Abstract
Aripiprazole is a dopamine (DA) D2 receptor partial agonist, approved by the Food and Drug Administration (FDA) for the treatment of schizophrenia. DA receptor partial agonists have been previously assessed as potential therapeutic agents for cocaine dependence. The present experiment examined the effect of aripiprazole on methamphetamine self-administration in a rodent model of an increasing drug self-administration with prolonged session duration. Wistar rats were allowed to self-administer methamphetamine (0.05 mg/kg/injection, intravenously) in either 1-h (short access: ShA rats) or 6-h sessions (long access: LgA rats). After 15 sessions, the dose–response function of methamphetamine was determined under either a progressive- or a fixed-ratio schedule. Next, the effect of aripiprazole (0.3–10 mg/kg, subcutaneuously (s.c.)) on the dose–response function was examined. LgA rats exhibited an increasing rate of methamphetamine self-administration. Responding for methamphetamine by LgA rats was higher than that of ShA rats under both schedules. Pretreatment with aripiprazole shifted the dose–response function of methamphetamine to the right in both LgA and ShA rats. However, the effect of aripiprazole was greater in LgA than ShA rats. In in vitro receptor binding assay, no change in the level of D2 DA receptors in the nucleus accumbens and the striatum was found in any group. The present data suggest increased sensitivity of the dopaminergic system to aripiprazole in LgA rats compared with ShA rats. However, mechanisms other than downregulation of D2 DA receptors in the nucleus accumbens and the striatum may be responsible for the increased sensitivity of the dopaminergic function in LgA rats.
Keywords: methamphetamine, self-administration, escalation, dopamine receptor partial agonist, rats
INTRODUCTION
Methamphetamine is an N-methyl amphetamine analog that can be easily synthesized and has high-abuse potential. A recent report by the Community Epidemiology Work Group (2004) indicated a notable increase in methamphetamine treatment admissions in Atlanta and Minneapolis/St Paul, besides San Diego and Hawaii, suggesting an eastward spread of the drug. Similarly, the National Survey on Drug Use and Health noted an increase from 27.5% (2002) to 59.3% (2004) in the number of past month methamphetamine users who met the criteria for dependence. This epidemic of methamphetamine abuse prompted the development of pharmacological interventions of methamphetamine abuse.
One of the characteristics of drug abuse by humans is an increasing amount, that is, rate of drug intake over time (American Psychiatric Association, 2000). Previous research in our laboratory with rats found that extending session duration to 6 h engendered an increasing rate of cocaine self-administration under a fixed-ratio (FR) schedule of reinforcement (Ahmed and Koob, 1998, 1999). Using similar procedures, we established a rodent model of an increasing rate of methamphetamine self-administration (Kitamura et al, 2006). This animal model appears to have face validity for modeling an increasing rate of drug intake by drug-dependent humans. Furthermore, the finding that escalating cocaine self-administration was correlated with an increasing threshold for intracranial self-stimulation, indicative of a reduced functional state of the reward circuit (Ahmed et al, 2002), may support the construct validity of this model (Koob and Le Moal, 1997; Sinha et al, 2000). Thus, the investigation of neurobiological changes during an increasing rate of self-administration in this model may help elucidate the neural mechanisms of the development of drug dependence.
Evidence supports the notion that the increased dopamine (DA) neurotransmission by psychostimulants is involved in the reinforcing effects of the drugs that contribute to their self-administration in laboratory animals and abuse in humans (see reviews Koob, 1992; Woolverton and Johnson, 1992). With respect to drug dependence, using positron emission tomography (PET), decreased radioligand binding at the DA D2 receptors in the caudate/putamen was found in humans with methamphetamine and cocaine dependence implying the decreased dopaminergic neurotransmission in these drug abusers (Volkow et al, 2001a, 2004). Similarly, decreased DA transporters and DA in the brain were noted in chronic methamphetamine abusers (Sekine et al, 2001; Volkow et al, 2001b; Wilson et al, 1996). Therefore, the reduced dopaminergic neurotransmission during prolonged drug abuse may be associated with psychostimulant dependence manifested in an increasing rate of drug intake in humans.
In the present study, we examined the effect of aripiprazole on methamphetamine self-administration using a rodent model of an increasing rate of self-administration. Aripiprazole is a potent DA D2 receptor partial agonist (Semba et al, 1995; Burris et al, 2002; Shapiro et al, 2003) that was recently approved by the FDA for treatment of schizophrenia. A partial receptor agonist is a ligand that binds to the receptors like an endogenous ligand, but with intermediate efficacy (O’Brien, 1996). Because of this intermediate efficacy, DA receptor partial agonists can act as agonists in states of low DA tone, such as in a cocaine withdrawal state (Parsons et al, 1991), whereas they can act as antagonists in states of high-DA tone, such as in the presence of psychostimulants (Clark et al, 1991; Pulvirenti and Koob, 1994; Svensson et al, 1991). DA receptor partial agonists have previously been hypothesized to be potential therapeutic agents for cocaine dependence (see review Pulvirenti and Koob, 1994). Thus, we were interested in the effect of aripiprazole on methamphetamine self-administration, particularly in rats that exhibit an increased rate of methamphetamine self-administration compared with rats with a stable rate of self-administration. An increased rate of drug intake is associated with drug dependence in humans. Therefore, any difference in the effect of aripiprazole on methamphetamine self-administration between rats with an increased rate of self-administration and those with a stable rate of self-administration may elucidate neural adaptations underlying an increasing rate of self-administration. Additionally, changes in levels of DA D2 receptors were evaluated to directly relate the effect of aripiprazole on methamphetamine self-administration to the D2 dopaminergic system.
METHODS
All animal use procedures were approved by The Scripps Research Institute Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines (NIH Publication no. 85-23, revised 1996).
Animals and Apparatus
Twenty-eight male Wistar rats (Charles River, Hollister, CA), each weighing between 228 and 255 g (progressive-ratio (PR) rats) and between 422 and 607 g (FR rats) at the beginning of the study, served as subjects. PR rats (n = 14) were experimentally naïve at the start of the experiment. FR rats (n = 14) had histories of sucrose self-administration (p.o.) under a PR schedule and self-administration of 0.1 mg/kg/injection of methamphetamine (intravenously (i.v.)) for 16 sessions under an FR schedule. The FR rats were drug free for 23 days before this experiment. They were housed in groups of three in plastic cages with a reversed 12: 12 h light/dark cycle with lights on at 2000 h. Food and water were available ad libitum throughout the study. During experimental sessions, each rat was placed in an operant chamber, which was placed in a light- and sound-attenuating cubicle (28 × 26 × 20 cm; Med Associates Inc., St Albans, VT). The front door and the back wall of the chamber were made of transparent plastic, and the sidewalls were stainless steel. The chamber had two retractable response levers mounted on a sidewall and a food hopper located between the levers. A stimulus light was mounted above each lever. A drug injection was delivered by a syringe pump (Razel™ Scientific Instruments, Georgia, VT) located on top of the cubicle. Experimental sessions were controlled and recorded by a PC computer with custom interface and software in the experimental room.
Self-Administration Procedure
For surgery, rats were anesthetized with 2–3% of isoflurane mixed in oxygen. They were implanted with a silastic catheter (0.3 × 0.64mm OD; Dow Corning Co. Midland, MI) into the right external jugular vein under aseptic conditions. The distal end of the catheter was s.c. threaded to the back of the rat where it exited the rat via a metal guide cannule (22G, Plastics One Inc., Roanoke, VA) that was anchored at the back of the rat. After surgery, rats were given analgesics (Flunixin®, 2.5 mg/kg, s.c.). Antiobiotic (Timentin®, 20 mg, i.v.; SmithKline Beecham, Philadelphia, PA) was administered to the rats at least for a week. The catheter was daily flushed with heparinized saline (30 U/ml). The patency of catheters in the rats was tested using an ultra short-acting barbiturate, Brevital® (methohexital sodium, 10 mg/ml, 2 mg/rat), whenever a catheter failure was suspected during the study. Methamphetamine maintained a stable pattern of responding within each session under the present FR condition, which made it easy to detect abnormal self-administration behavior caused by catheter leakage or blockage in rats. Generally, a total loss of a muscle tone within 3 s after a Brevital injection indicated the patency of a catheter.
Experimental sessions were conducted once a day, 7 days a week during the dark (active) cycle. Immediately before a session, rats were transferred from the vivarium to an experimental room where the operant chambers were located. After being flushed with 0.9% saline, a rat’s indwelling catheter was connected to a tube that exited the chamber through a metal spring and a swivel and was connected to a syringe pump. After the drug delivery system was connected to the rat, the chamber was closed, and the session started immediately. The start of a session was signaled by the presentation of two response levers into the chamber. Responding on the right lever resulted in the delivery of 0.1 ml of a drug injection over 4 s. During an injection, stimulus lights above both levers were illuminated and lasted throughout the time-out period (20 s) that followed each injection. Pressing the left lever was counted but had no other programmed consequences. The session ended by the withdrawal of the levers from the chamber. After the session, the catheter was filled with 0.9% saline containing heparin (30 units/ml), and the rat was returned to the home cage.
The PR group
Rats (n = 14) were implanted with intravenous catheters as described above. After recovery, the rats were trained to self-administer 0.2 mg/kg/injection of methamphetamine in 1-h sessions under an FR 1 schedule. The dose of methamphetamine was reduced to 0.1 mg/kg/injection and then 0.05 mg/kg/injection. This process took six sessions because we tried not to expose rats to a high dose of methamphetamine for long. When the rats reached the dose of 0.05 mg/kg/injection, they were allowed to selfa-dminister the dose of methamphetamine for six more sessions (baseline sessions). After these sessions, the rats were divided into two groups balanced by the number of injections/session during the last three baseline sessions. During the escalation period, one group of rats (LgA, n = 8) was allowed to self-administer 0.05 mg/kg/injection of methamphetamine for 6 h per day under an FR 1 schedule, whereas the other group (ShA, n = 6) was allowed to do so for 1 h per day. After 15 escalation sessions, the dose–response function of methamphetamine was determined in both groups under a PR schedule. That is, various doses of methamphetamine (0, 0.05, 0.1, 0.2 mg/kg/injection) were made available in each group of rats. For the PR schedule, the response requirement began at 1 response/injection and increased according to the following equation: responses/injection = [5 × e(injection number × 0.2)] − 5 (Richardson and Roberts, 1996). When a rat failed to achieve the response requirement within 1 h, the session ended. A session length under a PR schedule was always set at 12 h, and PR sessions lasted an average of 3 h across rats. Three escalation sessions (LgA, 6 h session; ShA, 1 h session under an FR 1 schedule) separated the two-test sessions under a PR schedule. Each dose of methamphetamine was tested once in each rat. Doses of methamphetamine were tested in a counterbalanced manner across rats. After the determination of a methamphetamine dose–response function, the effect of the pretreatment of aripiprazole (1–10 mg/kg) on the methamphetamine dose–response function was examined. Various combinations of doses of aripiprazole and methamphetamine were tested in a counterbalanced sequence across rats. Doses of aripiprazole were injected s.c. 1 h before a test session. The doses of aripiprazole and the pretreatment time were determined based on the literature and an abstract publication of the literature (Semba et al, 1995; Li et al, 2005; Natesan et al, 2006; Feltenstein et al, 2006).
The FR group
The rats were briefly involved in a study on the effect of the withdrawal from methamphetamine self-administration on the reinforcing effect of a natural reinforcer, sucrose. They had been drug free for 23 days. The rats were divided into two groups based on the previous history of methamphetamine self-administration. That is, rats that previously self-administered methamphetamine in a 1-h session were assigned to a short access group (ShA, n = 7), whereas rats previously tested in a 6-h session were assigned to a long access group (LgA, n = 7). The rats were allowed to self-administer 0.05 mg/kg/injection of methamphetamine in a 6-h session (LgA rats) or in a 1-h session (ShA rats) under an FR 1 schedule. After 15 sessions of an escalation period, a dose–response function of methamphetamine was determined in each group under an FR5 schedule. More specifically, various doses of methamphetamine (0.025, 0.05, and 0.1 mg/kg/injection) were made available in a 1-h session in each group of rats, and five responses on the right lever resulted in the delivery of methamphetamine. Two escalation sessions separated the two test sessions. The reason that two sessions, instead of three as under the PR schedule, separated test sessions was that we had to finish the study before moving our laboratory to a new location. With two sessions in between, we have not observed any carry-over effect of aripiprazole on the following test session. Doses of methamphetamine were examined in a counterbalanced manner across rats. After the determination of a methamphetamine dose–response function, the effect of the pretreatment of aripiprazole on the methamphetamine dose–response function was examined. Various combinations of doses of aripiprazole and methamphetamine were tested in a counterbalanced sequence across rats. Doses of aripiprazole (0.3–1 mg/kg) were injected s.c. 1 h before a test session.
Data Analysis
The data were expressed as the mean number of injections per session as well as the mean mg/kg per session for each group of rats. The effect of session duration on methamphetamine self-administration per session as well as in the first hour of a session was examined over the first 15 escalation sessions using a two-way repeated measures analysis of variance (ANOVA; session duration × daily session; Prism 4.0, GraphPad, San Diego, CA) with the Bonferroni post hoc test. The pattern of responding for methamphetamine was expressed as the mean number of injections per hour over 6-h sessions in LgA rats and compared between the first and the 15th escalation sessions. Differences in the rate of responding between the first and the 15th escalation sessions were evaluated using the paired t-test on self-administration at each hour after the data were transformed to log values because of unequal variance (Prism 4.0, GraphPad, San Diego, CA). The dose–response functions of methamphetamine between LgA and ShA rats under FR and PR schedules were compared using the Student’s t-test on responding at each dose of methamphetamine after the data were transformed to log values because of unequal variance. The effect of pretreatment of aripiprazole on methamphetamine self-administration was compared between LgA and ShA rats at each dose of methamphetamine using two-way repeated measures ANOVA (session duration × aripiprazole dose) with the Bonferroni post hoc test. For the data under an FR schedule, the effect of aripiprazole on the maximum self-administration of methamphetamine in the dose–response function was compared within a group of LgA or ShA rats using one-way repeated measures ANOVA (Prism 4.0, GraphPad, San Diego, CA). To calculate A50 doses of aripiprazole (a dose that inhibited 50% of the maximum rate of responding after the vehicle pretreatment) for 0.025 and 0.05 mg/kg/injection of methamphetamine, respectively, under an FR and PR schedule, the group mean number of injections of methamphetamine at each dose of aripiprazole was normalized as percent of the mean number of injections after the vehicle treatment. Using linear regression over the normalized data, we calculated an A50 dose of aripiprazole in each group. We calculated the A50 dose of aripiprazole from the group mean number of injections instead of in each rat because aripiprazole did not reduce responding at all in one ShA rat of the PR group and two ShA rats of the FR group, which is an important observation. However, a significant difference between the A50 doses of aripiprazole between LgA and ShA groups was assessed using Mann–Whitney Rank-Sum test after we ranked A50 doses of individual rats across groups and ranked the ShA rats without any aripiprazole effect as the highest (Prism 4.0, GraphPad, San Diego, CA).
In Vitro D2 DA Receptor Binding Procedure
One day after the last self-administration session, all the rats were killed by decapitation. The striatum and the nucleus accumbens were collected and were immediately frozen at −80°C until assay.
The binding method was described previously (Weed et al, 1998). Briefly, frozen tissue was thawed and homogenized in 100 volume (100 ml/g tissue) of 50mM potassium phosphate buffer (pH 7.4). The tissue was then centrifuged at 25 000 g at 4°C for 10 min. The resulting pellet was resuspended in 100 volume of the potassium phosphate buffer and centrifuged. The pellet was collected and suspended in the buffer at tissue concentration of 2.0 mg/ml. The tissue (1 mg/assay) was added to each assay containing various concentrations (0.01–1.25 nM) of [3H] spiperone (15 Ci/mmol), 0.1 μM of mianserin to block 5-HT2 receptors. The potassium phosphate buffer was added to arrive at a final volume of 2 ml. The assays were incubated at 37°C for 60 min. Nonspecific binding was determined in the presence of 100 μM unlabeled s-butaclamol in assays. Binding reactions were terminated by rapid vacuum filtration through Whatman GF/C filters using a 24-well Brandel cell harvester (Brandel Co., Gaithersburg, MD, USA) with an ice-cold 50mM potassium phosphate buffer. The collected tissues on the filters were deposited into Packard Top Count deep well plates and left to dry overnight. Five hundred microliters of Microscint-20 cocktail (Packard Instruments, Downers Grove, IL) was added to each well and the plate was allowed to stand for 4 h. Radioactivity was counted using a Packard Top Count scintillation counter. Protein levels in tissue homogenate samples were determined using the bicinchoninic acid protein assay method (Smith et al, 1985). Absorbance (at 560 nM) was measured on a Beckman spectrophotometer (Beckman, Palo Alto, CA).
Data Analysis
The data were analyzed using an iterative curve fitting (Prism4, Graphpad, San Diego, CA). Maximum radioligand binding (Bmax) and affinity of a radioligand (Kd) in tissue samples were calculated.
Drugs
For self-administration, d-methamphetamine hydrochloride was provided by the National Institute on Drug Abuse (Rockville, MD). Aripiprazole was purchased from Toronto Research Chemicals Inc. (Ontario, Canada). Methamphetamine was dissolved in sterile 0.9% saline for self-administration. Aripiprazole was dissolved in 30% dimethyl-foramide in sterile water that was acidified with glacial acetic acid (2%). All drug solutions were prepared for each rat based on its body weight and were updated every 2 or 3 days. Doses of methamphetamine were expressed as salt whereas aripiprazole was expressed as base. For in vitro D2 DA receptor binding, mianserin, and s-butaclamol were purchased from Research Biochemical International (Natick, MA). [3H]Spiperone was purchased from Perkin Elmer Life Sciences (Boston, MA). All drug solutions were freshly prepared for each experiment.
RESULTS
Self-Administration by the PR Group
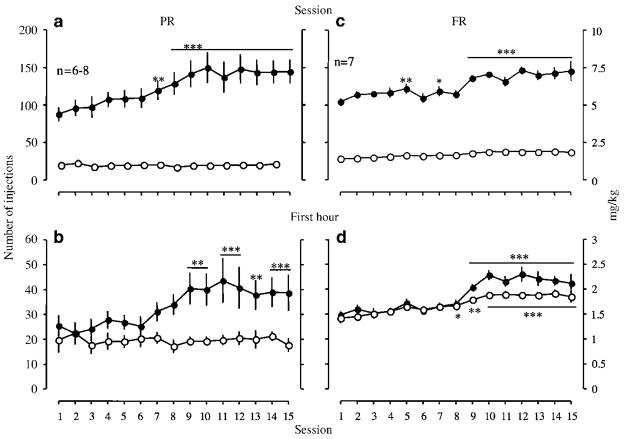
In LgA rats, the self-administration of 0.05 mg/kg/injection of methamphetamine per session (Figure 1a, solid symbols) as well as during the first hour of a session (Figure 1b, solid symbols) significantly increased over 15 days in 6-h sessions, compared with that in the first escalation session, starting in sessions 7 and 9, respectively. Self-administration of methamphetamine at this level by LgA rats was maintained over the course of the study. This effect was not seen in ShA rats responding in 1-h sessions. Although self-administration of methamphetamine by ShA rats remained stable during the 15 days of the escalation period, it showed a tendency to increase over the course of the study.
Figure 1.
Self-administration of methamphetamine by rats under a FR schedule during the escalation period. The two panels on the left (a, b) are the data from rats of the PR group, and the panels on the right (c, d) are the data from rats of the FR group. The upper panels (a, c) are the data from entire sessions, and the lower panels (b, d) are the data from the first hour of the sessions. Data are expressed as the number of injections on the left axis and mg/kg on the right axis. Error bars are SEM values for n = 6–8. Filled circles indicate self-administration by rats in 6-h sessions (LgA), and open circles indicate self-administration by rats in 1-h sessions (ShA). *p<0.05, **p<0.01, ***p<0.001 compared with session 1.
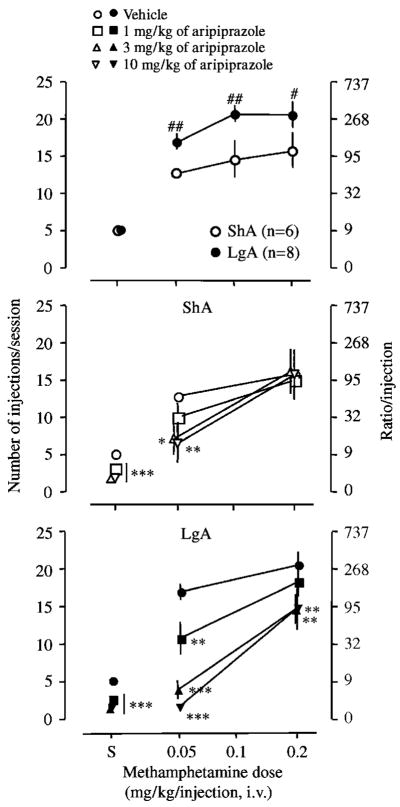
When the dose–response function of methamphetamine was determined under a PR schedule, methamphetamine maintained higher responding, that is, breakpoint, in the LgA than ShA rats (p<0.05, Figure 2, top). The pretreatment of aripiprazole decreased responding for 0.05 mg/kg/injection of methamphetamine in ShA rats without affecting responding for 0.2 mg/kg/injection of methamphetamine (Figure 2, middle). In LgA rats, aripiprazole decreased responding for both 0.05 and 0.2 mg/kg/injection of methamphetamine, shifting the dose–response function of methamphetamine to the right and downward (Figure 2, bottom). Furthermore, aripiprazole significantly reduced the maximum responding of methamphetamine in the LgA rats to a level similar to that of the ShA rats (Figure 2, bottom). The mean A50 dose of aripiprazole at 0.05 mg/kg/injection of methamphetamine were 4.72 and 8.37 mg/kg, respectively, in LgA and ShA rats, and the A50 dose of aripiprazole in LgA rats was significantly smaller than that of the ShA rats (Mann–Whitney U = 7.0, p<0.05). Aripiprazole also significantly decreased responding for saline in both groups of rats at all doses (Figure 2).
Figure 2.
Effect of aripiprazole on the dose–response function of methamphetamine self-administration under a PR schedule. Test sessions under a PR schedule ended when rats did not achieve reinforcement within an hour. The top panel is the dose–response function of methamphetamine determined after 15 escalation sessions. The middle panel is the data from ShA rats. The bottom panel is the data from LgA rats. Data are expressed as the number of injections/session on the left axis and the ratio per injection on the right axis. Error bars are SEM values. Filled symbols indicate the data of LgA rats, and open symbols indicate the data of ShA rats. Circles indicate the vehicle treatment; squares, 1 mg/kg of aripiprazole; triangles, 3 mg/kg of aripiprazole; inverted triangles, 10 mg/kg of aripiprazole. Aripiprazole was subcutaneuously injected 1 h before test sessions. S: saline. #p<0.05, ##p<0.01 compared with responding by ShA rats at the same dose of methamphetamine. *p<0.05, **p<0.01, ***p<0.001 compared with the vehicle treatment at the same dose of methamphetamine.
Self-Administration by the FR Group
In this group of rats, the self-administration of 0.05 mg/kg/injection of methamphetamine by LgA rats increased starting in session 5 with a 6-h session duration (Figure 1c). The self-administration of methamphetamine in the first hour of a session by LgA rats also significantly increased starting in session 9 (Figure 1d). The ShA rats also demonstrated a significant increase in the rate of self-administration starting in session 8 (Figure 1d). However, two-way ANOVA found a significant interaction between session duration and daily session on escalation in the rate of self-administration between ShA and LgA rats in the first hour (F(14, 168) = 2.12, p<0.05). Moreover, there was a nearly significant effect of session duration on escalation in the rate of self-administration (F (1, 168) = 4.36, p = 0.058). The increased level of self-administration during the escalation period was maintained in both LgA and ShA rats throughout the remainder of the study.
When responding for three doses of methamphetamine (0.025, 0.05, and 0.1 mg/kg/injection) was determined under an FR5 schedule, only the descending limb of the dose–response function of methamphetamine was observed in both LgA and ShA rats (Figure 3). Responding for methamphetamine was higher in LgA than ShA rats at all doses tested (p<0.05, Figure 3, top). In both ShA and LgA rats, the pretreatment with doses of aripiprazole (0, 0.3, 1 mg/kg) dose-dependently decreased responding for 0.025mg/kg/injection of methamphetamine, whereas it increased responding for 0.1 mg/kg/injection of methamphetamine. In addition, aripiprazole dose-dependently reduced the maximum self-administration of methamphetamine in the dose–response function in both ShA and LgA rats (ShA, 33.5 ± 5.0 to 20.4 ± 1.3 injections/session, p<0.05; LgA, 48.3 ± 4.1 to 25.8 ± 1.8 injections/session, p<0.01). The mean A50 doses of aripiprazole at 0.025 mg/kg/injection of methamphetamine were 0.62 and 1.02 mg/kg, respectively, in LgA and ShA rats, but with no significant difference between the groups (Mann–Whitney U = 15.0, p>0.1).
Figure 3.
Effect of aripiprazole on the dose–response function of methamphetamine under a FR schedule. Test sessions lasted 1 h. The top panel is the dose–response function of methamphetamine determined after 15 escalation sessions. The middle panel is the data from ShA rats. The bottom panel is the data from LgA rats. Data are expressed as the number of injections/session. Error bars are SEM values. Filled symbols indicate responding by LgA rats, and open symbols indicate responding by ShA rats. Circles indicate the vehicle treatment; squares, 0.3 mg/kg of aripiprazole; triangles, 1 mg/kg of aripiprazole. Aripiprazole was s.c. injected 1 h before test sessions. #p<0.05 compared with responding by ShA rats at the same dose of methamphetamine. *p<0.05, ***p<0.001 compared with the vehicle treatment at the same dose of methamphetamine.
In Vitro D2 DA Receptor Binding at the Striatum and the Nucleus Accumbens
The binding of [3H]spiperone in the nucleus accumbens and in the striatum fitted a one-site binding model (data not shown). The brain tissues of drug naïve rats that were littermates of FR rats served as control. The Bmax in all the groups was similar (Table 1; p>0.5). Likewise, the Kd at D2 DA receptors did not differ across groups (p>0.4).
Table 1.
In Vitro D2 Dopamine Receptor Binding
| Nucleus accumbens (n = 6–8) |
Striatum (n = 3) |
|||
|---|---|---|---|---|
| Bmax (fmol/mg protein) | Kd (nM) | Bmax (fmol/mg protein) | Kd (nM) | |
| Naive | 229.7 ± 19.5 | 0.037 ± 0.004 | 235.1 ± 11.3 | 0.036 ± 0.007 |
| FR ShA | 257.3 ± 1.8 | 0.052 ± 0.013 | 281.4 ± 36.2 | 0.028 ± 0.004 |
| FR LgA | 260.8 ± 16.7 | 0.057 ± 0.011 | 281.9 ± 8.1 | 0.032 ± 0.002 |
| PR ShA | 259.6 ± 13.9 | 0.056 ± 0.004 | 256.8 ± 22.8 | 0.031 ± 0.006 |
| PR LgA | 275.5 ± 40.2 | 0.043 ± 0.0007 | 242.8 ± 43.1 | 0.030 ± 0.003 |
Data represent mean ± SEM. The data are from two (nucleus accumbens) or three (striatum) experiments, each experiment performed in duplicate. Because of the small amount of nucleus accumbens tissue in each rat, one experiment was performed using the tissues pooled from three to four animals.
DISCUSSION
Under the present conditions, methamphetamine functioned as a positive reinforcer in all rats consistent with its abuse potential in humans. When the session duration was extended to 6 h, the increased rate of self-administration of methamphetamine in LgA rats was observed compared with that in ShA rats with 1-h session duration. The increased rate of self-administration of cocaine, heroin, and methamphetamine was previously demonstrated in rats with prolonged session duration (Ahmed and Koob, 1998; Ahmed et al, 2000; Kitamura et al, 2006). In human abusers, it was noted that the rate of drug intake increased during the development of drug dependence (American Psychiatric Association, 2000). In this regard, this animal model with extended session duration may mimic drug-taking behavior by drug-dependent humans.
ShA rats of the FR group also showed an increase in the rate of self-administration. The exact reasons are not clear. However, the experimental history of the rats may explain this observation. Several studies have shown that pre-exposure to either drugs or different schedules of reinforcement influenced cocaine self-administration (Panlilio et al, 2006; Morgan et al, 2002). The rats of the present FR group had previously been trained to respond for sucrose under a PR schedule where response requirement per reinforcement exponentially increased within a session. Moreover, the rats were exposed to self-administration of 0.1 mg/kg/injection of methamphetamine for 16 sessions. Thus, pre-exposure to a PR schedule and to a higher dose of methamphetamine (0.1 mg/kg/injection) than the baseline dose (0.05 mg/kg/injection) in the present study may have contributed to an increase in the rate of self-administration in ShA rats of the FR group, an effect producing a mild escalation-like action.
In agreement with earlier reports with cocaine (Ahmed and Koob, 1998; Paterson and Markou, 2003), doses of methamphetamine maintained higher responding in LgA than ShA rats under the present PR and FR schedules. This upward shift of the dose–response function of psychostimulants was previously suggested to indicate the development of a form of tolerance termed ‘reward allostasis’ (Ahmed and Koob, 2005). Although tolerance to a given effect of a drug is generally characterized by a rightward shift of the dose–response function of the drug (Hardman et al, 1996), not all tolerance is a competitive interaction (Colpaert, 1996). The upward shift of the dose–response function of cocaine may also reflect tolerance to a rate-decreasing effect of cocaine in LgA rats (Zernig et al, 2004). Several arguments have been raised in defense of both positions, and a parsimonious explanation is that both mechanisms play a role (Ahmed and Koob, 2004b).
The present data under PR and FR schedules suggest that pretreatment with aripiprazole, a DA D2 receptor partial agonist, shifted the dose–response function of methamphetamine to the right in both LgA and ShA rats. Additionally, the effects of aripiprazole were significantly greater in LgA than in ShA rats under the PR schedule. A rightward shift of dose–response functions of psychostimulants has been previously found with pretreatment of DA receptor antagonists (Depoortere et al, 1993; Bergman et al, 1990). As discussed above, a DA receptor partial agonist acts as an agonist under conditions of a low DA tone, whereas it acts as an antagonist under conditions of a high tone of DA (Clark et al, 1991; Pulvirenti and Koob, 1994; Svensson et al, 1991). Thus, in the presence of methamphetamine, a potent DA releaser, aripiprazole was likely to act as a DA receptor antagonist, which is in agreement with the present results.
One concern may be that the doses of aripiprazole (3 and 10 mg/kg) used under the PR schedule produced a rate-decreasing effect on operant responding. As mentioned above, aripiprazole appears to have a similar profile to DA D2 receptor antagonists in producing surmountable antagonism, that is, downward and rightward shifts in stimulant self-administration that are partly associated with direct rate-decreasing effects of the drug. Under the present PR schedule, there was decreased responding for saline in rats after the pretreatment of aripiprazole (1–10 mg/kg). Similarly, a modest rate-decreasing effect of aripiprazole (2.5–5 mg/kg) was observed on operant responding and locomotor activity in rats (Marona-Lewicka and Nichols, 2004; Schwabe and Koch, 2006; Feltenstein et al, 2006) although behavior-disrupting effect of aripiprazole appeared to depend on which kind of behaviors was analyzed (Li et al, 2005). Therefore, it is possible that a rate-decreasing effect of aripiprazole is associated with the data under a PR schedule. However, in the present study, aripiprazole did not decrease responding for 0.2 mg/kg/injection of methamphetamine in ShA rats under the PR schedule suggesting that the effect of aripiprazole in LgA rats did result not simply from a nonspecific rate-decreasing effect, but also from a specific effect of aripiprazole on methamphetamine self-administration.
A similar argument can be made regarding the dose of 1 mg/kg of arpiprazole. About 1 mg/kg of aripiprazole significantly decreased responding for 0.05 mg/kg/injection of methamphetamine in the LgA rats under the PR schedule. However, the same dose of aripiprazole significantly increased responding for 0.1 mg/kg/injection of methamphetamine in the FR group of rats suggesting that 1 mg/kg of aripiprazole exerted a specific effect on the reinforcing effect of methamphetamine. Consequently, although doses of aripiprazole may have some rate-decreasing effect on responding, the results still suggest that methamphetamine self-administration was more sensitive to aripiprazole pretreatment in LgA rats than in ShA rats and that this differential effect of aripiprazole on methamphetamine self-administration resulted from a specific interaction between aripiprazole and the reinforcing effect of methamphetamine.
Previously, PET studies showed decreased radioligand binding at DA D2 receptors in stimulant-, alcohol- and heroin-dependent humans, which has been interpreted as a constitutive decrease in the DA system function (Volkow et al, 2001a, 2004). Therefore, we hypothesized that the adaptation in dopaminergic function may be related to an increased rate of methamphetamine self-administration and, perhaps, the increased reinforcing effect of methamphetamine in LgA rats with prolonged session duration. In the present study, the effect of aripiprazole on responding was greater in LgA rats than in ShA rats under a PR schedule. This suggests that the dopaminergic system in LgA rats was more sensitive to the antagonizing action of aripiprazole than in ShA rats providing support for our hypothesis. The present results were consistent with those found with cocaine in that rats with extended access to cocaine exhibited increased sensitivity to pretreatment with cis-flupenthixol, a D1/D2 DA receptor antagonist, in cocaine self-administration (Ahmed and Koob, 2004a).
Previously, in our laboratory, no changes in DA transporter function and the level of DA release by cocaine were found in the nucleus accumbens of LgA rats compared with ShA rats (Ahmed et al, 2003). In humans, the decreased level of D2 receptors in the caudate/putamen was found with methamphetamine and cocaine dependence using PET (Volkow et al, 2001a, 2004). Accordingly, we speculated that the decreased level of D2 receptors in LgA rats may underlie the escalation of self-administration with extended access to methamphetamine. In the present study, however, neither the affinity nor the number of the D2 receptors was changed in the nucleus accumbens and the striatum of all rats compared with drug-naïve rats. The reasons for the discrepancy between the behavioral effect of aripiprazole and no change in D2 receptors in the nucleus accumbens and the striatum in LgA rats are speculative at this point. However, the dopaminergic system in other brain areas may be involved in the decreased dopaminergic function in LgA rats with prolonged access to methamphetamine. In fact, Stefanski et al (1999) have shown that daily 1.4–2 mg/kg of methamphetamine self-administration for 5 weeks produced a reduced level of D2 receptor binding in the substantia nigracompacta and the ventral tegmental area, whereas no change was observed in the nucleus accumbens and the caudate. Alternatively, there may be changes in transduction and intracellular signaling that mediate the increased sensitivity to aripiprazole. But, clearly, further research is needed.
One may speculate that no experience of operant responding in drug-naïve rats could affect D2 receptor binding compared with the ShA and LgA rats. Neural responses to psychostimulants may vary depending on a pattern of drug administration even between passive and self-administration of methamphetamine (Stefanski et al, 1999). Nonetheless, the lack of differences across the three groups of rats (naïve, ShA, LgA) in the present results appears to rule out this concern. Moreover, Ben-Shahar et al (2005, 2006) did not find differences in the level of DA transporters and c-fos level in various brain areas between ‘saline self-administered’ rats and cocaine self-administered LgA rats. The report that the DAT binding and DA level did not differ between naïve rats and rats with passive administration of gradually increasing doses of methamphetamine also supports our results (Segal et al, 2003).
In addition to its action at DA D2 receptors, aripiprazole has high affinity at 5-HT1A and 5-HT2A receptors (Burris et al, 2002; Shapiro et al, 2003; Marona-Lewicka and Nichols, 2004). Therefore, it is possible that aripiprazole influenced methamphetamine self-administration via an interaction with 5-HT1A and 5-HT2A receptors. However, Natesan et al (2006) demonstrated that doses up to 10 mg/kg of aripiprazole occupied less than 10% of 5-HT2 receptors in vivo in contrast to over 80% of the occupancy at DA D2 receptors in rats. This finding appears to minimize the role of 5-HT2A receptors in the action of aripiprazole in the present study. With respect to 5-HT1A receptors, aripiprazole was reported to be a partial or full agonist at the receptor (Bardin et al, 2006; Bruins Slot et al, 2006). Methamphetamine is also a weak 5-HT releaser suggesting that aripiprazole may have acted as an agonist at 5-HT1A receptors. To date, there is no clear evidence on the relationship between 5-HT1A receptors and psychostimulant self-administration. However, 8-OH DPAT, a 5-HT1A receptor agonist, was shown to increase responding for a low dose of cocaine in monkeys (Czoty et al, 2005). In the present study, aripiprazole decreased cocaine self-administration, an action not consistent with an agonist action at 5-HT1A receptors.
Another notable effect of aripiprazole on methamphetamine self-administration was a reduction in the maximum responding for methamphetamine in LgA rats under both PR and FR schedules, which was not apparent in ShA rats. Specifically, the break point for methamphetamine in LgA rats under a PR schedule was decreased to a level similar to that of ShA rats. This suggests that pretreatment with aripiprazole stabilized the increased self-administration in LgA rats to the level before escalation. Previously, it was shown that cis-flupenthixol, a DA receptor antagonist, completely reduced responding for cocaine in both LgA and ShA rats under an FR schedule (Ahmed and Koob, 2004a). In contrast, quinpirole, a full D2 receptor agonist, failed to alter the break point for amphetamine in rats under a PR schedule (Izzo et al, 2001). These data together support the hypothesis that a DA receptor partial agonist may stabilize dopaminergic neurotransmission in contrast to a direct agonist or antagonist.
Similar results were reported in humans in that 20 mg of aripiprazole decreased the subjective effects and the discriminative-stimulus effect of amphetamine (15 mg, p.o.; Lile et al, 2005). At this point, there is a paucity of data comparing aripirazole with other partial DA receptor agonists in relation to psychostimulant self-administration. However, the fact that, unlike BP 897 (a partial D3 receptor agonist, Garcia-Ladona and Cox, 2003), aripiprazole did not produce catalepsy in rats despite over 80% of D2 receptor occupancy (Natesan et al, 2006), and that aripiprazole is available in the market as an antipsychotic may make aripiprazole useful as proof of principle for the concept of partial agonists in the treatment of psychostimulant dependence.
Collectively, the data suggest that increased sensitivity to aripiprazole in LgA rats, compared with ShA rats, was related to escalation in the rate of methamphetamine self-administration with prolonged session duration. However, mechanisms other than downregulation of D2 receptors in the nucleus accumbens and the striatum may be responsible for the increased sensitivity to aripiprazole in LgA rats.
Acknowledgments
We thank Dr John C Kermode for suggestions on statistical analysis. We gratefully acknowledge the technical assistance of Mike Pham, who was an undergraduate student of the University of California in San Diego, in the self-administration study. We also thank Mike Arends for editorial assistance and Sheila Specio for the rats of the FR group. This is publication number 18280 from The Scripps Research Institute. This study was supported by the National Institute on Drug Abuse Grants DA010072 (GFK) and DA10352 (WLW).
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Changes in response to a dopamine receptor antagonist in rats with escalating cocaine intake. Psychopharmacology. 2004a;172:450–454. doi: 10.1007/s00213-003-1682-9. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Vertical shifts in dose–injection curves reflect reward allostasis, not sensitization (commentary on Zernig et al, ‘Do vertical shifts in dose–response rate-relationships in operant conditioning procedures indicate ‘sensitization’ to ‘drug wanting”) Psychopharmacology. 2004b;171:354–355. doi: 10.1007/s00213-003-1601-0. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology. 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lin D, Koob GF, Parsons LH. Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J Neurochem. 2003;86:102–113. doi: 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. IV-TR. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Bardin L, Kleven MS, Barret-Grevoz C, Depoortere R, Newman-Tancredi A. Antipsychotic-like vs cataleptogenic actions in mice of novel antipsychotics having D2 antagonist and 5-HT1A agonist properties. Neuropsychopharmacology. 2006;31:1869–1879. doi: 10.1038/sj.npp.1300940. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Ettenberg A. One hour, but not six hours, of daily access to self-administered cocaine results in elevated levels of the dopamine transporter. Brain Res. 2006;1095:148–153. doi: 10.1016/j.brainres.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Jacob B, Roarty MP, Ettenberg A. Prolonged daily exposure to i.v. cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav. 2005;82:411–416. doi: 10.1016/j.pbb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. Antagonism of cocaine self-administration by selective dopamine D(1) and D(2) antagonists. Behav Pharmacol. 1990;1:355–363. doi: 10.1097/00008877-199000140-00009. [DOI] [PubMed] [Google Scholar]
- Bruins Slot LA, De Vries L, Newman-Tancredi A, Cussac D. Differential profile of antipsychotics at serotonin 5-HT1A and dopamine D2S receptors coupled to extracellular signal-regulated kinase. Eur J Pharmacol. 2006;534:63–70. doi: 10.1016/j.ejphar.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- Clark D, Furmidge LJ, Petry N, Tong Z-Y, Ericsson M, Johnson D. Behavioural profile of partial D2 dopamine receptor agonists: 1. Atypical inhibition of d-amphetamine-induced locomotor hyperactivity and stereotypy. Psychopharmacology. 1991;105:381–392. doi: 10.1007/BF02244434. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. System theory of pain and of opiate analgesia: no tolerance to opiates. Pharmacol Rev. 1996;48:355–402. [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Effects of the 5-HT(1A) agonist (+/−)-8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on cocaine choice in cynomolgus monkeys. Behav Pharmacol. 2005;16:187–191. doi: 10.1097/00008877-200505000-00008. [DOI] [PubMed] [Google Scholar]
- Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav. 1993;45:539–548. doi: 10.1016/0091-3057(93)90503-l. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Altar CA, See RE. Aripiprazole blocks reinstatement of cocaine seeking in an animal model of relapse. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.04.010. in press. [DOI] [PubMed] [Google Scholar]
- Garcia-Ladona FJ, Cox BF. BP 897, a selective dopamine D3 receptor ligand with therapeutic potential for the treatment of cocaine-addiction. CNS Drug Rev. 2003;9:141–158. doi: 10.1111/j.1527-3458.2003.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 9. The McGraw-Hill Co; New York: 1996. pp. 557–560. [Google Scholar]
- Izzo E, Orsini C, Koob GF, Pulvirenti L. A dopamine partial agonist and antagonist block amphetamine self-administration in a progressive ratio schedule. Pharmacol Biochem Behav. 2001;68:701–708. doi: 10.1016/s0091-3057(01)00472-5. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose–effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann NY Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Li M, Budin R, Fleming AS, Kapur S. Effects of novel antipsychotics, amisulpiride and aripiprazole, on maternal behavior in rats. Psychopharmacology. 2005;181:600–610. doi: 10.1007/s00213-005-0091-7. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Vansickel AR, Glaser PE, Hays LR, Rush CR. Aripiprazole attenuates the discriminative-stimulus and subject-rated effects of D-amphetamine in humans. Neuropsychopharmacology. 2005;30:2103–2114. doi: 10.1038/sj.npp.1300803. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. Aripiprazole (OPC-14597) fully substitutes for the 5-HT1A receptor agonist LY293284 in the drug discrimination assay in rats. Psychopharmacology. 2004;172:415–421. doi: 10.1007/s00213-003-1677-6. [DOI] [PubMed] [Google Scholar]
- Morgan D, Brebner K, Lynch WJ, Roberts DC. Increases in the reinforcing efficacy of cocaine after particular histories of reinforcement. Behav Pharmacol. 2002;13:389–396. doi: 10.1097/00008877-200209000-00012. [DOI] [PubMed] [Google Scholar]
- Natesan S, Reckless GE, Nobrega JN, Fletcher PJ, Kapur S. Dissociation between in vivo occupancy and functional antagonism of dopamine D2 receptors: comparing aripiprazole to other antipsychotics in animal models. Neuropsychopharmacology. 2006;31:1854–1863. doi: 10.1038/sj.npp.1300983. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Drug addiction and drug abuse. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 9. The McGraw-Hill; New York: 1996. pp. 557–577. [Google Scholar]
- Panlilio LV, Solinas M, Matthews SA, Goldberg SR. Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301109. in press. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB., Jr Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Koob GF. Dopamine receptor agonists, partial agonists and psychostimulant addiction. Trends Pharmacol Sci. 1994;15:374–379. doi: 10.1016/0165-6147(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Schwabe K, Koch M. Effects of aripiprazole on operant responding for a natural reward after psychostimulant withdrawal in rats. Psychopharmacology. 2006 doi: 10.1007/s00213-006-0520-2. in press. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, O’Neil ML, Melega WP, Cho AK. Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical profiles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharmacology. 2003;28:1730–1740. doi: 10.1038/sj.npp.1300247. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, et al. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Semba J, Watanabe A, Kito S, Toru M. Behavioural and neurochemical effects of OPC-14597, a novel antipsychotic drug, on dopaminergic mechanisms in rat brain. Neuropharmacology. 1995;34:785–791. doi: 10.1016/0028-3908(95)00059-f. [DOI] [PubMed] [Google Scholar]
- Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- Svensson K, Ekman A, Piercey MF, Hoffmann WE, Lum JT, Carlsson A. Effects of the partial dopamine receptor agonists SDZ 208-911, SDZ 208-912 and terguride on central monoamine receptors: a behavioral, biochemical and electrophysiological study. Naunyn–Schmiedebergs Arch Pharmacol. 1991;344:263–274. doi: 10.1007/BF00182999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001b;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Weed MR, Woolverton WL, Paul IA. Dopamine D1 and D2 receptor selectivities of phenyl-benzazepines in rhesus monkey striata. Eur J Pharmacol. 1998;361:129–142. doi: 10.1016/s0014-2999(98)00669-4. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Johnson KM. Neurobiology of cocaine abuse. Trends Pharmacol Sci. 1992;13:193–200. doi: 10.1016/0165-6147(92)90063-c. [DOI] [PubMed] [Google Scholar]
- Zernig G, Wakonigg G, Madlung E, Haring C, Saria A. Do vertical shifts in dose–response rate-relationships in operant conditioning procedures indicate ‘sensitization’ to ‘drug wanting’? Psychopharmacology. 2004;171:349–351. doi: 10.1007/s00213-003-1601-0. [DOI] [PubMed] [Google Scholar]