Abstract
Background
The composition of atherosclerotic plaque affects the likelihood of an atherothrombotic event but prospective studies relating risk factors to carotid wall and plaque characteristics measured by MRI are lacking. We hypothesized that traditional risk factors are predictors of carotid wall and plaque characteristics measured two decades later.
Methods and Results
A high-resolution contrast-enhanced MRI exam of the carotid artery was performed in 1769 participants. Measures of carotid wall volume and maximum thickness; lipid core presence, volume and maximum area; and fibrous cap thickness were performed centrally. The average age of the sample was 70 years, 57% female, 81% white, and 19% African American. Greater age, total and LDL cholesterol, male gender, white race, diabetes, hypertension and smoking as measured at baseline, were all significant predictors of increased wall volume and maximum wall thickness 18 years later. An analysis of lipid core was restricted to the 1180 participants with maximum wall thickness ≥1.5mm. Lipid core was observed in 569 individuals (weighted percentage = 42%). Baseline age, total and LDL cholesterol were predictors of presence of lipid core 18 years later; however, these relationships were attenuated after adjustment for wall thickness. Concurrently measured LDL was associated with greater lipid core volume, independent of wall thickness. Concurrently measured glucose and body mass index were inversely associated fibrous cap thickness.
Conclusions
Traditional atherosclerosis risk factors are related to increased wall volume and wall thickness two decades later, but they do not discriminate characteristics of plaque composition (core and cap) independent of wall size.
Keywords: carotid arteries, epidemiology, magnetic resonance imaging, plaque
The composition of atherosclerotic plaque affects the likelihood of an atherothrombotic event but prospective studies relating risk factors to carotid wall and plaque characteristics measured by MRI are lacking. We hypothesized that traditional risk factors are predictors of carotid wall and plaque characteristics measured two decades later. We measured carotid wall volume and maximum thickness; lipid core presence, volume and maximum area; and fibrous cap thickness in an epidemiologic cohort of 1769 men and women, average age 70 years, using high-resolution contrast-enhanced MRI. Greater age, total and LDL cholesterol, male gender, white race, diabetes, hypertension and smoking as measured at baseline, were all predictors of increased wall volume and maximum wall thickness 18 years later. Age, total and LDL cholesterol were predictors of presence of lipid core 18 years later, primarily through their association of increased wall thickness. LDL was associated with greater lipid core volume. Increased fasting glucose and obesity were associated with a thin fibrous cap. These results confirm the importance of traditional risk factors in predicting atherosclerosis. Specifically, risk factors measured in mid-life predict atherosclerosis in late life. These findings have implications for risk factor modification in mid-life and future risk of cardiovascular disease.
The composition of atherosclerotic plaque plays a critical role in occurrence of clinical cardiovascular and cerebrovascular events (1, 2). The plaque is composed of distinct morphologic features including a fibrous cap comprised of smooth muscle cells and fibrotic tissue, and a lipid core containing fat-laden macrophages and extracellular lipids. Histologic studies have led to the recognition that plaque structure influences the risk of plaque rupture. Specifically, a plaque with a thin fibrous cap and a large lipid core is more prone to rupture (3). Because a major cause of ischemic cerebrovascular events is carotid plaque rupture, as observed in both histologic (4) and magnetic resonance imaging (MRI)-based studies (5), it is important to identify the factors that alter plaque structure, and those factors that lead to plaque rupture. MRI has enabled the noninvasive characterization of atherosclerotic plaque in population-based samples (6).
Few studies have characterized correlates of plaque structure of the carotid artery; most have examined correlates of the coronary arteries, and then usually as cross-sectional assessments in clinical or autopsied populations (7–9). Only one report has examined plaque characteristics and their determinants in a population-based or epidemiologic sample (10). The present report describes results from the Atherosclerosis Risk in Communities (ARIC) Study in which contrast enhanced MRI studies of the carotid artery were obtained approximately two decades after a baseline examination. Our study aim was to evaluate the ability of traditional risk factors for atherosclerosis, measured two decades previously and concurrent with the MRI exam, to predict MRI-detectable carotid wall and plaque characteristics.
Methods
The study sample consisted of 2066 members of the ARIC study cohort who participated in the Carotid MRI substudy in 2004–2005. ARIC is a cohort study of atherosclerosis among 15,792 African American and white adults initially examined between 1987 and 1989 (11, 12). For the substudy, a stratified sampling plan was used in order to increase the prevalence of informative plaques while maintaining the ability to make population-based inferences. The goal was to recruit 1200 participants with high values of maximum carotid artery intimal medial thickness (IMT) at their last or penultimate ultrasound examination, and 800 individuals randomly sampled from the remainder of the IMT distribution. Field-center-specific cutpoints of carotid IMT were adjusted over the recruitment period to approximately achieve this goal, with 100% sampling above the cutpoint, and a sampling fraction below the cutpoint to achieve the desired 800. By the end of the recruitment period, cutpoints ranged from 1.00 to 1.28 mm representing the 68th to the 73rd percentiles of maximum IMT. These cutpoints allowed for recruitment of similar numbers of participants with high IMT from each field center.
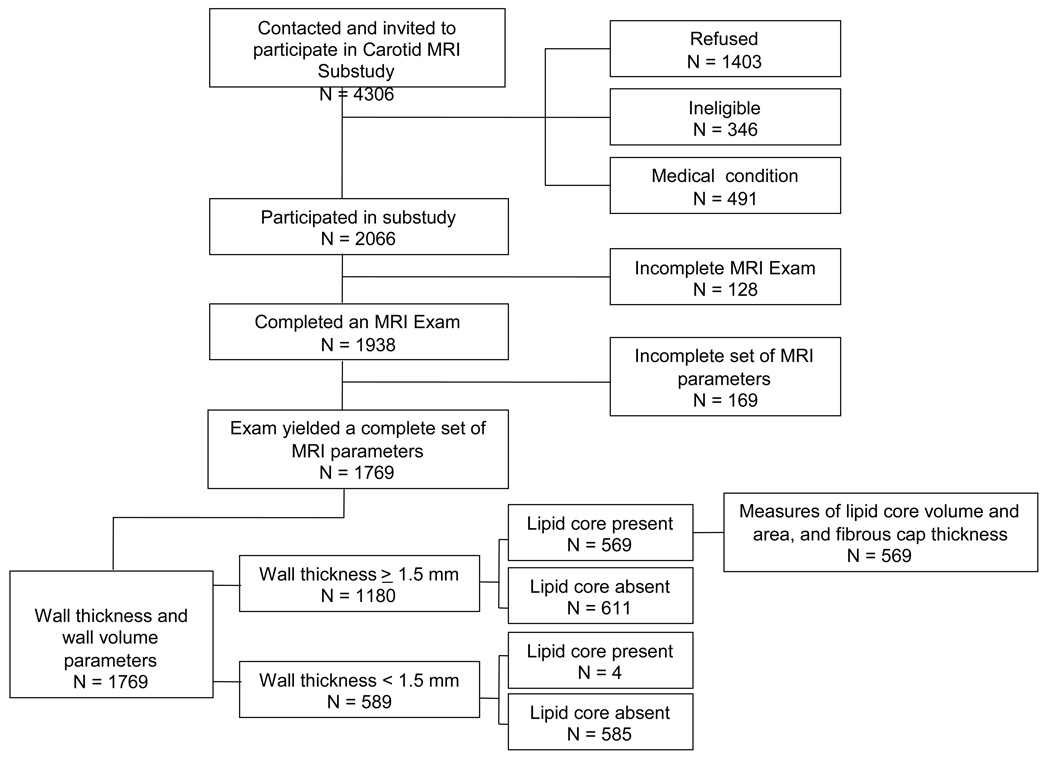
Recruitment lists were created by the coordinating center by sampling from above and below the IMT cutpoint as indicated by the sampling plan. Lists included an indicator for which carotid had the greatest IMT. Ineligibility criteria for the substudy included standard contraindications to the MRI exam or contrast agent, carotid revascularization on either side for the low IMT group or on the side selected for imaging for the high IMT group, and difficulties in completing informed consent. A total of 4306 persons were contacted and invited to participate in the substudy; 1403 refused, 346 were ineligible, 491 reported medical conditions which precluded their participation, and 2066 (48%) participated (Figure 1). Those who refused were more likely to be African American, and have diabetes, hypertension, and obesity, compared to those who participated.
Figure 1.
Schematic describing the ARIC Carotid MRI Substudy Sample and Resulting MRI Measures
Measurement protocols were identical at the baseline ARIC examination and the Carotid MRI substudy examination conducted approximately 18 years later. Smoking and prescription drug use were ascertained by interview. Fasting blood samples were assayed for total and HDL cholesterol, and glucose using conventional techniques. LDL cholesterol was calculated according to the Friedewald formula. High sensitivity c-reactive protein (CRP) and urinary albumin and creatinine were obtained only at the substudy time point. Albumin-creatinine ratio (ACR) was calculated as a measure of albuminuria. Resting blood pressure was determined using a random-zero sphygmomanometer. Hypertension was defined as systolic or diastolic blood pressure of 140 or 90 mmHg or greater, or use of antihypertensive medication. Diabetes was defined as a fasting glucose ≥7.0 mmol/L, a nonfasting glucose level ≥11.1 mmol/L, or a self-report of physician-diagnosed diabetes or treatment. Previous history of CVD included adjudicated myocardial infarction, stroke, and/or revascularization procedure (13). The study was approved by the institutional review committees and participants provided informed consent.
MRI Scanning
A contrast enhanced MRI exam was performed according to a standard protocol. Each study was acquired on a 1.5T whole body scanner equipped with a 4-element phased array carotid coil. A 3-dimensional time-of-flight MR angiogram (MRA) was acquired through both carotid bifurcations. Detailed black blood MRI (BBMRI) images were acquired through the extracranial carotid bifurcation known to have a thicker maximum wall, unless the contralateral carotid bifurcation wall appeared thicker on the MRA to the technologist, in which case the contralateral vessel was chosen for detailed imaging. BBMRI imaging was achieved using an ECG-gated, 2-dimensional double inversion recovery fast spin echo sequence with the inversion time set to suppress the signal of blood. The detailed images included 16 transverse T1-weighted, fat-suppressed BBMRI slices (repetition time/echo time, 1RR/5msec; thickness, 2mm; acquired in-plane resolution, 0.51×0.58mm2; total longitudinal coverage, 3.2cm) oriented perpendicular to the vessel and centered through the thickest segment of the artery or plaque, if present. These 16 slices were acquired five minutes after the intravenous injection of gadodiamide.
Of the 2066 individuals who participated in the Carotid MRI substudy, 1938 completed an MRI examination (Figure 1). Reasons for incomplete MRI examinations (N=128, 6%) included: ineligible at the time of the scan (n=5, 4%); inability to lie in the scanner (n=7, 5%); aborted scan (n=14, 11%); refusal (e.g., claustrophobia; n=38, 30%); and not recorded (n=64, 50%).
MRI Reading
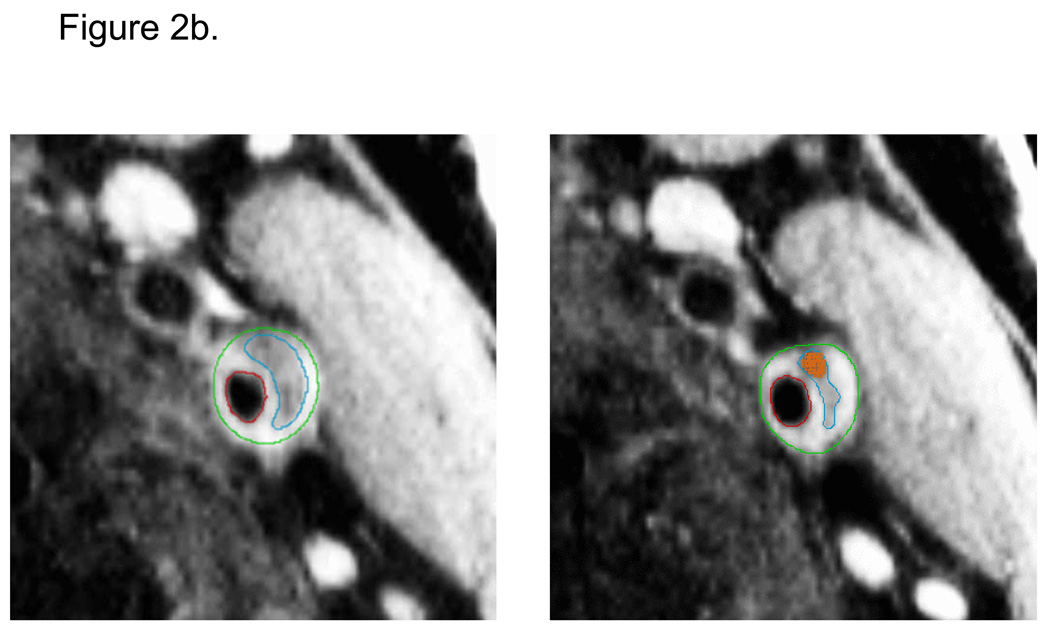
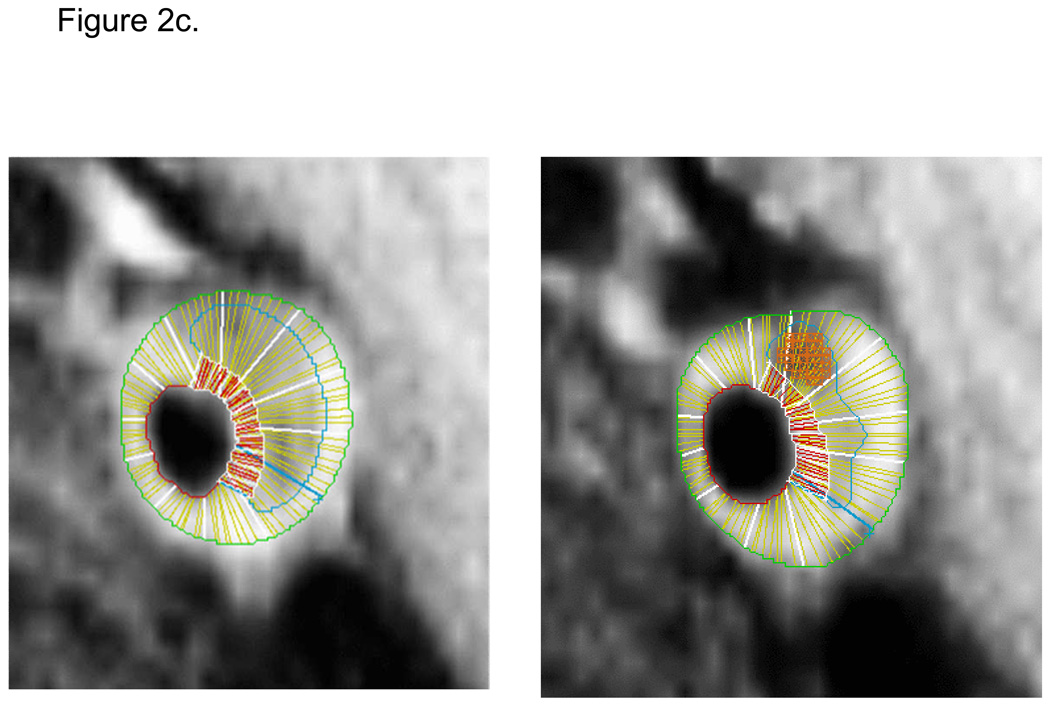
Seven readers were trained to interpret the MRI images and contour the wall components on the postcontrast BBMRI images using specialized software. Readers drew contours to delineate the lumen, outer wall, lipid core, and calcification (Figure 2). Calcification was identified as areas of signal loss on the corresponding TOF MRA images. Lipid core was delineated based on the preferential enhancement of the surrounding fibrous tissue (14, 15), and had intermediate signal on TOF images. The fibrous cap contour was automatically generated to approximate the lumen and lipid core contours. Only eight of 16 slices were analyzed (1.6cm segment), selected as those centered on the slice with the thickest wall. All exams were assigned quality scores (0, 1, or 2) based on image quality and protocol adherence. Exams receiving a quality score of zero were not analyzed.
Figure 2.
Contiguous transverse contrast-enhanced black blood MRI images through a left carotid artery plaque in an ARIC participant with (a) and without (b) manually-drawn contours. Contours delineate the lumen (red), outer wall (green), and lipid core (blue). Calcification was manually shaded (orange). Automated software divided the wall into 12 radial segments and the cap into radial segments at 15 degree increments (c). Segmental wall thickness values were determined by averaging the yellow thickness measurements within each wall segment, and segmental cap thickness values were determined by averaging the red thickness measurements within each cap segment (c).
Vessel walls were divided into 12 radial segments and mean thickness values were generated for each segment. Mean thickness values were also generated for the entire fibrous cap. Area measurements were calculated for the lipid core and calcification contours. Volumetric data were computed by integrating area measurements over all eight slices examined. Details regarding the MRI methods are contained in a supplement.
Attributes could not be ascertained in 1938 scans for the common carotid artery (CCA, n=18, 11%), internal carotid artery (ICA, n=87, 51%), or both (n=64, or 38%), because of protocol deviations or poor image quality (n=143, 7%).
Reliability Study
Reliability coefficients were obtained from an internal reliability study in which 130 scans were re-read by the same or a different reader, and 52 participants were re-scanned within two months. Reliability of lipid core and cap measurements was based on persons with lipid core: 40 repeat readings and 14 repeat scans. The inter- and intra-technician reliability was estimated using the intra-class correlation coefficient (16) and can be interpreted as: greater than 0.75, excellent; 0.4 to 0.75, fair to good; and less than 0.4, poor (17). Reliability based on repeated readings was excellent for wall volume, wall thickness, and lipid core volume (0.76 to 0.85), and fair to good for cap thickness measures (0.60) and lipid core area (0.72). Reliability based on repeated scans was also excellent for wall thickness and volume (0.77 and 0.79), fair to good for minimum cap thickness and lipid core area (0.49 and 0.66), and poor for lipid core volume and cap thickness (0.30 and 0.38). Kappa coefficients for repeated readings and repeated scans for lipid core presence were 0.61 and 0.45.
Statistical Methods
All analyses were based on methods appropriate for stratified random sampling. Analyses were weighted by the inverse of the sampling fractions in the eight sampling strata (2 IMT strata X 4 field centers). Analyses were conducted using SAS version 9.1 or SUDAAN. The analysis approach considered both baseline and concurrent (Year 18) measures of risk factors in relation to MRI measures in those 1769 participant exams in which a complete set of MRI parameters are available (Figure 1). Wall thickness and wall volume were analyzed in the full set. Due to the resolution constraints of the scan, we restricted consideration of lipid core to those 1180 participants whose maximum wall thickness was ≥1.5 mm (weighted percentage = 62%) Only four lipid cores were excluded using this cutpoint. Measures of lipid core volume and area, and fibrous cap thickness were analyzed as continuous variables among those 569 participants with a lipid core (weighted percentage = 42%). An additional analysis considered lipid core presence as a dichotomous variable. Standardized regression coefficients are presented for linear and logistic regression models, standardizing by one standard deviation of exposure and outcome (for continuous outcomes) with adjustment for age, race, and gender. Lipid core volume and areas were also adjusted for maximum wall thickness.
Results
The average age of the sample was 70 years, 57% female, and 19% African American. The average follow-up time between the baseline ARIC examination and the substudy was 18 years (range: 15 – 20 years). LDL and total cholesterol declined between baseline and year 18, consistent with an increase in the use of cholesterol lowering medications (Table 1). BMI increased over this period by approximately 1 to 2 kg/m2 in each gender-race group. Approximately 20% of white participants and 35% of African American participants had diabetes by year 18. Smoking declined by approximately half over this period.
Table 1.
Weighted Means (weighted standard deviations) and Weighted Percentages for Risk Factors at Baseline (BL) and Year 18
| White Female (N=674) |
White Male (N=698) |
African-American Female (N=234) |
African- American Male (N=163) |
||
|---|---|---|---|---|---|
| Age (years) | Y18 | 70.4 (5.4) | 71.0 (5.7) | 69.1 (5.2) | 68.9 (5.5) |
| LDL Cholesterol (mmol/L) | BL | 3.4 (1.0) | 3.5 (0.9) | 3.4 (1.1) | 3.6 (1.1) |
| Y18 | 3.0 (0.9) | 2.7 (0.9) | 3.3 (1.0) | 3.1 (0.8) | |
| HDL Cholesterol (mmol/L) | BL | 1.6 (0.5) | 1.1 (0.3) | 1.6 (0.5) | 1.3 (0.3) |
| Y18 | 1.4 (0.4) | 1.1 (0.3) | 1.4 (0.4) | 1.2 (0.3) | |
| Total Cholesterol (mmol/L) | BL | 5.6 (1.0) | 5.3 (1.0) | 5.5 (1.1) | 5.4 (1.1) |
| Y18 | 5.3 (1.0) | 4.7 (1.0) | 5.4 (1.1) | 4.9 (0.9) | |
| Glucose (mmol/L) | BL | 5.5 (1.3) | 5.6 (0.8) | 5.7 (2.0) | 5.8 (0.9) |
| Y18 | 5.8 (1.3) | 6.0 (1.2) | 6.3 (1.8) | 6.7 (2.2) | |
| Body mass index (kg/m2) | BL | 25.9 (4.8) | 26.8 (3.4) | 28.5 (5.1) | 26.9 (3.7) |
| Y18 | 28.1 (5.5) | 28.1 (4.0) | 30.5 (5.2) | 28.4 (4.1) | |
| Diabetes (%) | BL | 4.2 | 4.6 | 5.8 | 11.9 |
| Y18 | 18.5 | 21.8 | 30.7 | 39.1 | |
| Hypertension (%) | BL | 18.2 | 17.9 | 46.8 | 28.8 |
| Y18 | 62.3 | 57.5 | 78.7 | 67.4 | |
| Current Smoker (%) | BL | 17.1 | 14.7 | 20.6 | 28.7 |
| Y18 | 7.3 | 6.6 | 8.1 | 15.6 | |
| Cholesterol medication (%) | BL | 2.0 | 2.6 | 1.8 | 0.3 |
| Y18 | 42.1 | 48.2 | 36.5 | 37.9 | |
| History of CVD (%) | BL | 1.0 | 2.9 | 0.4 | 1.9 |
| Y18 | 6.3 | 18.0 | 4.6 | 12.7 | |
| Log CRP | BL | NA | NA | NA | NA |
| Y18 | 0.8 (1.0) | 0.5 (1.0) | 1.2 (1.1) | 0.8 (1.0) | |
| Log ACR | BL | NA | NA | NA | NA |
| Y18 | 2.3 (0.9) | 2.3 (1.0) | 2.4 (1.2) | 2.4 (1.3) |
ICA wall volume and maximum segmental wall thickness were markedly higher in men than women and in whites than African Americans (Table 2). Lipid cores were evaluated in carotid arteries in which the maximum wall thickness was equal to or exceeded 1.5mm. This corresponded to 62% of all arteries, with 42% containing lipid core. The volume and area of the lipid core was greatest in white men, corresponding to their larger wall volume. Calcium areas were also greater in white men. The mean fibrous cap thickness similarly was greatest in white men, with no difference observed across the groups for minimum cap thickness.
Table 2.
Weighted Means (weighted standard deviations) and Weighted Percentages for Selected MRI Measures at Year 18
| White | African American | ALL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Female | N | Male | N | Female | N | Male | ||
| Carotid Artery Wall Thickness Measures | |||||||||
| Total Wall Volume (ml) | 674 | 0.375 (0.140) | 698 | 0.480 (0.183) | 234 | 0.348 (0.118) | 163 | 0.420 (0.153) | 0.413 (0.165) |
| Maximum Segmental Wall Thickness | 674 | 1.84 (0.96) | 698 | 2.32 (1.29) | 234 | 1.65 (0.95) | 163 | 1.85 (1.07) | 1.99 (1.13) |
| Maximum Wall Thickness >=1.5 mm | 674 | 57.2% | 698 | 74.2% | 234 | 45.6% | 163 | 57.6% | 62.1% |
| Lipid Core Measures | |||||||||
| Lipid Core Present (%) * | 424 | 37.8% | 541 | 45.8% | 118 | 51.7% | 97 | 36.7% | 42.4% |
| Total Lipid Core Volume (ml) † | 190 | 0.05 (.06) | 281 | 0.08 (.1) | 54 | 0.04 (.04) | 44 | 0.05 (.08) | 0.06 (0.08) |
| Maximum Lipid Core Area (cm2) † | 190 | 0.09 (0.08) | 281 | 0.13 (0.13) | 54 | 0.08 (0.08) | 44 | 0.09 (0.08) | 0.11 (0.10) |
| (Median) Maximum Calcium Area (cm2) | 190 | 0.012 | 281 | 0.022 | 54 | 0.019 | 44 | 0.019 | 0.018 |
| Fibrous Cap Measures | |||||||||
| Cap Thickness (mm) † | 190 | 0.65 (0.28) | 281 | 0.70 (0.31) | 54 | 0.65 (0.29) | 44 | 0.66 (0.27) | 0.67 (0.30) |
| Minimum Cap Thickness (mm) † | 190 | 0.48 (0.25) | 281 | 0.49 (0.25) | 54 | 0.47 (0.25) | 44 | 0.48 (0.21) | 0.48 (0.25) |
restricted to participants with maximum wall thickness >=1.5 mm
restricted to participants with maximum wall thickness >=1.5 mm and lipid core present
Greater baseline age, male gender, white race, total cholesterol, LDL cholesterol, glucose, BMI, and presence of diabetes, hypertension, and smoking were all associated with increased total wall volume (Table 3). The same risk factors except BMI were associated with maximum segmental wall thickness. Some of these associations, when measured concurrently, persisted, particularly for wall volume. In other cases, concurrently measured risk factors were not associated with outcome even though the baseline risk factor was, e.g., glucose, BMI.
Table 3.
Relationship between continuous wall thickness measures and risk factors obtained at Baseline and Year 18, adjusting for age, race, and gender (standardized beta coefficients*, p-value in parentheses).
| Total wall volume N=1769 |
Maximum segmental wall thickness N=1769 |
|||
|---|---|---|---|---|
| Baseline | Year 18 | Baseline | Year 18 | |
| Age | 0.10 (<0.0001) | 0.09 (0.001) | 0.12 (<0.0001) | 0.11 (<0.0001) |
| Race (ref=white) | −0.21 (<0.0001) | −0.22 (<0.0001) | −0.23 (<0.0001) | −0.23 (<0.0001) |
| Gender (ref=female) | 0.60 (<0.0001) | 0.60 (<0.0001) | 0.37 (<0.0001) | 0.38 (<0.0001) |
| Total cholesterol | 0.07 (0.02) | 0.08 (0.003) | 0.12 (<0.0001) | 0.04 (0.12) |
| HDL | 0.04 (0.43) | 0.003 (0.92) | 0.006 (0.89) | −0.008 (0.77) |
| LDL | 0.06 (0.04) | 0.06 (0.02) | 0.12 (<0.0001) | 0.04 (0.15) |
| Glucose | 0.11 (0.01) | 0.02 (0.49) | 0.07 (0.01) | 0.04 (0.14) |
| BMI | 0.07 (0.02) | 0.04 (0.14) | 0.03 (0.29) | −0.005 (0.86) |
| Diabetes | 0.53 (0.01) | 0.20 (0.004) | 0.39 (0.03) | 0.16 (0.02) |
| Hypertension | 0.18 (0.009) | 0.16 (0.005) | 0.18 (0.007) | 0.19 (0.001) |
| Smoking | 0.18 (0.01) | 0.17 (0.07) | 0.27 (<0.0001) | 0.26 (0.01) |
| CRP | NA | 0.04 (0.18) | NA | 0.04 (0.19) |
| ACR | NA | 0.09 (0.002) | NA | 0.07 (0.01) |
Number of standard deviations differences in MRI variables associated with one standard deviation difference in continuous risk factors (from Table 1), or between categories of a dichotomous variable. Significant (p<0.05) results are indicated in bold italics.
Increased baseline levels of total cholesterol and LDL cholesterol, as well as age, were the only factors which predicted presence of lipid core 18 years later (Table 4). A one standard deviation increase in total cholesterol (approximately 1.0 mmol/L) increased the odds of lipid core presence by 26% (β=0.23, odds ratio=1.26, p=0.003). These relationships were attenuated with adjustment for wall thickness, which was itself strongly associated with presence of lipid core. The wall thickness adjusted odds of lipid core for a one standard deviation increase in total cholesterol was 1.16 (p=0.10). No risk factor measured at year 18 was associated with presence of lipid core.
Table 4.
Relationship between presence of lipid core and risk factors obtained at Baseline and Year 18, restricted to participants with maximum wall thickness >= 1.5 mm, adjusting for age, race, and gender, and adjusting for age, race, gender, and wall thickness (standardized beta coefficient s*, p-value in parentheses).
| Presence of Lipid Core N=1180 |
||||
|---|---|---|---|---|
| Unadjusted for wall thickness | Adjusted for wall thickness | |||
| Baseline | Year 18 | Baseline | Year 18 | |
| Age | 0.20 (0.01) | 0.21 (0.01) | 0.15 (0.07) | 0.16 (0.06) |
| Race (ref=white) | 0.19 (0.31) | 0.19 (0.32) | 0.34 (0.10) | 0.34 (0.10) |
| Gender (ref=female) | 0.17 (0.25) | 0.18 (0.25) | −0.05 (0.78) | −0.04 (0.79) |
| Wall thickness | NA | NA | 1.14 (<0.0001) | 1.14 (<0.0001) |
| Total cholesterol | 0.23 (0.003) | −0.10 (0.19) | 0.15 (0.10) | −0.09 (0.26) |
| HDL | −0.02 (0.86) | −0.13 (0.14) | −0.01 (0.90) | −0.12 (0.21) |
| LDL | 0.19 (0.02) | −0.07 (0.36) | 0.12 (0.16) | −0.07 (0.38) |
| Glucose | 0.08 (0.26) | 0.08 (0.30) | 0.02 (0.83) | 0.10 (0.24) |
| BMI | −0.02 (0.79) | −0.04 (0.62) | −0.10 (0.27) | −0.08 (0.37) |
| Diabetes | −0.11(0.74) | 0.08 (0.65) | −0.44 (0.23) | 0.06 (0.76) |
| Hypertension | 0.28 (0.12) | 0.16 (0.34) | 0.06 (0.75) | 0.06 (0.72) |
| Smoking | 0.23 (0.22) | 0.16 (0.56) | 0.01 (0.98) | −0.11 (0.68) |
| CRP | NA | 0.03 (0.71) | NA | 0.01 (0.90) |
| ACR | NA | 0.05 (0.48) | NA | 0.01 (0.93) |
Beta coefficient for presence of lipid core associated with one standard deviation difference in continuous risk factors (from Table 1), or between categories of a dichotomous variable. Significant (p<0.05) results are indicated in bold italics.
Two risk factors were significantly, albeit modestly associated with the size of the lipid core (Table 5). Younger age at baseline (p=0.03) and increased LDL at year 18 (p=0.02) were associated with greater lipid core volume at year 18, independent of wall thickness.
Table 5.
Relationship between continuous lipid core measures and risk factors obtained at Baseline and Year 18, restricted to participants with maximum wall thickness >= 1.5 mm who have lipid core present, adjusting for age, race, gender, and wall thickness (standardized beta coefficients*, p-value in parentheses).
| Total lipid core volume N=573 |
Maximum lipid core area N=573 |
|||
|---|---|---|---|---|
| Baseline | Year 18 | Baseline | Year 18 | |
| Age | −0.06 (0.03) | −0.04 (0.10) | −0.03 (0.32) | −0.02 (0.54) |
| Race (ref=white) | −0.06 (0.36) | −0.05 (0.41) | −0.02 (0.76) | −0.01 (0.81) |
| Gender (ref=female) | 0.05 (0.38) | 0.05 (0.37) | 0.03 (0.53) | 0.03 (0.52) |
| Wall thickness | 0.80 (<0.0001) | 0.80 (<0.0001) | 0.85 (<0.0001) | 0.85 (<0.0001) |
| Total cholesterol | −0.001 (0.98) | 0.04 (0.09) | −0.004 (0.84) | 0.04 (0.15) |
| HDL | 0.06 (0.14) | −0.003 (0.90) | 0.04 (0.29) | −0.01 (0.72) |
| LDL | −0.03 (0.32) | 0.06 (0.02) | −0.02 (0.39) | 0.04 (0.11) |
| Glucose | 0.03 (0.46) | 0.003 (0.90) | 0.03 (0.17) | 0.02 (0.38) |
| BMI | −0.03 (0.37) | −0.02 (0.64) | −0.02 (0.59) | 0.01 (0.87) |
| Diabetes | 0.17 (0.38) | −0.05 (0.45) | 0.20 (0.14) | −0.05 (0.40) |
| Hypertension | −0.09 (0.18) | 0.05 (0.44) | −0.08 (0.19) | 0.04 (0.50) |
| Smoking | −0.02 (0.86) | −0.12 (0.28) | −0.05 (0.53) | −0.19 (0.06) |
| CRP | NA | 0.02 (0.39) | NA | 0.02 (0.41) |
| ACR | NA | −0.02 (0.44) | NA | −0.03 (0.17) |
Number of standard deviations differences in MRI variables associated with one standard deviation difference in continuous risk factors (from Table 1), or between categories of a dichotomous variable. Significant (p<0.05) results are indicated in bold italics.
Two risk factors were associated with cap thickness measures (Supplemental Results Table). Glucose measured at Year 18 was inversely associated with cap thickness (β= −0.13, p=0.01) and BMI measured at Year 18 was inversely associated with minimum cap thickness (β= −0.13, p=0.04).
Discussion
We have examined the correlates of atherosclerotic plaque size and composition in the ICA in a population-based cohort. Our principal findings are that greater baseline levels of total cholesterol, LDL cholesterol, glucose, and presence of diabetes, hypertension, and smoking, are all significant predictors of increased total wall volume and maximum wall thickness 18 years later. In contrast, the only baseline risk factors that predicted the presence of a lipid core were age, total cholesterol and LDL cholesterol. Perhaps not surprisingly, these relationships were mediated through greater wall thickness resulting from the intra-wall accumulation of lipids. Few risk factors were associated with the size of the lipid core and the thickness of the fibrous cap; the associations were sporadic and modest in size. Of note, two metabolic factors, increased glucose levels and obesity, were associated with a thinner fibrous cap. These findings emphasize the importance of traditional risk factors in predicting carotid wall size, but not necessarily the composition of the carotid plaque.
We evaluated risk factors that are known to be associated with carotid IMT in ARIC and other studies (18–20) confirming the expected associations with total and LDL cholesterol, glucose, diabetes, hypertension, and smoking. A slight difference in risk factor profiles was observed between total wall volume and maximum wall thickness: in particular, baseline BMI predicted total wall volume but not maximum wall thickness. This might be explained by wall volume reflecting the vessel diameter, which depends on body size rather than CV risk factors. In addition, diabetes was a stronger predictor of wall volume than wall thickness. The latter finding may be supported by previous work in which diabetes was shown to increase total and distal plaque burden in dissected coronary arteries (21). MRI-measured wall volume may be a better measure of distal plaque burden than wall thickness.
Only one other previous study has examined the correlates of atherosclerotic plaque characteristics in a population-based cohort. The Multi-Ethnic Study of Atherosclerosis study has reported that total cholesterol was the sole cross-sectional correlate of lipid core presence in 151 individuals with a lipid core but free of clinically apparent cardiovascular disease (10). Compared to those in the lowest tertile of total cholesterol, and adjusted for wall thickness, the odds of lipid core presence for participants in the middle and highest tertiles were 2.76 and 4.63. Our findings differ from these in two ways. In ARIC, neither baseline nor year 18 wall thickness adjusted total cholesterol levels were significantly associated with presence of lipid core. Furthermore, the cross-sectional association between total cholesterol and presence of lipid core was inverse, albeit non-significant. (This inverse association was not explained by lipid lowering medications as it persisted in the subset not taking these medications; not shown). The difference in these study results may be explained by differences in the underlying cohorts including a higher prevalence of cores observed in MESA than in ARIC and greater use of lipid lowering medications in ARIC than in MESA.
To our knowledge, this is the first population-based study of risk factor associations of quantitative measure of fibrous cap thickness. Most other studies have been conducted in small clinical or autopsy samples, and have focused on a categorical (not quantitative) assessment of fibrous cap, i.e., whether the plaque is ruptured or intact (5, 9). In our study, the inverse associations of concurrently measured glucose and BMI with cap thickness are consistent with the hypothesis that risk factors are associated with a thin fibrous cap. These particular observations suggest a metabolic/inflammatory mechanism by which the composition of the plaque is modified.
The contrast between the large numbers of risk factors associated with wall thickness/volume versus the limited set of risk factors associated with plaque composition is striking. There are several possible explanations for this discrepancy. First, repeatability coefficients for plaque composition characteristics, especially for repeated scans, were modest. Thus, measurement error is a possible explanation for the weaker findings for the lipid core and fibrous cap thickness measures. However, we have only moderate confidence in the estimates of repeatability for plaque composition characteristics; the coefficients were based upon only 14 repeated studies. In contrast, the reliability estimates were excellent for wall thickness/volume measures. This is not unexpected. Repeatability depends upon the range of normal values. Consequently, repeatability was best for the largest structures (wall thickness) and poorest of the smallest structures (cap thickness). Despite weak reliability, the descriptive statistics that we report here for plaque composition characteristics are consistent with measures obtained from pathology studies (5, 22), giving us confidence in the validity of the measures. Our cap thickness measurements (Table 2) are marginally higher than those reported for non-ruptured sites in symptomatic plaque specimens (22). This difference was anticipated given that symptomatic would likely have thinner caps. Furthermore, with a linear resolution close to 500µ, we expect some overestimation of our cap thickness measurements. In conclusion, for the smaller structures in particular, measurements are accurate but not highly reproducible, possibly explaining the weaker relationships for plaque composition measures.
There are other possible explanations for the generally null findings. The resolution of the MRI images limits our ability to characterize small plaques, thus we were able to study only the largest plaques found within the thickest arterial walls. By truncating the distribution of lipid core volume and cap thickness, we have thereby restricted the range of values and possibly the association with risk factors. Another possibility is that risk factor profiles differ for calcified and non-calcified plaques. However, exclusion of heavily calcified plaques had no effect on the strength of the associations between risk factors and lipid core (not shown). Finally, risk factors for plaque composition may truly differ from risk factors for wall thickness and wall volume. Other factors may play a role in the development and progression of atherosclerotic plaque, particularly those in the inflammatory and coagulation pathways (23). For example, we have recently reported that variation within the promoter region of the matrix metalloproteinase-2 gene is associated with fibrous cap thickness (24).
A limitation is the characterization of a single plaque and the assumption that it represents the character of plaques systemically. The one plaque we characterized was the largest in the visualized carotid arteries. Consequently, we assume that the morphology of this plaque and its risk factor relationships are representative of plaques throughout. Indeed, there is evidence to support the contention that plaque characteristics are moderately correlated across major arterials beds (3, 25). Nonetheless, these studies are small and limited usually to homogeneous samples (e.g., symptomatic patients). Since the wall thickness/volume measures and the plaque composition measures were similarly assessed at one arterial location, this argument does not explain why only a limited set of risk factor relationships was observed for plaque composition.
There are many strengths of the present study including its being a well-characterized sample of a population-based cohort with data collected over an 18 year period. This has provided an opportunity to characterize contemporaneous as well as previous risk factors in relation to outcome measures. A standardized MRI protocol with central reading facility was utilized. Quality control data were collected allowing an assessment of the reliability of the MRI measures. Furthermore, this is the first population-based study of plaque in which all participants received a contrast-enhanced MRI examination, which improves reliability considerably (14, 26).
We have found that traditional CVD risk factors measured two decades previously can predict carotid wall thickness and volume. In contrast, only total cholesterol and LDL cholesterol were associated with the presence of a lipid core, a relationship that was mediated through greater wall thickness. Very few relationships were observed between risk factors and quantity of lipid core and thickness of fibrous cap. Notably, two metabolic factors, increased glucose levels and obesity, were associated with a thinner fibrous cap. In conclusion, traditional risk factors increase the extent of atherosclerosis, but in the presence of atherosclerosis they do not discriminate plaque composition as measured by MRI.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding Sources
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The Carotid MRI Substudy was supported by National Heart, Lung, and Blood Institute cooperative agreement U01-HL-075572.
The following individuals received grant funding from the National Heart Lung and Blood Institute to conduct the research described in this manuscript: Lynne E. Wagenknecht, Bruce A. Wasserman, Lloyd E. Chambless, Josef Coresh, Aaron R. Folsom, Thomas H. Mosley, Christie M. Ballantyne, A. Richey Sharrett, and Eric Boerwinkle.
Footnotes
Conflict of Interest Disclosures
Christie M. Ballantyne reports the following relationships: Grant/Research Support (all significant): Abbott, Astra Zeneca, GlaxoSmithKline, Merck, Pfizer, Sanofi-Synthelabo, Schering-Plough, Takeda. Consultant (modest): Abbott, Astra Zeneca, Atherogenics, GlaxoSmithKline, Merck, Merck Schering Plough, Novartis, Pfizer, Sanofi-Synthelabo, Schering-Plough, Takeda. Speakers Bureau (all modest): AstraZeneca, Glaxo Smith Kline, Merck, Merck Schering Plough, Pfizer, Reliant, Schering-Plough. Honorarium (significant): Merck, Astra Zeneca. Honorarium (modest): Abbott, Atherogenics, GlaxoSmithKline, Merck Schering Plough, Novartis, Pfizer, Sanofi-Synthelabo, Schering-Plough, Takeda.
Contributor Information
Lynne Wagenknecht, Wake Forest University School of Medicine.
Bruce Wasserman, Johns Hopkins University.
Lloyd Chambless, University of North Carolina at Chapel Hill.
Josef Coresh, Johns Hopkins University.
Aaron Folsom, University of Minnesota.
Thomas Mosley, University of Mississippi.
Christie Ballantyne, Baylor College of Medicine.
Richey Sharrett, Johns Hopkins University.
Eric Boerwinkle, University of Texas.
References
- 1.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47 Suppl:C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 2.Virmani R, Ladich ER, Burke AP, Kolodgie FD. Histopathology of carotid atherosclerotic disease. Neurosurgery. 2006;59 Suppl S3:219–227. doi: 10.1227/01.NEU.0000239895.00373.E4. [DOI] [PubMed] [Google Scholar]
- 3.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 4.Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: The Oxford plaque study. Circulation. 2006;113:2320–2328. doi: 10.1161/CIRCULATIONAHA.105.589044. [DOI] [PubMed] [Google Scholar]
- 5.Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, Tran N, Polissar NL, Isaac C, Ferguson MS, Garden GA, Cramer SC, Maravilla KR, Hashimoto B, Hatsukami TS. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events. A prospective assessment with MRI – Initial Results. Stroke. 2006;37:818–823. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 6.Saam T, Hatsukami TS, Takaya N, Chu B, Underhill H, Kerwin WS, Cai J, Ferguson MS, Yuan C. The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology. 2007;244:64–77. doi: 10.1148/radiol.2441051769. [DOI] [PubMed] [Google Scholar]
- 7.Gyongyosi M, Glogar D, Weidinger F, Domanovits H, Laggner A, Wojta J, Zorn G, Iordanova N, Huber K. Association between plasmin activation system and intravascular ultrasound signs of plaque instability in patients with unstable angina and non-st-segment elevation myocardial infarction. Am Heart J. 2004;147:158–164. doi: 10.1016/j.ahj.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Isoda K, Arakawa K, Kamezawa Y, Nishizawa K, Nishikawa K, Shibuya T, Ohsuzu F, Nakamura H. Effect of coronary risk factors on arterial compensatory enlargement in Japanese middle-aged patients with de novo single-vessel disease--an intravascular ultrasound study. Clin Cardiol. 2001;24:443–450. doi: 10.1002/clc.4960240605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–1282. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 10.Wasserman BA, Sharrett AR, Lai S, Gomes AS, Cushman M, Folsom AR, Bild DE, Kronmal RA, Sinha S, Bluemke DA. Risk factor associations with the presence of a lipid core in carotid plaque of asymptomatic individuals using high-resolution MRI. The Multi-Ethnic Study of Atherosclerosis (MESA) Stroke. 2008;39:329–335. doi: 10.1161/STROKEAHA.107.498634. [DOI] [PubMed] [Google Scholar]
- 11.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 12.Jackson R, Chambless L, Yang K. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender and ethnicity. J Clin Epidemiol. 1996;49:1441–1446. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 13.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclero HAsis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 14.Wasserman BA, Smith WI, Trout HH, 3rd, Cannon RO, 3rd, Balaban RS, Arai AE. Carotid artery atherosclerosis: in vivo morphologic characterization with gadolinium-enhanced double-oblique mr imaging initial results. Radiology. 2002;223:566–573. doi: 10.1148/radiol.2232010659. [DOI] [PubMed] [Google Scholar]
- 15.Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, Takaya N, Polissar NL, Yuan C. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: Comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circ. 2005;112:3437–3444. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 16.Fleiss JL. The Design and Analysis of Clinical Experiments. New York, NY: John Wiley & Sons; 1986. [Google Scholar]
- 17.Landis JR, Koch GG. The measure of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 18.Heiss G, Sharrett AR, Barnes RW, Chambless LE, Szklo M, Alzola C ARIC Investigators. Carotid atherosclerosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC Study. Am J Epidemiol. 1991;134:250–256. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- 19.O'Leary DH, Polak JF, Kronmal RA, Kittner SJ, Bond MG, Wolfson SK, Jr, Bommer W, Price TR, Gardin JM, Savage PJ The CHS Collaborative Research Group. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. Stroke. 1992;23:1752–1760. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 20.Sharrett AR, Ding J, Criqui MH, Saad MF, Liu K, Polak JF, Folsom AR, Tsai MY, Burke GL, Szklo M. Smoking, diabetes, and blood cholesterol differ in their associations with subclinical atherosclerosis: The Multiethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2006;186:441–447. doi: 10.1016/j.atherosclerosis.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics. A postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 22.Redgrave JN, Gallagher P, Lovett JK, Rothwell PM. Critical cap thickness and rupture in symptomatic carotid plaques: The Oxford Plaque Study. Stroke. 2008;39:1722–1729. doi: 10.1161/STROKEAHA.107.507988. [DOI] [PubMed] [Google Scholar]
- 23.Libby P. The molecular mechanisms of the thrombotic complications of atherosclerosis. J Int Med. 2008;263:517–527. doi: 10.1111/j.1365-2796.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volcik K, Chambless L, Coresh J, Folsom A, Campbell S, Mosley T, Ni H, Wagenknecht L, Wasserman B, Boerwinkle E. Matrix Metalloproteinase 2 genetic variation influences measures of fibrous cap thickness: The Atherosclerosis Risk in Communities (ARIC) Study. Circ. 2008;117:e198–e291. doi: 10.1016/j.atherosclerosis.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleiner M, Kummer M, Mirlacher M, Sauter G, Cathomas G, Krapf R, Biedermann BC. Arterial neovascularization and inflammation in vulnerable patients. Early and late signs of symptomatic atherosclerosis. Circ. 2004;110:2843–2850. doi: 10.1161/01.CIR.0000146787.16297.E8. [DOI] [PubMed] [Google Scholar]
- 26.Takaya N, Cai J, Ferguson MS, Yarnykh VL, Chu B, Saam T, Polissar NL, Sherwood J, Cury RC, Anders RJ, Broschat KO, Hinton D, Furie KL, Hatsukami TS, Yuan C. Intra- and inter-reader reproducibility of magnetic resonance imaging for quantifying the lipid-rich necrotic core is improved with gadolinium contrast enhancement. J Magn Reson Imaging. 2006;24:203–210. doi: 10.1002/jmri.20599. [DOI] [PubMed] [Google Scholar]