus mosquitoes were fed on two individuals with known mf density (two mf/ml [0.05 mf/20 μl] and 8,032 mf/ml [207 mf/20 μl]) and daily mortality recorded for each of the groups for the following 15 d. The survival curves of the two groups were fitted and compared by carrying out a log-rank test using the nonparametric survival analysis functions, survfit and survdiff, available in Splus (Tableman and Kim 2004).
Results
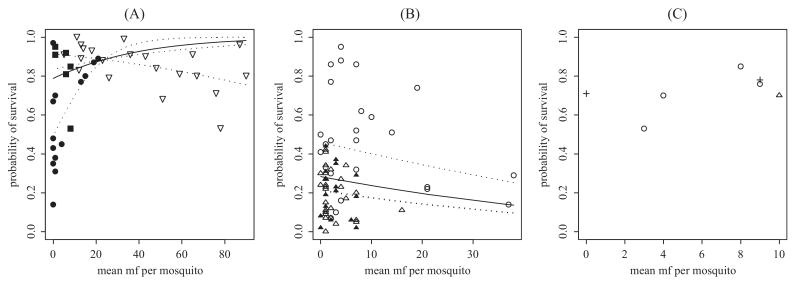
Details of the data from each of the studies used in this analysis are given in Table 1 and indicate that existing single studies on vector survival varied considerably with regard to both mosquito sample size and the range and levels of human mf density used in the survival experiments. Figure 1 shows the genera specific scatterplots of the associations between predicted mean mf intake by the mosquitoes and the proportion of vectors that survived (data from each study denoted by a separate symbol). The plotted raw data show not only the overall patterns of mosquito survival with estimated mf intake intensities for each vector genus but also the considerable effect that between-study heterogeneities may exert on these patterns. They also highlight the striking paucity of the available data for Anopheles (Fig. 1C). The overall mean genera-specific mortality was similar for Anopheles (24%) and Culex (27%) but seem to be significantly higher for the pooled Aedes data (71%).
Fig. 1.
Scatterplots of the relationship between the probability of mosquito survival and the predicted mean numbers of mf ingested per mosquito by study (each denoted by an individual symbol) for (A) Culex, (B) Aedes, and (C) Anopheles mosquitoes. The solid line represents the overall population model fit (GLMM), whereas the dashed lines denote the individual study level relationships (Pinheiro and Bates 2000). Data sources: (A) ∇, Jordan and Goatly (1962), Cx. quinquefasciatus; ●, McGreevy et al. (1982), Cx. quinquefasciatus; ■, Brito et al. (1997), Cx. quinquefasciatus; (B) Δ, Samarawickrema et al. (1985b), Ae. polynesiensis; ▲, Samarawickrema et al. (1985b), Ae. samoanus; ◯, Rosen (1955), Ae. polynesiensis; (C) ◯, Hicks (1932), An. costalis; Δ, Hicks (1932), An. funestus; +, Bockarie (unpublished data), An. punctulatus.
The results of the conventional logistic regression models used to study the effect of mf intensity on the survival rate of mosquitoes in each study are shown in Table 2. Estimated mean values and the 95% CLs of the intercept (β0) and slope (β1) parameters of the logistic linear regression model (with mf uptake as a single explanatory variable) are given for each study, along with the P values for the F-test when each full model is compared with the null model of no association between mf intensity and vector survival. It can be seen that in the case of the three studies using Aedes mosquitoes, there seems to be no significant association between mf intensity and the proportion of mosquitoes that survive the incubation period (P > 0.05 for all studies). This lack of a relationship together with the fact that the 95% CLs for the slope parameter (β1) either overlap or are very close, in contrast to the wide variation observed in the CL values of the intercept parameter (β0), led us to specify only the intercept as a random effect in the GLMM for this genus, thereby making the assumption that, while the pattern of the mf intensity–vector survival association is the same for all these studies, the level of mosquito survival may vary between studies. In contrast, the results for the three studies using Culex mosquitoes were more variable, with two of the studies showing a significant association between mf intensity and mosquito survival (Table 2). Indeed, although the largest of these studies, that of Jordan and Goatly (1962), showed a significant reduction in mosquito survival with increasing parasite intensity, the much smaller study (in terms of the range of mf uptakes studied) of McGreevy et al. (1982) paradoxically showed the opposite, with vector survival appearing to increase with increasing infection levels (Fig. 1A). These observed between-study differences in the vector survival relationship with parasitic infection intensity, and the lack of overlaps in the 95% CLs for both the parameters of each of the fitted study-specific linear logistic regressions, thus led us to incorporate both the intercept and slope as random effects in our GLMM for this genus. The analysis of the pooled Anopheles data showed mf intensity to have no significant effect on vector survival (P = 0.152); however, the paucity of the data for this genus makes drawing firm conclusions regarding the potential impact of infection intensity on vector survival difficult, and therefore we relied on the longitudinal analysis of An. punctulatus survival described below to draw firmer conclusions regarding parasite-induced vector mortality for this genus.
Table 2. Results of the generalized logistic linear model (GLM) fits to individual studies.
| Study | Valuea | 95% CL | F-value | P value |
|---|---|---|---|---|
| Culex | ||||
| Brito et al. 1997 | ||||
| B0 (intercept) | 3.299 | 2.164–4.434 | ||
| B1 (slope) | −0.262 | −0.431 to −0.093 | 2.235 | 0.209 |
| McGreevy et al. 1982 | ||||
| B0 (intercept) | −0.089 | −0.205 to −0.027 | ||
| B1 (slope) | 0.101 | 0.081–0.121 | 6.937 | 0.022 |
| Jordan and Goatly 1962 | ||||
| B0 (intercept) | 2.514 | 2.230–2.798 | ||
| B1 (slope) | −0.016 | −0.022 to −0.010 | 6.835 | 0.0177 |
| Aedes | ||||
| Rosen 1955 | ||||
| B0 (intercept) | −0.090 | −0.123 to −0.057 | ||
| B1 (slope) | −0.024 | −0.030 to −0.018 | 1.573 | 0.222 |
| Samarawickrema et al. 1985ab | ||||
| B0 (intercept) | −1.253 | − 1.331 to −1.175 | ||
| B1 (slope) | −0.060 | −0.084 to −0.036 | 1.639 | 0.211 |
| Samarawickrema et al. 1985ac | ||||
| B0 (intercept) | −1.230 | −1.363 to −1.099 | ||
| B1 (slope) | −0.072 | −0.107 to −0.037 | 0.729 | 0.406 |
| Anopheles | ||||
| Pooled data | ||||
| B0 (intercept) | 0.726 | 0.287–1.165 | ||
| B1 (slope) | 0.052 | −0.013 to 0.117 | 2.852 | 0.152 |
Presented in log odds form.
Ae. polynesiensis.
Ae. samoanus.
Table 3 portrays the results of the mixed effects logistic regression analysis carried out on the Culex and Aedes data using the glmmPQL routine described above, with model fits shown in Fig. 1. The solid lines in the graphs correspond to the overall genus-specific mf intensity–vector survival relationship as estimated by the fixed part of each of the mixed effects model described in Table 3, whereas the dashed lines show the study-specific fixed effect relationships for each genus. The results for Culex clearly indicate a lack of a significant overall relationship between mf intensity and mosquito survival in the combined data (slope of the fixed effects model component statistically insignificant, P = 0.296; Table 3), most probably as a direct result of the marked variability observed for this relationship between individual studies (as highlighted by the dashed lines in Fig. 1A). Similarly, even when the slopes of the relationships between mf intensity and vector survival were held to be similar with only the levels of mortality assumed to vary between studies for the Aedes data, the overall mean relationship for this genus was also not statistically significant (P > 0.05; Table 3; Fig. 1B).
Table 3. Results of generalized linear mixed model (GLMM) fits to Culex and Aedes mosquitoes with overall population (fixed effects) and study-specific estimates for each genus.
| Model | Value | 95% CL | df | t-value | P value |
|---|---|---|---|---|---|
| Culex | |||||
| Random slope and intercept | |||||
| B0 (intercept) | 1.304 | −0.017 to 2.625 | 37 | 1.935 | 0.061 |
| B1 (slope) | 0.032 | −0.031 to 0.095 | 37 | 1.018 | 0.315 |
| Study-specific fits | B0 (intercept) | B1 (slope) | |||
| Jordan and Goatly 1962 | 2.363 | −0.014 | |||
| McGreevy et al. 1982 | 1.616 | 0.0177 | |||
| Brito et al. 1997 | −0.034 | 0.087 | |||
| Aedes | |||||
| Random intercept | |||||
| B0 (intercept) | −0.926 | −1.627 to −0.224 | 68 | −2.589 | 0.012 |
| B1 (slope) | −0.024 | −0.053 to 0.005 | 68 | −1.588 | 0.117 |
| Study-specific fits | B0 (intercept) | B1 (slope) | |||
| Rosen 1955 | −0.158 | −0.024 | |||
| Samarawickrema et al. 1985a | −1.301 | −0.024 | |||
| Ae. polynesiensis | |||||
| Samarawickrema et al. 1985a | −1.320 | −0.024 | |||
| Ae. samoanus |
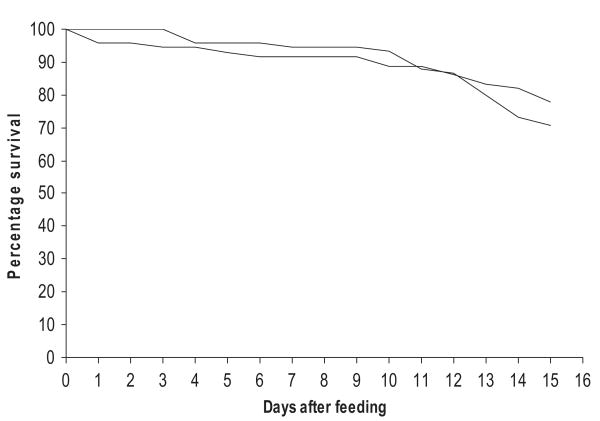
The second part of our analysis sought to supplement the modeling of the Anopheles data described above and relied on unpublished data from feeding experiments carried out using An. punctualtus s.l. in PNG. This study recorded daily survival for two batches of mosquitoes carrying high and low level infections respectively over the course of the extrinsic incubation period. Figure 2 shows the daily survival for both groups, with total mortality over the whole 15 d being 29% for the low infection group and 22% for the high. A log-rank test showed no significant difference in survival between the two groups (χ2 = 0.8, P = 0.382), supporting the above findings for Anopheles that higher loads of infection at least for the levels studied here do not cause increased mortality.
Fig. 2.
Daily mortality of two groups of An. punctulatus s.l. with different levels of infection (solid line = 0.5mf/20 μl [2 mf/ml], dashed = 207 mf/20 μl [8,032 mf/ml]). Lines represent predictions of the fits of the nonparametric survival model described in the text.
Discussion
The coevolution of parasites and their hosts is complex, but in general, natural selection would be expected to favor a parasite that did not kill its host for at least for as long as it takes for the parasite to mature and achieve successful transmission. Our results, based on combining evidence across a range of similar studies, would thus seem, on the surface, to support this outcome in the case of the survival of mosquito hosts infected with W. bancrofti. However, it is pertinent to note in this regard that for the studies using Culex mosquitoes, the largest single study, in terms of both the number of data points and range of mf intensities investigated, showed a significant study-level positive association between mf intensity and mosquito mortality (Jordan and Goatly 1962). Samarawickrema and Laurence (1978) also working with Wuchereria-infected Cx. quinquefasciatus noted a similar detrimental effect of infection on vector survival but only above intakes of 20 mf per mosquito. Jordan and Goatly (1962) is the only study to cover this range in the data included here, and hence their study result could be more representative of the true relationship for this mosquito genus. Further support that Culex requires additional investigation comes from a more recent study (Krishnamoorthy et al. 2004), in which Cx. quinquefasciatus were found to suffer 21% higher mortality after feeding on a microfilaremic volunteer as opposed to an amicrofilarmeic one, with excess mortality risk presumably related to the level of infection in the mosquitoes. The studies of McGreevy et al. (1982) and Brito et al. (1997) were also carried out using Cx. quinquefasciatus mosquitoes but covered much smaller ranges of mf intakes and showed contrastingly a decrease in mortality over this range. This result is a paradox much discussed in previous papers (Brengues and Coz 1972, Pichon et al. 1980, Bryan and Southgate 1988), with one explanation suggested being that this observed increase in survival of the infected vectors could be caused by infected mosquitoes becoming less mobile (e.g., because of the effect of infection on flight capability; Townson 1970) and hence causing themselves less injury in the cage than their lightly or uninfected counterparts (Brengues and Coz 1972, Pichon et al. 1980, Bryan and Southgate 1988). The inconsistency in the results for Culex could thus be related to both experimental artifacts and between-study differences in the ranges of human mf densities studied (and hence mf uptakes); however, overall, the results suggest that, in this genus at least, the detrimental effect of infection if or when it occurs may be manifest only at the highest mf intakes.
The situation for the other two vectors is less clear. The comparison of overall mosquito survival over the entire incubation period suggests that, on the whole, Aedes mosquitoes suffer a much higher level of mortality (71%) over all mf intensities compared with either Anopheles (24%) and Culex (27%). This supports other findings (Rosen 1955), in which this excess mortality in Aedes was attributed to the higher numbers of infective third-stage larvae (L3) developing within these vectors. We have previously shown that the maximum numbers of L3 developing in Culex and Anopheles are much lower than in Aedes as a result of stronger regulation of mf uptake and L3 development in the former mosquitoes (≈5 versus 20 L3; Snow et al. 2006), indicating that the higher levels of mosquito mortality observed in the Aedes mosquitoes may be caused by a greater development or migration of larvae through the hemocele in this mosquito. However, an intriguing finding from our study is that the higher mortality of infected Aedes mosquitoes occurred over mf intensity ranges that did not produce a similar mortality rate in Culex and Anopheles (Fig. 1), suggesting that it is unlikely that the higher mortality of these mosquitoes is related to infection intensity per se. Instead, this outcome may represent either a higher natural or experimentally induced susceptibility of these mosquitoes to carrying filarial infection. This is further supported by the fact that there seems to be little evidence of density dependence in the observed mortality in these mosquitoes with increasing infection intensity, although the data on survival for this genus is also only available up to 40 mf per host, the same burden at which detrimental effects in Culex appear.
A major limitation as far as the analysis for Anopheles is concerned is the lack of available published data making conclusions regarding infection-induced mortality difficult presently for this genus. Thus, our finding of no association between infection intensity and mortality could imply that the severe regulation in mf intake and hence restricted development of L3 seen in this genus may mean that fewer larvae are likely to be present in the mosquito to cause excess motality (Pichon 1974, Southgate and Bryan 1992, Snow and Michael 2002) or that this outcome may be an artifact of severely limited data.
Although mixed effects meta-analytical frameworks as used here may clearly provide a powerful tool for resolving questions not fully evident from single smaller studies, by essentially facilitating the combination of data appropriately from separate studies and thereby increasing analytical power to detect ecological relationships (Michael et al. 1994, Anrnqvist and Wooster 1995, Meyers and Mertz 1998, Snow and Michael 2002, Snow et al. 2006), our results support the view that the success of this approach is ultimately related to the quality of the data in primary studies (Osenberg et al. 1999). A major problem in this regard in this study was clearly the lack of large published datasets exploring parasite-induced mosquito mortality, particularly with respect to Anopheles. In addition, we showed that smaller studies are likely to describe only a small part of the entire relationship, and in particular, we highlight how the restricted ranges of x variables (here mf uptake intensities) typically covered by such experiments (McGreevy et al. 1982, Brito et al. 1997) may contribute to wide variations in study-specific survival for the same genus and even species (e.g., opposing relationships for Cx. quinquefasciatus). These findings show that future studies must use sufficiently large sample sizes and address vector survival particularly at higher mf intakes if we are to clarify and confirm the finding in this study that detrimental effects of filarial infection on mosquito survival may occur only at the higher infection intensities that may arise in filarial vectors.
Undoubtedly, an important consideration in the interpretation of these findings is the relevance of laboratory-based experiments on mosquito survival to the corresponding effects of infection under natural transmission settings. Laboratory experiments ignore many indirect costs of infection, such as reduced flight capability (Townson 1970) or reduced feeding ability (Molyneux and Jeffries 1986). Furthermore, laboratory studies often use unnatural vector–parasite couples, or laboratory colonies, removing any evolutionary resistance to the detrimental effects of infection that mosquitoes may have evolved to local parasites. Thus, infective rates seen in the laboratory may be far higher than those seen in field studies (Pichon 2002). However, although field studies may provide a more realistic estimate of mortality rates under natural conditions, there are also a number of limitations involved in the investigation of this question using wild mosquitoes. First, it is difficult to distinguish between parasite mortality versus mosquito mortality because of the added effect of age, because the operations of all of these three variables will manifest as a reduction in the numbers of infected mosquitoes, although using uninfected age-matched controls will mitigate against this problem to a significant extent. Second, there is no way of accurately estimating the density of mf in the blood of the human host on which mosquitoes fed, leading to estimates of infection levels in investigated mosquitoes to be based either on mean mf levels in the human populations or means in sampled, wild caught mosquitoes. Furthermore, factors such as multiple filarial infections (Samarawickrema 1967, Krafsur and Garrett-Jones 1977), climatic factors, and co-infection with malaria parasites (in Anopheles) could have competing detrimental effects on survival (Gad et al. 1979, Ferguson and Read 2002a), and hence confound any survival analysis. Note also that most experimental studies typically investigate vector mortality either only over or at the end of a single extrinsic incubation period. Thus, although these experiments provide information on the effect of parasite-induced vector mortality on the level of transmission from a single transmission cycle arising from mf uptake to output of L3, they provide little data on any effects that infection may have on the long-term survival rate of infected mosquitoes.
The importance of the finding of a lack of an infection effect on mosquito survival, not only for W. bancrofti but perhaps also in the case of Plasmodium (Ferguson and Read 2002b), is that it implies that interventions against both parasites are unlikely to result in enhanced parasite transmission as a result of increased vector host survival as infection intensity falls. In the case of Anopheles, it also suggests that reduction in levels of one parasite will not enhance the transmission of the other. If true, these results will clearly increase the prospects of parasite control. However, this study has highlighted how the existence of between-study heterogeneities in methodology and experimental conditions can severely confound the outcomes of experiments aiming to investigate this topic. Future studies must not only be based on well-designed studies that use standardized methods and cover relevant mf intake ranges, but must crucially also develop and apply frameworks that allow the design and interpretation of data from comparable field and laboratory studies, if we are to reliably quantify the effects of parasitic infection on vector mortality.
Acknowledgments
The authors thank the Medical Research Council, United Kingdom, for studentship support of L.S. E.M. gratefully acknowledges the financial support of U.S. Public Health Service NIH Grant R01 AI69387–01A1 for facilitating this work. M.J.B. is thankful for the financial support of U.S. Public Health Service NIH ICIDR Grant U19 AI33061 during the writing of this manuscript.
References Cited
- Anrnqvist G, Wooster D. Meta-analysis—synthesizing research findings in ecology and evolution. Trends Ecol Evol. 1995;10:236–240. doi: 10.1016/S0169-5347(00)89073-4. [DOI] [PubMed] [Google Scholar]
- Brengues J, Coz J. Receptivite comparee de trois especes du complex Anopheles gambiae Giles presentes en Afrique de l'Ouest, vis-a-vis de Wuchereria bancrofti Cobbold. Cahiers ORSTOM Ser Entomol Med Parasitol. 1972;10:207–215. [Google Scholar]
- Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88:9–25. [Google Scholar]
- Brito AC, Williams P, Fontes G, Rocha EMM. A comparison of two Brazilian populations of Culex quinquefasciatus (Say, 1823) from endemic and non-endemic areas to infection with Wuchereria bancrofti (Cobbold 1877) Mem Inst Oswaldo Cruz. 1997;92:33–36. doi: 10.1590/s0074-02761997000100007. [DOI] [PubMed] [Google Scholar]
- Bryan JH, Southgate BA. Factors affecting the transmission of Wuchereria bancrofti by anopheline mosquitoes. 2. Damage to ingested microfilariae by mosquito foregut armatures and development of filarial larvae in mosquitoes. Trans R Soc Trop Med Hyg. 1988;82:138–145. doi: 10.1016/0035-9203(88)90288-x. [DOI] [PubMed] [Google Scholar]
- Bryan JH, Oothman P, Andrews BJ, McGreevy PB. Effects of pharyngeal armature of mosquitoes on microfilariae of Brugia pahangi. Trans R Soc Trop Med Hyg. 1976;68:14. doi: 10.1016/0035-9203(74)90241-7. [DOI] [PubMed] [Google Scholar]
- Burkot TR, Durrheim DN, Metrose WD, Speare R, Ichimori K. The argument for integrating vector control with multiple drug administration campaigns to ensure elimination of lymphatic filariasis. Filaria J. 2006;5:1–7. doi: 10.1186/1475-2883-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crans JW. Experimental infection of Anopheles gambiae and Culex pipiens fatigans with Wuchereria bancrofti in coastal east Africa. J Med Entomol. 1973;10:189–193. doi: 10.1093/jmedent/10.2.189. [DOI] [PubMed] [Google Scholar]
- Das PK, Subramanian S, Manoharan A, Ramaiah KD, Vanamail P, Grenfell BT, Bundy DAP, Michael E. Frequency distribution of Wuchereria bancrofti infection in the vector host in relation to human host: evidence for density dependence. Acta Trop. 1995;60:159–165. doi: 10.1016/0001-706x(95)00123-v. [DOI] [PubMed] [Google Scholar]
- Dye C. Vectorial capacity: must we measure all its components? Parasitol Today. 1986;2:203–209. doi: 10.1016/0169-4758(86)90082-7. [DOI] [PubMed] [Google Scholar]
- Dye C. The analysis of parasite transmission by blood-sucking insects. Annu Rev Entomol. 1992;37:1–19. doi: 10.1146/annurev.en.37.010192.000245. [DOI] [PubMed] [Google Scholar]
- Ellrot D. The effect of different microfilarial densities of Brugia malayi in Mastomys natalensis on the mortality of the vector Aedes aegpti. Trop Med Parasitol. 1987;38:344. [Google Scholar]
- Failloux AB, Raymond M, Ung A, Glaziou P, Martin PMV, Pasteur N. Variation in the vector competence of Aedes polynesiensis for Wucherreria bancrofti. Parasitology. 1995;111:19–29. doi: 10.1017/s0031182000064568. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, Read AF. Genetic and environmental determinants of malaria parasite virulence in mosquitoes. Proc R Soc Lond Ser B. 2002a;269:1217–1224. doi: 10.1098/rspb.2002.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson HM, Read AF. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 2002b;18:256–261. doi: 10.1016/s1471-4922(02)02281-x. [DOI] [PubMed] [Google Scholar]
- Gad AM, Maier WA, Piekarski G. Pathology of Anopheles stephensi after infection with Plasmodium berghei. Zietschrift Parasitenkunde. 1979;60:249–261. doi: 10.1007/BF00929172. [DOI] [PubMed] [Google Scholar]
- Hicks EP. The transmission of Wuchereria bancrofti in Sierra Leone. Ann Trop Med Parasitol. 1932;26:407–422. [Google Scholar]
- Jordan P, Goatly KD. Bancroftian filariasis in Tanganyika: a quantative study of the uptake, fate and development of microfilariae of Wuchereria bancrofti in Culex fatigans. Ann Trop Med Parasitol. 1962;56:173–187. [Google Scholar]
- Krafsur ES, Garrett-Jones C. The survival in nature of Wuchereria-infected Anopheles funestus Giles in north-eastern Tanzania. Trans R Soc Trop Med Hyg. 1977;71:155–160. doi: 10.1016/0035-9203(77)90086-4. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy K, Subramanian S, van Oortmarssen GJ, Habbema JDF, Das PK. Vector survival and parasite infection: the effect of Wuchereria bancrofti on its vector Culex quinquefasciatus. Parasitology. 2004;129:1–8. doi: 10.1017/s0031182004005153. [DOI] [PubMed] [Google Scholar]
- Laurence BR. Natural mortality in two filarial vectors. Bull WHO. 1963;28:229–234. [PMC free article] [PubMed] [Google Scholar]
- Lindsay SW, Denham DA. The ability of Ae. aegypti mosquitoes to survive and transmit infective larvae of Brugia pahangi over successive blood meals J. Helminthol. 1986;60:159–168. doi: 10.1017/s0022149x00026031. [DOI] [PubMed] [Google Scholar]
- Manga L. Vector-control synergies between ‘roll back malaria’ and the Global Programme to Eliminate Lymphatic Filariasis in the African region. Ann Trop Med Parasitol. 2002;96:S129–S132. doi: 10.1179/000349802125002473. [DOI] [PubMed] [Google Scholar]
- McGreevy PB, Kolstrup N, Tao J, McGreevy MM, Marshall TF. Ingestion and development of Wuchereria bancrofti in Culex quinquefasciatus, Anopheles gambiae and Aedes aegpti after feeding on humans with varying densities of microfilariae in Tanzania. Trans R Soc Trop Med Hyg. 1982;76:288–296. doi: 10.1016/0035-9203(82)90170-5. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Mertz G. Reducing uncertainty in the bilogical basis of fisheries management by meta-analysis of data from many populations: a synthesis. Fisheries Res. 1998;37:51–60. [Google Scholar]
- Michael E, Grenfell BT, Bundy DAP. The association between microfilaraemia and disease in lymphatic filariasis. Proc R Soc Lond Ser B. 1994;256:33–40. doi: 10.1098/rspb.1994.0045. [DOI] [PubMed] [Google Scholar]
- Molyneux DH, Jeffries D. Feeding behaviour of pathogen infected vectors. Parasitology. 1986;92:721–736. doi: 10.1017/s0031182000065574. [DOI] [PubMed] [Google Scholar]
- Osenberg CW, Sarnelle O, Cooper SD, Holt RD. Resolving ecological questions through meta-analysis: goals, metrics and models. Ecology. 1999;80:1105–1117. [Google Scholar]
- Pichon G. Relations mathematiques entre le nombre des microfilares ingerees et le nombre des parasites chez differents vecteurs naturels ou experimentaux de filarioses. Cahiers ORSTOM Ser Entomol Med Parasitol. 1974;4:199–216. [Google Scholar]
- Pichon G. Limitation and facilitation in the vectors and other aspects of the dynamics of filarial transmission: the need for vector control against Anopheles-transmitted filariasis. Ann Trop Med Parasitol. 2002;96:S143–S152. doi: 10.1179/000349802125002509. [DOI] [PubMed] [Google Scholar]
- Pichon G, Prodhon J, Riviere F. Filarioses: surdispersion parasitaire et surinfection de l'hote invertebre. Cahiers ORSTOM Ser Entomol Med Parasitol. 1980;18:27–47. [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. Springer; New York: 2000. [Google Scholar]
- Rosen L. Observations on the epidemiology of human filariasis in French Oceania. Am J Hyg. 1955;61:219–248. [Google Scholar]
- Samarawickrema WA. A study of the age-composition of natural populations of Culex pipiens fatigans Wiederman in relation to the transmission of filariasis due to Wuchereria bancroft (Cobbold) in Ceylon. Bull WHO. 1967;37:117–137. [PMC free article] [PubMed] [Google Scholar]
- Samarawickrema WA, Laurence BR. Loss of filarial larvae in a natural mosquito population. Ann Trop Med Parasitol. 1978;72:561–565. doi: 10.1080/00034983.1978.11719361. [DOI] [PubMed] [Google Scholar]
- Samarawickrema WA, Spears GFS, Sone F, Ichimori K, Cummings RF. Filariasis transmission in Samoa. I. Relation between density of microfilariae and larval density in laboratory-bred and wild-caught Aedes (Stegomyia) polymesiensis (Marks) and wild-caught Aedes (Finlaya) samoanus (Gruenberg) Ann Trop Med Parasitol. 1985a;79:89–100. [PubMed] [Google Scholar]
- Samarawickrema WA, Spears GFS, Sone F, Ichimori K, Cummings RF. Filariasis transmission in Samoa. II. Some factors related to the development of microfilariae in the intermediate host. Ann Trop Med Parasitol. 1985b;79:101–107. [PubMed] [Google Scholar]
- Schall R. Estimation in generalised linear models with random effects. Biometrika. 1991;78:719–727. [Google Scholar]
- Snow LC, Michael E. Transmission dynamics of lymphatic filariasis: density-dependence in the uptake of Wuchereria bancrofti microfilariae by vector mosquitoes. Med Vet Entomol. 2002;16:409–423. doi: 10.1046/j.1365-2915.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- Snow LC, Bockarie MJ, Michael E. Transmission dynamics of lymphatic filariasis: vector-specific density dependence in the development of Wuchereria bancrofti infective larvae in mosquitoes. Med Vet Entomol. 2006;20:261–272. doi: 10.1111/j.1365-2915.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- Southgate BA, Bryan JH. Factors affecting transmission of Wuchereria bancrofti by anopheline mosquitoes. 4. Facilitation, limitation, proportionality and their epidemiological significance. Trans R Soc Trop Med Hyg. 1992;86:523–530. doi: 10.1016/0035-9203(92)90096-u. [DOI] [PubMed] [Google Scholar]
- Tableman M, Kim JS. Survival analysis using S Analysis of time-to-event data. Chapman & Hall/CRC; Boca Raton, FL: 2004. [Google Scholar]
- Townson H. The effect of infection with Brugia pahangi on the flight of Ae. aegpti. Ann Trop Med Parasitol. 1970;64:411–420. doi: 10.1080/00034983.1970.11686712. [DOI] [PubMed] [Google Scholar]
- Wharton RH. Studies on filariasis in Malaya: observations on the development of Wuchereria malayi in Mansonia (Mansonioides) longipalpis. Ann Trop Med Parasitol. 1957a;51:278–296. doi: 10.1080/00034983.1957.11685817. [DOI] [PubMed] [Google Scholar]
- Wharton RH. Studies on filariasis in Malaya: the efficiency of Mansonia longipalpis as an experimental vector of Wuchereria malayi. Ann Trop Med Parasitol. 1957b;51:422–439. doi: 10.1080/00034983.1957.11685832. [DOI] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Smith GM. Analysing ecological data. Springer; New York: 2007. [Google Scholar]