Abstract
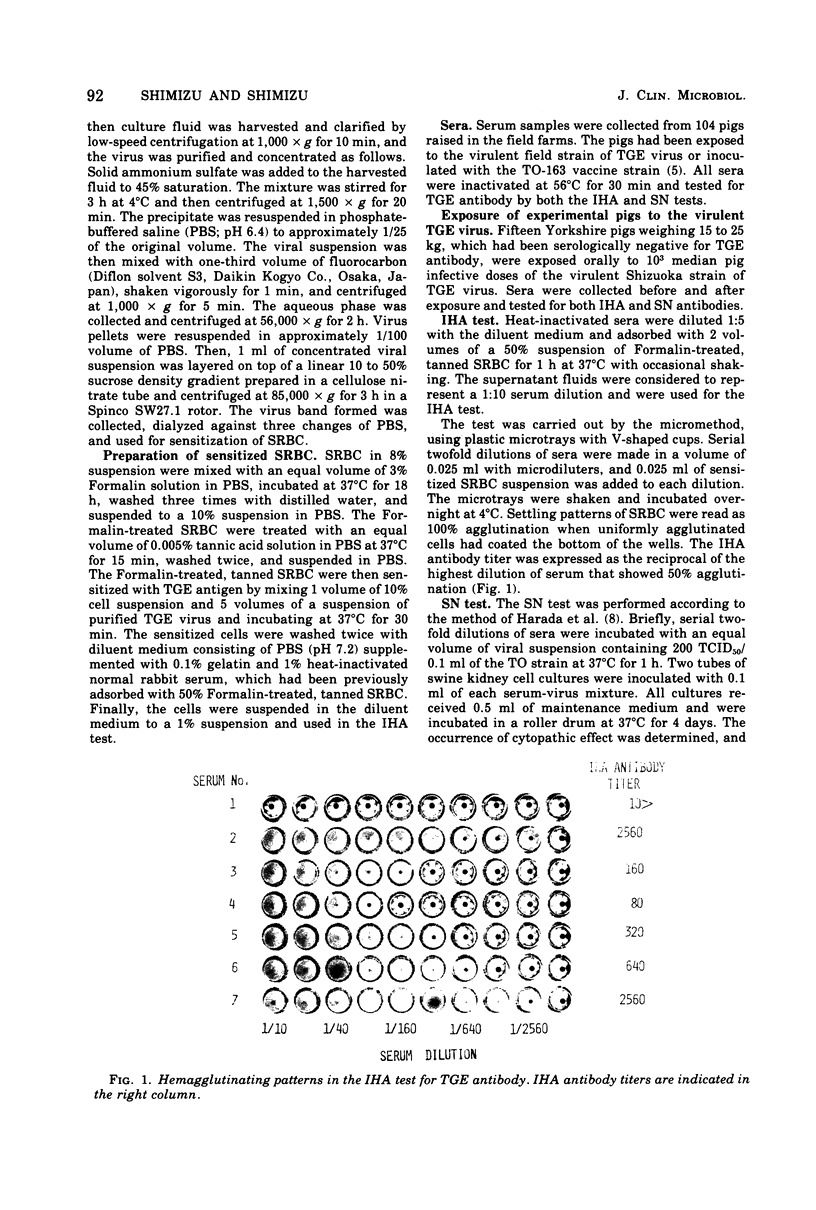
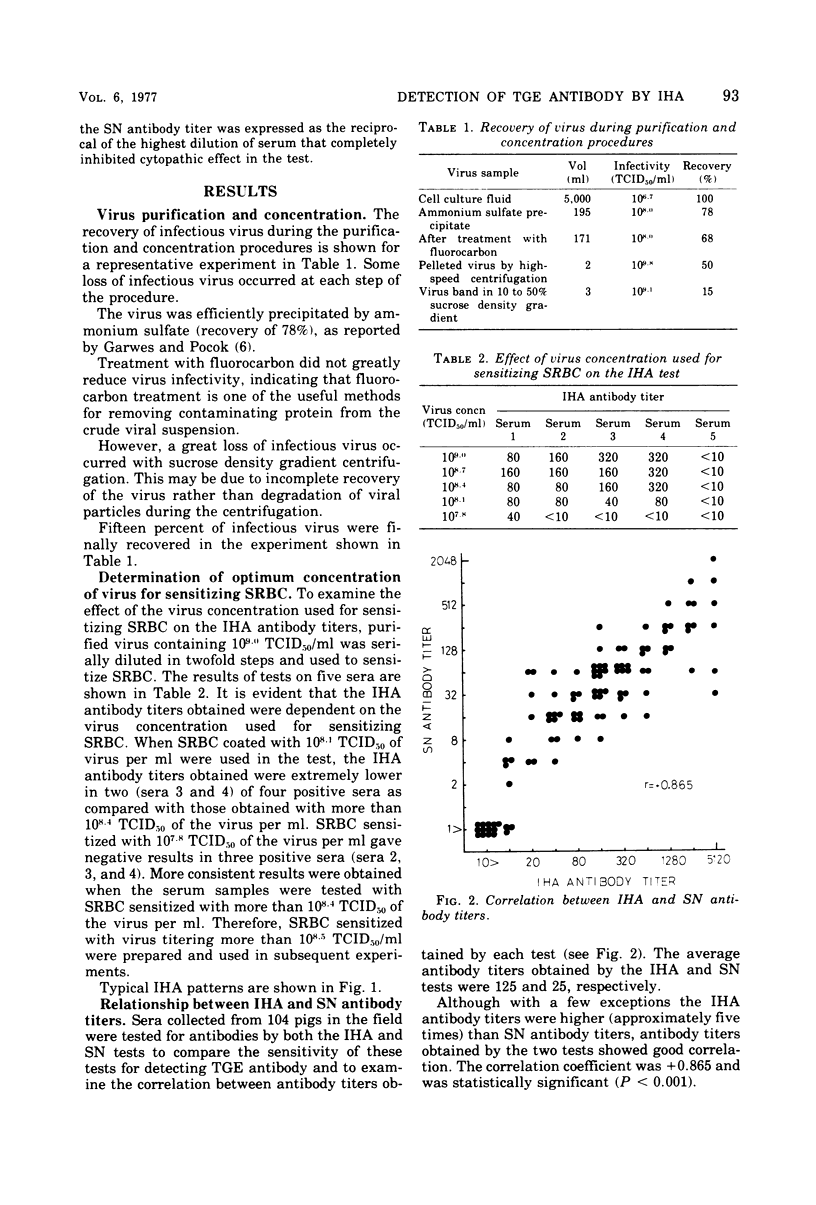
A micro-indirect hemagglutination (IHA) test was developed for detecting antibody against transmissible gastroenteritis (TGE) virus of pigs. TGE virus propagated in swine kidney cell cultures was highly purified and concentrated by the combination of ammonium sulfate precipitation, treatment with fluorocarbon, and sucrose density gradient centrifugation. Tanned sheep erythrocytes were sensitized with purified virus for use in the IHA test. The results of testing 104 serum samples collected from pigs in the field indicated that the IHA antibody titers were approximately five times higher than those obtained by a serum neutralization test and that there was good correlation between the antibody titers determined by the two tests. High IHA antibody titers developed in pigs experimentally exposed to virulent TGE virus. Sensitized sheep erythrocytes were stable under long-term storage at 4 degrees C (at least for 50 days). The conclusions made are that the IHA test described is more sensitive than the serum neutralization test for the detection of TGE antibody and may be of value for serodiagnosis of TGE.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohac J., Derbyshire J. B. The detection of transmissible gastroenteritis viral antibodies by immunodiffusion. Can J Comp Med. 1976 Apr;40(2):161–165. [PMC free article] [PubMed] [Google Scholar]
- Bohac J., Derbyshire J. B., Thorsen J. The detection of transmissible gastroenteritis viral antigens by immunodiffusion. Can J Comp Med. 1975 Jan;39(1):67–75. [PMC free article] [PubMed] [Google Scholar]
- Fuccillo D. A., Moder F., Traub R. G., Hensen S., Sever J. L. Micro indirect hemagglutination test for Cytomegalovirus. Appl Microbiol. 1971 Jan;21(1):104–107. doi: 10.1128/am.21.1.104-107.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuuchi S., Shimizu Y., Kumagai T. Vaccination of newborn pigs with an attenuated strain of transmissible gastroenteritis virus. Am J Vet Res. 1976 Dec;37(12):1401–1404. [PubMed] [Google Scholar]
- Garwes D. J., Pocock D. H. The polypeptide structure of transmissible gastroenteritis virus. J Gen Virol. 1975 Oct;29(1):25–34. doi: 10.1099/0022-1317-29-1-25. [DOI] [PubMed] [Google Scholar]
- Harada K., Furuuchi S., Kumagai T., Sasahara J. Pathogenicity, immunogenicity and distribution of transmissible gastroenteritis virus in pigs. Natl Inst Anim Health Q (Tokyo) 1969 Winter;9(4):185–192. [PubMed] [Google Scholar]
- Harada K., Kumagai T., Sasahara J. Studies on transmissible gastroenteritis in pigs. 3. Isolation of cytopathogenic viurs and its use for serological investigation. Natl Inst Anim Health Q (Tokyo) 1967 Fall;7(3):127–137. [PubMed] [Google Scholar]
- Ishii S., Watanabe M. An indirect hemagglutination test of rinderpest virus. Natl Inst Anim Health Q (Tokyo) 1971 Summer;11(2):55–63. [PubMed] [Google Scholar]
- McKENNA J. M., ZUSCHEK F., FRANKEL J. W. An hemagglutination test for titration of antibodies to polioviruses. Proc Soc Exp Biol Med. 1958 Jan;97(1):160–163. doi: 10.3181/00379727-97-23675. [DOI] [PubMed] [Google Scholar]
- Phillip J. I., Cartwright S. F., Scott A. C. The size and morphology of T.G.E. and vomiting and wasting disease viruses of pigs. Vet Rec. 1971 Mar 20;88(12):311–312. doi: 10.1136/vr.88.12.311. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Isayama Y., Kawakami Y., Murase N., Kawano T. Micro indirect hemagglutination test for detecting antibody against infectious bovine rhinotracheitis virus. Natl Inst Anim Health Q (Tokyo) 1972 Spring;12(1):1–7. [PubMed] [Google Scholar]
- Stone S. S., Kemeny L. J., Jensen M. T. Partial characterization of the principal soluble antigens associated with the coronavirus of transmissible gastroenteritis by complement fixation and immunodiffusion. Infect Immun. 1976 Feb;13(2):521–526. doi: 10.1128/iai.13.2.521-526.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



