Abstract
Insect populations may differ in several life history traits, including behavioural ones such as sexual signalling. We tested whether male Mediterranean fruit fly (medlfy), Ceratitis capitata (Wiedemann) (Diptera: Tephritidae), populations obtained from geographically isolated areas exhibit differences in quantitative and qualitative aspects of male sexual signalling. Male sexual signalling was studied in four medfly populations (originating from Brazil, Portugal, Kenya, and Greece) under identical laboratory conditions (25 °C, 60% r.h., and L14:D10). The four populations had been reared for one generation in the laboratory. Sexual signalling was studied in the F1 progeny that were fed one of two diets (yeast hydrolysate plus sugar or sugar only). On both diets, the four populations differed significantly in the progress of maturity (indicated by the average number of males exhibiting sexual signalling) and in the quantity of signalling after attaining maturity. Yeast availability significantly increased sexual signalling; however, it had a different impact on the quantity of signalling in the different populations. A bimodal pattern of sexual signalling, with one peak at approximately 08:00–09:00 hours and the second at approximately 13:00–14:00 hours, was recorded for all populations and diets. However, quantitative differences among the populations within the ‘sexually active’ period of the day resulted in significant differences in the daily pattern of sexual signalling. The significance of these findings for understanding adaptations of geographically-isolated medfly populations to different ecosystems, as well as its practical importance for the application of the Sterile Insect Technique (SIT) against C. capitata, is discussed.
Keywords: sexual behaviour, sexual calling, mating behaviour, daily rhythm, Diptera, Tephritidae, Ceratitis capitata, Sterile Insect Technique
Introduction
Signalling, defined as “an act or structure that alters the behaviour of another organism, which evolved of that effect, and which is effective because the receiver’s response has also evolved” (Smith & Harper, 2003), is considered to be the most common form of communication throughout the living world. For insects, the exchange of chemical messages via pheromones plays an essential role in their mating behaviour. Female attraction and copulation for males of many polygynous insect species hinge on their ability to sexually advertise themselves in the mating arena, using pheromones and other signals (Alcock, 1997). However, signalling and other reproductive activities (courtship or mating) may have a cost in other fitness components, such as longevity (Cordts & Partridge, 1996; Papadopoulos et al., 2004). Signalling in the wild is also associated with increased predation risk (Hendrichs et al., 1994).
In the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) (Diptera: Tephritidae), pheromone emission by males is an integral part of a complex mating behaviour based on leks (Prokopy & Hendrichs, 1979; Arita & Kaneshiro, 1985; Whittier et al., 1992). Males form loose aggregations on leaf undersurfaces of preferred host trees and defend territories (Whittier et al., 1992). While perched in these positions, they display signalling behaviour, which involves the emission of a sex pheromone attractive to females, by curling their abdomen upward and extruding the terminal end of the rectal epithelium (Arita & Kaneshiro, 1986). Upon the arrival of a potential mate, a complex array of behavioural patterns takes place, including (1) male wing fanning that provides visual, acoustical, and pheromonal cues, and (2) rapid rotations of the male’s head (‘head rocking’) (Webb et al., 1983; Arita & Kaneshiro, 1989; Briceňo et al., 1996, 2002). This sequence of specific stimulus-response interactions between the two mating partners, referred to as courtship, leads to the male attempting to mount the female and finally copulation. The mating behaviour of male medflies has been a topic of extensive research in the last few decades (Eberhard, 1999; Yuval & Hendrichs, 1999), including studies of the sexual compatibility and variation in courtship songs among medfly strains from different origins and mass rearing facilities (Briceño et al., 2002; Cayol et al., 2002). However, comparative studies of signalling activity among geographically isolated populations are still limited.
During the last few centuries, C. capitata has expanded beyond its ancestral region of origin located in central Africa, invading a multitude of areas worldwide. Thus, it exhibits a wide geographical distribution in which a variety of environmental conditions are encountered. Differences among ecosystems related to abiotic factors, such as temperature, relative humidity, and light intensity or biotic ones, such as vegetational properties, distribution of resources, or predator pressure can cause shifts in both natural and sexual selection influencing the evolution of the mating system of the species (Thornhill & Alcock, 1983). Therefore, life history traits, including behavioural ones such as signalling activity, might undergo different adaptations within the geographical range of the species. Analyzing the courtship behaviour of males from different origins, Briceňo et al. (2002) and Lux et al. (2002) reported differences among the populations studied.
In long-lived insect species, energy reserves carried over from the immature stages are not enough to support adult survival and reproduction. Hence, males in these species must invest significant time on a daily basis foraging for various nutritional resourses to ensure survival and reproductive capability. In order to perform sexual signalling, males of C. capitata, must go through a sexual maturation period during which feeding on carbohydrate and protein food is necessary (Christenson & Foote, 1960; Webster & Stoffolano, 1978). Typical feeding substrates for adult fruit flies in nature include fruit juices oozing from ripe fruit, nectar, pollen, droplets of insect honeydews, and bird droppings (Tsiropoulos, 1977; Hendrichs et al., 1991). Strong evidence from a series of studies indicate that participation in the lekking arena and subsequent pheromone emission by male Mediterranean fruit flies is an activity that demands high amounts of energy and that only males with adequate nutritional reserves can obtain mates (Warburg & Yuval, 1997a; Yuval et al., 1998; Kaspi et al., 2000). The effect of adult food on sexual signalling of wild and mass-reared male medflies has been studied in the last few years (Papadopoulos et al., 1998; Kaspi & Yuval, 2000; Shelly et al., 2002; Shelly & Kennelly, 2002; Shelly & McInnis, 2003). Although protein availability increases sexual signalling in wild males, it is not known whether it has similar quantitative effects in different wild populations.
Recently the use of the Sterile Insect Technique (SIT) as a method of C. capitata control has expanded in many areas worldwide, due to the target-specific and environmental friendly character of the method (Hendrichs et al., 1995, 2002). The successful application of the SIT depends on the ability of the laboratory-reared sterile males to disperse effectively and compete against wild fertile males for mating opportunities with native females. Operational SIT programs are currently utilizing genetic sexing strains (GSS). A single wild medfly population contributes the raw genetic material for the development of a GSS. Because a GSS is ‘assembled from specific components’ (Cayol et al., 2002) it is not feasible to develop such a strain for each country that requests the application of SIT against the Mediterranean fruit fly. Instead, sterile males of a specific GSS are shipped as irradiated pupae from rearing facilities to different SIT operations around the world. The above strategy has resulted in wide use of a GSS derived from the same genetic material. For a comprehensive discussion on the use of one strain for all medfly sterile insect release programs see Robinson et al. (2002). However, concerns about possible behavioural non-homogeneity and sexual incompatibility among wild medfly populations question the implementation of this strategy on a worldwide level (Cayol et al., 2002). Hence, comparative studies among populations from different geographic regions, focusing on important aspects of the medfly’s mating behaviour, such as sexual signalling, have great practical importance.
Sexual signalling constitutes a major fitness component for male medflies, as it is positively correlated with lifespan (Papadopoulos et al., 2004) and mating success (Whittier et al., 1994; Shelly, 2000). Selection pressures might cause shifts in major components of sexual signalling resulting in local adaptations and therefore geographical variation among isolated populations. Here, we tested the null hypothesis that medfly populations from isolated geographic regions exhibit similar qualitative (daily pattern of sexual signalling) and quantitative sexual signalling features. To accept or reject the above hypothesis we compared the age-specific sexual signalling performance among medfly populations from different geographic regions, while attempting to understand evolutionarily-driven adaptations within the variety of environmental conditions that the species encountered. We also tested the effect of food on the maturation process and on quantitative and qualitative elements of male sexual signalling using geographically isolated medfly populations.
Materials and methods
Experimental conditions and flies used
The experiments were conducted in the laboratory of Entomology and Agricultural Zoology of the University of Thessaly, Greece, during the autumn-spring of 2005–2006 at 25 ± 1 °C, 65 ± 5% r.h., and L14:D10 photoperiod, with the photophase starting at 07:00 hours. Light was provided by daylight fluorescent tubes with the intensity inside the test cages ranging from 1500 to 2000 lux.
We tested four medfly populations, originating from Brazil [Petrolina (lat: − 9.40, lon: − 40.49, host: Psidium guajava L. (Myrtaceae)], Portugal [Madeira (lat: 32.74, lon: − 16.98), host: Prunus persica (L.) Batsch (Rosaceae)], Kenya [Nairobi (lat: − 1.27, lon: 36.80), host: Coffea arabica L. (Rubiaceae)] and Greece [Chios (lat: 38.47, lon: 25.99), host: Citrus aurantium L.(Rutaceae)]. Pupae retrieved from field-infested fruits were transported by a courier agency to our laboratory. Because host fruit species may affect several biological parameters of the medfly (Krainacker et al., 1987), we reared all four populations for one generation under identical lab conditions and used the F1 progeny in our experiments. Rearing of wild flies was done by keeping adults in groups of about 100 individuals in wooden, wire-screened cages (30 × 30 × 30 cm) provided with water and a standard adult diet (YS) consisting of a mixture of yeast hydrolysate, sugar, and water at a 4:1:5 ratio. Females were allowed to oviposit into 5-cm hollow, red plastic hemispheres (domes) that were artificially punctured with 40–50 evenly distributed holes. Eggs were deposited on the inner surface of the dome. Each dome was fitted into a hole (5-cm in diameter) in the cover of a 5.5-cm plastic Petri dish. Water was placed in the Petri dish in order to maintain humidity levels beneath the dome to an adequate level for female oviposition (Boller, 1985). A plastic cup containing 0.5 ml of orange juice was also placed in the Petri dish to stimulate oviposition. Immatures were reared (same density of 50–100 eggs per food amount for all populations) on an artificial diet consisting of 200 g sugar, 200 g brewer’s yeast, 100 g soybean flour, 4 g salt mixture, 16 g ascorbic acid, 16 g citric acid, 3 g sodium propionate, and 1 l water (Boller, 1985).
Effect of age and food type on signalling performance
Soon after emergence, males from each population were transferred into cubic cages (20 × 20 × 20 cm) of transparent Plexiglas with a mesh window for ventilation, provided with water, and randomly assigned to one of the two food treatments, either standard adult diet (YS) or sugar only (S). Each test cage (replicate) contained 10 males. The effects of age and food type on male signalling activity of the different populations were determined by counting the number of males displaying sexual signalling (emitting sexual pheromone by extruding the terminal end of the rectal epithelium as described by Arita & Kaneshiro; 1986, 1989). One observer recorded sexual signalling from 07:00–20:00 hours daily from adult emergence until day 20 of age. Each hourly observation included three records with a lag period of approximately 4 min between each record. The average of these three counts was used in subsequent analyses. Mortality rates during the testing period were below 5% for all four medfly populations. When an individual died, it was replaced with another of the same age and food treatment. We ran 10 replicates for each population and each food treatment.
Daily pattern of signalling activity
The daily pattern of sexual signalling activity for each population and food treatment was determined by analyzing the hourly records (07:00–20:00 hours) from day 13 through day 16. Within this age group, sexual signalling reached peak rates. We considered that most of the tested males had attained sexual maturity when the average age-specific signalling rates reached a plateau (Papadopoulos et al., 2004).
Statistical analysis
The effect of population (first factor), food (second factor), and age (repeated factor) on male sexual signalling was determined by repeated measures analysis of variance (ANOVA) (Sokal & Rolf, 1995). As signalling activity was zero through day 2 of age for all populations and both food treatments, only data from day 3–20 were considered in the analysis. The effect of population (first factor), food (second factor) and time of the day (repeated factor) on the daily pattern of male sexual signalling was also determined by repeated measures ANOVA performed separately for each of the adult days 13–16 (plateau of sexual signalling). As the results obtained from those four analyses were similar we present data only for adult day 15. To quantify the positive effect of YS on the signalling levels for each population, we calculated the average daily signalling ratio between YS- and S-fed males in relation to the age of males. These data were analyzed by a multiple linear regression analysis (ANCOVA) with ratio (YS/S) as a dependent variable, and age after attaining maturing (12–20 d) and population as independent variables. The data analysis was done using SPSS 15.0 (SPSS Inc., Chicago, IL, USA)
Results
Effect of population, age, and food regime
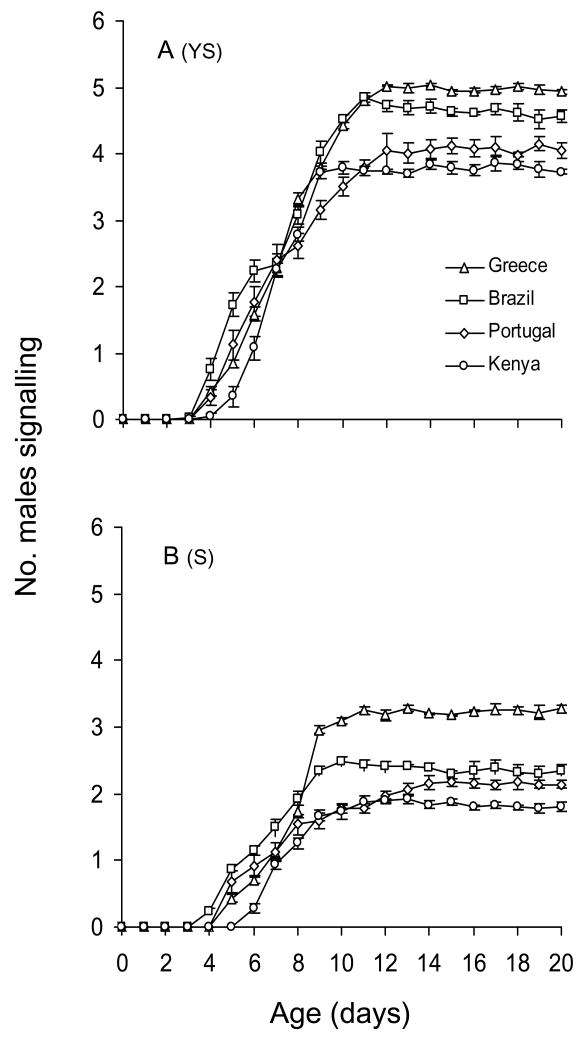
Considering both food regimes, we found significant differences among the four populations in the overall level of sexual signalling activity and the amount of signalling after attaining maturity (Figure 1A, B; Table 1). The progress of maturity (indicated by the average number of males performing sexual signalling) was also significantly different among the four populations tested (significant interaction between age and population). Similar results regarding the progress of maturity were obtained when only days 4–10 were considered (population*age: F18,420 = 14.1, P<0.01). Both food (F1,70 = 237.1, P<0.01) and population (F3,70 = 18.8, P<0.01) had significant effects in this time period, as did age (F6,420 = 1099.3, P<0.01). However, performing the same analysis considering only days 12–20 (mature flies), we found no significant interaction between age and population (F24,560 = 1.5, P = 0.20). Population (F3,70 = 393.3, P<0.01) and food (F1,70 = 4738.4, P<0.01) were found to significantly affect sexual signalling in this analysis; however, because flies had attained maturity, age had no significant effects (F8,560 = 0.8, P = 0.55). Males obtained from Brazil performed significantly higher sexual signalling at younger ages (days 4–10), followed by Greek and Portuguese males (no significant difference between them), whereas those obtained from Kenya performed the lowest sexual signalling (Tukey’s Honestly Significant Difference test: P<0.05). Maturity, indicated by peak signalling rates, was generally attained between days 9 and 11 of age over all populations and both food regimes. Greek males had significantly higher signalling rates after attaining maturity (days 12–20) in both food regimes, followed by Brazilian and Portuguese males, while Kenyan males performed the lowest sexual signalling (Tukey’s HSD: P<0.05).
Figure 1.
Age-specific sexual signalling of males of the four Ceratitis capitata populations fed on (A) yeast plus sugar (YS) and (B) sugar-only (S). On each day of age, observations were conducted hourly from 07:00–20:00 hours in 10 cages (replicates) containing 10 males each. Values on y-axis are mean numbers (± SE) of males signalling per cage per hourly observation.
Table 1.
Repeated measures ANOVA on the effect of population (first factor), food (second factor), and age (repeated factor) on male Ceratitis capitata sexual signalling. Ages from adult-day 3 (beginning of signalling) to adult-day 20 were considered in the analysis
| Source of variation | d.f. | MS | F | P |
|---|---|---|---|---|
| Population | 3 | 60.0 | 183.5 | <0.001 |
| Food | 1 | 807.9 | 2469.9 | <0.001 |
| Population*food | 3 | 2.4 | 7.4 | <0.001 |
| Error (between subjects) | 70 | 0.3 | ||
| Age | 17 | 113.7 | 1423.34 | <0.001 |
| Age*population | 51 | 1.4 | 18.3 | <0.001 |
| Age*food | 17 | 8.5 | 106.6 | <0.001 |
| Age*population*food | 51 | 0.2 | 2.6 | <0.001 |
| Error (age) | 1190 | 0.08 |
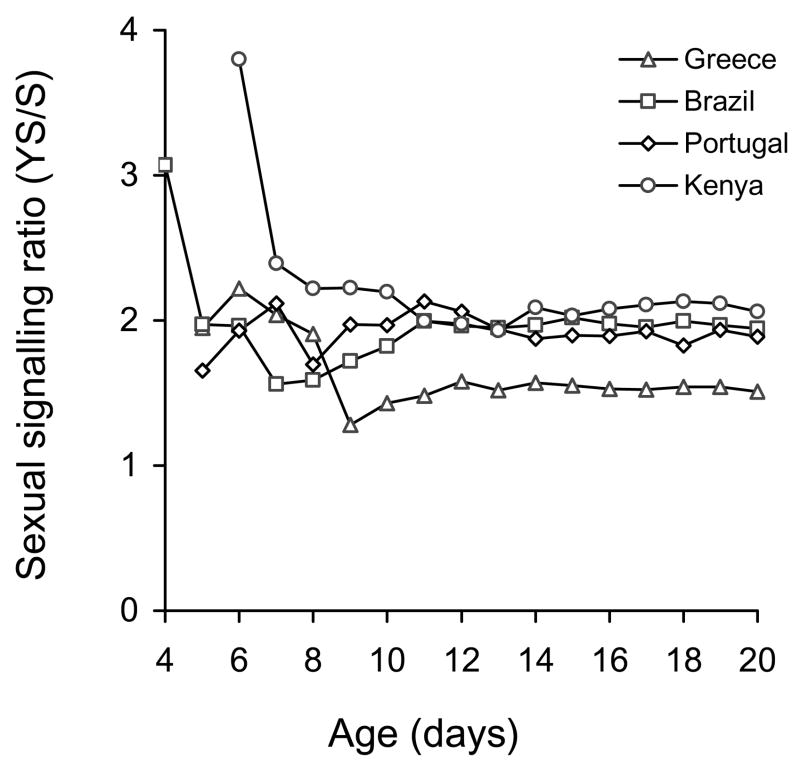
Yeast hydrolysate significantly increased sexual signalling in all four populations (Table 1, Figure 1). The significant interaction between age and food indicates the significant effect of food regime on the progress of sexual maturation in male medflies. With the exception of the Brazilian population, males provided with YS initiated sexual signalling 1 day earlier than S-fed males (Figure 1). For Brazilian males, signalling started on the same day for YS and S diets. The significant interaction between food and population (Table 1) suggests a different impact of food on the quantity of signalling of the various populations. The positive effect of YS on the signalling levels for each population in relation to the age of males is given in Figure 2. After males had attained sexual maturity (adult day 12–20), protein availability boosted signalling levels to almost double for the populations from Kenya, Brazil, and Portugal and to approximately 1.5 for the Greek population. Multiple regression analysis revealed that population was a significant predictor of the YS/S ratio of sexual signalling (F3,31 = 186.9, P<0.0001), while age was not (F1,31 = 0.006, P = 0.938). The positive effect of YS on sexual signalling was significantly higher in Kenyan males, followed by Brazilian and Portuguese (no significant differences between them), whereas it was significantly lower in the Greek males (Tukey’s HSD test: P<0.05).
Figure 2.
Ratio between signalling rates of Ceratitis capitata males fed on either yeast + sugar or sugar only, in relation to the age of flies for four fly populations. Data used in ratios were obtained from Figure 1.
Effect of population, food, and time of day
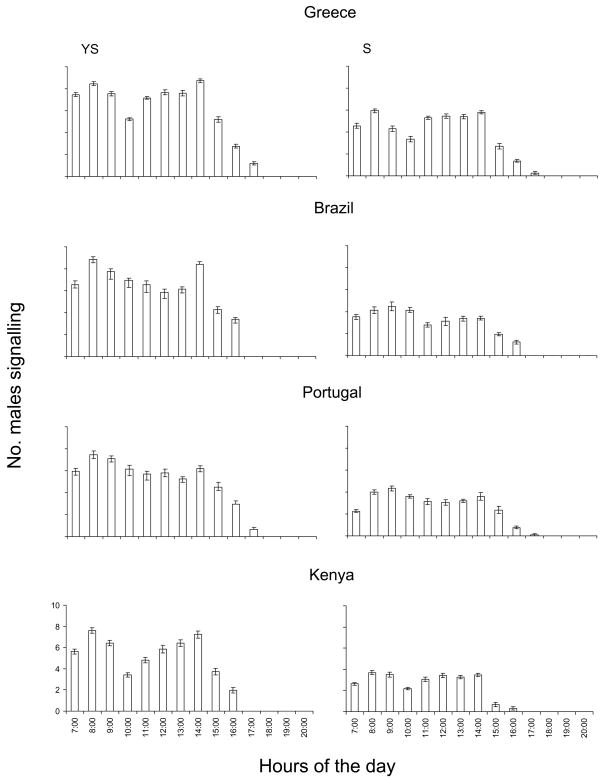
Both population and food significantly affected sexual signalling considering only those counts at day 15 of age (Table 2). The population*food interaction was also significant. Regardless of the population and food, time of day significantly affected sexual signalling (Table 2). The interaction between time of day and population was significant suggesting different daily patterns of signalling among the four populations. Food may also cause shifts in the daily pattern of signalling as evidenced by the significant time of day*food interaction (Table 2). YS-fed males exhibited higher signalling activity than S-fed ones during all hours of the sexually active period of the day (07:00–17:00 hours) for all four populations (Figure 3). In both food treatments and all four populations, sexual signalling was performed from 07:00 hours (beginning of photophase) to 16:00 hours for Kenyan and Brazilian males, and to 17:00 hours for Greek and Portuguese ones. Sexual signalling activity followed a bimodal pattern for all populations (weakly bimodal for the Portuguese males) and food regimes, with one clear peak at approximately 08:00–09:00 hours and the second at approximately 13:00–14:00 hours. However, there were quantitative differences among the populations that resulted in significant differences in the daily pattern as it is evidenced by the significant time of the day*population interaction. For example, in the YS food regime (Figure 3), the lowest signalling rates between the two peaks was observed at 10:00 hours for the Greek and Kenyan flies, but at 12:00 and 13:00 hours for the Brazilian and Portuguese flies, respectively.
Table 2.
Repeated measures ANOVA on the effect of population (first factor), food (second factor), and time of day (repeated factor) on daily rhythm of male Ceratitis capitata sexual signalling. Times of the day between 07:00 and 17:00 hours (sexually active period of the day) of adult-day 15 were considered in the analysis
| Source of variation | d.f. | MS | F | P |
|---|---|---|---|---|
| Population | 3 | 96.3 | 124.1 | <0.001 |
| Food | 1 | 1375.2 | 1771.6 | <0.001 |
| Population*food | 3 | 5.0 | 6.5 | 0.001 |
| Error (between subjects) | 70 | 0.7 | ||
| Time of the day | 10 | 260.0 | 529.1 | <0.001 |
| Time of the day*population | 30 | 6.1 | 12.5 | <0.001 |
| Time of the day*food | 10 | 16.1 | 32.9 | <0.001 |
| Time of the day*population*food | 30 | 1.5 | 3.3 | <0.001 |
| Error (time of the day) | 700 | 0.4 |
Figure 3.
Daily rhythm of sexual signalling on adult-day 15 of Ceratitis capitata males from four different populations fed on yeast + sugar (left column) and sugar only (right column). Values on y-axis are mean numbers (± SE) of males signalling per cage.
Discussion
Our results reveal significant differences among the four medfly populations originated from Kenya, Brazil, Portugal, and Greece in both progress of maturity and signalling levels after attaining maturity. Therefore, the null hypothesis of no variation in sexual signalling among populations was rejected. The above differences were apparent with both food regimes. As a big portion of the environmentally-induced variability was removed by using the F1 offspring reared on the same larval diet, and all populations experienced identical laboratory conditions, these findings suggest underlying genetic differences among the populations tested. Differences in genotypes may affect the expression of behavioural traits, such as sexual signalling (Alcock, 1997), and particularities of different environments related to biotic and abiotic factors may cause geographic variation among populations as a result of natural selection (Mousseau & Olvido, 2001). Therefore, it seems that adaptive mechanisms operating in the wild account for the differences in signalling performance among medfly populations observed in our study.
Under constant conditions in the laboratory, and within the sexually active period of the day, males of the four populations exhibited differences in the daily rhythm of sexual signalling. Nonetheless, our results indicate that the observed bimodality in the daily pattern of sexual signalling, reported in both food regimes, seems to be a common property for male medflies. The bimodal pattern of signalling activity was also observed in a laboratory study with a wild Greek population tested under similar laboratory conditions to those in our study (Papadopoulos et al., 1998). However, wild flies from Guatemala held under a different photoperiod (L12:D12, instead of L14:D10) did not display a bimodal signalling rhythm (Landolt et al., 1992). Apparently, photoperiod may affect an endogenously regulated trait of expressing daily rhythms of sexual signalling.
Prevailing environmental conditions in natural settings, such as temperature and photoperiod may mask the expression of an intrinsic behavioural trait (i.e., bimodality in the daily pattern of signalling activity) causing shifts in the daily rhythms of its expression. For example, data obtained under semi-field or field conditions (fluctuating abiotic factors) indicated that signalling activity in C. capitata follows either a bimodal or a unimodal pattern. Prokopy & Hendrichs (1979,) in a field cage study in Guatemala, recorded a normal distribution of signalling activity throughout the day with only one peak around noon. Similar unimodal patterns were observed by Hendrichs et al. (1991) and Warburg & Yuval (1997b) in open-field studies conducted in the island of Chios, Greece, and a coastal area of Israel, respectively. However, field observations in Egypt showed two peaks in sexual signalling activity, one before the hottest part of the day and a smaller one in the afternoon (Hendrichs & Hendrichs, 1990). It was hot midday temperatures (>36 ºC) that caused suspension of any behavioural activity including sexual signalling that resulted in a bimodal daily pattern of sexual signalling in the above study. It seems that in the wild, temperature and photophase are the two dominant factors regulating the daily pattern of sexual signalling in male medflies.
The bimodal activity observed in our study might be attributed to the cost of sexual signalling that is well known to be a highly demanding activity in both terms of time and energy for male medflies (Warburg & Yuval, 1997a). Therefore, pheromone emission of the optimum quality and quantity might not be performed on a constant basis, and males may temporarily withdraw from lekking sites, engaging in other activities (feeding, resting, etc.) and resume signalling later. To elucidate this hypothesis, the duration of sexual signalling for each individual should be determined in different populations and for many individuals.
We have also demonstrated a significant effect of protein availability on the daily pattern of sexual signalling (significant interaction between food and time of the day). It seems that YS-fed males invest more in signalling, and proportionally for longer periods of time, than S-fed ones, resulting in temporal shifts on the expression of sexual signalling in relation to flies fed on sugar only. The energetic demands for signalling activity may also account for this effect.
Our results show a different impact of protein diet on signalling levels among medfly populations. Differences between ecosystems in the spatial and temporal distribution of critical feeding resources, such as protein, may result in corresponding changes in the time invested by males on different activities such as signalling (Aluja et al., 2001). Hence, it seems plausible that in ecosystems with limited protein availability, males that are able to locate and exploit such resources acquire an important adaptive advantage that may be favoured by natural selection. Thus, it is expected that males in such environments may have adapted to use protein more efficiently and invest more of the ingested protein on sexual activities. However, little is known of protein resources and their distribution in the ecosystems from which the flies were obtained (for discussion on resource distribution in various ecosystems, see Prokopy, 1980).
Males of all populations that were fed on a diet consisting of protein and sugar (YS) signalled on a more frequent basis, started signalling earlier, and attained sexual maturity sooner than sugar only-fed ones. Our findings are in agreement with a previous laboratory study reporting positive effects of protein adult food on both signalling frequency and rate of sexual maturation in a wild Greek population (Papadopoulos et al., 1998). Several other studies correlate nutrition with reproductive success of male medflies. For example, Warburg & Yuval (1996) reported higher levels of lipids in males fed only with carbohydrates than males fed on a diet consisting of protein plus sugar. They attributed the above decline of energetic reserves of YS-fed males on their increased sexual activity, suggesting that feeding on a diet of high nutritional value allows males to use their lipids at a greater rate than poorly nourished ones. Adequate nutrition is also reported as a necessary condition to initiate sexual behaviour (Yuval et al., 1998). Male copulatory success was positively correlated with the overall level of sexual activity (defined as number of courtships performed) number of attempted copulations, and number of females courted (Whittier et al., 1994), and protein-fed males were more likely to mate than protein-deprived ones (Blay & Yuval, 1997).
The relationship between quality of food and pheromone emission has been investigated for other tephritids as well. A varying quality of diets has been found to affect several aspects of the sexual behaviour of Anastrepha obliqua, Anastrepha serpentina, and Anastrepha striata, but had no significant effects on Anastrepha ludens (Aluja et al., 2001). Anastrepha suspensa males feeding on yeast and sugar or sugar alone exhibited similar signalling levels and quantities of pheromone production (Landolt & Sivinsky, 1992; Epsky & Heath, 1993). Contrary to our findings, Epsky & Heath (1993) suggested possible depression of signalling activity due to adult dietary protein, or interference of protein with pheromone emission. In both above studies, males provided with water only (no food at all) exhibited reduced sexual signalling and pheromone emission compared with males fed on sugar or yeast plus sugar.
Homogeneity in terms of mating behaviour and sexual compatibility among medfly populations has received increased attention recently, due to their high importance for SIT operations. Briceňo et al. (2002) found significant differences in male courtship songs among wild populations originating from Costa Rica, Argentina, and Hawaii. However, Lux et al. (2002) analyzing courtship data, found no major differences among wild medfly populations originating from a number of geographic regions world-wide. Moreover, mating compatibility tests among wild medfly strains from nine countries showed that medfly populations worldwide had not yet evolved specific kinds of sexual behaviours that could lead to pre-mating isolation (Cayol et al., 2002). Based on the above findings, Robinson et al. (2002) suggest that for the SIT “…a particular mass reared strain can be used at any location”. Our study reveals quantitative differences among the four medfly populations in terms of signalling intensity and daily rhythm of signalling activity. However, males from all populations express sexual signalling within the same period of the photophase. Therefore, a GSS strain showing a broad overlap in daily signalling with the wild males (independent of the wild males’ origin) could be suitable for SIT programs in various parts of the world.
Acknowledgments
This study was supported by the National Institute on Aging (P01-AG022500-01; P01-AG08761-10). The authors are grateful to A. Malavasi (Moscamed, Brazil), B. Paranhos (Embrapa Semi-Árido, Brazil), L. Dantas (Madeira-Med, Portugal), and S. Ekesi (ICIPE, Kenya) for providing wild material. We also thank C. Nakas (University of Thessaly) for his advice on the statistical analyses. Critical comments from two anonymous reviewers greatly improved this paper.
References
- Alcock J. Animal Behaviour. 6. Sinauer; Sunderland, MA, USA: 1997. [Google Scholar]
- Aluja M, Jácome I, Ordóñez RM. Effect of adult nutrition on male sexual performance in four neotropical fruit fly species of the genus Anastrepha (Diptera: Tephritidae) Journal of Insect Behavior. 2001;14:759–775. [Google Scholar]
- Arita LH, Kaneshiro KY. The dynamics of the lek system and mating success in males of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) Proceedings of the Hawaiian Entomological Society. 1985;25:39–48. [Google Scholar]
- Arita LH, Kaneshiro KY. Structure and function of the rectal epithelium and anal glands during mating behavior in the Mediterranean fruit fly male. Proceedings of the Hawaiian Entomological Society. 1986;26:27–30. [Google Scholar]
- Arita LH, Kaneshiro KY. Sexual selection and lek behavior in Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae) Pacific Science. 1989;43:135–143. [Google Scholar]
- Blay S, Yuval B. Nutritional correlates of reproductive success of male Mediterranean fruit flies (Diptera: Tephritidae) Animal Behaviour. 1997;54:59–66. doi: 10.1006/anbe.1996.0445. [DOI] [PubMed] [Google Scholar]
- Boller EF. Rhagoletis cerasi and Ceratitis capitata. In: Sing P, Moore RF, editors. Handbook of Insect Rearing. Vol. 2. Elsevier; Amsterdam, The Netherlands: 1985. pp. 135–144. [Google Scholar]
- Briceňo RD, Ramos D, Eberhard WG. Courtship behavior of male Ceratitis capitata (Diptera: Tephritidae) in captivity. Florida Entomologist. 1996;79:130–143. [Google Scholar]
- Briceňo RD, Eberhard WG, Vilardi JC, Liedo P, Shelly TE. Variation in the intermittent buzzing songs of male medflies (Diptera: Tephritidae) associated with geography, mass-rearing, and courtship success. Florida Entomologist. 2002;85:32–40. [Google Scholar]
- Cayol JP, Coronado P, Taher M. Sexual compatibility in medfly (Diptera: Tephritidae) from different origins. Florida Entomologist. 2002;85:51–57. [Google Scholar]
- Christenson LD, Foote RH. Biology of fruit flies. Annual Review of Entomology. 1960;5:171–192. [Google Scholar]
- Cordts R, Partridge L. Courtship reduces longevity of male Drosophila melanogaster. Animal Behaviour. 1996;52:269–278. [Google Scholar]
- Eberhard WG. Sexual behavior and sexual selection in the medfly, Ceratitis capitata. In: Aluja M, Norrbom A, editors. Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior. CRC Press; Boca Raton, FL, USA: 1999. pp. 459–490. [Google Scholar]
- Epsky ND, Heath RR. Food availability and pheromone production by males of Anastrepha suspensa (Diptera, Tephritidae) Environmental Entomology. 1993;22:942–947. [Google Scholar]
- Hendrichs J, Hendrichs MA. Mediterranean fruit fly (Diptera, Tephritidae) in nature: Location and diel pattern of feeding and other activities on fruiting and nonfruiting hosts and nonhosts. Annals of the Entomological Society of America. 1990;83:632–641. [Google Scholar]
- Hendrichs J, Katsoyannos BI, Papaj DR, Prokopy RJ. Sex differences in movement between natural feeding and mating sites and tradeoffs between food consumption, mating success and predator evasion in Mediterranean fruit flies (Diptera: Tephritidae) Oecologia. 1991;86:223–231. doi: 10.1007/BF00317534. [DOI] [PubMed] [Google Scholar]
- Hendrichs J, Katsoyannos BI, Wornoayporn V, Hendrichs MA. Odor mediated foraging by yellowjacket wasps (Hymenoptera, Vespidae): Predation on leks of pheromone calling Mediterranean fruit fly males (Diptera, Tephritidae) Oecologia. 1994;99:88–94. doi: 10.1007/BF00317087. [DOI] [PubMed] [Google Scholar]
- Hendrichs J, Franz G, Rendon P. Increased effectiveness and applicability of the Sterile Insect Technique through male-only releases for control of Mediterranean fruit flies during fruiting seasons. Journal of Applied Entomology. 1995;119:371–377. [Google Scholar]
- Hendrichs J, Robinson AS, Cayol JP, Enkerlin W. Medfly area wide sterile insect technique programmes for prevention, suppression or eradication: The importance of mating behavior studies. Florida Entomologist. 2002;85:1–13. [Google Scholar]
- Kaspi R, Taylor PW, Yuval B. Diet and size influence sexual advertisement and copulatory success of males in Mediterranean fruit fly leks. Ecological Entomology. 2000;25:279–284. [Google Scholar]
- Krainacker DA, Carey JR, Vargas RI. Effect of larval host on life history traits of the Mediterranean fruit fly, Ceratitis capitata. Oecologia. 1987;73:583–590. doi: 10.1007/BF00379420. [DOI] [PubMed] [Google Scholar]
- Landolt PJ, Sivinski J. Effects of time of day, adult food, and host fruit on incidence of calling by male Caribbean fruit flies (Diptera: Tephritidae) Environmental Entomology. 1992;21:382–387. [Google Scholar]
- Landolt PJ, Heath RR, Chambers DL. Oriented flight responses of female Mediterranean fruit flies to calling males odor of calling males and a synthetic pheromone blend. Entomologia Experimentalis et Applicata. 1992;65:259–266. [Google Scholar]
- Lux SA, Munyiri FN, Vilardi JC, Liedo P, Economopoulos A, et al. Consistency in courtship pattern among populations of medfly (Diptera: Tephritidae): Comparisons among wild strains and strains mass reared for sit operations. Florida Entomologist. 2002;85:113–125. [Google Scholar]
- Mousseau TA, Olivido AE. Nature Encyclopedia of Life Sciences. Nature Publishing Group; London, UK: 2001. Geographical Variation; pp. 1–8. [Google Scholar]
- Papadopoulos NT, Katsoyannos BI, Kouloussis NA, Economopoulos AP, Carey JR. Effect of adult age, food, and time of day on sexual calling incidence of wild and mass-reared Ceratitis capitata males. Entomologia Experimentalis et Applicata. 1998;89:175–182. [Google Scholar]
- Papadopoulos NT, Katsoyannos BI, Kouloussis NA, Carey JR, Muller HG, Zhang Y. High sexual signalling rates of young individuals predict extended life span in male Mediterranean fruit flies. Oecologia. 2004;138:127–134. doi: 10.1007/s00442-003-1392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price PW. Insect Ecology. Wiley; New York, NY, USA: 1984. [Google Scholar]
- Prokopy RJ, Hendrichs J. Mating behaviour of Ceratitis capitata (Diptera, Tephritidae) on a field caged host tree. Annals of the Entomological Society of America. 1979;72:642–648. [Google Scholar]
- Prokopy RJ. Fruit Fly Problems. National Institute of Agricultural Sciences; Kyoto and Naha, Japan: 1980. Mating behavior of frugivorous Tephritidae in nature; pp. 37–46. [Google Scholar]
- Robinson AS, Cayol JP, Hendrichs J. Recent findings on medfly sexual behavior: Implications for SIT. Florida Entomologist. 2002;85:171–181. [Google Scholar]
- Shelly TE. Male signalling and lek attractiveness in the Mediterranean fruit fly. Animal Behaviour. 2000;60:245–251. doi: 10.1006/anbe.2000.1470. [DOI] [PubMed] [Google Scholar]
- Shelly TE, Kennelly SS, McInnis DO. Effect of adult diet on signalling activity, mate attraction, and mating success in male Mediterranean fruit flies (Diptera: Tephritidae) Florida Entomologist. 2002;85:150–155. [Google Scholar]
- Shelly TE, Kennelly S. Influence of male diet on male mating success and longevity and female remating in the Mediterranean fruit fly (Diptera: Tephritidae) under laboratory conditions. Florida Entomologist. 2002;85:572–579. [Google Scholar]
- Shelly TE, McInnis DO. Influence of adult diet on the mating success and survival of male Mediterranean fruit flies (Diptera: Tephritidae) from two mass -rearing strains on field-caged host trees. Florida Entomologist. 2003;86:340–344. [Google Scholar]
- Smith JM, Harper D. Animal Signals. Oxford University Press; New York, NY, USA: 2003. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 3. Freeman; New York, NY, USA: 1995. [Google Scholar]
- Thornhill R, Alcock J. The Evolution of Insect Mating Systems. Harvard University Press; Cambridge, MA, USA: 1983. [Google Scholar]
- Tsiropoulos GJ. Reproduction and survival of the adult Dacus oleae feeding on pollen and honeydews. Environmental Entomology. 1977;6:390–392. [Google Scholar]
- Warburg MS, Yuval B. Effects of diet and activity on lipid levels of adult Mediterranean fruit flies. Physiological Entomology. 1996;21:151–158. [Google Scholar]
- Warburg MS, Yuval B. Effects of energetic reserves on behavioural patterns of Mediterranean fruit flies (Diptera: Tephritidae) Oecologia. 1997a;112:314–319. doi: 10.1007/s004420050314. [DOI] [PubMed] [Google Scholar]
- Warburg MS, Yuval B. Circadian patterns of feeding and reproductive activities of Mediterranean fruit flies (Diptera: Tephritidae) on various hosts in Israel. Annals of the Entomological Society of America. 1997b;90:487–495. [Google Scholar]
- Webb JC, Calkins CO, Chambers DL, Schwienbacher W, Russ K. Acoustical aspects of behaviour of Mediterranean fruit-fly, Ceratitis capitata - Analysis and identification of courtship sounds. Entomologia Experimentalis et Applicata. 1983;33:1–8. [Google Scholar]
- Webster RP, Stoffolano JG. Influence of diet on maturation of reproductive system of apple maggot, Rhagoletis pomonella (Diptera-Tephritidae) Annals of the Entomological Society of America. 1978;71:844–849. [Google Scholar]
- Whittier TS, Kaneshiro KY, Prescott LD. Mating behavior of Mediterranean fruit flies (Diptera: Tephritidae) in a natural environment. Annals of the Entomological Society of America. 1992;85:214–218. [Google Scholar]
- Whittier TS, Nam FY, Shelly TE, Kaneshiro KY. Male courtship success and female discrimination in the Mediterranean fruit fly (Diptera, Tephritidae) Journal of Insect Behavior. 1994;7:159–170. [Google Scholar]
- Yuval B, Kaspi R, Shloush S, Warburg MS. Nutritional reserves regulate male participation in Mediterranean fruit fly leks. Ecological Entomology. 1998;23:211–215. [Google Scholar]
- Yuval B, Hendrichs J. Behavior of flies in the genus Ceratitis. In: Aluja M, Norrbom A, editors. Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior. CRC Press; Boca Raton, FL, USA: 1999. pp. 429–456. [Google Scholar]