Abstract
Marginal zone (MZ) B cells are absent in CD19-/- mice. Possible causes include an intrinsic defect in B cells and/or a secondary defect in the extrinsic MZ microenvironment as a result of changes in B cell differentiation in mice lacking CD19. Cells in the MZ also include MZ macrophages (MZM) and MZ dendritic cells (DC). Although CD19 is only expressed on B cells, SIGN-R1+ MZM are absent and CD11c+ MZ DC distribution is abnormal in CD19-/- mice. Adoptively transferred B cells from normal mice are able to reconstitute MZ B cells in CD19-/- mice. In contrast, CD19-/- B cells could not enter the MZ of the normal mice. Furthermore, MZM distribution and MZ DC distribution are restored following MZ B cell reconstitution in CD19-/- mice. Thus, MZ B cells are required for MZM differentiation and MZ DC localization, but the deficiency of MZ B cells in CD19-/- mice is caused by a defect of intrinsic B cell signaling.
In CD19-deficient mice, B cells complete differentiation in the bone marrow to become mature follicular (FO)4 B cells, but marginal zone (MZ) B cells are largely absent (1). Mice without MZ B cells show impaired pathogen defense (2, 3). How CD19 regulates MZ B cells is unknown. Both transitional and mature FO B cells are able to differentiate into MZ B cells (4, 5). The transition to the MZ compartment is governed in part by BCR signals (6). Lack of CD19 may render B cells unable to generate the BCR signal necessary for MZ B cell differentiation. This would represent a primary B cell-intrinsic defect. In contrast, the MZ consists of a complex of structures and cells in addition to B cells, including mucosal addressin cell adhesion molecule (MAdCAM)-1+ sinus lining cells, ERTR7+ stromal cells, sialic acid-binding Ig-like lectin (SIGLEC)-1+ marginal metallophilic macrophages (MMM), specific intracellular adhesion molecule-grabbing nonintegrin receptor (SIGNR)1+ MZ macrophages (MZM), and CD11c+ MZ dendritic cells (DC) (7, 8). Previous studies have shown that the MZ environment fails to form properly in mice that lack all B cells (9, 10). Thus, an alternative hypothesis is that the absence of CD19 on B cells results in developmental defects in other MZ structures such that the MZ is incapable of hosting MZ B cells, a secondary, B cell-extrinsic defect. The goals of this study are to determine whether there are secondary defects in the MZ in CD19-deficient mice and, if so, whether these defects are the cause of the failure to form mature MZ B cells.
Materials and Methods
C57BL/6 CD45.1 and CD45.2 mice were obtained from The Jackson Laboratory. The CD19 knockout (CD19ko) was crossed from 129Sv/J to CD57Bl/6 for 14 generations (11). CD45.1, CD19ko mice were created by further crosses. All experiments were performed with age- and sex-matched mice. The University of Alabama at Birmingham Institutional Animal Care and Use Committee approved all mouse protocols. Splenic B cells were enriched by negative selection before an adoptive transfer procedure as previous described (12). Purity of B cells was typically 97% by flow cytometry analysis. Twenty million purified B cells from donor mice (8-12 wk) were transferred i.v. into recipient mice. Recipient mice were sacrificed 7 or 17 days after transfer. Cells and tissues were analyzed by flow cytometry and immunofluorescence microscopy as previously described (13, 14). Abs to the following targets were purchased and, in some cases, conjugated to Alexa fluorochromes: C1qRp (AA4.1-allophycocyanin, eBioscience); IgM (II-41-FITC, BD Pharmingen; II-41-PE-Cy7, eBioscience); CD23 (B3B4-PE, BD Pharmingen); B220 (RA3-6B2-PerCP and allophycocyanin-Cy7, BD Pharmingen); CD1d (1B1-FITC, BD Pharmingen), CD19 (6D5-SPRD, SouthernBiotech); CD21/35 (7G6-FITC, BD Pharmingen; eBio4E3-Pacific Blue, eBioscience); CD45.1 (A20-Alexa Fluor 488, BioLegend); IgM (goat anti-mouse Alexa Fluor 555, Invitrogen); CD4 (Alexa Fluor 647, Caltag Laboratories); Moma-1 (anti-SIGLEC-1-biotin or purified, BMA Biomedicals), peanut agglutinin (Alexa Fluor 488, Vector Laboratories), ER-TR9 (anti-mouse SIGN-R1-biotin; BMA Biomedicals), CD11c (N418-Alexa Fluor 488, BioLegend; HL3-allophycocyanin, BD Pharmingen), CD11b (allophycocyanin, SouthernBiotech), MAdCAM-1 (MECA-367-purified, BD Pharmingen), ER-TR7 (rat anti-mouse reticular fibroblast-purified; BMA Biomedicals), CR-Fc (containing the cysteine-rich domain of the mannose-binding receptor) was a gift from Dr. L. Martinez-Pomares (University of Nottingham, Nottingham, U.K.). Except as stated otherwise, green represents signals for Alexa Fluor 488 staining, blue for Alexa Fluor 350, red for Alexa Fluor 555, and magenta for Alexa Fluor 647. ImageJ version 1.37 was used to count fluorescence from SIGN-R1+ staining of cells in follicles. Ten follicles of three representative mice were chosen in each group. Statistical comparisons between groups were made using a two-tailed Student's t test.
Results and Discussion
Precursors of MZ B cells exist in CD19ko mice
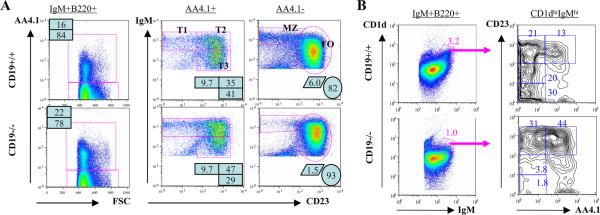
One hypothesis for the lack of MZ B cells in CD19ko mice is that precursor cells fail to differentiate normally. To determine whether CD19 is required for B cell differentiation in the periphery, we analyzed splenic B cell subsets in CD19ko mice. T1, T2, and T3 subsets are all present in mice that lack CD19 (Fig. 1A). Given that transitional B cells were present, we then used CD1d, which is expressed on both transitional and FO precursors of MZ B cells as well as on mature MZ B cells (4, 15), to examine each of these compartments in CD19ko mice. The percentage of CD1d-high, IgM-high B cells is reduced in CD19ko mice, consistent with the loss of mature MZ B cells in these mice (Fig. 1B and supplemental Fig. 1).5 This population could be further subdivided into immature and mature fractions, based on staining with AA4.1. Classical mature MZ B cells (AA4.1-low, CD23-low cells within the CD1d-high, IgM-high subset) are lacking in the CD19ko mice, as expected. In contrast, the percentage of transitional MZ B cell precursors (CD23-high, AA4.1-high cells within the CD1d-high, IgM-high subset) in mice that lack CD19 is comparable to that in wild type (WT) mice (0.44 and 0.42%, respectively). Furthermore, the follicular precursors of MZ B cells (AA4.1-low, CD23-high cells within the CD1d-high, IgM-high subset) are also preserved (0.3 and 0.67%, respectively). The overall frequency of combined transitional and follicular MZ B precursors is reduced by 30% in CD19ko mice. Thus, CD19 contributes to but is not required for the selection of cells into the CD1d-high, IgM-high, CD23+ MZ precursor populations.
FIGURE 1.
Transitional and FO MZ B cell precursors in CD19ko mice. A, IgM+B220+ splenocytes from the indicated mice were analyzed for the expression of AA4.1 (left). Subsets of transitional AA4.1+ B cells (middle) and mature AA4.1- B cells (right) were identified by the expression of IgM and CD23. B, Mature and precursor MZ B cells in a CD1d-high, IgM-high gate (left) were further analyzed (right) to identify transitional (AA4.1+) and mature follicular (AA4.1-, CD23+) precursors, as well as mature MZ B cells (AA4.1-, CD23-).
The MZ microenvironment is altered in CD19ko mice
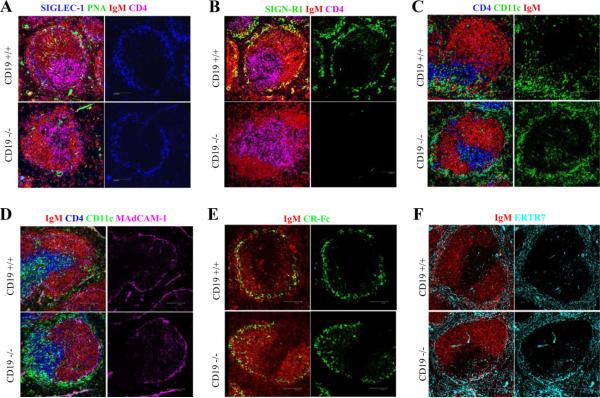
A second hypothesis for the absence of mature MZ B cells in the CD19ko is that defects in other MZ cells or structures prevent the capture of MZ B cells in this region, based on previous studies that demonstrate that the MZ microenvironment is defective in mice with a total lack of all B cells (9). In such mice, MZM and MMM are absent and CD11c DC encircle the MZ rather than concentrate in bridging channels as in normal mice. MZM are reported to be required for the retention of MZ B cells (16), and stromal cells in MZ are thought to retain MZ B cells through integrins (17). Therefore, we examined macrophages, DC, and other components of the MZ. Although SIGLEC-1+ MMM are present normally, SIGN-R1+ MZM are absent in CD19ko mice (Fig. 2, A and B). In addition, CD11c+ DC are found circumferentially around the MZ in CD19ko mice rather than in the polar distribution in bridging channels found in WT mice (Fig. 2C). MAdCAM-1+ sinus lining cells, CR-Fc+ DC, CD11b+ macrophages, and ERTR7+ stromal cells are intact in CD19ko mice (Fig. 2, D-F and data not shown). Thus, the absence of CD19 results in selective changes in the MZ microenvironment. Previous reports showing that CD19ko mice are susceptible to bacteria and unable to mount effective T-dependent and T-independent responses (18, 19) could be due to not only the known defects on B cells but also to the abnormal MZ microenvironment.
FIGURE 2.
Altered MZ microenvironment in CD19ko mice. Spleen sections were stained for MMM (SIGLEC-1; blue in A), B cells (anti-IgM; red in all panels), T cells (anti-CD4; purple or blue in A-D), MZM (anti-SIGN-R1; green in B), CD11c DC (anti-CD11c; green in C and D), marginal sinus lining cells (anti-MAdCAM-1; purple in D), CR-Fc DC (anti-CR-Fc; green in E), stromal cells (ERTR7; cyan in F), and peanut agglutinin (green in A).
The defect in differentiation of mature MZ B cells in CD19ko mice is B cell intrinsic
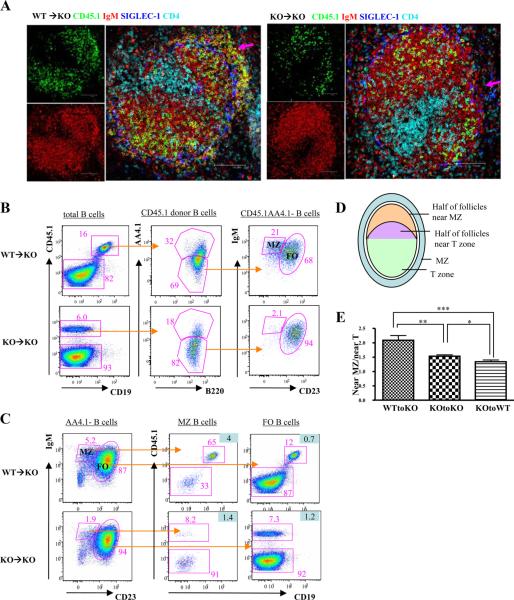
To determine whether extrinsic, secondary changes in the MZ prevent MZ B cell differentiation in CD19ko mice, purified splenic B cells from either WT or CD19ko, CD45.1 donor mice were transferred into CD19ko, CD45.2 recipients. Seven days after transfer of B cells from WT mice, donor-derived, IgM-bright B cells were present in the MZ, outside the marginal sinuses, in the recipient mice (Fig. 3A, arrow). In contrast, donor-derived B cells were present only in the FO region after the transfer of B cells from CD19ko donors. The presence or absence of donor-derived B cells with phenotypic characteristics of MZ B cells was confirmed by flow cytometry (Fig. 3, B and C). Thus, despite the presence of both transitional and FO donor-derived precursors of MZ B cells in the mice receiving either CD19ko or WT B cells, (supplemental Fig. 2), only WT B cells are able to repopulate the MZ of the CD19ko recipients. After the transfer of B cells from WT mice into CD19ko mice, the percentage of donor B cells with a MZ phenotype is expanded relative to the percentage in a normal mouse (21% and 5.6%, respectively). In contrast, this does not occur after the transfer of B cells from CD19ko mice into CD19ko recipients, indicating that the expansion of the MZ compartment requires CD19 and is not simply induced by the transfer itself. Similarly, purified CD19ko B cells transferred into WT recipients are excluded from the MZ (supplemental Fig. 3, A and B). Irradiation of the recipients to reduce the numbers of competing B cells in the recipient or the cotransfer of WT B cells with CD19ko B cells into the irradiated recipients to create a more normal MZ environment also failed to rescue the ability of CD19ko B cells to enter the MZ (supplemental Fig. 3, C and D). Thus, the microenvironment in WT mice could not rescue the differentiation of CD19ko B cells. Therefore, we conclude that it is the lack of an intrinsic CD19 signal on B cells rather than the defects of the MZ microenvironment in CD19ko mice that results in the deficiency of mature MZ B cells in CD19ko mice.
FIGURE 3.
B cell-intrinsic CD19 signal is required for MZ B cell differentiation. Spleens from CD19ko, CD45.2 recipients were analyzed 7 days after adoptive transfer of B cells from CD45.1 WT or CD19ko (KO) donors. A, Spleen sections were stained for B cells (anti-IgM; red), donor cells (anti-CD45.1; green), MMM (anti-SIGLEC-1, which delineate the inner boundary of the MZ; blue), and T cells (anti-CD4; cyan). Arrows indicate that MZ B cells are reconstituted in mice receiving WT B cells but not CD19ko B cells. B and C, B cells in the spleens of CD19ko, CD45.2 recipient mice 7 days after adoptive transfer of CD45.1 B cells from WT or CD19ko mice were analyzed by flow cytometry. B, CD45.1 donor B cells were analyzed for the distribution of mature (AA4.1-) MZ and FO phenotypes. C, all mature B cells in the MZ and FO compartments in recipient mice were analyzed for donor and recipient B cells. The figures highlighted in light blue are the ratios of the frequencies of donor cells in MZ B cells (middle column) or FO B cells (right column) to the frequencies of donor cells in total B cells. D and E, The ratios of the numbers of donor B cells in the inner and outer halves of follicles, as illustrated (D), of the indicated recipient mice that received either WT or CD19ko B cells were calculated using image analysis software (E). *, p < 0.05; **, p < 0.005; ***, p < 0.001.
Following transfer into CD19ko recipients, WT donor cells in the follicle (i.e., not in the MZ) appeared to concentrate more in the region of the B follicle near the MZ rather than near the T cell zone, whereas CD19ko donor cells distribute more evenly across the follicle (Fig. 3A). We calculated the ratio of donor B cells (CD45.1+) to total B cells (IgM+) in the central and distal halves of follicles as illustrated (Fig. 3D) and confirmed that the transferred WT donor cells were more likely to reside in the region of the follicle adjacent to the MZ than the CD19ko donor cells (p < 0.005; Fig. 3E). Thus, even those WT donor B cells that did not enter the MZ after transfer into CD19ko recipients were more likely to reside in the region of the follicle adjacent to the MZ. This emphasizes that MZ differentiation is a continuum regulated by CD19. Therefore, an intrinsic CD19 signal is essential in the differentiation of mature MZ B cells from MZ B precursors.
CD19+ B cells induce the reconstitution of other MZ components in CD19ko mice
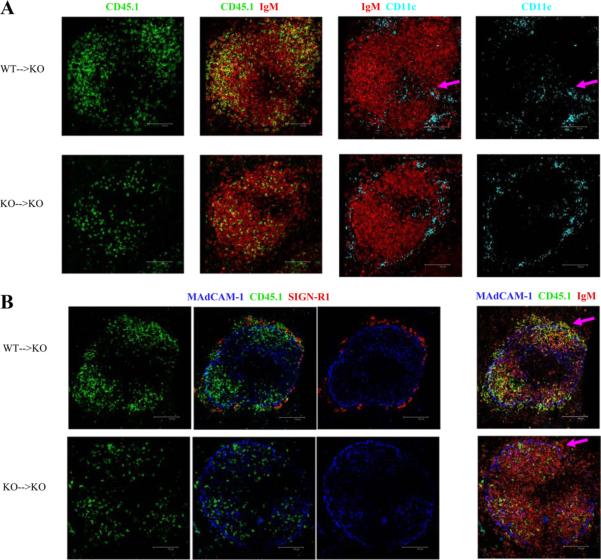
The above experiments indicate that although the MZ in the mice that lack CD19 is abnormal, the failure of MZ B cell differentiation is due to defects in the B cells themselves. Thus, we could determine whether the other abnormalities in the MZ could be corrected by reconstitution of MZ B cells. We tested whether the normal localization of MZM and CD11c+ DC was restored in CD19ko mice after the adoptive transfer of purified B cells from WT mice. At 7 days after transfer, CD11c+ DC were relocated into the bridging channels, as in WT mice, rather than circumferentially around the MZ, as in CD19ko mice (Fig. 4A). At this time point, SIGN-R1+ MZM were not detectable (not shown). However, 17 days after transfer of WT B cells, SIGN-R1+ MZM were also reconstituted (Fig. 4B). Thus, the proper differentiation of and/or localization of MZM and CD11c+ DC were restored following reconstitution of the MZ by adoptive transfer of WT B cells. Therefore, CD19-dependent B cell functions control the localization and/or differentiation of subsets of macrophages and DC.
FIGURE 4.
Splenic B cells from WT or CD19ko (KO) CD45.1 mice were adoptively transferred into CD19ko, CD45.2 recipients. Spleen sections of recipient mice, sacrificed 7 days (A)or 17 days later (B), were stained for histological analysis. A, IgM (B cells), CD45.1 (donor cells), and CD11c (CD11c DC) were examined. Arrows indicate the restored localization of CD11c DC in bridging channel in mice that received WT B cells. B, MAdCAM-1 (sinus lining cells), SIGN-R1 (MZM), IgM (B cells), and CD45.1 (donor cells) were examined. Arrows indicate donor-derived MZ B cells (green) and reconstitution of SIGN-R1+ MZM (red) in the MZ of mice that received WT B cells.
In summary, CD19 on B cells is required for the normal differentiation of other cells in the MZ microenvironment, demonstrating crosstalk between B cells and other cells in the MZ. However, lack of intrinsic CD19 signals in B cells, rather than the secondary effects on other cells, results in the failure to form mature MZ B cells in CD19ko mice.
Supplementary Material
Acknowledgments
We are grateful to Dr. L. Martinez-Pomares for CR-Fc, Dr. Z. Carlene, and Dr. S. Swindle for technical advice, L. Freeberg for maintenance of mice, and the flow cytometry and imaging cores of the University of Alabama at Birmingham Rheumatic Diseases Core Center P30 AR048311.
This work was supported by the National Institute of Arthritis, Musculoskeletal, and Skin Diseases, National Institutes of Health Grant RO1 AI 46225 (to R.H.C.) and the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (to R.H.C.).
Footnotes
Disclosures Yue Wang is now an employee of MedImmune, LLC.
Abbreviations used in this paper: FO, follicular; CD19ko, CD19 knockout; DC, dendritic cell; MMM, marginal metallophilic macrophage; MZ, marginal zone; MZM, marginal zone macrophage; WT, wild type.
The online version of this article contains supplemental material.
References
- 1.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 2000;12:39–49. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- 2.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 3.Belperron AA, Dailey CM, Booth CJ, Bockenstedt LK. Marginal zone B-cell depletion impairs murine host defense against Borrelia burgdorferi infection. Infect. Immun. 2007;75:3354–3360. doi: 10.1128/IAI.00422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava B, Quinn WJ, III, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J. Exp. Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cariappa A, Boboila C, Moran ST, Liu H, Shi HN, Pillai S. The recirculating B cell pool contains two functionally distinct, long-lived, posttransitional, follicular B cell populations. J. Immunol. 2007;179:2270–2281. doi: 10.4049/jimmunol.179.4.2270. [DOI] [PubMed] [Google Scholar]
- 6.Wen L, Brill-Dashoff J, Shinton SA, Asano M, Hardy RR, Hayakawa K. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 2005;23:297–308. doi: 10.1016/j.immuni.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Kraal G. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 8.Kraal G, Mebius R. New insights into the cell biology of the marginal zone of the spleen. Int. Rev. Cytol. 2006;250:175–215. doi: 10.1016/S0074-7696(06)50005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolte MA, Arens R, Kraus M, van Oers MH, Kraal G, van Lier RA, Mebius RE. B cells are crucial for both development and maintenance of the splenic marginal zone. J. Immunol. 2004;172:3620–3627. doi: 10.4049/jimmunol.172.6.3620. [DOI] [PubMed] [Google Scholar]
- 10.Crowley MT, Reilly CR, Lo D. Influence of lymphocytes on the presence and organization of dendritic cell subsets in the spleen. J. Immunol. 1999;163:4894–4900. [PubMed] [Google Scholar]
- 11.Wang Y, Brooks SR, Li X, Anzelon AN, Rickert RC, Carter RH. The physiologic role of CD19 cytoplasmic tyrosines. Immunity. 2002;17:501–514. doi: 10.1016/s1074-7613(02)00426-0. [DOI] [PubMed] [Google Scholar]
- 12.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J. Immunol. 2004;172:803–811. doi: 10.4049/jimmunol.172.2.803. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Carter RH. CD19 regulates B cell maturation, proliferation, and positive selection in the FDC zone of murine splenic germinal centers. Immunity. 2005;22:749–761. doi: 10.1016/j.immuni.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway J. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren's syndrome. J. Clin. Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amano M, Baumgarth N, Dick MD, Brossay L, Kronenberg M, Herzenberg LA, Strober S. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: β2-microglobulin-dependent and independent forms. J. Immunol. 1998;161:1710–1717. [PubMed] [Google Scholar]
- 16.Karlsson MC, Guinamard R, Bolland S, Sankala M, Steinman RM, Ravetch JV. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J. Exp. Med. 2003;198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–412. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 18.Rickert RC, Rajewsky K, Roes J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 19.Gardby E, Chen XJ, Lycke NY. Impaired CD40-signalling in CD19-deficient mice selectively affects Th2-dependent isotype switching. Scand. J. Immunol. 2001;53:13–23. doi: 10.1046/j.1365-3083.2001.00824.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.