Abstract
The nitrile (C≡N) stretching vibration is sensitive to environment, making nitrile-derivatized amino acids an increasingly utilized tool to study various biological processes. Herein, we show that the bandwidth of the C≡N stretching vibration of 5-cyanotryptophan is particularly sensitive to water, rendering it an attractive infrared probe of local hydration status. We confirm the utility of this probe in biological applications by using it to examine how the hydration status of individual tryptophan sidechains of an antimicrobial peptide, indolicidin, changes upon peptide binding to model membranes. Furthermore, we show that p-cyanophenylalanine and 5-cyanotryptophan constitute a useful fluorescence energy transfer pair.
1. Introduction
The stretching frequency of a nitrile (C≡N) group is sensitive to its environment, determined jointly by the ‘solvent’ and the parent molecule [1–12]. In addition, the C≡N stretching vibration is largely decoupled from other vibrational modes of biological molecules and thus, has been used to probe various properties of a wide variety of biological systems. For example, it has been used to (a) report on the orientation and hydration status of individual sidechains of a peptide that is bound to lipid membranes [13,14] or AOT reverse micelles [15], (b) probe the structure of amyloid fibers [16], (c) examine the binding of a ligand [17] or anesthetic halothane [18,19] to a protein, (d) probe the dynamics of an HIV-1 reverse transcriptase inhibitor via two-dimension infrared spectroscopy [20], (e) investigate local electric fields in proteins by means of vibrational Stark spectroscopy [21–23], and (f) study the local environments of DNA fragments [24–26].
For studies involving peptides and/or proteins, a nitrile group is typically introduced by incorporating a nitrile-derivatized amino acid into the targeted polypeptide sequence using either an in vitro [3,22] or in vivo synthesis method [17]. So far, three nitrile-derivatized amino acids, i.e., p-cyanophenylalanine (PheCN) [3,17], cyanoalanine [3] and thiocyanatoalanine [22], have been used for this purpose. However, in light of concerns that substitution of a native residue with a non-native amino acid, especially a non-natural amino acid, could lead to undesirable structural perturbations to the native fold, it would be beneficial to have other nitrile-derivatized amino acids available to replace a targeted native residue with the respective nitrile-containing derivative in order to minimize any potential structural perturbations. Herein, we investigate the potential utility of 5-cyanotryptophan (TrpCN) as an infrared reporter of local environment of proteins and/or peptides. Our motives for choosing this non-natural amino acid include the following: (a) tryptophan (Trp) is often located at sites that are important for either function or stability of proteins, (b) the Stark spectroscopic study of Boxer and coworkers [27] suggests that the nitrile group in TrpCN might be a more sensitive reporter of the local electrostatic environment than those in other nitrile-derivatized amino acids, and (c) the molar absorption coefficient of the C≡N stretching vibration of TrpCN is expected to be about 1.5 times larger than that of PheCN [27], an added advantage for studies wherein relatively low peptide/protein concentration is required. Finally, besides its potential usefulness as an infrared reporter, TrpCN could exhibit interesting fluorescence properties, akin to its structure analog, 5-hydroxytryptophan [28].
To test the utility of TrpCN as an infrared probe of local environment, we investigate how the C≡N stretching band of the free amino acid changes with solvent and how that of two TrpCN-containing mutants of a cationic antimicrobial peptide [29,30], indolicidin (sequence: ILPWKWPWWPWRR-NH2), varies in response to the binding of the peptide to DPC micelles and POPG lipid bilayers. Our results show that the bandwidth of the C≡N stretching vibration of TrpCN is especially sensitive to the degree of hydration of the amino acid, thus making it a very attractive infrared probe of local hydration status. In addition, our results indicate that while the fluorescence quantum yield of TrpCN is approximately one order of magnitude smaller than that of Trp, rendering it less useful as a stand-alone fluorescent probe, it can still be used together with PheCN [31] for florescence energy transfer applications.
2. Experimental section
2.1. Materials
5-cyanoindole (99%) was obtained from Aldrich (St. Louis, MO). Fmoc-L-TrpCN (98%, 99% ee 2S) was purchased from RSP Amino Acids (Shirley, MA). Chloroform solutions of dodecylphosphocholine (DPC) and the sodium salt of 1-hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phospho-(1'-sn-glycerol) (POPG) were purchased form Avanti Polar Lipids (Alabaster, AL). Millipore water was used to prepare aqueous solutions. Peptides were synthesized using standard Fmoc-protocols employing Rink resin. All peptide samples were purified to homogeneity by reverse-phase chromatography and characterized by mass-spectrometry. Following purification, peptides solutions were lyophilized and subsequently re-dissolved in H2O and titrated to pH 7.0. Chloroform solutions of DPC and POPG were dried under a stream of nitrogen, followed by dissication under vacuum to remove residual solvent. Dried DPC or POPG samples were re-dissolved in 20 mM phosphate buffer solution (pH 7.0) and then sonicated for about 10 min. Lyophilized peptides were then added to the resultant micelle or vesicle solution, where the total DPC or POPG concentration was 250 mM. The mixture was further sonicated for about 5 min and then used in infrared measurements involving micelle- or membrane-bound peptides.
The concentration of free TrpCN amino acid was estimated by weight, whereas that of the two indolicidin mutants was determined optically via the sample absorbance at 280 nm, which was measured on a Perkin–Elmer Lambda 25 UV-Vis spectrometer (Fremont, CA). The molar absorption coefficient of TrpCN was assumed to be the same as that of Trp (i.e., ε280 = 5500 M−1 cm−1).
2.2. FTIR spectroscopy
FTIR spectra were collected on a Nicolet Magna-IR 860 spectrometer using 1 cm−1 resolution. A CaF2 sample cell that is divided into two compartments using a Teflon spacer (56 µm unless explicitly specified) was employed to allow the separate measurements of the sample and reference (i.e., buffer) under identical conditions. The temperature was maintained at 20 °C for all measurements. To correct for slow instrument drift, an automated translation stage was used to move both the sample and reference sides in and out of the IR beam alternately, and each time a single-beam spectrum corresponding to an average of 16 scans was collected. The final result was usually an average of 64–128 such spectra. The spectra shown correspond to the net absorbance of the solutes, which were obtained by subtracting a linear background in the C≡N stretching spectral region.
2.3. Fluorescence and circular dichroism measurements
Fluorescence spectra were obtained at 20 °C on a Fluorolog 3.10 spectrofluorometer (Jobin Yvon Horiba, Edison, NJ) with a 1 cm quartz sample holder. Temperature was regulated using a TLC 50 Peltier temperature controller (Quantum Northwest, Spokane, WA). An integration time of 1.5 s/nm was used in all measurements. Far-UV circular dichroism (CD) spectra were collected on an Aviv Model 410 CD spectrometer (Aviv Biomedical, NJ).
3. Results and discussion
3.1. 5-cyanoindole and 5-cyanotryptophan
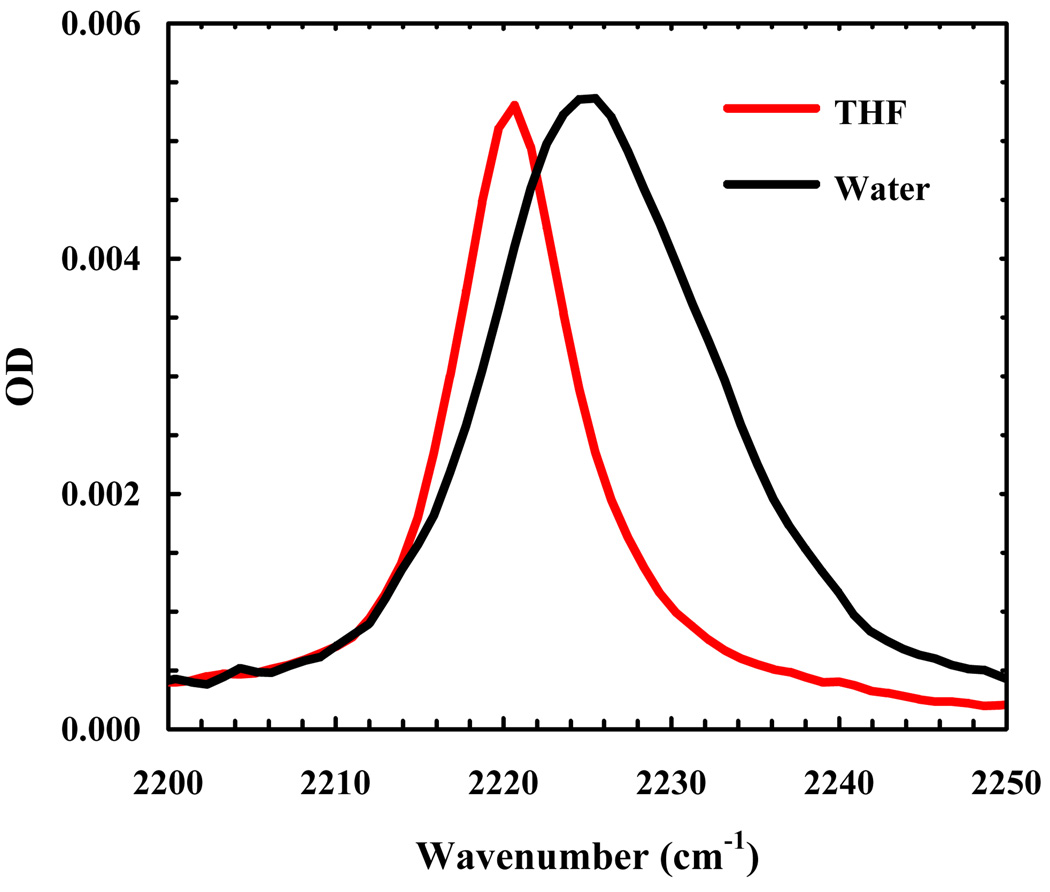
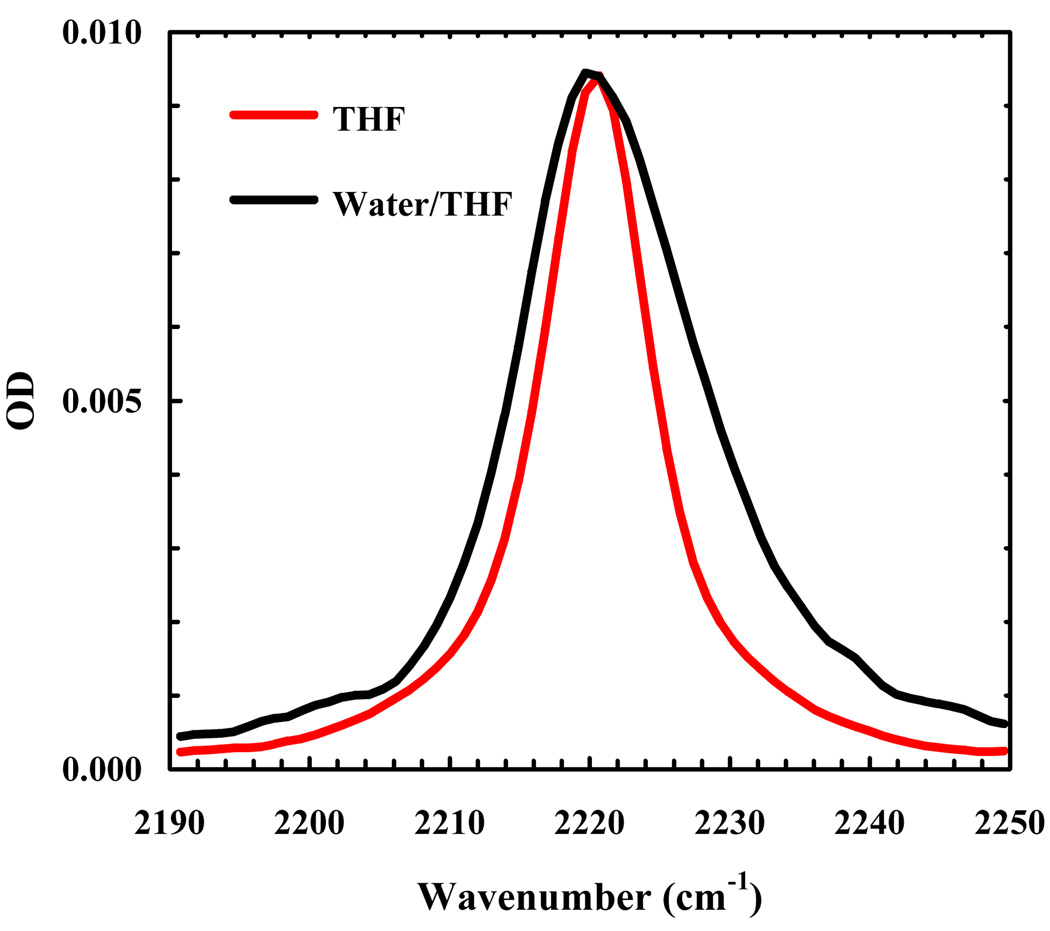
The FTIR spectra of 5-cyanoindole and the amine protected form of TrpCN (Fmoc-L-TrpCN) were measured in H2O, tetrahydrofuran (THF) and THF-H2O mixtures. Here, THF was used to mimic the hydrophobic interior of proteins because its dielectric constant is roughly ten times smaller than that of H2O. As shown (Figure 1), the peak position of the C≡N stretching band of 5-cyanoindole in THF is centered at about 2220 cm−1, which is only about 4 cm−1 apart from that in H2O. However, the bandwidth (FWHM) of the C≡N stretching vibration in THF (~8 cm−1) is significantly narrower than that in H2O (~18 cm−1). As indicated (Figure 2), the C≡N stretching vibration of Fmoc-L-TrpCN shows a similar trend. Moreover, in agreement with the study of Suydam and Boxer [27], the vibrational transition dipole moment of Fmoc-L-TrpCN is estimated to be a factor of about 1.8 larger than that of Fmoc-L-PheCN [3], calculated using the integrated areas of the respective C≡N stretching bands. Taken together, these results indicate that the C≡N stretching vibration of TrpCN, especially its bandwidth, is sensitive to solvent and thus, could serve as an infrared reporter of the local hydration status of peptides and proteins.
Figure 1.
C≡N stretching bands of 5-cyanoindole in THF and a water/methanol mixture (95/5, v/v), as indicated.
Figure 2.
C≡N stretching bands of Fmoc-L-TrpCN in THF and a water/THF mixture (60/40, v/v), as indicated. The concentration of Fmoc-L-TrpCN was estimated to be ~3.3 mM based on weight, and the optical pathlength was about 130 µm. For easy comparison, the spectrum measured in water/THF mixture has been scaled by a factor of 1.3.
Suydam and Boxer have shown that the C≡N stretching vibration of 5-cyanoindole has a larger Stark tuning rate than that of p-tolunitrile [27]. Thus, the seemingly low sensitivity of the peak frequency of the nitrile stretching band of 5-cyanoindole to solvent is somewhat surprising. However, this finding is consistent with the notion put forward by Webb and Boxer [23], as well as Cho and coworkers [7,32] that the Stark tuning rate is not necessarily a quantitative indicator of how the C≡N stretching vibration is affected by changes in solvation, due to the complexity of the interactions between solvent molecules and the C≡N moiety. For example, Cho and coworkers found that the C≡N stretching frequency of cyanomethane (CH3CN) is determined by the precise configuration of water molecules surrounding the C≡N group, namely, a linear arrangement of CH3CN-H2O (termed σ-bonding with nitrogen lone-pair) will result in a blue-shift, whereas interactions of water molecules with the π-orbital of the nitrile moiety (termed π-bonding) will lead to a red-shift [7]. In other words, the solvent-induced vibrational frequency shift for aliphatic nitriles is determined by an intricate balance between the σ-bonding and π-bonding interactions.
In the present case the indole ring is an additional, and perhaps more important, factor that needs to be considered. As shown (Figure 1), the bandwidth (FWHM) of the C≡N stretching vibration of 5-cyanoindole in water is about 8 cm−1 broader than that of p-tolunitrile [3], indicating that the structure of the parent molecule is an important determinant of the bandwidth (and peak frequency) of the C≡N stretching vibration. In other words, any interactions between the parent and solvent molecules that can affect the C≡N bond strength would result in a change in its vibrational frequency. Therefore, it is not surprising that an indole ring (in 5-cyanoindole and TrpCN), which is large and aromatic and thus capable of forming various water-indole complexes with different C≡N stretching frequencies, can lead to a broad C≡N stretching vibrational band. On the other hand, a smaller phenyl group (in p-tolunitrile and PheCN) would support a smaller number of static solute-solvent complexes on the time scale of the C≡N stretching vibration compared to the indole ring, thus a narrower C≡N stretching vibrational band. While a change in the lifetime of the excited vibrational state can also lead to spectral broadening/narrowing, the reported lifetimes of the first excited state of the C≡N stretching vibration of various nitriles [11,12] suggest that lifetime broadening is not a dominating factor in the current case.
3.2. Indolicidin
To test the utility of TrpCN as an infrared reporter of local hydration environment in the context of a biological system, we used it to probe how an individual Trp residue in indolicidin changes its hydration status upon peptide binding to micelles or membranes from the aqueous phase. Indolicidin is a Trp rich antimicrobial peptide, which is unstructured in aqueous solution but adopts a wedge-shaped conformation when bound to the membrane-water interface region of DPC micelles or POPG lipid bilayers [30]. Thus, indolicidin constitutes a good model system to test the applicability of TrpCN in monitoring changes in local hydration status at the single-residue level. Specifically, we prepared two indolicidin mutants, namely Trp11/TrpCN and Trp9/TrpCN, and measured their C≡N stretching bands in the presence or absence of those model membranes.
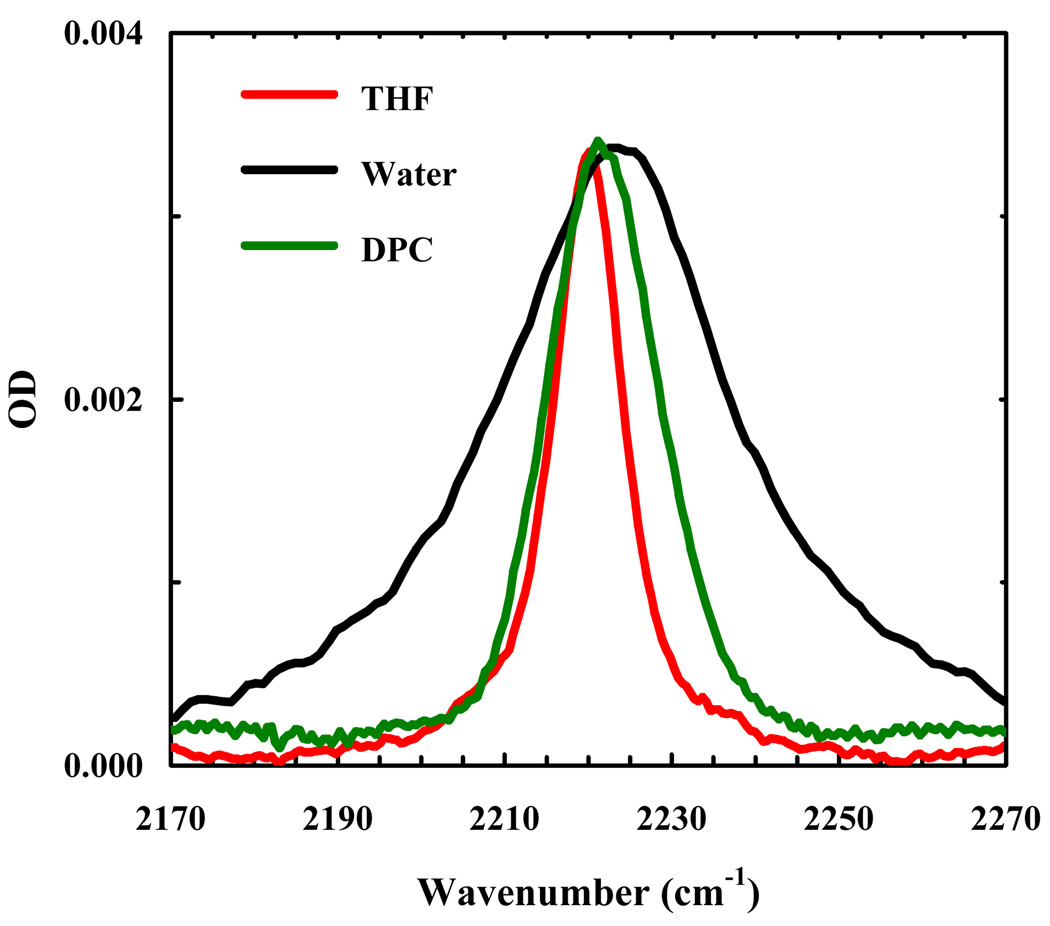
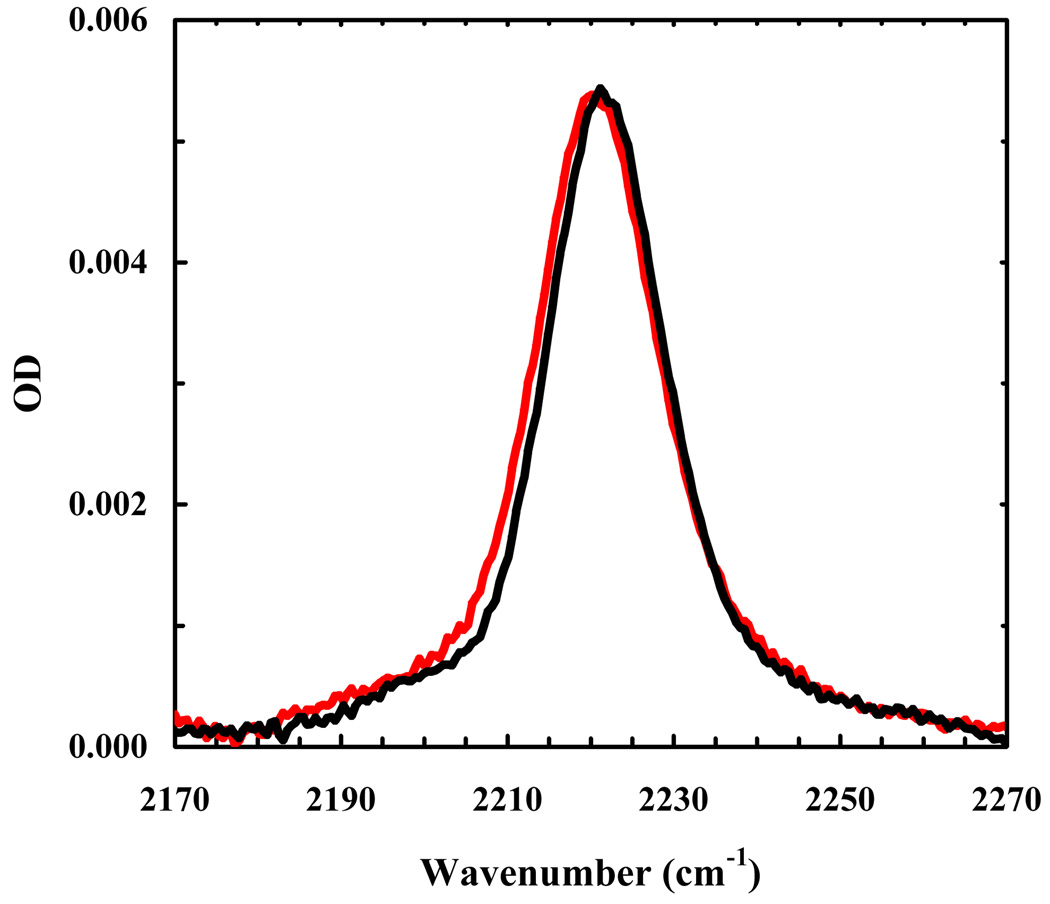
As shown (Figure 3), the C≡N stretching band of Trp9/TrpCN in water is very broad (the FWHM is approximately 34 cm−1). This result is consistent with the aforementioned picture that in aqueous solution the bandwidth of the C≡N stretching vibration of TrpCN is dominated by the mechanism of inhomogeneous broadening and also the fact that the unstructured indolicidin polypeptide chain molecule can sample a vastly large number of conformations. As shown (Figure 3), the C≡N stretching band of Trp9/TrpCN becomes significantly narrower upon binding to DPC micelles, indicating that the corresponding Trp sidechain (i.e., Trp9) is less hydrated in the micelle-bound state of indolicidin. Similarly, the C≡N stretching band of Trp11/TrpCN also indicates that Trp11 becomes less exposed to water upon peptide-micelle association. In fact, the C≡N stretching vibrational bands of the micelle-bound Trp11/TrpCN and Trp9/TrpCN are practically superimposable (Figure 4). Taken together, these results indicate that on average Trp9 and Trp11 sample a similar environment (in terms of hydration), even though they may show different orientations with respect to the peptide backbone and depths of membrane insertion according to molecular dynamics simulations [33,34]. While it is not a direct proof, the NMR study by Hancock and coworkers [30], which showed that a spin label residing mostly at the surface of the DPC micelles reduces the 1H NMR signal intensity of Trp9 and Trp11 by 35% and 22%, respectively, is nevertheless consistent with our findings.
Figure 3.
C≡N stretching bands of Trp9/TrpCN in THF, water, and DPC micelles, as indicated. The peptide concentration was ~2 mM in all solvents, except water where the concentration was not determined. For easy comparison, the spectra obtained in water and DPC micelles have been scaled, by a factor of 2.2 and 0.62, respectively.
Figure 4.
Comparison of the C≡N stretching bands of Trp11/TrpCN (red) and Trp9/TrpCN (blue) in DPC micelles. The Trp9/TrpCN data are identical to those used in Figure 3 and the Trp11/TrpCN spectrum was scaled by a factor of 1.3.
To verify that the Trp-to-TrpCN mutation does not cause significant conformational changes of the micelle-bound peptides, we further carried out CD studies. The CD spectrum of Trp11/TrpCN in the presence of DPC micelles (data not shown) shows that the peptide backbone gives rise to a negative CD band centered at 205 nm, as observed for the wild-type indolicidin [30], suggesting that the structural perturbation arising from the TrpCN mutation is insignificant. Thus, given the rather distinct role of Trp in many antimicrobial peptides and membrane proteins, TrpCN may prove to be a valuable infrared reporter that complements other probes for studying peptide-membrane interactions. In addition, these results validate the utility of TrpCN as an infrared probe of local hydration status or proteins, a subject of great importance [35,36].
It has been shown that indolicidin binds more strongly to lipid bilayers than to micelles [29,30]. Thus, we further measured the C≡N stretching bands of both indolicidin mutants in the presence of POPG bilayers (data not shown). Indeed, the C≡N stretching band of the POPG bilayer-bound peptides exhibits a small but measurable decrease (about 1–2 cm−1) in its bandwidth. This result is in agreement with the NMR study indicating that the Trp sidechains of the membrane-bound peptide are less disordered [30], further underscoring the sensitivity of the respective infrared probe. Finally, it is worth noting that the C≡N stretching band of these peptides measured in the aforementioned membrane environment is considerably broader than that measured in THF, suggesting that the corresponding Trp sidechains still sample a rather heterogeneous environment, consistent with the fact that the membrane-bound indolicidin molecules lie at the interfacial region of the membrane and, as a result, the Trp residues are situated in a partially hydrated and dynamic environment.
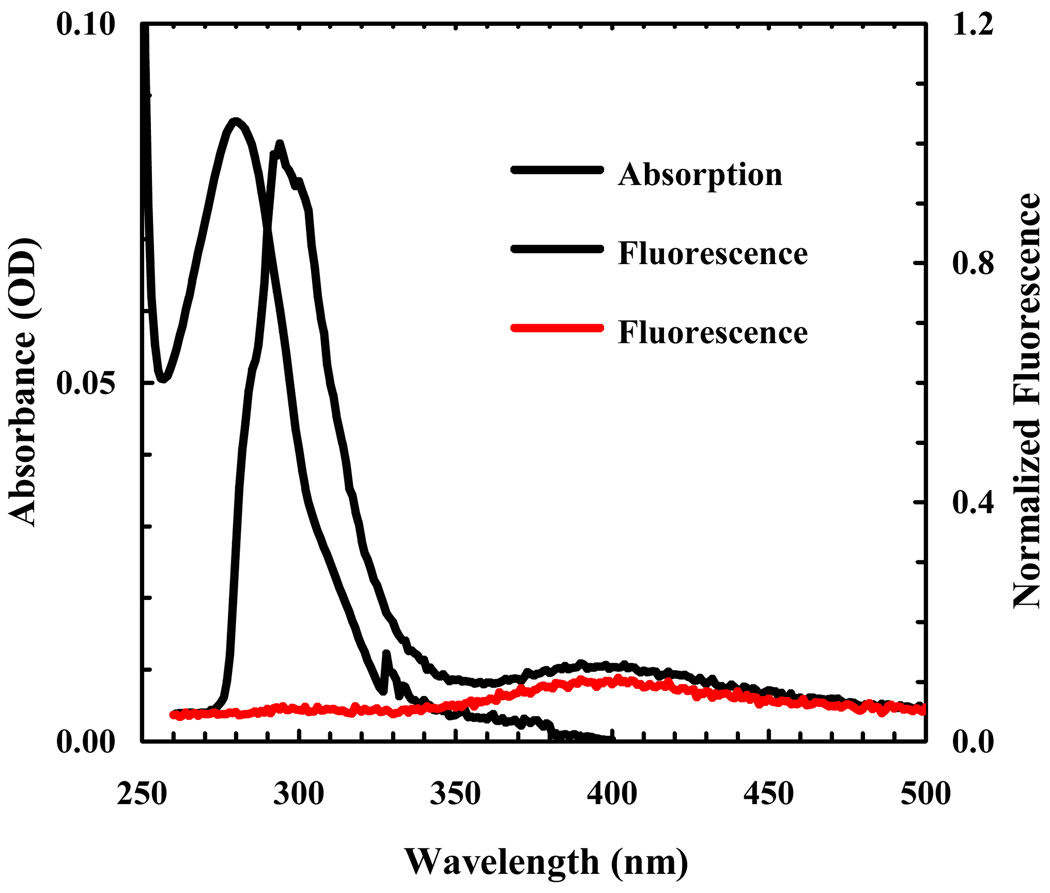
3.3. 5-cyanotryptophan as a fluorescence quencher of p-cyanophenylalanine
5-hydroxytrptophan, an analog of TrpCN, has been widely used as a fluorescence probe of protein structure and dynamics. This is because the fluorescence quantum yield of 5-hydroxytrptophan is higher than that of tryptophan and is also much less sensitive to solvent polarity [28]. In light of the usefulness of TrpCN as an infrared probe, it would be beneficial to further explore whether this chromophore exhibits similar fluorescent properties as 5-hydroxytrptophan. To our surprise, the acetylated and aminated form of TrpCN (i.e., Acetyl-TrpCN-NH2) is only weakly fluorescent in aqueous solution (Figure 5), making it less attractive to be used as a fluorescence reporter. However, the absorption spectrum of TrpCN overlaps significantly with the fluorescence emission spectrum of PheCN (Figure 5), suggesting that it is a viable candidate to serve as a FRET acceptor to PheCN. Indeed, the fluorescence spectrum of an amidated tripeptide, PheCN-Ala-TrpCN-NH2, shows that the PheCN emission is almost completely quenched by TrpCN (Figure 5). Assuming that the mechanism of the observed fluorescence quenching can be described within the framework of Förster theory and that at 280 nm the molar absorptivity of TrpCN is equal to that of Trp, we estimated the Förster radius of this pair to be 17.4 ± 1.0 Å. Previously, we have shown that PheCN is not only a useful fluorescent probe [37–39], but can also be used together with Trp for FRET applications where Trp serves as the acceptor [31,40]. Compared to the PheCN-Trp FRET pair, whose fluorescence spectra overlap, the PheCN-TrpCN FRET system is more convenient to use. This is because their fluorescence emissions are well separated and thus, in conjunction with the low fluorescence quantum yield of TrpCN, making the extraction of the donor fluorescence intensity from a measured FRET spectrum straightforward, a feature particularly useful in time-resolved FRET measurements.
Figure 5.
Absorption spectrum of Ac-TrpCN-NH2 in water (black), fluorescence spectrum of an equimolar (~20 µM) aqueous solution of PheCN and Ac-TrpCN-NH2 (blue), and fluorescence spectrum of PheCN-Ala-TrpCN-NH2 (red) in water (~16 µM). For fluorescence measurements, the excitation wavelength was 232 nm.
4. Conclusion
In summary, we demonstrate that the C≡N stretching vibration of TrpCN is a useful infrared probe of local environment. Measurements involving binding of indolicidin to model membranes indicate that the bandwidth of this vibrational mode is particularly sensitive to the degree of hydration of the respective Trp sidechains. Thus, we believe that this infrared reporter is especially useful in probing biological processes, such as protein folding and binding, wherein a Trp residue is becoming more or less hydrated. In addition, we show that, similar to the PheCN-Trp FRET pair, PheCN-TrpCN also constitutes a useful fluorescence donor-acceptor pair. The advantage of the PheCN-TrpCN pair is that their fluorescence emissions are well separated, thus making decomposition of a FRET spectrum into its constituent donor and acceptor contributions straightforward.
Acknowledgements
We gratefully acknowledge financial support from the National Institutes of Health (GM-065978 and RR-01348).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reimers JR, Hall LE. J. Am. Chem. Soc. 1999;121:3730. [Google Scholar]
- 2.Andrews SS, Boxer SG. J. Phys. Chem. A. 2000;104:11853. [Google Scholar]
- 3.Getahun Z, Huang C-Y, Wang T, De Leon B, DeGrado WF, Gai F. J. Am. Chem. Soc. 2003;125:405. doi: 10.1021/ja0285262. [DOI] [PubMed] [Google Scholar]
- 4.Dalosto SD, Vanderkooi JM, Sharp KA. J. Phys. Chem. B. 2004;108:6450. doi: 10.1021/jp0310697. [DOI] [PubMed] [Google Scholar]
- 5.Kurochkin DV, Naraharisetty SRG, Rubtsov IV. J. Phys. Chem. A. 2005;109:10799. doi: 10.1021/jp055811+. [DOI] [PubMed] [Google Scholar]
- 6.Maienschein-Cline MG, Londergan CH. J. Phys. Chem. A. 2007;111:10020. doi: 10.1021/jp0761158. [DOI] [PubMed] [Google Scholar]
- 7.Choi J-H, Oh K-I, Lee H, Lee C, Cho M. J. Chem. Phys. 2008;128:134506. doi: 10.1063/1.2844787. [DOI] [PubMed] [Google Scholar]
- 8.Oh K-I, Choi J-H, Lee J-H, Han J-B, Lee H, Cho M. J. Chem. Phys. 2008;128:154504. doi: 10.1063/1.2904558. [DOI] [PubMed] [Google Scholar]
- 9.Lindquist BA, Corcelli SA. J. Phys. Chem. B. 2008;112:6301. doi: 10.1021/jp802039e. [DOI] [PubMed] [Google Scholar]
- 10.Lindquist BA, Haws RT, Corcelli SA. J. Phys. Chem. B. 2008;112:13991. doi: 10.1021/jp804900u. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh A, Remorino A, Tucker MJ, Hochstrasser RM. Chem. Phys. Lett. 2009;469:325. doi: 10.1016/j.cplett.2008.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha J-H, Lee K-K, Park K-H, Choi J-H, Jeon S-J, Cho M. J. Chem. Phys. 2009;130:204509. doi: 10.1063/1.3140402. [DOI] [PubMed] [Google Scholar]
- 13.Tucker MJ, Getahun Z, Nanda V, DeGrado WF, Gai F. J. Am. Chem. Soc. 2004;126:5078. doi: 10.1021/ja032015d. [DOI] [PubMed] [Google Scholar]
- 14.Weeks CL, Polishchuk A, Getahun Z, DeGrado WF, Spiro TG. J. Raman Spectrosc. 2008;39:1606. doi: 10.1002/jrs.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee S, Chowdhury P, DeGrado WF, Gai F. Langmuir. 2007;23:11174. doi: 10.1021/la701686g. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Decatur SM. Biophys. J. 2007:559A. [Google Scholar]
- 17.Schultz KC, Supekova L, Ryu Y, Xie J, Perera R, Schultz PG. J. Am. Chem. Soc. 2006;128:13984. doi: 10.1021/ja0636690. [DOI] [PubMed] [Google Scholar]
- 18.Zou H, Liu J, Blasie JK. Biophys. J. 2009;96:4188. doi: 10.1016/j.bpj.2009.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Strzalka J, Tronin A, Johansson JS, Blasie JK. Biophys. J. 2009;96:4176. doi: 10.1016/j.bpj.2009.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang C, Bauman JD, Das K, Remorino A, Arnold E, Hochstrasser RM. Proc. Natl. Acad. Sci. USA. 2008;105:1472. doi: 10.1073/pnas.0709320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suydam IT, Snow CD, Pande VS, Boxer SG. Science. 2006;313:200. doi: 10.1126/science.1127159. [DOI] [PubMed] [Google Scholar]
- 22.Fafarman AT, Webb LJ, Chuang JI, Boxer SG. J. Am. Chem. Soc. 2006;128:13356. doi: 10.1021/ja0650403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb LJ, Boxer SG. Biochemistry. 2008;47:1588. doi: 10.1021/bi701708u. [DOI] [PubMed] [Google Scholar]
- 24.Silverman LN, Pitzer ME, Ankomah PO, Boxer SG, Fenlon EE. J. Phys. Chem. B. 2007;111:11611. doi: 10.1021/jp0750912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krummel AT, Zanni MT. J. Phys. Chem. B. 2008;112:1336. doi: 10.1021/jp711558a. [DOI] [PubMed] [Google Scholar]
- 26.Watson MD, Gai XS, Gillies AT, Brewer SH, Fenlon EE. J. Phys. Chem. B. 2008;112:13188. doi: 10.1021/jp8067238. [DOI] [PubMed] [Google Scholar]
- 27.Suydam IT, Boxer SG. Biochemistry. 2003;42:12050. doi: 10.1021/bi0352926. [DOI] [PubMed] [Google Scholar]
- 28.Twine SM, Szabo AG. Methods Enzymol. 2003;360:104. doi: 10.1016/s0076-6879(03)60108-4. [DOI] [PubMed] [Google Scholar]
- 29.Ladokhin AS, Selsted ME, White SH. Biophys. J. 1997;72:794. doi: 10.1016/s0006-3495(97)78713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozek A, Friedrich CL, Hancock REW. Biochemistry. 2000;39:15765. [PubMed] [Google Scholar]
- 31.Tucker MJ, Oyola R, Gai F. J. Phys. Chem. B. 2005;109:4788. doi: 10.1021/jp044347q. [DOI] [PubMed] [Google Scholar]
- 32.Cho M. J. Chem. Phys. 2009;130:094505. doi: 10.1063/1.3079609. [DOI] [PubMed] [Google Scholar]
- 33.Khandelia H, Kaznessis YN. J. Phys. Chem. B. 2007;111:242. doi: 10.1021/jp064776j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu JCY, Yip CM. Biophys. J. 2007;92:L100. doi: 10.1529/biophysj.107.108050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li T, Hassanali AA, Kao Y-T, Zhong D, Singer SJ. J. Am. Chem. Soc. 2007;129:3376. doi: 10.1021/ja0685957. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Wang L, Kao Y-T, Qiu W, Yang Y, Okobiah O, Zhong D. Proc. Natl. Acad. Sci. USA. 2007;104:18461. doi: 10.1073/pnas.0707647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tucker MJ, Oyola R, Gai F. Biopolymers. 2006;83:571. doi: 10.1002/bip.20587. [DOI] [PubMed] [Google Scholar]
- 38.Aprilakis KN, Taskent H, Raleigh DP. Biochemistry. 2007;46:12308. doi: 10.1021/bi7010674. [DOI] [PubMed] [Google Scholar]
- 39.Tang J, Yin H, Qiu J, Tucker MJ, DeGrado WF, Gai F. J. Am. Chem. Soc. 2009;131:3816. doi: 10.1021/ja809007f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glasscock JM, Zhu Y, Chowdhury P, Tang J, Gai F. Biochemistry. 2008;47:11070. doi: 10.1021/bi8012406. [DOI] [PMC free article] [PubMed] [Google Scholar]