Abstract
There is increasing evidence that activation of inflammatory responses in a variety of tissues is mediated co-operatively by the actions of more than one cell type. In particular, the monocyte has been implicated as a potentially important cell in the initiation of inflammatory responses to Toll-like receptor (TLR)-activating signals. To determine the potential for monocyte-regulated activation of tissue cells to underpin inflammatory responses in the vasculature, we established cocultures of primary human endothelial cells and monocytes and dissected the inflammatory responses of these systems following activation with TLR agonists. We observed that effective activation of inflammatory responses required bidirectional signalling between the monocyte and the tissue cell. Activation of cocultures was dependent on interleukin-1 (IL-1). Although monocyte-mediated IL-1β production was crucial to the activation of cocultures, TLR specificity to these responses was also provided by the endothelial cells, which served to regulate the signalling of the monocytes. TLR4-induced IL-1β production by monocytes was increased by TLR4-dependent endothelial activation in coculture, and was associated with increased monocyte CD14 expression. Activation of this inflammatory network also supported the potential for downstream monocyte-dependent T helper type 17 activation. These data define co-operative networks regulating inflammatory responses to TLR agonists, identify points amenable to targeting for the amelioration of vascular inflammation, and offer the potential to modify atherosclerotic plaque instability after a severe infection.
Keywords: atherosclerosis, endothelium, inflammation, monocyte, TLR
Introduction
There is increasing evidence that effective responses to inflammatory stimuli are generated through the actions of cell networks. In models of airway inflammation we have shown that tissue cells such as airway smooth muscle and epithelial cells have marked potential to contribute to establishment of inflammation. However, the responses of tissue cells to Toll-like receptor (TLR) agonists are markedly amplified in the presence of monocytes, whose secretion of interleukin-1β (IL-1β) in response to the engagement of a range of TLRs is crucial to an effective induction of many pro-inflammatory mediators.1,2 We have shown that these networks are therapeutically targetable through the blockade of either TLR signalling using a novel disruptor of lipid raft function3 or by the targeting of IL-1β signalling.1,2 The sensitivity of tissue cells to IL-1-dependent signalling is also important in inflammatory responses to mediators of sterile inflammation, such as necrotic cell debris,4 with the potential for the sensing of a variety of damage-associated signals by the monocyte.5
Atherosclerosis is an inflammatory condition.6,7 Strikingly, severe respiratory tract infections are associated with a marked but transient increase in the risk of a myocardial infarction.8 Levels of circulating lipopolysaccharide (LPS) are a risk factor in atherosclerotic disease,9,10 and polymorphisms encoding an impaired TLR4 (the LPS receptor) reduce the risk of atherosclerosis development in humans.11 ApoE−/− TLR4−/− mice that are fed on a high cholesterol diet also show reduced lesional size and lower numbers of plaque macrophages compared with mice that are wild-type for TLR4.12 Bacterial stimuli are sensed by many pattern recognition receptors.13,14 Of these, TLR4 and TLR2 mediate responses to LPS and bacterial lipoproteins, respectively,14 and in the context of atherosclerosis are variably expressed by monocytes,15 endothelial cells,16 vascular smooth muscle cells17 and platelets.18 Expression of TLR2 and TLR4 is also increased in atherosclerosis.19
Our groups have shown that IL-1 is a crucial regulator of vascular inflammation, and that inhibition of IL-1 signalling has therapeutic benefit in murine and porcine models of vascular disease.20,21 The expression of TLRs in the vessel wall, the amplification of TLR responses by monocytes in models of airway inflammation in an IL-1β-dependent fashion, and the potential for acute infections to destabilize atherosclerotic plaques indicated that such inflammatory networks may also be critical to the regulation of inflammation in the vessel. To examine these possibilities in detail, we exploited a coculture model of primary endothelial cells in coculture with monocytes. Here, we define a complex, TLR-specific, co-operative relationship between endothelial cells and monocytes in which IL-1 is pivotal to the mounting of effective responses. Specificity of responses to the TLR agonist was given by both the monocyte and the tissue cell. We also demonstrate that this network is amenable to therapeutic targeting at varying levels, with the potential to ameliorate vascular inflammation and potentially decrease the risk of plaque instability following acute infection.
Materials and methods
Cells and reagents
Reagents were obtained from Invitrogen (Paisley, UK) unless otherwise stated. Purified LPS from Escherichia coli strain R515 was purchased from Axxora (Nottingham, UK), Pam3CSK4 was from EMC Microcollections (Tübingen, Germany), hydrocortisone was from PromoCell (Heidelberg, Germany) and transwells (0·4-μm pore size) were from BD Biosciences (Cowley, UK). All antibodies were from eBioscience (San Diego, CA), Annexin-V–phycoerythrin (PE) was from BD Biosciences and ToPro-3 was from Invitrogen. CountBright absolute counting beads were from Invitrogen.
Human umbilical vein endothelial cells (HUVEC) were from umbilical cords using a protocol approved by North Sheffield Research Ethics Committee and involving written consent. The endothelial cells (EC) were used at passages 2–3 for all experiments and were isolated using 0·1% type IV collagenase (Sigma-Aldrich, Poole, UK), and grown on tissue culture plates or flasks coated with 0·2% gelatin. Cells were maintained in RPMI-1640 supplemented with 2 mm l-glutamine, 20 μg/ml endothelial cell growth supplement (Harbour Bio-Products, Norwood, MA), 95 μg/ml heparin (Sigma-Aldrich), 0·225% sodium bicarbonate, 100 U/ml penicillin, 100 μg/ml streptomycin, 10% fetal calf serum (FCS) and 10% newborn calf serum.
Monocytes were prepared by negative magnetic selection. Peripheral venous blood was taken from healthy volunteers with written consent using protocols approved by the South Sheffield and King’s College Research Ethics Committees as appropriate. In most experiments, peripheral blood mononuclear cells (PBMC) were prepared by density centrifugation from plasma/Percoll gradients as described elsewhere,22 or using OptiPrep™ (Axis-Shield, Upton Huntingdon, UK). For OptiPrep separations, following dextran sedimentation to remove erythrocytes, the leucocyte-rich plasma was resuspended in 6 ml of 20% platelet poor plasma (PPP) in 1 × Hanks’ buffered salt solution (HBSS) and 4 ml OptiPrep to give the cell suspension a density of 1·13 g/l. OptiPrep was made up at densities of 1·0925 and 1·078 g/l and layered over the 1·13 g/l layer, with a top layer of PPP/HBSS, before centrifugation at 700 g for 30 min. The PBMC were taken from the 1·078/PPP/HBSS interface. Enriched monocytes [81·4 ± 1·4 % CD14 positive (n = 45)] were prepared from PBMC using the negative magnetic selection Monocyte isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Germany).
For experiments investigating IL-17 production, PBMC were isolated using density gradient centrifugation (lymphocyte separation media; PAA, Pasching, Austria) from human peripheral blood of healthy donors. Purification of the various cell subsets was performed by magnetic antibody cell sorting (MACS) and enriched by positive selection using the QuadroMACS separator system (MACS; Miltenyi Biotec), according to the manufacturer’s instructions. Purity was confirmed by flow cytometry. CD4+ cells (99% pure) were selected with anti-CD4 beads and monocytes (> 97% pure) were isolated by positive selection using anti-CD14 microbeads (Miltenyi Biotec).
EC/monocyte cocultures
The EC were cultured in gelatin-coated 12-well plates and grown to 70–90% confluence. The cells were washed and the media were replaced with low-serum media (2% FCS). Twenty-four hours later, the cells were washed and cultured in low-serum media, in the presence or absence of monocytes at 20 000 cells/ml (approximately one monocyte for every five EC). Monocultures and cocultures were treated as indicated for 24 hr, after which a cell-free supernatant was prepared and stored at −80° until the concentrations of cytokines were determined by enzyme-linked immunosorbent assay (ELISA). To determine the source of released cytokines and any soluble mediators, further experiments were performed in which media from LPS-treated cells were transferred onto untreated cells. Cell-free media were added to the indicated cell type at a one in two dilution in fresh media, and the cells were cultured for 24 hr before preparation of a cell-free supernatant.
In vitro stimulation of T helper type 17 cells
Induction of IL-17 production from activated T cells and phenotyping of IL-17-producing T cells was carried out as described previously.23 Cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated FCS (Cambrex, Nottingham, UK), 1%l-glutamine, 1% sodium pyruvate, 1% HEPES, 1% penicillin/streptomycin, 1% non-essential amino acids (PAA Laboratories, Yeovil, Somerset, UK). One million monocytes were cultured at a 1 : 1 ratio with CD4+ T cells for 4 days in a 24-well plate in a total volume of 1 ml. A further 1 ml of either HUVEC LPS-treated or HUVEC buffer-treated supernatant from five EC donors was added to make up a total of 2 ml/well. Control wells had an extra 1 ml RPMI-1640 medium instead of HUVEC supernatants. Cultures were stimulated with 100 ng/ml anti-CD3 (R&D Systems, Abingdon, UK) and 100 ng/ml LPS (Sigma, St Louis, MO). Cells were cultured for a total of 4 days. Supernatants were collected on day 4 of cell culture and stored at −20° until analysis.
Flow cytometry
The ability of media from LPS-treated EC to modulate the expression of TLR4 and CD14 on the surface monocytes was determined by flow cytometry. The EC were seeded in 12-well plates as described and treated with buffer or LPS (1 ng/ml) for 24 hr, before collection of a cell-free supernatant, which was stored at −80° until required. Monocytes were prepared as described and seeded at 100 000 cells/ml in 12-well plates. Monocytes were then treated for 24 hr with buffer or LPS (0·5 ng/ml), or media from buffer or LPS-treated EC (at a one in two dilution). Following this, non-adherent cells were collected, and adherent cells were removed by gentle scraping in ice-cold PBS (without Ca2+ and Mg2+) containing 2% FCS and 5 mm ethylenediaminetetraacetic acid. Adherent and suspended cells were then pooled together and centrifuged at 2000 g for 5 min before resuspension in 100 μl fluorescence-activated cell sorting (FACS) buffer (comprising phosphate-buffered saline without Ca2+ and Mg2+, + 0·25% bovine serum albumin + 10 mm HEPES, pH 7·3–7·4). Non-specific antibody binding was blocked by incubating the cells on ice in 50 μg/ml mouse immunoglobulin G for 10 min. Cells were then labelled with anti-TLR4, anti-CD14 or relevant isotype controls (all PE-labelled) at a one in 10 dilution before incubation on ice for 30 min in the dark. One millilitre of ice-cold FACS buffer was then added and the cells were centrifuged at 2000 g for 5 min before resuspension in CellFIX™ (BD Biosciences). Samples were acquired on a BD FACScan (BD Biosciences, Mountain View, CA) and data were analysed using FlowJo (Tree Star Inc, Ashland, OR). Dead cells were excluded from these analyses on the basis of their forward and side scatter characteristics, validated by annexin V/To-Pro-3 staining (not shown).
To study monocyte viability in culture, monocytes were seeded and treated as above. After 24 hr, both suspended and adherent cells were pooled together as above and the cells were resuspended in annexin binding buffer (BD Biosciences) and labelled with PE-labelled annexin V for 20 min at room temperature in the dark. Immediately before analysis, an aliquot of CountBright beads was added to the sample. Viable monocytes were identified by their forward and side scatter characteristics and lack of staining for annexin V. Absolute numbers of viable monocytes in these samples were quantified by reference to the CountBright beads.
ELISA
All antibodies and detection reagents were purchased from R&D Systems. The ELISA were carried out according to the manufacturer’s instructions. Samples were diluted so that the optical density fell within the optimal portion of a log-l standard curve. The limits of detection for the ELISA were 78·125 pg/ml for IL-6 and tumour necrosis factor-α (TNF-α), 62·5 pg/ml for CXCL8, 19·5 pg/ml for IL-1β and 15 pg/ml for IL-17. If the concentrations of the cytokines were below the limit of detection they were assigned the above values.
The presence of IL-17 was also measured by ELISA. Samples were added to triplicate wells and diluted 1 : 2 with 1% bovine serum albumin/phosphate-buffered saline. The plate was read on an EL800 ELISA plate reader (BioTek Instruments Ltd., Potton, UK).
Statistics and analyses
All data were analysed using the prism 5 program (GraphPad, San Diego, CA).
Results
The importance of co-operative signalling between monocytes and tissue cells in the generation of responses to TLR stimuli is becoming increasingly evident. Endothelial cells are poor releasers of IL-1β,24 yet IL-1β is thought to be a critical mediator of vascular inflammation.21 Accordingly, we investigated the roles for monocytes in the activation of vascular inflammation, and the mechanisms by which IL-1β is implicated in the response to TLR signalling. In models of monocyte/EC coculture we observed that specificity of responses to TLR agonists was governed by the monocyte and the EC, through the regulation of monocyte IL-1β production and survival.
Specific patterns of cytokine production in LPS-activated cocultures
Initial experiments used a purified, TLR4-selective LPS to stimulate cocultures of EC and monocytes. Responses were compared with those of EC or monocyte monocultures at the same densities. Three patterns of cytokine release were observed.
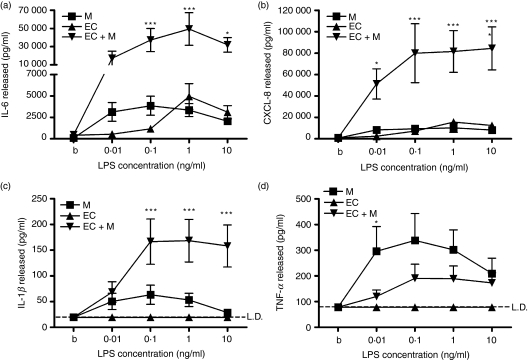
First, EC/monocyte cocultures treated with LPS released more IL-6 and CXCL8 than either cell type alone (Fig. 1). The LPS-mediated IL-6 production was still markedly enhanced in cocultures in which the EC and monocytes were kept apart by the use of transwell filters (data not shown).
Figure 1.
Differential release of cytokines from lipopolysaccharide (LPS)-treated endothelial cell (EC)/monocyte cocultures. Cocultures of EC and monocytes were formed, stimulated with LPS for 24 hr, and their production of cytokines was measured by enzyme-linked immunosorbent assay (ELISA). (a–c) LPS-treated EC/monocyte cocultures released more interleukin-6 (IL-6) (a), CXCL-8 (b) and interleukin-1β (IL-1β) (c) than either cell type alone. (d) LPS-treated EC/monocyte cocultures released less tumour necrosis factor-α (TNF-α) than LPS-treated monocytes. M indicates monocytes, EC indicates EC and EC + M indicates EC/monocyte cocultures. Data are ± SEM (n = 5), with each experiment using cells from independent human umbilical vein EC and monocyte donors. ***P < 0·001 and *P < 0·05, analysed using two-way anova with Bonferroni’s post-test. LD indicates the limit of detection of the ELISA.
The second pattern was seen in the production of IL-1β. In systems using airway cells, production of monocyte-derived IL-1β is necessary for TLR4-mediated activation of cocultures.1,2 Interleukin-1β was also released at greater levels from activated cocultures than from the individual cell types alone, but in contrast to the production of IL-6 and CXCL8, no IL-1β was released by EC monocultures (Fig. 1c).
Finally, and at odds with the patterns seen for IL-6/CXCL8 and IL-1β, less TNF-α was detected in the supernatants from stimulated cocultures than was produced from stimulated enriched monocytes alone (Fig. 1).
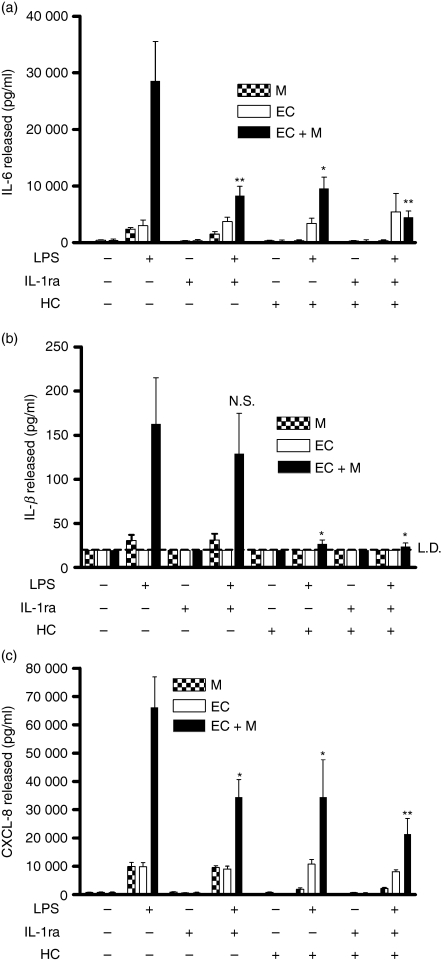
To dissect control of this system further, we blocked IL-1 signalling in cocultures using an IL-1 receptor antagonist (IL-1ra). The presence of IL-1ra significantly reduced the LPS-induced release of IL-6 and CXCL8 from cocultures, but did not affect the release of IL-1β (Fig. 2). Treatment of cocultures with hydrocortisone inhibited the subsequent detection of all three cytokines. These data identified IL-1β generation from the monocyte as an important signal resulting in the stimulation of IL-6 and CXCL8 generation from EC. However, the data displayed in Fig. 1 demonstrated that EC did not release IL-1β, implying that in activated cocultures, the increased IL-1β production was likely to originate not from the EC but from the monocytes. Further experiments investigated this possibility.
Figure 2.
Inhibition of coculture activation reveals differential regulation of cytokine production. Cocultures of endothelial cells (EC) and monocytes were formed, stimulated with lipopolysaccharide (LPS; 10 ng/ml) for 24 hr, and their production of cytokines determined by enzyme-linked immunosorbent assay (ELISA). (a) Both interleukin-1 receptor anatagonist (IL-1ra) and hydrocortisone reduced the release of IL-6 from the LPS-treated cocultures. (b) Hydrocortisone, but not IL-1ra, reduced the release of IL-1β from the LPS-treated cocultures. (c) Both IL-1ra and hydrocortisone reduced the release of CXCL8 from the LPS-treated cocultures. M indicates monocytes, EC indicates EC and EC + M indicates an EC/monocyte coculture. HC indicates hydrocortisone. Data are ± SEM (n = 4), with each experiment using cells from independent donors. **P < 0·01, and *P < 0·05, analysed using one-way anova with Tukey’s post-test. LD indicates the limit of detection of the ELISA.
EC are not passive downstream targets of IL-1, but regulate its production from monocytes
We subsequently determined the cellular sources of IL-1β and IL-6 in cocultures activated with LPS. To achieve this, media were transferred from LPS-activated monocytes onto fresh EC, or from LPS-activated EC onto fresh monocytes, and the consequences upon activation of the recipient cell type were determined.
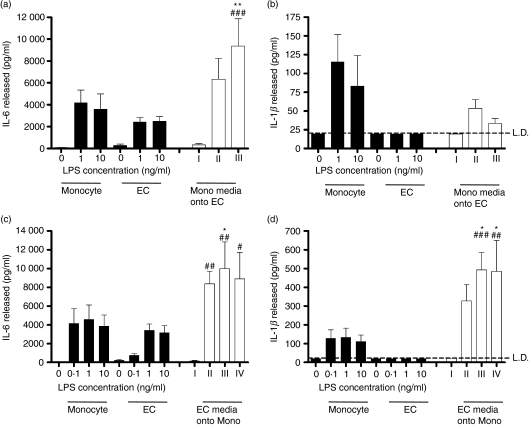
First, monocytes were activated with purified LPS. Supernatants from these cells contained IL-1β and IL-6. When these supernatants were applied at a 1 : 2 dilution in fresh media to EC, they caused the release of IL-6 (Fig. 3a) but not IL-1β from EC (Fig. 3b). Amounts of IL-6 generated from the EC were greater than seen when EC were stimulated with LPS directly (Fig. 3a).
Figure 3.
Endothelial cells (EC) are a source of interleukin-6 (IL-6) but not IL-1β in the cocultures, whereas as monocytes are a source of both cytokines. Monocytes or EC were cultured in isolation, at the same densities and conditions as used in cocultures. Filled bars show IL-6 and IL-1β production after direct activation of monocytes or EC by the indicated concentrations of lipopolysaccharide (LPS). In open bars, supernatants from LPS-treated monocytes were transferred onto fresh EC, or vice versa. (a, b) Bar I indicates the effects of addition of supernatants (diluted one in two in fresh media) from buffer-treated monocytes on EC. Bars II and III indicate the addition of media from monocytes treated with 1 or 10 ng/ml LPS, respectively, similarly diluted 1 : 2 in fresh media. (a) Release of IL-6 from EC treated with media from LPS-stimulated monocytes, and (b) media from LPS-stimulated monocytes was unable to induce the release of IL-1β from EC. (c, d) Bar I indicates the addition of supernatants (diluted one in two in fresh media) from buffer-treated EC onto monocytes; bars II, III and IV indicate the addition of media from EC treated with 0·1, 1 or 10 ng/ml LPS, respectively, similarly diluted 1 : 2 in fresh media. (c, d) Media from LPS-treated EC enhanced the release of both IL-6 and IL-1β from monocytes. Data are ± SEM (n = 4–8), each experiment using cells from independent donors. **P < 0·01 and *P < 0·05 (comparing the indicated bars with cytokine levels generated by monocytes alone). ###P < 0·001, ##P < 0·01 and #P < 0·05 (comparing the indicated bars with cytokine levels generated by EC alone). Data were analysed using two-way anova with Bonferroni’s post-test. LD indicates the limit of detection of the enzyme-linked immunosorbent assay.
In contrast, when EC were activated with LPS in the absence of monocytes, they made IL-6 but not IL-1β (Fig. 3c,d). Application of supernatants from LPS-activated EC, when applied at a 1 : 2 dilution in fresh medium to monocytes, caused the release of both IL-6 and IL-1β from the monocytes (Fig. 3d). Amounts of these cytokines generated from the monocytes were greater than seen when monocytes were stimulated with LPS directly (Fig. 3c,d).
Monocytes, but not EC, were also the predominant source of TNF-α (data not shown). In addition, whereas supernatants from LPS-stimulated EC activated monocytes to produce IL-1β, they did little to enhance the production of TNF-α (data not shown).
Activation of cocultures by a TLR2 agonist failed to recapitulate responses seen to LPS
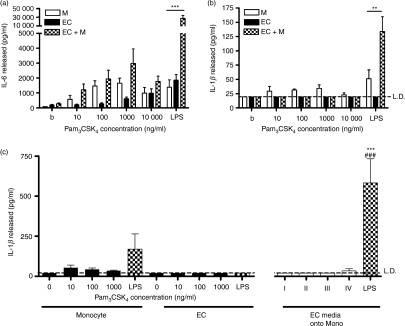
The data above showed the existence of bidirectional signals between monocytes and EC in the generation of cytokines in response to TLR4 agonists. Stimulation of monocytes was required for IL-1β production and subsequent activation of the EC, but activation of TLR4 on the EC also caused release of factor(s) that served to enhance IL-1β production from the monocyte. Because EC are thought to express TLR4 but not TLR2,25 we compared the responses of cocultures to Pam3CSK4, a specific agonist of TLR2. When used to activate monocytes alone, Pam3CSK4 induced the production of IL-6 that reached a similar maximum to the amount induced by LPS (Fig. 4). In contrast, treatment of cocultures with Pam3CSK4 did not cause the marked augmentation of the release of IL-6 and IL-1β that was observed upon treatment with LPS (Fig. 4). The data in Fig. 3 showed that activation of EC by LPS facilitated IL-1β production from monocytes and activation of cocultures. Pam3CSK4 caused IL-6 production from monocytes, but not activation of cocultures, so we hypothesized that this agonist failed to activate EC. In keeping with this hypothesis, we found that supernatants generated from EC treated with Pam3CSK4 did not induce the release of significant amounts of IL-1β from monocytes.
Figure 4.
Pam3CSK4-treated endothelial cells (EC) do not induce interleukin-1β (IL-1β) release from monocytes. Cocultures of EC and monocytes were formed, stimulated with different concentrations of Pam3CSK4 or lipopolysacchairde (LPS; 10 ng/ml) for 24 hr, and their production of cytokines was determined by enzyme-linked immunosorbent assay (ELISA). (a, b) EC/monocyte cocultures treated with Pam3CSK4 do not show the increased release of IL-6 and IL-1β that was observed with LPS treatment. (c) Bar I indicates the addition of supernatants (diluted one in two in fresh media) from buffer-treated EC onto monocytes; bars II, III and IV indicate the addition of media from EC treated with 10, 100 or 1000 ng/ml Pam3CSK4, respectively, similarly diluted 1 : 2 in fresh media. (c) Media from Pam3CSK4-treated EC did not enhance the release of IL-1β from monocytes, unlike LPS. Data are ± SEM (n = 3–5). ***P < 0·001 and **P < 0·01 [comparing the indicated bars with cytokine levels generated by monocytes alone (a–c)]. ###P < 0·001 [comparing the indicated bars with cytokine levels generated by EC alone (c)]. Data were analysed using two-way anova with Bonferroni’s post-test (a, b), or one-way anova with Tukey’s post-test (c). Data are from four or five different human umbilical vein EC and monocyte donors. LD indicates the limit of detection of the ELISA.
EC activation causes enhanced monocyte CD14 expression and survival
We investigated the mechanism through which EC activation by LPS resulted in enhanced production of IL-1β by monocytes. We hypothesized that EC might release ATP and enhance monocyte IL-1β production through P2X7-mediated activation of IL-1β secretion, but ATP levels were not increased in cocultures (n = 3, data not shown). In addition, the enhanced IL-1β release from monocytes induced by supernatants taken from activated EC was not inhibited when the supernatants were added to the monocytes in the presence of the P2X7 antagonist, KN62 (n = 3, data not shown).
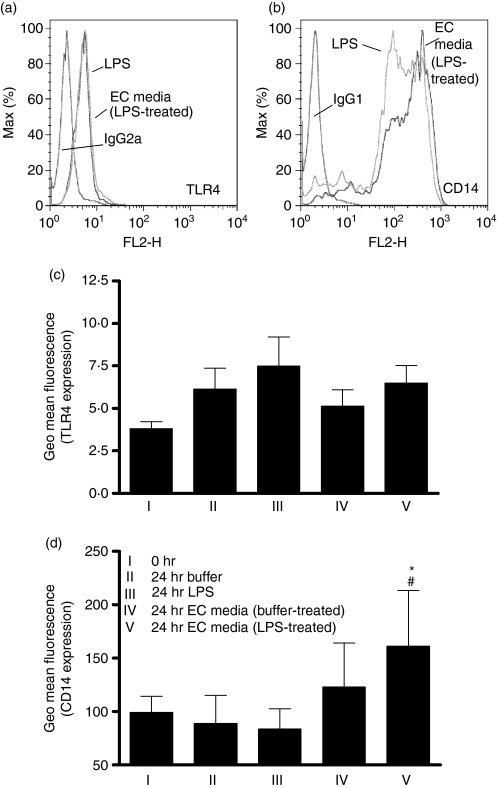
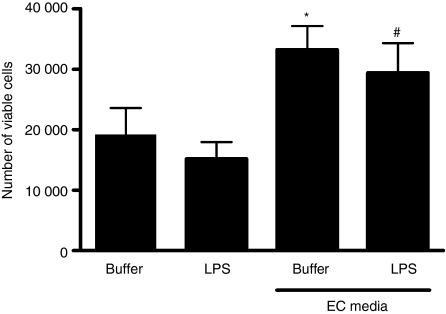
We therefore investigated the regulation of monocyte survival and the expression of the LPS receptor complex. Figure 5 shows that treatment of monocytes with media from LPS-activated EC, but not from EC treated with media alone, resulted in enhancement of CD14 expression without changes in TLR4 expression. Furthermore, media from EC (cultured either in the presence or absence of LPS) caused an increased survival of monocytes over 24 hr of culture (Fig. 6).
Figure 5.
Enhancement of monocyte CD14, but not Toll-like receptor 4 (TLR4), surface expression by endothelial cells (EC). The EC were cultured in 12-well plates and treated with buffer or lipopolysaccharide (LPS; 1 ng/ml) for 24 hr, before collection of a cell-free supernatant, which was stored at −80° until required. Monocytes (cultured at 100 000 cells/ml) were treated for 24 hr with buffer or LPS (0·5 ng/ml), or media from EC that had been treated with buffer or LPS (at a one in two dilution, allowing a maximum transferred LPS of 0·5 ng/ml). Following this, expression of CD14 and TLR4 by the monocytes was determined by flow cytometry. (a) A typical histogram showing no change in surface TLR4 expression on monocytes and (b) an increase in CD14 expression when monocytes were treated with media from LPS-treated EC. Mean data are shown in (c, d). Data are ± SEM (n = 4–5) *P < 0·05 (in comparison to buffer-treated cells) and #P < 0·05 (in comparison to LPS-treated cells). Data were analysed using one-way anova with Tukey’s post-test.
Figure 6.
Enhanced monocyte survival induced by endothelial cells (EC). Cell-free supernatants from EC stimulated with buffer or lipopolysaccharide (LPS; 1 ng/ml) were prepared. Monocytes were seeded at 100 000 cells/ml in 12-well plates and then treated for 24 hr with buffer or LPS (0·5 ng/ml), or the EC supernatants (at a one in two dilution, thus allowing a maximum transferred LPS of 0·5 ng/ml). Viable monocytes were identified by flow cytometry using forward/side scatter gating and annexin V staining, and quantified using CountBright Absolute Counting Beads. The data show that media from EC caused enhanced monocyte survival. Data are ± SEM (n = 5) for each condition. *P < 0·05 (in comparison to buffer-treated cells) and #P < 0·05 (in comparison to LPS-treated cells), analysed using one-way anova with Tukey’s post-test.
EC activation facilitates induction of a T helper type 17 response
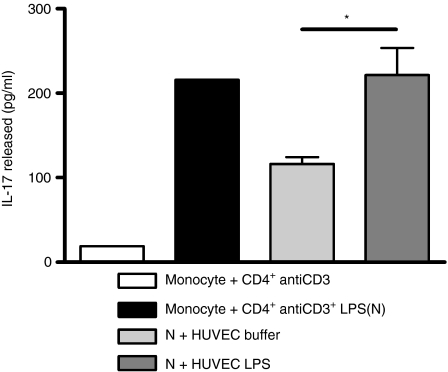
Recent work has shown that activation of human monocytes by TLR agonists such as LPS is required for effective induction of IL-17 secretion from T helper type 17 (Th17) cells.23 Our data show that endothelial cells regulated monocyte expression of CD14, activation by LPS and lifespan, so we speculated that they would also regulate the induction of effective Th17 responses. We therefore exploited a well-characterized system in which monocyte/lymphocyte cocultures were activated with LPS, resulting in induction of Th17 cell activation and IL-17 production.23 The data in Fig. 7 showed that supernatants generated from non-activated EC suppressed induction of IL-17 when added to these monocyte/lymphocyte cocultures. This suppression of Th17 immunity was not seen when monocyte/lymphocyte cocultures were treated with supernatants from LPS-activated HUVEC. The concentrations of LPS used to activate the EC (1 ng/ml) were orders of magnitude lower than those already present in the monocyte/lymphocyte coculture (100 ng/ml). Consequently, the transferred supernatants were not mediating their effects by the addition of more LPS, but through their actions to support monocyte survival and activation responses.
Figure 7.
Regulation of interleukin-17 (IL-17) release. The ability of cell-free supernatants from endothelial cells treated with either buffer or lipopolysaccharide (LPS) to support IL-17 production was determined. Lymphocytes were stimulated with anti-CD3 in coculture with monocytes and 100 ng/ml LPS, and the resulting IL-17 generation was measured by enzyme-linked immunosorbent assay. Addition of media from endothelial cells (EC) treated with buffer alone caused suppression of IL-17 generation from this activated coculture, whereas media from EC activated with 1 ng/ml LPS did not suppress IL-17 production. EC media were added at a 1 : 2 dilution. Data are ± SEM. Effects of supernatants from five EC donors, each treated with buffer or LPS, were tested on one monocyte/lymphocyte donor. *P < 0·05, analysed by t-test.
Discussion
In this study, we investigated the mechanisms by which TLR signalling may cause vascular inflammation. The potential importance of IL-1β as a mediator of vascular inflammation is well established. Here, we show that the release and function of IL-1β as induced by TLR agonists requires a cell network that shows features of a bidirectional, TLR-selective, system that is amenable to therapeutic targeting, and which may go on to regulate more complex inflammatory pathways involving Th17 systems.
Cocultures of ECs and monocytes treated with a TLR4-selective LPS, but not the TLR2 agonist Pam3CSK4, showed marked co-operative responses and differential regulation of cytokine production. Interleukin-6, CXCL8 and IL-1β were released in increased concentrations from LPS-activated cocultures, but in contrast, TNF-α production was suppressed. These data lend support to TLR4 being of major importance in sepsis and a potential therapeutic target for treatment of acute and sub-acute vascular inflammation.26 The relevance of such responses extends beyond inflammation caused by pathogens because multiple endogenous mediators of inflammation acting via TLR4 are also likely to be able to activate such co-operative signalling mechanisms.27
Having shown TLR4-selective activation of cocultures, we investigated the control of these systems in more detail. Amplification of IL-6 release from the LPS-stimulated cocultures occurred via an IL-1-dependent mechanism and was blocked by IL-1ra. These results place IL-1β as a central mediator of vascular inflammation, in keeping with previous observations in vitro,28,29 and in mouse and porcine models.20,21 Previous work by our group has shown that IL-1β is necessary for, but not generated at high enough levels to completely explain, the induction of cytokine production in cocultures of monocytes and airway smooth muscle cells activated with LPS.2 The levels of IL-1 generated here are consistent with these previous observations, and suggest once again that IL-1 is a critical cytokine in these systems, activating tissue cells and contributing to the induction of cytokine production by them, but it is likely that other monocyte-derived mediators also contribute to the total response observed. Endothelial cell/monocyte cocultures treated with LPS were found to release lower concentrations of TNF-α than monocytes alone. Similar results have been seen previously,30 indicating that the TLR4- and IL-1-dependent activation shown here is a highly specific pathway in the network of vascular inflammation. Of note, therefore, are recent data showing an increased circulating concentration of IL-1β in patients suffering from unstable angina,31 and that targeting of IL-1β results in decreased size of myocardial infarction.32
We have recently shown that, although EC are capable of making IL-1β in response to proinflammatory stimuli, its release from these cells is only at very low level and is often accompanied by IL-1ra, potentially resulting in suppression rather than activation of inflammation.24 In our data here, we found that EC did not release detectable IL-1β, but IL-1β production in cocultures was nonetheless regulated at two levels: direct TLR4-driven activation of monocytes, and TLR4-driven release of an EC-derived factor that enhances monocyte IL-1 release. Neither of these responses was inhibited by IL-1ra. The HUVEC express little functional TLR225 so the lack of an EC response to TLR2 agonists with the subsequent failure to enhance monocyte IL-1 release is likely to explain the observed TLR selectivity of responses. The importance of EC expression of TLR4 has also been seen in relevant in vivo models because in the absence of TLR4 on the endothelium responses to systemic LPS administration are markedly impaired.33
Despite their lack of release of IL-1β, the work presented here show that EC nonetheless actively use the IL-1 system to regulate local inflammation, but do this indirectly. We observed two distinct effects of EC-derived supernatants on monocytes potentially capable of resulting in increased IL-1 production in response to LPS in cocultures: enhancement of monocyte survival and CD14 expression. Monocyte survival was preserved both by supernatants from quiescent or LPS-activated EC, whereas enhancement of monocyte CD14 expression only occurred when cells were treated with media from LPS-activated EC. The TLR signalling is regulated at multiple levels beyond the control of receptor and coreceptor expression,34 and the mechanisms by which EC regulated monocyte function are the subject of ongoing work in our group. Monocytic cells release IL-1β in a mechanism involving P2X7 signalling,35 and we postulated that LPS-activated EC might release ATP, which would enhance the release of IL-1 from monocytes. We found, however, that ATP levels were not increased in activated cocultures (data not shown), nor did a P2X7 antagonist impair the ability of LPS-activated EC supernatants to enhance monocyte IL-1β release (not shown). Candidate cytokines and inflammatory mediators were also targeted, but pretreatment of monocytes with an anti-granulocyte–macrophage colony-stimulating factor receptor monoclonal antibody did not prevent the IL-1β-releasing activity of LPS-activated EC supernatants, nor did addition of IL-6 at levels found in these EC-derived supernatants act to enhance LPS-stimulated monocyte IL-1β production (data not shown). We are currently exploring the nature of the EC-derived monocyte activation signal through a proteomic approach.
Recent work has shown that activation of optimal Th17 responses in human cells requires not just T-cell receptor signalling, but the close proximity of LPS-activated monocytes. Th17 signalling serves as a potentially important component of the Th1-type immune response,36 playing important roles in the handling of some pathogens and the regulation of autoimmune inflammation and neutrophilic responses.36–38 Furthermore, Th17-type inflammatory responses are evident in patients with acute coronary syndromes.39 We observed that supernatants from quiescent EC suppressed the ability of LPS-activated monocytes to induce the activation of Th17 cells, which has the potential to represent an important control point in the regulation of vascular inflammation and atherogenesis. In contrast, supernatants from LPS-activated EC allowed monocytes to activate Th17 cells. Therefore EC, when activated with LPS, exert a range of activities, causing monocytes to up-regulate IL-1β production, increasing their survival, their CD14 expression, and their ability to support Th17 activation.
Our data describe an intricate network operating in a simple coculture. The function of this network has broad relevance in sepsis and may explain the basis of increased risk of myocardial infarction after pneumonia, where the release of endogenous TLR agonists, the presence of pathogen-related TLR4 agonists such as LPS or streptococcal pneumolysin,40 and the generation of a microenvironment that may activate monocytes in transit through the pulmonary microvascular bed, may all cause an inflammatory process that can destabilize atherosclerotic plaques. This requirement for a co-operative network controlling inflammation may feasibly also be an important mechanism to limit the spread of inflammation in the vasculature because were EC to release IL-1 on their own, this could result in rapid downstream activation of further stretches of vessel. In our model, the requirement for close proximity of monocytes (which release their IL-1 in microvesicles likely to exert a strong local effect35) may result in a more contained inflammatory response.
These observations suggest that neutralization of IL-1β, specific targeting of EC-mediated TLR4 signalling, or the factors released from these cells that are responsible for monocyte activation, survival and enhanced IL-1 release, represent important opportunities to intervene in vascular inflammation. Such therapies given around the time of major systemic inflammatory insults may act to reduce the increased risk of myocardial infarction or stroke seen in these settings.
Acknowledgments
We thank all those people in the Academic Unit of Respiratory Medicine who helped in the preparation of PBMC from whole blood, and all those that helped with the preparation of HUVEC. This work was funded by the British Heart Foundation (grant FS/06/004), and by a Medical Research Council Senior Clinical Fellowship (G116/170) to IS.
Disclosures
The authors have no conflicts on interests to declare.
References
- 1.Morris GE, Parker LC, Ward JR, et al. Cooperative molecular and cellular networks regulate Toll-like receptor-dependent inflammatory responses. FASEB J. 2006;20:2153–5. doi: 10.1096/fj.06-5910fje. [DOI] [PubMed] [Google Scholar]
- 2.Morris GE, Whyte MKB, Martin GF, Jose PJ, Dower SK, Sabroe I. Agonists of Toll-like receptors 2 and 4 activate airway smooth muscle via mononuclear leukocytes. Am J Respir Crit Care Med. 2005;171:814–22. doi: 10.1164/rccm.200403-406OC. [DOI] [PubMed] [Google Scholar]
- 3.Parker LC, Prestwich EC, Ward JR, Smythe E, Berry A, Triantafilou M, Triantafilou K, Sabroe I. A phosphatidylserine species inhibits a range of TLR, but not IL-1β, induced inflammatory responses by disruption of membrane microdomains. J Immunol. 2008;181:5606–17. doi: 10.4049/jimmunol.181.8.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–6. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 5.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satterthwaite G, Francis SE, Suvarna K, Blakemore S, Ward C, Wallace D, Braddock M, Crossman D. Differential gene expression in coronary arteries from patients presenting with ischemic heart disease: further evidence for the inflammatory basis of atherosclerosis. Am Heart J. 2005;150:488–99. doi: 10.1016/j.ahj.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 8.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–8. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 9.Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J Am Coll Cardiol. 1999;34:1975–81. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 10.Wiedermann CJ, Kiechl S, Schratzberger P, Dunzendorfer S, Weiss G, Willeit J. The role of immune activation in endotoxin-induced atherogenesis. J Endotoxin Res. 2001;7:322–6. [PubMed] [Google Scholar]
- 11.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–92. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 12.Michelsen KS, Wong MH, Shah PK, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–84. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker LC, Prince LR, Sabroe I. Translational mini-review series on Toll-like receptors: networks regulated by toll-like receptors mediate innate and adaptive immunity. Clin Exp Immunol. 2007;147:199–207. doi: 10.1111/j.1365-2249.2006.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabroe I, Read RC, Whyte MKB, Dockrell DH, Vogel SN, Dower SK. Toll-like receptors in health and disease: complex questions remain. J Immunol. 2003;171:1630–5. doi: 10.4049/jimmunol.171.4.1630. [DOI] [PubMed] [Google Scholar]
- 15.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-Like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–55. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 16.Faure E, Equils O, Sieling PA, et al. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–63. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 17.Sasu S, LaVerda D, Qureshi N, Golenbock DT, Beasley D. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ Res. 2001;89:244–50. doi: 10.1161/hh1501.094184. [DOI] [PubMed] [Google Scholar]
- 18.Ward JR, Bingle L, Judge HM, et al. Agonists of toll-like receptor (TLR)2 and TLR4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb Haemost. 2005;94:831–8. [PubMed] [Google Scholar]
- 19.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–61. [PubMed] [Google Scholar]
- 20.Chamberlain J, Evans D, King A, Dewberry R, Dower S, Crossman D, Francis S. Interleukin-1beta and signaling of interleukin-1 in vascular wall and circulating cells modulates the extent of neointima formation in mice. Am J Pathol. 2006;168:1396–403. doi: 10.2353/ajpath.2006.051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morton AC, Arnold ND, Gunn J, Varcoe R, Francis SE, Dower SK, Crossman DC. Interleukin-1 receptor antagonist alters the response to vessel wall injury in a porcine coronary artery model. Cardiovasc Res. 2005;68:493–501. doi: 10.1016/j.cardiores.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Sabroe I, Jones EC, Usher LR, Whyte MKB, Dower SK. Toll-Like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–10. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 23.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci USA. 2007;104:17034–9. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson HL, Varcoe RW, Stokes L, Holland KL, Francis SE, Dower SK, Surprenant A, Crossman DC. P2X receptor characterization and IL-1/IL-1Ra release from human endothelial cells. Br J Pharmacol. 2007;151:115–27. doi: 10.1038/sj.bjp.0707213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elson G, Dunn-Siegrist I, Daubeuf B, Pugin J. Contribution of toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood. 2007;109:1574–83. doi: 10.1182/blood-2006-06-032961. [DOI] [PubMed] [Google Scholar]
- 26.Rossignol DP, Lynn M. TLR4 antagonists for endotoxemia and beyond. Curr Opin Investig Drugs. 2005;6:496–502. [PubMed] [Google Scholar]
- 27.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–9. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 28.Wharram BL, Fitting K, Kunkel SL, Remick DG, Merritt SE, Wiggins RC. Tissue factor expression in endothelial cell/monocyte cocultures stimulated by lipopolysaccharide and/or aggregated IgG. Mechanisms of cell : cell communication. J Immunol. 1991;146:1437–45. [PubMed] [Google Scholar]
- 29.Pugin J, Ulevitch RJ, Tobias PS. A critical role for monocytes and CD14 in endotoxin-induced endothelial cell activation. J Exp Med. 1993;178:2193–200. doi: 10.1084/jem.178.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houston DS, Carson CW, Esmon CT. Endothelial cells and extracellular calmodulin inhibit monocyte tumor necrosis factor release and augment neutrophil elastase release. J Biol Chem. 1997;272:11778–85. doi: 10.1074/jbc.272.18.11778. [DOI] [PubMed] [Google Scholar]
- 31.Steppich BA, Moog P, Matissek C, et al. Cytokine profiles and T cell function in acute coronary syndromes. Atherosclerosis. 2007;190:443–51. doi: 10.1016/j.atherosclerosis.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 32.Abbate A, Salloum FN, Vecile E, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–83. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 33.Andonegui G, Bonder CS, Green F, Mullaly SC, Zbytnuik L, Raharjo E, Kubes P. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J Clin Invest. 2003;111:1011–20. doi: 10.1172/JCI16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12:133–50. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
- 35.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–35. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 36.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120:247–54. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 38.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–6. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Cheng X, Yu X, Ding YJ, et al. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97. doi: 10.1016/j.clim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Malley R, Henneke P, Morse SC, et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci USA. 2003;100:1966–71. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]