Abstract
Natural killer (NK) cells target and kill aberrant cells, such as virally infected and tumorigenic cells. Killing is mediated by cytotoxic molecules which are stored within secretory lysosomes, a specialized exocytic organelle found in NK cells. Target cell recognition induces the formation of a lytic immunological synapse between the NK cell and its target. The polarized exocytosis of secretory lysosomes is then activated and these organelles release their cytotoxic contents at the lytic synapse, specifically killing the target cell. The essential role that secretory lysosome exocytosis plays in the cytotoxic function of NK cells is highlighted by immune disorders that are caused by the mutation of critical components of the exocytic machinery. This review will discuss recent studies on the molecular basis for NK cell secretory lysosome exocytosis and the immunological consequences of defects in the exocytic machinery.
Keywords: exocytosis, immunodeficiency, immunological synapse, innate immunity, natural killer cells, secretory lysosome
Introduction
Natural killer (NK) cells are part of the innate arm of the immune system. Their function is to eliminate aberrant cells, including virally infected and tumorigenic cells. For this purpose NK cells store cytotoxic proteins within secretory lysosomes, specialized exocytic organelles that are also known as lytic granules. Analogous to an assassin pulling the trigger of his gun, the recognition of an aberrant target cell activates the polarized exocytosis of secretory lysosomes releasing cytotoxic molecules that kill the target cell. To understand the cytotoxic function of NK cells it is, therefore, important to determine how secretory lysosomes are mobilized to release their cytotoxic contents. Recently, significant progress has been made in understanding the molecular basis for secretory lysosome exocytosis in NK cells. In particular, the characterization of immune disorders in which NK cytotoxicity is impaired has led to the identification of novel components of the exocytic machinery (Table 1). These immune disorders also highlight the essential role that secretory lysosome exocytosis plays in NK cell cytotoxicity and demonstrate that the loss of the exocytic activity of NK cells has profound immunological consequences. This review summarizes these findings and highlights areas where our understanding of secretory lysosome exocytosis is limited.
Table 1.
Proteins with putative functions in the exocytosis of secretory lysosomes by natural killer cells
| Protein | Putative function | Associated immune disorder | References |
|---|---|---|---|
| Wiskott–Aldrich Syndrome protein (WASp) | Actin polymerization at the lytic synapse (Fig. 1a) | Wiskott–Aldrich Syndrome (WAS) | 23–27 |
| WASp Interacting protein (WIP) | Linking of actin cytoskeletal reorganization with the later stages of secretory lysosome exocytosis (Fig. 1b) | Not applicable | 29,30 |
| Cdc42 Interacting protein-4 (CIP) | Movement of microtubule organizing centre (MTOC) to the lytic synapse (Fig. 1b) | Not applicable | 28 |
| Adaptor protein 3 complex (AP-3) | Sorting of protein(s) to secretory lysosomes that are required for the movement of this organelle along microtubules (Fig. 1b) | Hermansky–Pudlak syndrome subset 2 (HPS2) | 34–36 |
| Rab7 interacting lysosomal protein (RILP)/ Rab7 | Movement of secretory lysosomes along microtubules towards the MTOC (Fig. 1b) | Not applicable | 33,37–39 |
| Rab27a | Docking of secretory lysosomes with the plasma membrane (Figs 1c and 2a). | Griscelli syndrome 2 (GS2) | 40–47 |
| Myosin IIa | Movement of secretory lysosomes into close apposition with plasma membrane (Figs 1c and 2a) | Not applicable | 29,50,51 |
| Munc13-4 | Priming of secretory lysosomes for fusion with the plasma membrane (Figs 1d and 2b) | Familial haemophagocytic lymphohistiocytosis subset 3 (FHL3) | 44,53–56 |
| Syntaxin 11 | Fusion of secretory lysosomes with the plasma membrane (Figs 1d and 2c) | Familial haemophagocytic lymphohistiocytosis subset 4 (FHL4) | 37,57–61 |
| VAMP7 | Fusion of secretory lysosomes with the plasma membrane or transport of proteins to secretory lysosomes (Figs 1d and 2c) | Not applicable | 37,62 |
| Syntaxin 7 | Fusion of secretory lysosomes with the plasma membrane or transport of proteins to secretory lysosomes (Figs 1d and 2c) | Not applicable | 37 |
| Dynamin 2 | Recapture of spent secretory lysosomes from the immunological synapse | Not applicable | 65 |
NK cells and cytotoxic T lymphocytes: brothers in arms
Natural killer cells and cytotoxic T lymphocytes (CTLs) perform complementary roles in immune responses directed against viruses and tumours. CTLs are antigen specific and recognize peptides derived from virus and tumour antigens presented by major histocompatibility complex (MHC) class I molecules.1,2 Not surprisingly, the cell surface expression of MHC class I is often down-regulated by tumours and virus-infected cells, enabling these cells to escape CTL killing.3,4 However, NK cells can recognize and kill cells that have down-regulated MHC class I molecules from their cell surface.5–7 The MHC class I molecules are recognized by NK cell inhibitory receptors and the ligation of these receptors inhibits the activation of NK cells. Conversely the lack of engagement of these receptors can activate NK cytotoxicity.5–7 In addition, NK cells recognize other signals associated with aberrant cells. Viral infection and malignant transformation of cells can induce the expression of the MHC class I chain-related (MIC) molecules MICA and MICB as well as the UL16-binding proteins.8 These molecules are recognized by the NK cell activating receptor NKG2D and ligand binding by this receptor can signal target cell killing.5–8 The natural cytotoxicity receptors (NCRs) represent a second class of activating receptors and include NKp46, which binds to influenza haemagglutinin.9 Furthermore, NK cells express the low-affinity IgG receptor CD16, which enables them to recognize and kill target cells opsonized with antibodies by antibody-dependent cell-mediated cytotoxicity.5–7 Yet despite the profound differences in how they recognize virus-infected and tumorigenic cells, secretory lysosome exocytosis is required for target cell killing by both NK cells and CTLs.
Pulling the trigger: secretory lysosome exocytosis
Secretory lysosomes are dual function organelles that combine the degradative function of conventional lysosomes with the capacity to undergo regulated exocytosis.10–12 The major cytotoxic proteins contained within secretory lysosomes in NK cells and CTLs are the granzymes and perforin.12–14 Target cell recognition induces secretory lysosome exocytosis and the release of the cytotoxic contents of this organelle. Perforin then facilitates the entry of the granzymes into the target cell cytoplasm, where they cleave a variety of targets, such as caspases, resulting in cell death.15,16
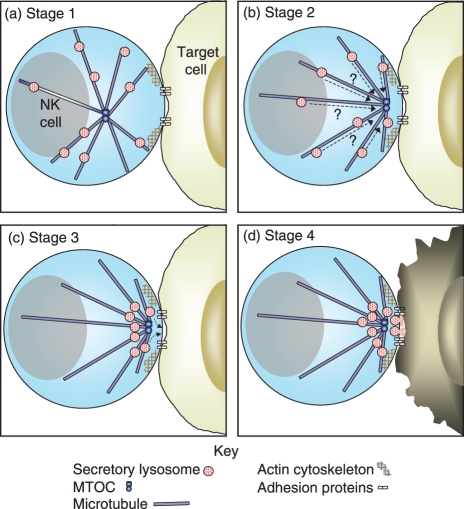
To ensure that NK cells do not kill indiscriminately, the exocytosis of secretory lysosomes is a tightly regulated and highly ordered process. For the purposes of this review it can be divided into four stages (Fig. 1). First, an activating, lytic immunological synapse forms at the point of contact with the target cell, and there is a rearrangement of the actin cytoskeleton. Second, the microtubule-organizing centre (MTOC) of the NK cell and the secretory lysosomes are polarized towards the lytic synapse. In the third stage secretory lysosomes dock with the plasma membrane at the lytic synapse, before finally in the fourth stage fusing with the plasma membrane and releasing their cytotoxic contents. Each of these steps is discussed below.
Figure 1.
Secretory lysosome exocytosis. (a) Stage 1: on recognition of a target cell by the natural killer (NK) cell a lytic immunological synapse forms at the point of contact with the target cell, and the actin cytoskeleton is reorganized, forming a ring of F-actin around the pSMAC. (b) Stage 2: the MTOC and secretory lysosomes become polarized towards the lytic synapse. It is likely that the secretory lysosomes move along microtubules to the lytic synapse, but this has not been confirmed in NK cells. (c) Stage 3: secretory lysosomes then move into close apposition with the plasma membrane, a process known as docking. (d) Stage 4: finally, the secretory lysosomes fuse with the plasma membrane, releasing their cytotoxic contents towards the target cell plasma membrane.
Stage 1: Formation of a lytic immunological synapse and reorganization of the actin cytoskeleton
Upon recognition of a target cell, a lytic synapse develops at the point of contact between the target and the NK cell (Fig. 1a).17–19 Imaging experiments demonstrate that the NK lytic synapse can be divided into two distinct domains.17–19 The peripheral supramolecular activation cluster (pSMAC) forms a ring at the point of contact and contains adhesion molecules including lymphocyte function-associated antigen 1 (LFA-1, CD11a/CD18). The central SMAC (cSMAC) is found within this ring and is thought to be the focal point for the exocytosis of secretory lysosomes, allowing them to release their content towards the target cell. Indeed, this is analogous to the lytic synapse formed between a CTL and its target, in which the cSMAC also corresponds to the site of secretory lysosome exocytosis.10,19 As such, the polarized exocytosis of secretory lysosomes at the lytic synapse represents an important functional specialization of cytotoxic cells, as it is predicted to enable NK cells and CTLs to kill aberrant cells, while normal cells that are in close proximity remain unharmed.
In addition to acting as the focus of secretory lysosome exocytosis, the lytic synapse is the major site of NK receptor signalling that is initiated upon target cell recognition.17 Little is known about how NK receptor signalling cascades integrate with the polarized exocytosis of secretory lysosomes, although multiple signals may be required. For example, interaction of intercellular adhesion molecule 1 (CD54) with its NK cell ligand LFA-1 induces the polarization of secretory lysosomes to the lytic synapse, but not their exocytosis.20 Conversely, binding of the NK receptor CD16 to IgG on opsonized target cells induces exocytosis, but not secretory lysosome polarization.20
Actin polymerization and cytoskeletal reorganization at the lytic synapse are required for secretory lysosome exocytosis. Disruption of actin cytoskeletal rearrangements, such as inhibition of actin polymerization or depolymerization by treatment with cytochalasin D and jasplakinolide, respectively, blocks NK cytotoxicity and impairs the mobilization of secretory lysosomes to the lytic synapse.18,21,22 This cytoskeletal reorganization involves the accumulation of filamentous-actin (F-actin) in the pSMAC region of the lytic synapse, whereas the cSMAC is largely free of actin, enabling secretory lysosomes to come into intimate contact with the plasma membrane in this region.17,18 The Wiskott–Aldrich syndrome protein (WASp) is required for the rearrangement of actin at the lytic synapse.18,23 Expression of WASp is restricted to cells of the haemopoietic lineage and it acts on the actin cytoskeleton through the Arp2/3 complex, to induce actin nucleation and branching.24 The gene encoding WASp is mutated in the immune disorder Wiskott–Aldrich syndrome (WAS) and NK cells isolated from these patients show a reduced ability to kill target cells, although this effect is often compensated for by increased numbers of NK cells.23–25 WASp co-localizes with F-actin at the lytic synapse, and correspondingly in NK cells lacking functional WASp, F-actin accumulation at the lytic synapse and secretory lysosome polarization are both diminished.23,26 Furthermore, the ability of NK cells from WAS patients to form conjugates with target cells is reduced, suggesting that this process also requires actin polymerization.26 WASp is regulated by the GTPase Cdc42, which in its GTP bound form binds to WASp inducing a conformational change that allows WASp to interact with the Arp2/3 complex and initiate actin polymerization.24 Crucially target cell recognition by NK cells promotes GTP binding by Cdc42, suggesting that WASp acts as an interface between NK receptor signalling pathways and secretory lysosome exocytosis.26 Furthermore, and consistent with this notion, WASp is tyrosine phosphorylated in activated NK cells, which may also promote its ability to stimulate actin polymerization.26,27
Stage 2: Polarization of the MTOC and secretory lysosomes to the lytic synapse
In the next stage of secretory lysosome exocytosis, the MTOC and secretory lysosomes polarize towards the lytic synapse (Fig. 1b). This process must be co-ordinated with the formation of the lytic synapse and the reorganization of the actin cytoskeleton. Two proteins that interact with WASP may play this role, namely WASp interacting protein (WIP) and Cdc42 interacting protein-4 (CIP4).28–30 WIP is associated with secretory lysosomes and upon NK cell activation it polarizes with WASP towards the lytic synapse.30 At this site, in the human NK cell line YTS, WIP and WASp form a complex with F-actin and Myosin IIa, which is dependent on protein kinase C-θ activation of WIP.29 The precise role of WIP and this complex are not known, although depletion of WIP by RNA interference (RNAi) leads to loss of cytotoxicity.30 Intriguingly, whereas WIP depletion has only a minor effect on F-actin accumulation at the lytic synapse, secretory lysosomes are unable to polarize to the lytic synapse in WIP-deficient cells.30 Together these observations suggest that WIP may play a role in linking actin cytoskeletal changes to the later stages in secretory lysosome exocytosis.
Likewise CIP4 may link the reorganization of the actin cytoskeleton and the movement of the MTOC to the lytic synapse. CIP4 interacts with the microtubule component α-tubulin in the human NK cell line YTS.28 On activation, CIP4 is polarized to the lytic synapse, where it co-localizes with the MTOC and interacts with WASp.28 Moreover, depletion of CIP4 by RNAi inhibits MTOC polarization and reduces the cytotoxicity of the YTS cells, but CIP4 depletion has no effect on F-actin accumulation at the lytic synapse.28 The implication is that CIP4 acts after the reorganization of F-actin, to facilitate the movement of the MTOC to the lytic synapse.
Movement of the MTOC to the lytic synapse is thought to be a prerequisite for the polarization of secretory lysosomes. Indeed, if NK cells cannot polarize the MTOC to the lytic synapse then secretory lysosomes also fail to become polarized to this site.31,32 In CTLs, and presumably in NK cells, secretory lysosomes are transported along microtubules in a minus-end direction from the cell periphery to the MTOC.33 As the MTOC moves to the lytic synapse, this brings secretory lysosomes into close proximity with the plasma membrane, and in CTLs the MTOC contacts the plasma membrane.33 The motor proteins required to transport secretory lysosomes to the lytic synapse in NK cells are unknown, although the adaptor protein 3 (AP-3) complex is required for this step in CTLs.34 The β-subunit of the AP-3 complex is mutated in the immune disorder Hermansky–Pudlak syndrome subset 2 (HPS2) and correspondingly CTLs and NK cells isolated from HPS2 patients have reduced cytotoxicity.10,34,35 In HPS2 CTLs, the secretory lysosomes are enlarged and unable to move along microtubules towards the MTOC and lytic synapse.34 Precisely why the AP-3 complex is required for secretory lysosome exocytosis is unclear, although in other cells the AP-3 complex functions in protein sorting to conventional lysosomes.36 The reduction in exocytosis may, therefore, be secondary to a defect in secretory lysosome formation. Indeed, the secretory lysosome protein CD63 is mis-sorted to the plasma membrane in HPS2 CTLs.34 One intriguing possibility is that AP-3 may sort one or more proteins to secretory lysosomes that are required for the movement of this organelle to the lytic synapse.
Another protein that may play a role in the movement of secretory lysosomes to the lytic synapse is the small GTPase Rab7, which was identified in secretory lysosome fractions from the human NK cell line YTS by proteomic analysis.37 Rab7 is involved in the movement of conventional lysosomes, in a minus-end direction, along microtubules, through its effector Rab7 interacting lysosomal protein (RILP), which recruits dynein–dynactin motor complexes to lysosomes.38,39 Furthermore, and consistent with a role for Rab7 in the movement of secretory lysosomes, over-expression of RILP in CTLs promotes clustering of secretory lysosomes around the MTOC.33 The role of Rab7 and RILP in NK cells is unknown, but clearly warrants investigation.
Stage 3: Docking of secretory lysosomes with the plasma membrane
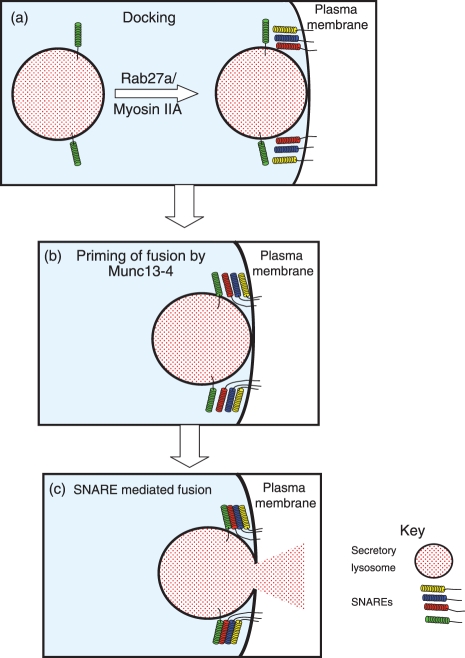
Docking of secretory lysosomes with the plasma membrane precedes the fusion of these two membranes (Figs 1c and 2a), and in CTLs this requires the small GTPase Rab27a.40 The gene encoding Rab27a is mutated in the immunodeficiency Griscelli syndrome 2 (GS2), and in Ashen mice, which results in severely reduced cytotoxic activity in CTLs.40–43 In CTLs that lack functional Rab27a, secretory lysosomes cluster around the MTOC, but are unable to fuse with the plasma membrane.40 Rab27a is localized to the late endosomes of CTLs, although upon activation by target cell recognition it becomes co-localized with secretory lysosomes at the lytic synapse.44 Although initial reports demonstrated that Rab27a is required for NK cell cytotoxicity, this GTPase is dispensable under certain circumstances for secretory lysosome exocytosis in NK cells.43,45–47 Target cell killing activated by CD16 is unaltered in GS2 NK cells, although cytotoxicity induced by the NCR receptor NKp30 is impaired in the same cells.46 This suggests that there are both Rab27a-dependent and -independent pathways for exocytosis in NK cells. The closely related GTPase Rab27b has also been detected in NK cells by microarray analysis, raising the possibility that Rab27b could play a complimentary role to Rab27a in NK cell secretory lysosome exocytosis.48 Indeed, these proteins may have some degree of functional redundancy, as mast cells from the double Rab27a/Rab27b knockout mice have a greater defect in regulated exocytosis than those from either of the individual knockouts.49
Figure 2.
The docking and fusion of secretory lysosomes with the plasma membrane. (a) Docking: secretory lysosomes move into close apposition with the plasma membrane. In cytotoxic T lymphocytes (CTLs), and most likely in natural killer (NK) cells, this step requires Rab27a.40–47 Myosin IIa is also required for the docking of secretory lysosomes with the plasma membrane.29,50,51 (b) Priming: Munc13-4 is required in CTLs immediately before membrane fusion to prime secretory lysosomes for fusion and may act by enabling soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) proteins to interact with each other.44,53–56 Munc13-4 is likely to perform the same function in NK cells. (c) Fusion: the subsequent fusion of the secretory lysosome with the plasma membrane will require the formation of a trans-SNARE complex that bridges the opposing membranes and drives their fusion. The SNAREs that act at this step in NK cells are unknown, but may include syntaxin 11 and VAMP7.37,57–61
Myosin IIa may also play a role in the later stages of secretory lysosome exocytosis. This protein is recruited by WIP to a WASp–WIP-F-Actin complex at the lytic synapse on NK cell activation.29 Surprisingly, considering its early recruitment to the lytic synapse, depletion or inhibition of myosin IIa has no effect on movement of either the MTOC or secretory lysosomes to the lytic synapse.50 However, secretory lysosomes are unable to fuse with the plasma membrane and cytolytic activity is severely impaired.50,51 The precise role of myosin IIa is unclear, although it may act to move secretory lysosomes from around the MTOC into close apposition with the plasma membrane.
Stage 4: Fusion of secretory lysosomes with the plasma membrane
To release their cytotoxic contents, the secretory lysosomes mobilized to the lytic synapse must fuse with the plasma membrane (Figs 1d and 2c). Fusion between two cellular membranes in the exocytic and endocytic pathways is catalysed by soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs).52 SNAREs have a modular structure comprising one or two SNARE domains, varying N-terminal domains and either a transmembrane domain or a lipid tail to anchor them to a membrane. A SNARE complex is formed when four SNARE domains come together across two membranes to form a four-helical bundle, bringing the membranes into close proximity and driving their fusion (Fig. 2c).52
The Rab27a binding partner Munc13-4 is required immediately before fusion of secretory lysosomes with the plasma membrane in CTLs and it may perform the same function in NK cells (Fig. 2b).44,53,54 Munc13-4 is mutated in familial haemophagocytic lymphohistiocytosis subset 3 (FHL3) and both NK cells and CTLs isolated from these patients have reduced cytotoxicity.53–55 Analysis by electron microscopy has revealed that in FHL3 CTLs cells secretory lysosomes can dock at the plasma membrane, but do not fuse.54 Munc13-1, the neuronal homologue of Munc13-4, primes vesicles for fusion by releasing syntaxin 1A from Munc-18, allowing syntaxin 1A to form a functioning SNARE complex and so induce fusion.56 Munc13-4 may perform a similar function in NK cells and CTLs, priming SNARE-mediated fusion.
The SNAREs responsible for secretory lysosome fusion with the plasma membrane in NK cells are poorly defined, although syntaxin 11 is required for the exocytosis of secretory lysosomes in these cells.57–60 This SNARE is mutated in FHL subset 4 (FHL4), and NK cells isolated from FHL4 patients, or in which syntaxin 11 expression has been depleted by RNAi, have severely impaired secretory lysosome exocytosis and correspondingly are unable to kill target cells.57–60 Surprisingly, in NK cells this SNARE is associated with cytoplasmic puncta that are distinct from the secretory lysosomes and the plasma membrane.37 As such, the requirement of syntaxin 11 for secretory lysosome exocytosis is puzzling. However, in macrophages, which also express syntaxin 11, this SNARE is mobilized to the plasma membrane during phagocytosis.61 One intriguing possibility is that syntaxin 11 may be recruited to the exocytic site upon NK cell activation whereupon it may promote membrane fusion. Syntaxin 11 is not an absolute requirement for secretory lysosome exocytosis in NK cells, as both secretory lysosome exocytosis and cytotoxicity in cells isolated from FHL4 patients can be partially restored after culturing the cells for 2–3 days in high levels of interleukin-2 (IL-2).60 The implication is that there may be an alternative syntaxin-11-independent pathway that is up-regulated in NK cells by IL-2. Indeed, syntaxin 11 is not consistently detected in CTLs, which presumably can also use a syntaxin-11-independent pathway for secretory lysosome exocytosis.60
In addition to syntaxin 11, other SNAREs will be required for secretory lysosome exocytosis. The SNAREs vesicle-associated membrane protein 7 (VAMP7) and syntaxin 7 are both localized, at least in part, to NK cell secretory lysosome membranes.37 The role of syntaxin 7 in NK cells has not been defined, but RNAi knockdown of VAMP7 inhibits granzyme B release and target cell killing by the NK cell line YT-Indy.62 These data suggest that VAMP7 is required for a membrane fusion reaction that either occurs during secretory lysosome exocytosis or that is required for the delivery of proteins to this organelle. More work is, however, needed to determine whether any of the aforementioned SNAREs form part of the trans-SNARE complex that drives fusion of the secretory lysosome with the plasma membrane.
Replenishing the NK cell secretory lysosome arsenal
Little is known about what happens after the fusion of secretory lysosomes with the plasma membrane. Are secretory lysosomes retained, as in the ‘kiss and run’ model of neuronal vesicle fusion, or do they become incorporated into the plasma membrane?63 NK cells are able to kill an average of four target cells each, but appear to become ‘exhausted’ after this and have diminished perforin and granzyme B levels.64 Treatment with IL-2 can replenish the cells’ cytotoxicity after 48 hr, presumably through increased expression of new perforin and granzymes, whose levels increase again.64 It is not clear from this work whether the secretory lysosomes themselves are lost and replenished, or simply their content. Not surprisingly, little is known about the machinery involved in the replenishment of secretory lysosomes or their content, although dynamin 2 may be involved. Dynamin 2 is a large GTPase that is required for cytotoxicity in NK cells.65 In cells in which dynamin 2 is inhibited using a chemical inhibitor, or depleted by RNAi, secretory lysosomes polarize to the lytic synapse but fusion with the plasma membrane is reduced, suggesting that dynamin 2 may play a role in the late stages of secretory lysosome exocytosis. In other cell types dynamin has been found to play a role in secretory granule recapture and in membrane fission.66,67 Dynamin 2 may play an analogous role in recapture of secretory lysosomes from the plasma membrane, and promote exocytosis by removing spent secretory lysosome membranes from the secretory domain so that new ones can dock.65
Unable to pull the trigger: the immunological consequences of mutations in the NK cell exocytic machinery
As outlined above, NK cell cytotoxicity is severely impaired in cells that have mutations in proteins that are required for secretory lysosome exocytosis (Table 1). The consequences of the resultant loss in NK cell cytotoxicity are difficult to dissect as WASP, the AP-3 complex, Rab27a, Munc13-4 and syntaxin 11 are expressed in a variety of other cell types. Nonetheless, the loss of NK cytotoxicity is likely to make a significant contribution to the pathology of the immune disorders in which these proteins are mutated. HPS2, GS2, FHL-3 and FHL-4 are all characterized by haemophagocytic lymphohistiocytosis, which has also been observed in WAS.68–70 Haemophagocytic lymphohistiocytosis is an exaggerated inflammatory response that is normally triggered by viral infection.68,69 In the aforementioned disorders NK cells cannot kill the cells that promote the immune response to infection, so the resultant inflammation cannot be resolved and becomes life threatening.68,69 Consequently, despite haemophagocytic lymphohistiocytosis being at least partly caused by the loss of cytotoxic function of NK cells, treatment for this condition requires immunosuppressive agents to reduce the inflammatory process.68,69
Concluding remarks
In recent years much has been learnt about the exocytosis of secretory lysosomes and the essential role of this process in NK cell cytotoxicity. These studies demonstrate that secretory lysosome exocytosis is a tightly regulated and coordinated process. Many of the proteins that facilitate secretory lysosome exocytosis have been identified, with much of this information coming from the study of immune disorders in which NK cell cytotoxicity is impaired. However, more research is required to fully understand this complex molecular process and in particular how it is integrated with the signalling pathways of the multiple NK cell receptors.
Acknowledgments
Nicola Topham is funded by a BBSRC PhD studentship. We are grateful to Andrew MacDonald and Martin Stacey for comments on the manuscript. We would also like to apologize to the authors of the many relevant works that could not be cited owing to space constraints.
Glossary
Abbreviations:
- AP-3
adaptor protein 3
- CIP4
Cdc42 interacting protein-4
- cSMAC
central supramolecular activation cluster
- CTL
cytotoxic T lymphocyte
- F-actin
filamentous-actin
- FHL3
familial haemophagocytic lymphohistiocytosis subset 3
- FHL4
familial haemophagocytic lymphohistiocytosis subset 4
- GS2
Griscelli syndrome 2
- HPS2
Hermansky–Pudlak syndrome subset 2
- IL-2
interleukin 2
- LFA-1
lymphocyte function-associated antigen 1
- MHC
major histocompatibility complex
- MTOC
microtubule organizing centre
- NCR
natural cytotoxicity receptor
- NK
natural killer
- pSMAC
peripheral supramolecular activation cluster
- RILP
Rab7 interacting lysosomal protein
- RNAi
RNA interference
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptors
- VAMP7
vesicle-associated membrane protein 7
- WAS
Wiskott–Aldrich syndrome
- WASp
Wiskott–Aldrich syndrome protein
- WIP
WASp interacting protein
Disclosures
The authors have no conflicts of interest.
References
- 1.Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Annu Rev Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- 2.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–65. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt EW. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology. 2003;110:163–9. doi: 10.1046/j.1365-2567.2003.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol. 2003;195:346–55. doi: 10.1002/jcp.10290. [DOI] [PubMed] [Google Scholar]
- 5.Moretta L, Biassoni R, Bottino C, Cantoni C, Pende D, Mingari MC, Moretta A. Human NK cells and their receptors. Microbes Infect. 2002;4:1539–44. doi: 10.1016/s1286-4579(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 6.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–43. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 7.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 8.Mistry AR, O’Callaghan CA. Regulation of ligands for the activating receptor NKG2D. Immunology. 2007;121:439–47. doi: 10.1111/j.1365-2567.2007.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandelboim O, Lieberman N, Lev M, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–60. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 10.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annu Rev Cell Dev Biol. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 11.Burkhardt JK, Hester S, Lapham CK, Argon Y. The lytic granules of natural killer cells are dual-function organelles combining secretory and pre-lysosomal compartments. J Cell Biol. 1990;111:2327–40. doi: 10.1083/jcb.111.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lettau M, Schmidt H, Kabelitz D, Janssen O. Secretory lysosomes and their cargo in T and NK cells. Immunol Lett. 2007;108:10–9. doi: 10.1016/j.imlet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury D, Lieberman J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu Rev Immunol. 2008;26:389–420. doi: 10.1146/annurev.immunol.26.021607.090404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pipkin ME, Lieberman J. Delivering the kiss of death: progress on understanding how perforin works. Curr Opin Immunol. 2007;19:301–8. doi: 10.1016/j.coi.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–70. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 16.Trapani JA, Bird PI. A renaissance in understanding the multiple and diverse functions of granzymes? Immunity. 2008;29:665–7. doi: 10.1016/j.immuni.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Vyas YM, Mehta KM, Morgan M, Maniar H, Butros L, Jung S, Burkhardt JK, Dupont B. Spatial organization of signal transduction molecules in the NK cell immune synapses during MHC class I-regulated noncytolytic and cytolytic interactions. J Immunol. 2001;167:4358–67. doi: 10.4049/jimmunol.167.8.4358. [DOI] [PubMed] [Google Scholar]
- 18.Orange JS, Harris KE, Andzelm MM, Valter MM, Geha RS, Strominger JL. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci USA. 2003;100:14151–6. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orange JS. The lytic NK cell immunological synapse and sequential steps in its formation. Adv Exp Med Biol. 2007;601:225–33. doi: 10.1007/978-0-387-72005-0_23. [DOI] [PubMed] [Google Scholar]
- 20.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–12. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz P, Zaytoun AM, Lee JH., Jr Mechanisms of human cell-mediated cytotoxicity. III. Dependence of natural killing on microtubule and microfilament integrity. J Immunol. 1982;129:2816–25. [PubMed] [Google Scholar]
- 22.Wulfing C, Purtic B, Klem J, Schatzle JD. Stepwise cytoskeletal polarization as a series of checkpoints in innate but not adaptive cytolytic killing. Proc Natl Acad Sci USA. 2003;100:7767–72. doi: 10.1073/pnas.1336920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orange JS, Ramesh N, Remold-O’Donnell E, et al. Wiskott–Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci USA. 2002;99:11351–6. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stradal TE, Rottner K, Disanza A, Confalonieri S, Innocenti M, Scita G. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 2004;14:303–11. doi: 10.1016/j.tcb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott–Aldrich syndrome. Cell. 1994;78:635–44. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- 26.Gismondi A, Cifaldi L, Mazza C, et al. Impaired natural and CD16-mediated NK cell cytotoxicity in patients with WAS and XLT: ability of IL-2 to correct NK cell functional defect. Blood. 2004;104:436–43. doi: 10.1182/blood-2003-07-2621. [DOI] [PubMed] [Google Scholar]
- 27.Cory GO, Garg R, Cramer R, Ridley AJ. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. Wiskott–Aldrich Syndrome protein. J Biol Chem. 2002;277:45115–21. doi: 10.1074/jbc.M203346200. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee PP, Pandey R, Zheng R, Suhoski MM, Monaco-Shawver L, Orange JS. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J Exp Med. 2007;204:2305–20. doi: 10.1084/jem.20061893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krzewski K, Chen X, Orange JS, Strominger JL. Formation of a WIP-, WASp-, actin-, and myosin IIA-containing multiprotein complex in activated NK cells and its alteration by KIR inhibitory signaling. J Cell Biol. 2006;173:121–32. doi: 10.1083/jcb.200509076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krzewski K, Chen X, Strominger JL. WIP is essential for lytic granule polarization and NK cell cytotoxicity. Proc Natl Acad Sci USA. 2008;105:2568–73. doi: 10.1073/pnas.0711593105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Trivedi PP, Ge B, Krzewski K, Strominger JL. Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc Natl Acad Sci USA. 2007;104:6329–34. doi: 10.1073/pnas.0611655104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopcow HD, Allan DS, Chen X, Rybalov B, Andzelm MM, Ge B, Strominger JL. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci USA. 2005;102:15563–8. doi: 10.1073/pnas.0507835102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–5. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 34.Clark RH, Stinchcombe JC, Day A, et al. Adaptor protein 3-dependent microtubule-mediated movement of lytic granules to the immunological synapse. Nat Immunol. 2003;4:1111–20. doi: 10.1038/ni1000. [DOI] [PubMed] [Google Scholar]
- 35.Fontana S, Parolini S, Vermi W, et al. Innate immunity defects in Hermansky–Pudlak type 2 syndrome. Blood. 2006;107:4857–64. doi: 10.1182/blood-2005-11-4398. [DOI] [PubMed] [Google Scholar]
- 36.Newell-Litwa K, Seong E, Burmeister M, Faundez V. Neuronal and non-neuronal functions of the AP-3 sorting machinery. J Cell Sci. 2007;120:531–41. doi: 10.1242/jcs.03365. [DOI] [PubMed] [Google Scholar]
- 37.Casey TM, Meade JL, Hewitt EW. Organelle proteomics: identification of the exocytic machinery associated with the natural killer cell secretory lysosome. Mol Cell Proteomics. 2007;6:767–80. doi: 10.1074/mcp.M600365-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Cantalupo G, Alifano P, Roberti V, Bruni CB, Bucci C. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 2001;20:683–93. doi: 10.1093/emboj/20.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordens I, Fernandez-Borja M, Marsman M, et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein–dynactin motors. Curr Biol. 2001;11:1680–5. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 40.Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152:825–34. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson SM, Yip R, Swing DA, et al. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci USA. 2000;97:7933–8. doi: 10.1073/pnas.140212797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menasche G, Pastural E, Feldmann J, et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173–6. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 43.Haddad EK, Wu X, Hammer JA, 3rd, Henkart PA. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J Cell Biol. 2001;152:835–42. doi: 10.1083/jcb.152.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menager MM, Menasche G, Romao M, et al. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat Immunol. 2007;8:257–67. doi: 10.1038/ni1431. [DOI] [PubMed] [Google Scholar]
- 45.Bizario JC, Feldmann J, Castro FA, et al. Griscelli syndrome: characterization of a new mutation and rescue of T-cytotoxic activity by retroviral transfer of RAB27A gene. J Clin Immunol. 2004;24:397–410. doi: 10.1023/B:JOCI.0000029119.83799.cb. [DOI] [PubMed] [Google Scholar]
- 46.Gazit R, Aker M, Elboim M, Achdout H, Katz G, Wolf DG, Katzav S, Mandelboim O. NK cytotoxicity mediated by CD16 but not by NKp30 is functional in Griscelli syndrome. Blood. 2007;109:4306–12. doi: 10.1182/blood-2006-09-047159. [DOI] [PubMed] [Google Scholar]
- 47.Klein C, Philippe N, Le Deist F, Fraitag S, Prost C, Durandy A, Fischer A, Griscelli C. Partial albinism with immunodeficiency (Griscelli syndrome) J Pediatr. 1994;125:886–95. doi: 10.1016/s0022-3476(05)82003-7. [DOI] [PubMed] [Google Scholar]
- 48.Dybkaer K, Iqbal J, Zhou G, Geng H, Xiao L, Schmitz A, d’Amore F, Chan WC. Genome wide transcriptional analysis of resting and IL2 activated human natural killer cells: gene expression signatures indicative of novel molecular signaling pathways. BMC Genomics. 2007;8:230. doi: 10.1186/1471-2164-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizuno K, Tolmachova T, Ushakov DS, Romao M, Abrink M, Ferenczi MA, Raposo G, Seabra MC. Rab27b regulates mast cell granule dynamics and secretion. Traffic. 2007;8:883–92. doi: 10.1111/j.1600-0854.2007.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andzelm MM, Chen X, Krzewski K, Orange JS, Strominger JL. Myosin IIA is required for cytolytic granule exocytosis in human NK cells. J Exp Med. 2007;204:2285–91. doi: 10.1084/jem.20071143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ito M, Tanabe F, Sato A, Ishida E, Takami Y, Shigeta S. Inhibition of natural killer cell-mediated cytotoxicity by ML-9, a selective inhibitor of myosin light chain kinase. Int J Immunopharmacol. 1989;11:185–90. doi: 10.1016/0192-0561(89)90070-2. [DOI] [PubMed] [Google Scholar]
- 52.Jahn R, Scheller RH. SNAREs – engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–43. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 53.Neeft M, Wieffer M, de Jong AS, et al. Munc13-4 is an effector of rab27a and controls secretion of lysosomes in hematopoietic cells. Mol Biol Cell. 2005;16:731–41. doi: 10.1091/mbc.E04-10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feldmann J, Callebaut I, Raposo G, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–73. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 55.Marcenaro S, Gallo F, Martini S, Santoro A, Griffiths GM, Arico M, Moretta L, Pende D. Analysis of natural killer-cell function in familial hemophagocytic lymphohistiocytosis (FHL): defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108:2316–23. doi: 10.1182/blood-2006-04-015693. [DOI] [PubMed] [Google Scholar]
- 56.Gladycheva SE, Ho CS, Lee YY, Stuenkel EL. Regulation of syntaxin1A-munc18 complex for SNARE pairing in HEK293 cells. J Physiol. 2004;558:857–71. doi: 10.1113/jphysiol.2004.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prekeris R, Klumperman J, Scheller RH. Syntaxin 11 is an atypical SNARE abundant in the immune system. Eur J Cell Biol. 2000;79:771–80. doi: 10.1078/0171-9335-00109. [DOI] [PubMed] [Google Scholar]
- 58.zur Stadt U, Schmidt S, Kasper B, et al. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14:827–34. doi: 10.1093/hmg/ddi076. [DOI] [PubMed] [Google Scholar]
- 59.Arneson LN, Brickshawana A, Segovis CM, Schoon RA, Dick CJ, Leibson PJ. Cutting edge: syntaxin 11 regulates lymphocyte-mediated secretion and cytotoxicity. J Immunol. 2007;179:3397–401. doi: 10.4049/jimmunol.179.6.3397. [DOI] [PubMed] [Google Scholar]
- 60.Bryceson YT, Rudd E, Zheng C, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110:1906–15. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang S, Ma D, Wang X, Celkan T, Nordenskjold M, Henter JI, Fadeel B, Zheng C. Syntaxin-11 is expressed in primary human monocytes/macrophages and acts as a negative regulator of macrophage engulfment of apoptotic cells and IgG-opsonized target cells. Br J Haematol. 2008;142:469–79. doi: 10.1111/j.1365-2141.2008.07191.x. [DOI] [PubMed] [Google Scholar]
- 62.Marcet-Palacios M, Odemuyiwa SO, Coughlin JJ, et al. Vesicle-associated membrane protein 7 (VAMP-7) is essential for target cell killing in a natural killer cell line. Biochem Biophys Res Commun. 2008;366:617–23. doi: 10.1016/j.bbrc.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 63.Rutter GA, Tsuboi T. Kiss and run exocytosis of dense core secretory vesicles. Neuroreport. 2004;15:79–81. doi: 10.1097/00001756-200401190-00016. [DOI] [PubMed] [Google Scholar]
- 64.Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells – enhancement by therapeutic antibodies. PLoS ONE. 2007;2:e326. doi: 10.1371/journal.pone.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arneson LN, Segovis CM, Gomez TS, Schoon RA, Dick CJ, Lou Z, Billadeau DD, Leibson PJ. Dynamin 2 regulates granule exocytosis during NK cell-mediated cytotoxicity. J Immunol. 2008;181:6995–7001. doi: 10.4049/jimmunol.181.10.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holroyd P, Lang T, Wenzel D, De Camilli P, Jahn R. Imaging direct, dynamin-dependent recapture of fusing secretory granules on plasma membrane lawns from PC12 cells. Proc Natl Acad Sci USA. 2002;99:16806–11. doi: 10.1073/pnas.222677399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verbsky JW, Grossman WJ. Hemophagocytic lymphohistiocytosis: diagnosis, pathophysiology, treatment, and future perspectives. Ann Med. 2006;38:20–31. doi: 10.1080/07853890500465189. [DOI] [PubMed] [Google Scholar]
- 69.Janka G, Zur Stadt U. Familial and acquired hemophagocytic lymphohistiocytosis. Hematology Am Soc Hematol Educ Program. 2005:82–8. doi: 10.1182/asheducation-2005.1.82. [DOI] [PubMed] [Google Scholar]
- 70.Pasic S, Micic D, Kuzmanovic M. Epstein–Barr virus-associated haemophagocytic lymphohistiocytosis in Wiskott–Aldrich syndrome. Acta Paediatr. 2003;92:859–61. doi: 10.1080/08035250310003631. [DOI] [PubMed] [Google Scholar]