Abstract
Although CD8+ cytotoxic T lymphocytes (CTL) exhibit both Fas ligand (FasL) -based and perforin-based lytic activities, the accepted hallmark of a fully active CTL remains its perforin killing machinery. Yet the origin, rationale for possessing both a slow-acting (FasL) and a fast-acting (perforin) killing mechanism has remained enigmatic. Here we have investigated perforin expression in CTL directly involved in acute tumour (i.e. leukaemias EL4 and L1210) allograft rejection occurring within the peritoneal cavity. We show that at the height of the immune response, the majority of conjugate-forming CD8+ CTL express high levels of perforin messenger RNA and protein, and kill essentially via perforin. Later however, coinciding with complete rejection, fully cytocidal CTL emerge which exhibit a stark decrease in perforin and now kill preferentially via constitutively expressed FasL. Although late in emergence, and persistent, these powerful CTL are neither effector-memory nor memory CTL. This finding has implications for the monitoring of anti-transplant responses in clinical settings, based on assessing perforin expression in graft infiltrating CD8+ T cells. The results show that as the immune response progresses in vivo, targeted cellular suicide mainly prunes high perforin-expressing CD8+ cells, resulting in the gradual switch in effector CTL, from mostly perforin-based to largely Fas/FasL-based killers. Hence, two kinds of CD8+ CTL have two killing strategies.
Keywords: allograft rejection, CD8 T cells, cytotoxic T lymphocyte, Fas, Fas ligand, perforin
Introduction
CD8+ cytotoxic T lymphocytes (CTL) are key players in adaptive immune responses against certain viruses and intracellular parasites, in the rejection of transplants and in tumour immune responses.1–5 They become cytokine-secreting and cytocidal through a complex sequence of events involving clonal activation of CTL precursors to rapidly dividing lymphoblasts that express Fas ligand (FasL), as well as perforin-containing and granzyme-containing lytic granules. With some 10–20 cell divisions occurring during the first week of immunization, millions of such lymphoblasts can emerge from a handful of CD8+ precursor cells;6 however, the vast majority of these die en route, presumably as a result of activation-induced cell death (AICD), which is both perforin/granzyme7–9 and Fas/FasL dependent.10
The lytic protein perforin has traditionally been regarded as the hallmark of CTL action,2,11,12 and the ‘lethal hit’ has been linked to the combined action of perforin and granzymes, cosecreted upon cognate recognition, for immune synapse formation.4,13–15 On the other hand, CTL activity against certain target cells in calcium-free medium16–18 (where perforin is neither secreted nor lytic), as well as killing by CTL derived from perforin-deficient mice19,20 indicated that CTL must possess an additional, non-perforin killing mechanism shown to be mediated by FasL.21–23 Although FasL-based killing is slower than that induced by secreted perforin and granzymes,24 an adequate explanation to account for the cellular origin, specific purpose and rationale for these two distinct pathways has not been forthcoming. It has recently been proposed that FasL is stored in vesicles distinct from the traditional perforin-containing and granzyme-containing lytic granules (ref. 25 but see also ref. 26), suggesting that FasL and perforin expression, and consequently their deployment, are controlled differently.
The aim of the present study has been to define the origin, regulation of expression and possible role of the two cytocidal mechanisms in CTL generated in vivo in the course of allograft rejection. Here, based on findings with in vivo-primed CTL procured from the site of allograft rejection, we show that perforin (and FasL)-expressing CTL are generated early in the course of the allograft response. However, as the immune response progresses, and most perforin-expressing lymphoblasts have been pruned (probably by AICD), the evolving CTL population has now switched their killing phenotype, from mostly perforin-based to powerful Fas/FasL-based killers. The finding that mature CTL express little if any perforin and yet kill efficiently also has implications for predicting the onset of an anti-transplant response based on assessing perforin expression in graft-infiltrating CD8+ T cells.
Materials and methods
Mice
Mice were bred, supplied and maintained by the Weizmann Institute. The strains used included C57BL/6 and BALB/c mice, which were major histocompatibility complex (MHC) H-2b and H-2d, respectively. Other mice included perforin-knockout mice on a C57BL/6 (H-2b) background27 and transgenic 2C mice.28 Mice were between 8 and 10 weeks of age. All animal and procedures used in this study were approved by the Weizmann Animal Care and Use Committee.
Tumour cell lines culture medium and the generation of alloreactive CTL in vivo
Tumour cell lines included sub-lines LF+ and LF− derived from leukaemia L1210 of DBA/2 (H-2d) mice and leukaemia EL4 of C57BL/6 (H-2b) mice. LF+ and LF− are L1210 variants, high and low Fas, transfected with either a Fas-expression construct21 or a Fas anti-sense expression vector,27 respectively. Cultured cells were maintained in a RHFM medium consisting of RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mm glutamine, 1% non-essential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, 5 × 10−5 mβ-mercaptoethanol and 1 mm sodium pyruvate. Cells were cultured at 37° in a 5·0% CO2 humidified atmosphere.
Suspensions containing 25 × 106 tumour cells suspended in 0·5 ml phosphate-buffered saline (PBS) were injected intraperitoneally (i.p.) into allogeneic recipient mice. Alloreactive peritoneal exudate lymphocytes (PEL) and splenocytes were extracted and purified, as described elsewhere in detail.29,30 In short, following their inoculation with allogeneic tumour cells, mice were killed at various time-points. Their spleens and peritoneal exudate cells were collected in PBS supplemented with 5% heat-inactivated newborn calf serum (PBS-NCS). Splenocytes were obtained by mincing the organ through a stainless steel screen into PBS-NCS, and vigorously pipetting the resulting suspension to disassociate large cellular clumps. The PEL were isolated by resuspending the peritoneal cells in medium and incubating them at 37° for 1 hr, allowing adherent cells to adhere to culture dishes or to nylon wool columns. Finally, CD8+ cells were sorted using a fluorescence-activated cell sorter (FACS) with an appropriate anti-CD8+ fluorescent antibody, as described next.
Flow cytometry and cell sorting
Cells were suspended with the appropriate concentration of monoclonal antibody in PBS containing 1% bovine serum albumin. Goat anti-mouse immunoglobulin G (#5000003; Jackson-Immune Research Laboratories Inc, West Grove, PA) was used to prevent non-specific labelling. The following monoclonal antibodies were employed for flow cytometry and cell sorting: allophycocyanin-Cy7-conjugated anti-CD8+a/lyt-2 (100714, Biolegend, San Diego, CA), fluorescein isothiocyanate (FITC)-conjugated anti-mouse H-2Kb (116506; Biolegend, San Diego, CA), FITC-conjugated anti-CD44/Pgp-1 (1500-02; Southern Biotechnology Associates, Inc.), R-phycoerythrin-conjugated anti-CD62L/l-selectin (1705-09; Southern Biotechnology Associates, Inc., Birmingham, AL), FITC-conjugated anti-mouse CD95/Fas (554257; BD Pharmingen Inc, San Jose, CA), biotinylated rat anti-mouse 2C-TCR 1B2 (obtained from Dr Reisner, of the Weizmann Institute), mouse anti-perforin CB5.4 (804-057; Alexis Biochemicals, San Diego, CA). Propidium iodide (PI) (P-4170, Sigma) staining was used to exclude dead cells. The FACS analysis was performed using a FACScan (Becton Dickinson, San Jose, CA); cell sorting was carried out with an Aria FACS (Becton Dickinson) with cell quest software.
Conjugate formation and cytotoxicity assays
Conjugates were formed by mixing 106 CD8+ cells with an equal number of target cells suspended in 1 ml PBS-NCS. The suspension was allowed to stand for 10 min at room temperature and then centrifuged at 1000 g for 10 min at this temperature. The cells were then resuspended vigorously, placed on ice and the number of conjugates was recorded as described previously.31
A 51Cr-release assay was used to determine lytic activity. Target cells were labelled for 1·5 hr at 37° with Na251CrO4 (Chromium-51; CJS11; GE Healthcare, Haifa, Israel) and washed three times with cold PBS-NCS. Lytic assays were conducted in either U-shaped or 96-well microtitre plates, or 5-ml polystyrene round-bottom tubes (BD Pharmingen Inc, San Jose). CD8+ T cells and labelled target cells were mixed and then centrifuged at 1000 g for 5 min to promote conjugate formation. The mixture of cells was then incubated at 37° for specified times, allowing cytotoxic activity to take place. To terminate the assay, plates were recentrifuged at 2000 g for 10 min at 4°. One hundred microlitres of supernatant was then harvested from each well, and its radioactivity was determined with a COBRAII gamma-counter. The percentage lysis (cytotoxicity) was calculated as follows:
Total release was the amount of radioactivity released by 1 N HCl; spontaneous release was usually below 10%.
RNA preparation and reverse transcription–polymerase chain reaction
RNA was extracted from CD8+ cells using TRI Reagent according to the manufacturer’s protocol (MRC Molecular Research Center, Cincinnati, OH). Reverse transcription–polymerase chain reaction (RT-PCR) was performed by mixing 5 μg total RNA with a cocktail containing 5× buffer (Promega, Madison, WI), 10 mm dNTP mixture (Gene Craft, Koln, Germany), 10 U RNAase inhibitor (Takara BIO INC, Otsu, Shiga, Japan), Random Primer oligo-DT (Promega) and 0·125 U AMV reverse transcriptase (Promega). Diethylpyrocarbonate was added to bring the final volume to 50 μl, and the mixture was incubated at 42° for 50 min. The reaction was terminated by incubating the mixture at 70° for 15 min and then chilling it on ice. Five microlitres of the resultant RT-PCR product was amplified for 30 cycles using the ReddyMix PCR Master Mix (ABgene, Surrey, UK) with 30 ng of the following primers: perforin primer, forward 5′-GGG AAC CAA GCT ACA CCA GA-3′, reverse 5′-AAA CCA GAG TGG GGA GAC CT-3′; FasL primer, forward 5′-CTT GGG CTC CTC CAG GGT CAG T-3′, reverse, 5′-TCT CCT CCA TTA GCA CCA GAT CC-3′; granzyme-B primer, forward 5′-TCG ACC CTA CAT GGC CTT AC-3′, reverse, 5′-CAC ACT CCC GAT CCT TCT GT-3′. The sample undergoing PCR was first incubated at 94° for 2 min. This was followed by 30 cycles at 94° for 30 seconds, at 57–64° (depending on the annealing temperature of the specific primers) for 1 min and at 72° for 1 min. Finally, the sample underwent one cycle of incubation at 94° for 30 seconds and 72° for 7 min. Five microlitres of each PCR product was resolved by electrophoresis on 1·5% agarose gel and visualized using ethidium bromide staining.
Western blotting
Cells lysates were obtained by incubating cells in RIPA buffer containing 1% phenylmethylsulphonyl fluoride, a protease inhibitor, at room temperature for 20 min. Twenty micrograms of the extracted protein was electrophoresed on a 10% sodium dodecyl sulphate–polyacrylamide gel and then transferred to a nitrocellulose membrane (#BA85 Schleicher & Schuell Bioscience, Inc., Keene, NH). The resulting protein blot was blocked with goat anti-mouse immunoglobulin G (#5000003; Jackson-Iimmuno Research). Perforin was detected using the monoclonal antibody CB5.4 (#804057F; Alexis Biochemicals). Blots were developed by SuperSignal west pico chemiluminescence substrate (#34080 Thermo Fisher Scientific Inc, Rockford, IL) and exposed to superRX film (FUJIFILM Global, Tirat Carmel, Israel).
Immunoperoxidase staining
CD8+ cells (105) in PBS were placed on poly-l-lysine-treated glass slides and left to adhere for 1 hr at 4°. The slides were washed in PBS, fixed in 4% paraformaldehyde for 10 min at room temperature, washed in PBS, permeabilized by 0·2% Triton-X for 10 min at room temperature, washed in Tris–HCl 50 mm, pH 7·6, denatured with 0·5% HIO4 for 10 min at room temperature, washed in Tris–HCl, quenched with 0·3% H2O2 for 15 min at room temperature, and washed again with Tris–HCl. Peroxidase staining was performed using the ABC staining system kit (SC-2019; Santa Cruz Biotechnology, Santa Cruz, CA) in conjunction with the P1-8 perforin antibody. Cover glasses were mounted with Entellan. Perforin (protein) expression was evaluated on a Nikon E800 light microscope (Nikon Instruments, Inc, Melville, NY).
Inhibition of protein synthesis by emetine
The PEL were pretreated with the non-reversible inhibitor of protein synthesis emetine (Sigma, E2375), 0–2·5 μm for 2 hr at 37°. Incorporation of [35S]methionine (0·2 ml containing 1 × 106 PEL, plus 2 μCi [35S]methionine, for 4 hr) into ice-cold 10% trichloroacetic acid-insoluble materials collected on glass fibre discs, was measured by a liquid scintillation counter.32
Results
Responding CD8+ T cells found at the site of graft rejection
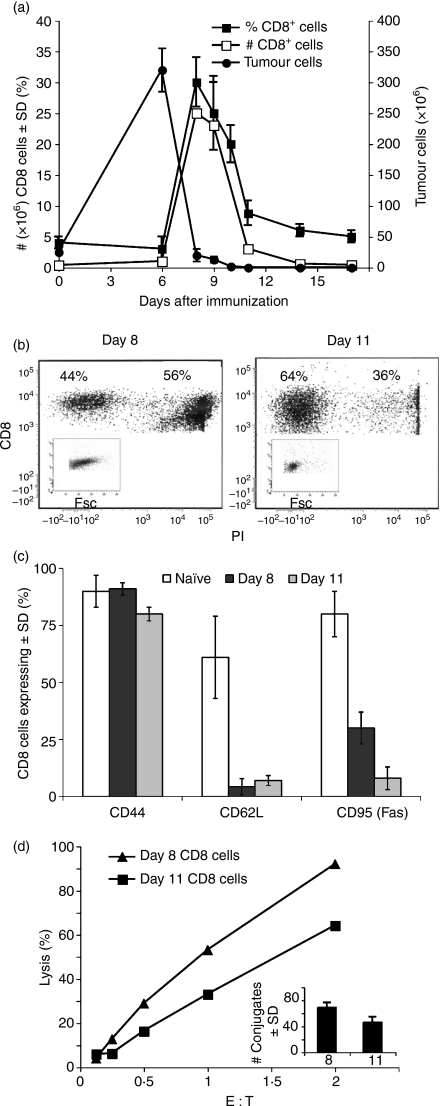
It was found that 25 × 106 EL4 tumour cells injected i.p. into allogeneic BALB/c mice grew progressively, reaching 300 × 106 to 400 × 106 cells per mouse on the 6th day (Fig. 1a). From that point on, a drop in their number could be seen, coinciding with a marked rise in CD8+ T cells infiltrating the peritoneal cavity (Fig. 1a). Tumour rejection was almost always complete around day 8–10 post-injection, the time at which the CD8+ T-cell population reached its peak and began to decline, tapering off around day 11 (Fig. 1a), with specific CD8+ T cells lingering in the peritoneal cavity for around a month.31 Day 8 CD8+ cells comprised 25–35% of the total peritoneal cells; decreasing to 8–11% on day 11 (Fig. 1a). In addition, 56% of day 8 CD8+ PEL were found to be dead (PI-stained), in contrast to only 36% of day 11 cells (Fig. 1b). Day 8 CD8+ cells varied in size widely, consisting of some larger (lymphoblast) cells, in contrast to the uniformly small cells of day 11 (Fig. 1b). Overall, the majority of CD8+ cells from either day were CD44high and CD62Llow, indicating the presence of a class of lymphocytes active in the periphery (Fig. 1c). CD95 (Fas) expression varied greatly, with 25–35% of day 8 CD8+ cells expressing this death receptor, compared with only 5–10% of day 11 cells (Fig. 1c). Although displaying an apparently higher killing activity, day 8 CD8+ PEL formed more conjugates than day 11 PEL, (Fig. 1d), which could account for the difference, for in this system, almost all conjugates have been shown to proceed to lysis.33 Similar results were obtained in a C57BL/6 anti-LF PEL system.
Figure 1.
CD8+ T-cell response during allograft rejection in the peritoneal cavity. (a) Kinetics of tumour growth and CD8+ T-cell response. BALB/c mice were injected intraperitoneally with allogeneic EL4 tumour cells. Peritoneal exudate lymphocytes (PEL) were, stained for CD8+, the allogeneic tumour for H-2b. Mean ± SD of five animals; one out of three experiments with similar results. (b) CD8 and propidium iodide (PI) staining of responding peritoneal cells. Day 8 and 11 PEL were stained by a CD8+ antibody and analysed by fluorescence-activated cell sorting (FACS) for CD8+ expression. PI staining was used to discern dead cells. Insert depicts forward scatter of CD8+, PI-negative cells. (c) Cell surface markers of responding CD8+ cells. PEL of naïve, day 8 and day 11 immunized mice were stained for CD8+, CD44, CD62L and CD95 (Fas), and analysed by FACS. The mean of four repeat experiments ± SD is shown. (d) Cytotoxicity and conjugate formation by peritoneal CD8+ T cells. Days 8 and 11 immune CD8+ PEL were subjected to a 4-hr cytotoxic assay at different effector-to-target cell (E : T) ratios and % lysis was recorded. In parallel, a fraction of the CD8+ cells was allowed to conjugate at an E : T ratio of 1/1 and the number of conjugates was determined (insert).
Expression of cytolytic molecules in CD8+ PEL
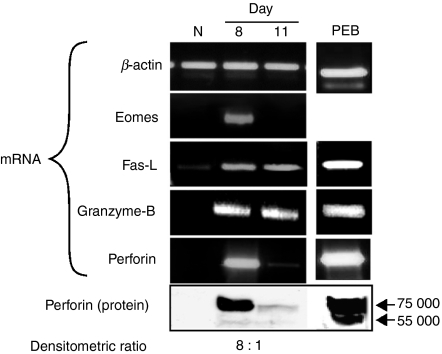
The lytic activity of day 8 and day 11 BALB/c anti-EL4 CD8+ T cells (Fig. 1d) suggested proportional expression of messenger RNA (mRNA) levels of perforin, eomesodermin (Eomes), granzyme-B and FasL, molecules traditionally considered staples of effector CD8+ cells. FasL and granzyme-B mRNA was almost equally expressed in day 8 and day 11 CD8+ PEL (Fig. 2). In contrast, whereas massive levels of perforin mRNA were found in day 8 CD8+ cells, the amounts found in day 11 CD8+ cells were almost undetectable. Similarly, the mRNA levels of Eomes (a transcription factor that regulates T-cell lytic functions),34,35 were considerable in day 8 cells, but undetectable in day 11 CD8+ CTL (Fig. 2). Results of Western blot analysis were in accord with the mRNA data; an abundance of perforin in day 8 CD8+ cells, in contrast to only trace amounts in day 11 CD8+ CTL. Similar results were obtained with C57BL/6 anti-LF CD8+ PEL.
Figure 2.
Expression of Eomes, Granzyme-B, FasL and perforin in CD8+ T cells. CD8+ peritoneal exudate lymphocytes (PEL) were derived from naive BALB/c mice as well as 8 and 11 days post-injection with allogeneic EL4 tumour cells. Reverse transcription–polymerase chain reaction and Western blot were performed. RNA and protein extracted from PEL blasts (PEB), provided positive controls for FasL, granzyme-B and perforin messenger RNA and protein.
Immunocytochemistry of perforin expression in CD8+ PEL
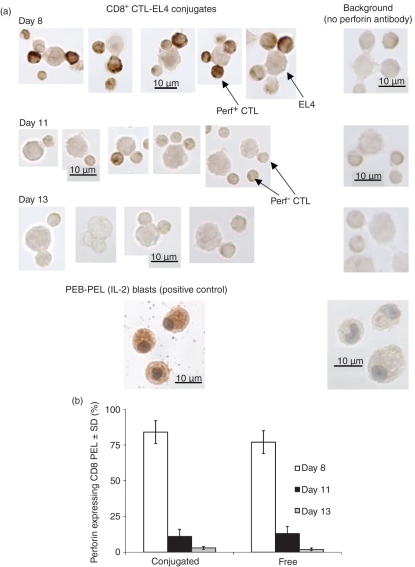
The aforementioned experiments revealed that during allograft rejection, the responding CD8+ CTL populations emerging on days 8 and 11 differed greatly in perforin mRNA and protein expression, with the latter cells expressing only trace amounts of perforin protein and mRNA (Fig. 2). To determine whether day 8 and day 11 CD8+ CTL were uniformly expressing perforin at high or low levels, respectively, we employed perforin immunocytochemistry (ICC) on conjugate-forming CD8+ PEL (Fig. 3). In general, within a given perforin-expressing cell, ICC revealed punctate-shape staining, representative of perforin storage granules typical of CTL (Fig. 3a). As expected, there was a far higher degree of staining with day 8 cells. Whereas around 80–90% of the day 8-conjugated cells were perforin positive, only 11% of day 11-conjugated cells stained positive (Fig. 3b), these showed a faint, homogeneous (less punctate) pattern of staining. Interestingly, there was also a significant minority (ca. 17%) of day 8-conjugated CD8+ CTL that stained negative for perforin, indicating the emergence of perforin-deficient CTL already at this early stage of CTL development. These results show that in the course of an allograft response the responding CD8+ peritoneal cells switch to a perforin-deficient phenotype from day 8 to day 11.
Figure 3.
Perforin expression in CD8+ cytotoxic T lymphocytes. Peritoneal exudate lymphocytes (PEL) were extracted from BALB/c anti-EL4 mice 8, 11 or 13 days after tumour injection, CD8+ cells were sorted, excluding propidium iodide-positive cells. (a) CD8+-EL4 conjugates were stained (immunocytochemsitry) using perforin antibody. PEL blasts (PEB) were used as a positive control. Antibody controls were conjugates reacted without the perforin antibody. (b) % Perforin staining of free and conjugated CD8+ sorted cells. Mean ± SD of four repeat experiments, each with cells procured from five animals P(v) > 0·05).
The influence of antigen on perforin expression
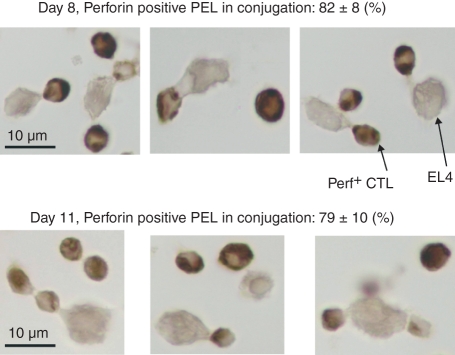
The switch from perforin-expressing to perforin-deficient CTL could simply be related to the elimination of antigen, in the form of live tumour cells in the peritoneal cavity. To test this hypothesis, we injected BALB/c mice with allogeneic EL4 tumour cells. Eight days later, to prolong the presence of antigen in the peritoneal cavity, the mice were again injected with EL4 tumour cells every day, for six consecutive days. CD8+ PEL were then collected, and conjugates were formed and tested for perforin expression. The results showed that the majority of conjugate-forming cells, even as late as 14 days after the initial injection, expressed perforin (Fig. 4). In fact, the percentage of conjugated cells expressing perforin on day 14 was similar to that seen in day 8 cells (Figs 3, 4). These results indicated that in the continuous presence of stimulating antigen, responding CD8+ cells continued to express the lytic protein perforin. Hence the switch from perforin-expressing to perforin-deficient CTL is likely to be the result of the elimination of antigen.
Figure 4.
Perforin expression in conjugated CD8+ cytotoxic T lymphocytes obtained after multiple tumour injections. EL4 tumour cells were injected intraperitoenally into BALB/c mice. Eight days later, an additional 25 × 106 EL4 cells were injected daily for 6 days. CD8+ EL4 conjugates were stained (immunocytochemsitry) using perforin antibody.
Day 8 and day 11 CTL employ alternate killing mechanisms
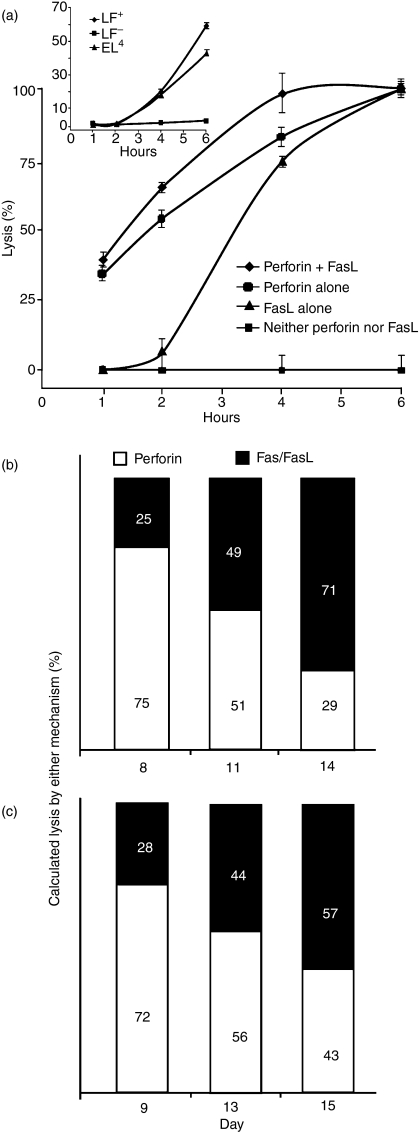
Although expressing almost equal amounts of FasL mRNA (Fig. 2), day 8 and day 11 CTL differed considerably in their perforin contents (Figs 2, 3). We next determined how this influenced the overall killing by CTL, as the immune response progressed. Specifically, we examined if, as predicted, CD8+ T cells lose their perforin-based lytic activity, along with down-regulation of perforin expression (Figs 1d, 2, 3). To this end, Fas/FasL-induced lysis was studied with alloreactive PEL-CTL derived from perforin-mutant (knockout) mice (Fig. 5a). The perforin/granzyme pathway alone was assessed by employing Fas-deficient target cells (LF−). At each time-point tested, cytotoxicity was higher when the two mechanisms operated in concert (Fig. 5a). Unlike the perforin/granzyme pathway, which brought about fast killing, almost 2 hr elapsed before Fas/FasL-based killing became evident. Eventually, killing by both mechanisms reached the same plateau (Fig. 5a).To determine whether the 2-hr delay in FasL-based killing of Fas-expressing LF+ cells was related to the CTL themselves, we examined the killing of LF+, LF− and EL4 cells induced by soluble recombinant FasL trimmers instead of CTL; the results again indicated a lag of 2 hr, similar to that observed with CTL-FasL with no lytic activity against Fas-deficient LF− cells (Fig. 5a). Exploiting the 2-hr delay in FasL-based killing, the relative contribution of either killing mechanism in the course of CTL development was tested. To this end, CD8+ sorted BALB/c anti-EL4 PEL collected on days 8, 11 and 14, were subjected to a lytic assay. Lysis recorded after 1 hr reflected mainly perforin-based killing, whereas that determined after 3 hr represented the combined effects of perforin-based and FasL-based killing, with the fraction indicating the relative contribution of perforin on each day (Fig. 5b). Whereas day 8 CD8+ CTL killed mostly via perforin, a smaller proportion of killing by day 11 cells was perforin-based, the remaining being FasL-mediated. Day 14 CTL showed an even greater reliance upon FasL (Fig. 5b).
Figure 5.
Differential contribution of the perforin- and Fas-based lytic mechanisms. (a) C57BL/6 and perforin-deficient (PO) anti-LF peritoneal exudate lymphocytes (PEL) were extracted 10 days after immunization and subjected to a 6-hr lytic assay against LF+ and LF– cells. Insert: Strictly Fas/FasL-based killing: soluble recombinant FasL trimmer-mediated lysis of LF+ and EL4 commences with a lag of 2 hr; LF– cells were not lysed by rFasL. Mean ± SD of three repeat experiments, each with cells procured from three animals. (b) BALB/c anti-EL4 CD8+ PEL subjected to a 1- and 3-hr lytic assay against EL4. Perforin contribution to total killing was calculated: 1 hr/3 hr % lysis; accordingly, FasL-based killing was 100 – (1 hr/3 hr %). One out of three experiments with similar results. (c) C57BL/6 anti-LF CD8+ PEL subjected to a 4-hr lytic assay against LF+ and LF−. Lysis of LF− was affected by perforin only. Lysis of LF+ was affected by combination of perforin and FasL-based killing. % Perforin killing was calculated as follows: (% LF− lysis/% LF+ lysis) × 100, FasL-based killing was calculated: 100 − (% LF− lysis/% LF+ lysis) × 100
An alternative strategy employed to demonstrate the switch in perforin utilization over time was by averting FasL action. CD8+ sorted C57BL/6 anti-LF PEL collected on days 9, 13 and 15, were subjected to a 4 hr-lytic assay against LF+ or LF− target cells. Lysis of LF+ reflected the combined action of perforin and FasL, lysis of LF− was primarily the result of perforin. The difference between these two values indicated lysis by FasL. Whereas day 8 CTL produced virtually total lysis by perforin alone, day 11 cells only displayed about half their efficacy when low-Fas LF targets were employed. Day 14 CTL exhibited an even greater reliance upon FasL when their 4-hr lytic activity was assessed (Fig. 5c). Both models indicate that the CD8+ CTL population switched its killing phenotype, from mostly perforin-based to Fas/FasL-based killers. In addition to expressing FasL mRNA transcripts (Fig 2), day 11 PEL displayed cell-surface FasL (detectable by Fas L antibodies and FACS), and their Fas-based killing by PEL was almost fully blocked by either the Fas antibody Jo2 or by soluble recombinant Fas-Fc, but not with Mg2 ethyleneglycoltetraacetic acid, which blocks perforin-mediated killing.32
De novo synthesis of perforin does not account for lysis induced by perforin-deficient PEL
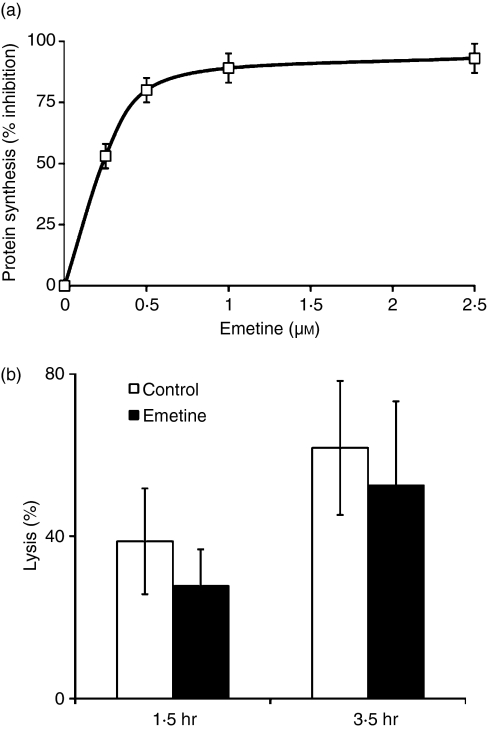
Although a large proportion of day 11 CD8+ cells were found to be perforin deficient by RT-PCR, Western blotting and ICC (Figs 2, 3), they still killed well (Figs 1d, 5b, c). Yet day 11 PEL could still kill via perforin synthesized de novo upon TCR-mediated binding to the cognate target cells. To examine this possibility, we employed emetine, a potent, non-reversible inhibitor of protein synthesis, at concentrations that blocked virtually all protein synthesis sparing conjugate formation (Fig. 6a). Day 11 PEL exposed to emetine (at 2·5 μm, 93% inhibition of protein synthesis) still killed efficiently (Fig. 6b), as has been found before for a purely FasL-based killing by CTL derived from perforin-deficient mice.32 Emetine exhibited a more profound inhibitory effect within the first 1·5 hr, a time at which killing was largely the result of perforin (Fig. 6b). These results indicate that killing by day 11 CD8+ cells does not rely on de novo protein (including perforin) synthesis.
Figure 6.
Emetine effects on protein synthesis and on lysis induced by day 11 peritoneal exudate lymphocytes (PEL). (a) Emetine, 0–2·5 μm, inhibition of protein synthesis in BALB/c anti-EL4 PEL. (b) Inhibition of lysis by day 11 PEL pre-incubated with Emetine (2·5 μm, for 2 hr) before a 1·5-hr or 3·45-hr lytic assay in the presence of Emetine; effector-to-target ratio of 10 : 1. Mean ± SD of three repeat experiments, each with cells procured from three animals is shown. P(v) < 0·05).
The fate of day 8 perforin-rich CTL and the origin of day 11 CTL
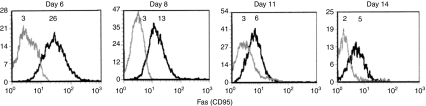
We next explored whether day 11 perforin-deficient CTL were derived from the perforin-expressing CTL population present in the peritoneal cavity 3 days earlier. To this end, day 8 BALB/c anti-EL4 PEL were cultured in vitro for 48 hr, which excludes the impact of cells newly arriving in the peritoneal cavity. Remaining cells were counted, CD8+/PI-negative sorted, conjugated with cognate target cells, and stained for perforin, both before and after the 48-hr incubation. Overall, 37% of the original day 8 CD8+ CTL survived the 2-day culture period, and a significant change in perforin expression occurred in comparison with the precultured CD8+ cells (Table 1). The disappearance of CD8+ cells over time could be the result of AICD, supported by the finding that early in the immune response, day 6 and day 8 CD8+ cells coexpressed high Fas (Figs 1c, 7), and so could be subject to AICD, compared with low Fas-expressing day 11 and day 14 CD8+ PEL (Fig. 7). Elsewhere we have shown Fas-dependence (and blocking by the Fas antibody JO2) of AICD induced upon prolonged incubation of PEL with cognate target cells.30 To further establish a connection between Fas expression and enhanced elimination of perforin-expressing CTL, day 8 CD8+ PEL were sorted by their Fas expression and incubated in vitro for 24 hr. Most (97%) of the Fashigh cells were found to be dead (eosin-stained) compared with only 23% of the Faslow cells.
Table 1.
Cytotoxic T lymphocytes change from perforin-expressing to perforin-deficient cells in vitro
| 0 hr | 48 hr | Survived | |||
|---|---|---|---|---|---|
| Cells | ×106 | (%) | ×106 | (%) | (%) |
| CD8+ cells | 162 | – | 60 | – | 37 |
| Perforin-positive | 116 | 72 | 19 | 31 | 16 |
| Perforin-deficient | 45 | 28 | 41 | 61 | 91 |
Figure 7.
Fas expression on responding CD8+ peritoneal exudate lymphocytes (PEL). Mean fluorescence intensity (MFI, in italics) of Fas+ CD8+ PEL extracted from the peritoneal cavity of C57BL/6 anti-LF mice 6, 8, 11 and 14 days after immunization. Animals were killed and the cells were analysed on the same day. Cells were stained with CD8+ and Fas (CD95) antibodies and analysed by fluorescence-activated cell sorting. The grey line depicts cells stained for CD8+ only and serve as a control. One of three experiments with similar though variable patterns is shown.
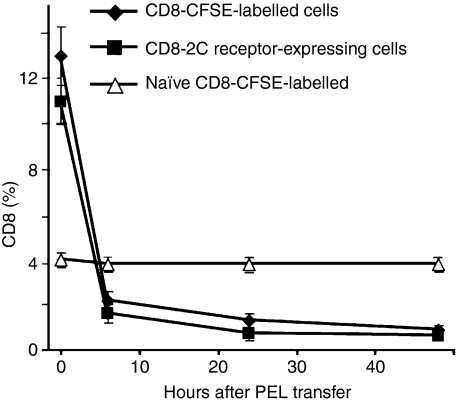
We then determined whether the disappearance of day 8 CD8+ cells observed in culture (Table 1), could be demonstrated in vivo. To this end, day 8 PEL were collected from some immunized mice, then reinjected i.p. into the remaining mice. Parallel experiments were conducted with transgenic 2C anti-LF PEL. PEL were recovered from recipient mice and the CD8+/CFSE+ or 2C CD8+ populations were analysed by FACS. In control experiments, PEL from naïve mice were injected into other naïve mice. In contrast to transferred naïve PEL, which were almost completely accounted for, transferred immune PEL took a different course. Already after 6 hr, the majority of the transferred cells could not be traced, and virtually no transferred cells were found after 24 and 48 hr (Fig. 8). No transferred CD8+ PEL were found in the spleen or mesenteric nodes either. This indicated that the majority of perforin-containing day 8 cells vanished by day 11, possibly as a result of AICD, consistent with their high cell surface Fas expression (Fig. 7), known to be associated with AICD.
Figure 8.
The fate of day 8 CD8+ peritoneal exudate lymphocytes (PEL) in vivo. Day 8 BALB/c anti-EL4 PEL were stained with CFSE, analysed for CD8+ CFSE+ cells, and then reinjected intraperitoneally (i.p.) into remaining mice. Parallel experiments were conducted with immunized transgenic 2C and C57BL/6 anti-LF mice. PEL from the 2C mice were extracted 8 or 11 days after immunization and reinjected i.p. into C57BL/6 mice of the same day, i.e. day 8 PEL were injected into day 8 mice. PEL were removed from the latter mice at different times and the CD8+/CFSE+ or 2C CD8+ population was analysed by fluorescence-activated cell sorting. Each experiment was performed twice.
Are perforin-deficient PEL-CTL memory cells?
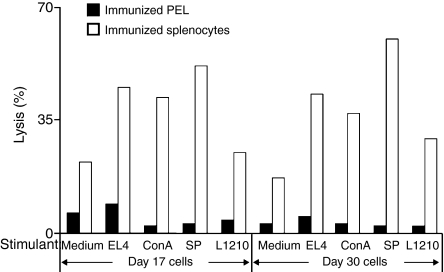
Finally we explored whether the small, perforin-deficient CD8+ PEL, which linger in the peritoneal cavity, could be regarded as memory cells. Memory T cells exhibit an enhanced response upon restimulation with cognate antigen or with polyclonal T-cell activators, so we subjected PEL and splenocytes procured from BALB/c anti-EL4 mice, 17 and 30 days after tumour injection, to a 3-day restimulation in vitro. Cognate EL4 cells, cognate-irradiated spleen cells, non-cognate LF tumour cells (H2d), or concanavalin A, a powerful polyclonal activator of T cells including of memory CTL,36,37 were used as stimulators. Stimulated cells were then subjected to a 4-hr lytic assay. Compared with the control, non-stimulated PEL, no enhancement in killing ability was seen with day 17 or 30 PEL, incubated with any of the stimulants (Fig. 9). This was in contrast to the enhanced response of splenocytes derived from the same mice, which exhibited a substantial increase in lytic activity upon stimulation. Mature CD8+ CTL, obtained at the time of complete rejection (day 11 and onward), are small, non-dividing lymphoid cells that exhibit powerful specific conjugation and lytic activity and express CD44high and CD62Llow activated CTL markers. Combined with phenotype analyses of PEL cell surface markers (Fig. 1c), these data indicated that the perforin-deficient cytocidal CD8+ PEL, found in the peritoneal cavity, at least at the tail end of an allogeneic immune response, could be regarded as bona fide effector cells and not memory cells, at least of a known type.
Figure 9.
Restimulation of primed splenocytes and peritoneal exudate lymphocytes (PEL). PEL and splenocytes were collected from BALB/c anti-EL4 mice injected 17 and 30 days before were subjected to a 3-day restimulation with LF+, EL4, irradiated C57BL/6 splenocytes, concanavalin A (2 μg/ml), and with medium as control. Cells were then tested in a 4-hr lytic assay against EL4 and % lysis was recorded. One out of three experiments performed is illustrated.
Discussion
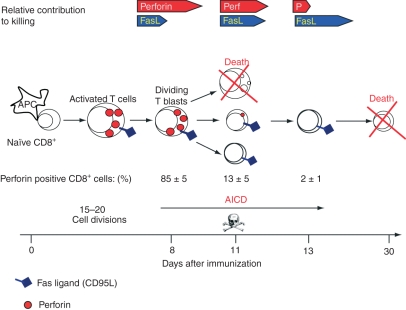
Although known to copossess a FasL-based killing mechanism,21 the accepted hallmark of effector CTL continues to be its perforin (and granzyme) killing machinery. Yet the rationale for having both a slow-acting (FasL-mediated) and a fast-acting (perforin-based) killing mechanism at their disposal has remained unexplained at both the cellular and molecular levels. Here we have addressed these issues using in vivo-primed CTL involved in allograft rejection in the peritoneal cavity.18,38 We have found that at the height of the response, the majority of conjugate-forming CD8+ CTL indeed express high levels of perforin mRNA and protein, and kill preferentially through perforin, as expected. Later, however, equally cytocidal CD8+ CTL are found which exhibit a stark decrease in the expression of perforin and its transcription factor eomesodermin and yet kill efficiently by means of FasL. The results suggest that targeted cellular suicide, or fratricide, prunes the high perforin-expressing CD8+ CTL, resulting in the gradual emergence of ‘mature’, perforin-deficient CD8+ CTL, which are still highly cytotoxic but now kill largely via FasL. As an immune response progresses in vivo, therefore, the responding CD8+ CTL population gradually switches killing phenotype, from mostly perforin-based to FasL-based killers (Fig. 10).
Figure 10.
The transition from mostly perforin to FasL cytotoxic T lymphocytes (CTL). The CTL start off as naïve CD8+ T cells, which upon activation by antigen-laden antigen-presenting cells, and cytokines develop into dividing CTL blasts that continue to proliferate and differentiate into effector CTL of decreasing sizes. Initially, the responding CTL possess both the perforin and FasL killing mechanisms. As the immune reaction progresses, and antigen becomes limiting, most CTL blasts are pruned (probably by activation-induced cell death); the remaining CTL, now of decreasing sizes, gradually stop expressing perforin, relying mainly on FasL activity. These CTL are not memory cells however.
Mature PEL CTL are small, non-dividing, non-granular lymphoid cells that nevertheless exhibit powerful, specific conjugation and lytic activities.5,29,31,39,40 That PEL CTL and not natural killer (NK) cells are responsible for the PEL action reported here is supported by the strict specificity of their lytic activity and conjugate formation, inhibition by MHC antibodies, and expression of markers. The PEL CTL exhibit rigorous lytic specificity, including even recognition of MHC mutations,41 and their binding to and lytic action against target cells is blocked by MHC antibodies.42 Further, NK-like activity against classical NK target cells such as lymphoma YAC of A/J mice (KkDd) is close to background; yet effective cytocidal PEL CTL can be generated in response to YAC cells.17,43 The results with PEL conjugate formation are particularly revealing: unlike NK cells, PEL form conjugates essentially with MHC cognate target cells; but little PEL conjugate formation was detected with non-cognate YAC or other allogeneic cells.31,44 As perforin expression in PEL has been targeted to conjugated PEL (Fig. 3), it follows that the vast majority of the CD8 cells examined were CTL and not NK cells. Finally, we have shown that the majority of CD8 sorted PEL (or CTL-hybridomas thereof exhibiting the authentic PEL specificity) express CD3.43
Consideration must be given to indirect killing by PEL, possibly mediated by TNF or interferon-γ (IFN-γ). However, no cytocidal activity against Fas-deficient target cells was effected by perforin-deficient CTL, yet Fas-expressing target cells were lysed by FasL-expressing, perforin-deficient PEL (Fig. 5a). As PEL killing is a relatively fast process, occurring equally well under conditions where macromolecule (RNA, DNA and protein) biosynthesis is fully blocked (Fig. 6b),30 and as TNF and IFN-γ antibodies do not block short-term (up to 6 hr) PEL-mediated killing (unpublished results), the involvement of these cytokines in PEL killing proper seems unlikely. Interestingly, coincubation of PEL and Fas-deficient target cells (such as Fas-antisense expressing cells) produced up-regulation of Fas expression, which appears to involve inducible nitric oxide synthase stimulated by IFN-γ.45 Up-regulation of Fas expression detected on initially low Fas-expressing tumours injected in vivo is compatible with the observed switch from mostly perforin-based to a largely Fas-based CTL mechanism as reported here.
Despite their considerable lytic activity, characteristic perforin-containing lytic granules were rarely observed in or isolated from mature PEL, or from the cytolytic hybridomas.17,27,46–48 Moreover, only close to background levels of perforin mRNA and protein have been detected in PEL,17,43,46 although this is contrasted by the findings described later and in ref.49. Unlike earlier studies, performed wholly on mature (day 11) PEL, here we have examined perforin expression in CD8+ PEL at early as well as late stages of their development in vivo. The largely perforin-deficient, FasL-expressing CTL procured 11 days after injection succeeded earlier CD8+ CTL that expressed high levels of perforin (Figs 2, 3). Compared with only traces of perforin mRNA and protein evident in day 11 PEL, the time at which they exhibited potent lytic activity (Fig. 1d), considerable amounts of perforin mRNA and protein were found in CD8+ CTL isolated on day 8 and before. In studies performed at the single cell level, Kelso et al.50 have shown that CD8+ lymphocytes stimulated in vitro with IL-2 and anti-CD3 expressed high levels of perforin, granzyme-B and IFN-γ mRNA.50 It is noteworthy that perforin-deficient PEL also expressed massive quantities of perforin-containing lytic granules upon their exposure to IL-2 in vitro.17 [Hence cessation of perforin expression in mature PEL in vivo is likely to be the result of a shortage of antigen, IL-2 and other signals known to influence gene (including perforin) expression at earlier stages of CTL development.) In fact, repeated injections of antigen (cognate allogeneic cells) helped maintain high levels of perforin expression, preventing the switch to perforin-deficient cells (Fig. 4).
Given only traces of perforin mRNA detected in day 11 CTL, we hypothesized that a few, residual, perforin-expressing day 8 CD8+ CTL could account for that. This is why we turned to explore perforin expression in individually conjugated PEL, shown all to be effector CTL33 (see below). Using perforin ICC, we showed that while the majority of conjugated day 8 CD8+ CTL expressed perforin, only a minority of conjugated day 11 (mature) CTL stained positive for perforin (Fig. 3), which could explain the detection of a small perforin RNA signal by Northern blots in bulk CD8+-sorted PEL reported previously.45 In an earlier communication, we showed that neither continuous new gene expression nor protein synthesis were required for FasL-based lytic action of in vivo-primed PEL derived from perforin-deficient (PO) mice.32 That lytic activity of day 11 CTL took place even when protein synthesis was blocked by emetine (Fig. 6), excluded the possibility that de novo synthesized perforin (as well as other proteins), triggered upon CTL–target interaction, accounted for the lytic action of such cells (Fig. 6b).
As discussed above, micromanipulation of individual CTL–target conjugates showed that close to 100% of all conjugate-forming PEL were killer cells; yet some conjugates lysed quickly (within 5–25 min), whereas the lysis of others took up to 2–3 hr to complete.33 It is reasonable to assume that the fast killing seen with some PEL was perforin-mediated, whereas the slower killer PEL lysed via the FasL pathway.24 Based on that premise, we examined the contribution of perforin to the overall killing observed in the course of CD8+ PEL activation and progression in vivo.
We first confirmed that perforin-mediated killing brought about lysis shortly after conjugation; and that almost 2 hr elapsed before Fas/FasL-based killing became evident. Eventually, killing by either mechanism reached a plateau through different kinetics (Fig. 5a). Kinetic analysis revealed that CD8+ CTL lose their perforin-based lytic activity simultaneously with down-regulation of perforin expression. Whereas day 8 CTL were mostly perforin-based killers, only a small proportion of killing by day 11 cells could be attributed to perforin, the remaining being FasL-based. Day 14 CTL showed an even greater reliance upon FasL (Figs 5b, c).
A small proportion of day 8 conjugate-forming CD8+ cells did not express perforin (Fig. 3). These cells may have escaped AICD, eventually giving rise to the (mostly) perforin-deficient CTL seen later on (e.g. day 11). Furthermore, day 6 and perforin-expressing day 8 CTL coexpressed high Fas on their cell surface membrane (Fig. 7), which presumably played a role in their demise, induced by AICD. Conversely, day 11 CD8+ CTL expressed neither (Figs 1c and 7). This observation may be related to and could explain excessive (apoptotic) cell death of early but not late CTL (Fig. 1b). Alternatively, it is possible that a fraction of perforin-expressing PEL lymphoblasts seen earlier subsequently develop into progressively smaller (Fig. 1b), non-dividing lymphoid cells, which express powerful lytic activity but virtually no perforin (see Fig. 10). These CTL kill preferentially and repeatedly via FasL40 even when protein synthesis is fully blocked (Fig. 6). Together with differential perforin expression data in day 8 and day 11 PEL (Fig. 3), and continuous FasL mRNA expression along the immune reaction (Fig. 2), these results suggest that mature (day 11) PEL-CTL are largely but not only FasL-based killers, unlike their day 8 predecessors, which are mainly but not only perforin-based killers. When combined, these results support a switch from perforin to Fas/FasL-based killers in the course of CTL development in vivo. Hence two cell types, two killing strategies.
The precise function(s) of the two (possibly more) CTL lytic mechanisms continues to be an open question in CTL biology. An obvious explanation is that there are complementary or fallback mechanisms. For example, virus-infected cells and some tumours can evade CTL action by interfering with a particular step of the perforin/granzyme-mediated killing process.5,51,52 Alternatively, Fas expression in malignant tissues can be down-regulated or deranged by the expression of Fas spliced, inactive variants.53 Two mechanisms instead of one killing mechanism, which is perforin-based and FasL-based, would still enable CTL to function. An alternative hypothesis has been that the FasL-based CTL mechanism mainly plays an immunoregulatory role (e.g. in AICD), and to a lesser extent, in killing cognate target cells proper.54 The foregoing studies suggest differential, temporal expression of the two CTL killing mechanisms, with perforin dominating early blastoid and dividing stages of responding CTL, and FasL dominating the mature stages of CTL development. A related issue pertains to the possibility that mature, perforin-deficient PEL in fact represent some sort of memory CTL or a class of effector memory CTL.55,6 Whereas there is much discussion of what sort of markers really define memory T cells, including CTL, there is no doubt that an enhanced functional response to the original antigen best reflects a state of memory T cells. We tested PEL for memory-marker expression as well as anamnestic responses to antigens, mitogenic lectins, with an equally negative outcome. Unlike memory CTL, PEL do not require the conventional 2- to 3-day restimulatory period typical of memory CTL to exhibit full cytolytic activity. In fact, PEL were not restimulated by either cognate antigen or polyclonal stimulators (Fig. 9), and so could not be regarded as memory CTL, in contrast to a strong memory CTL response of lymphoid cells procured from the lymph nodes and spleen of the same mice (Fig. 9). Lack of a memory-like response may be the result of a shortage of auxiliary cells and factors required for restimulation of purified PEL. In fact, mature (day 11) PEL responded favourably to externally added, high-dose IL-2, resulting in the production of high perforin-expressing CTL which exhibited potent, specific lytic activity indistinguishable from their PEL-CTL of origin.17 On the other end, cell surface marker analysis (Fig. 1c) has shown that mature CD8+ PEL are CD44high and CD62Llow, unlike memory cells, which are traditionally regarded as being CD44high CD62Lhigh.56 Similar perforin-deficient cytocidal CD8+ cells have been found in lymph nodes as well as in the blood of immunized animals even a month after immunization (data not shown). These observations would also argue against an accumulation of perforin-expressing CTL or memory CTL at the anatomical site of allograft rejection. Accordingly allospecific CD8+ memory cells reside in peripheral lymphoid organs rather than the site of the graft, which is the peritoneal cavity in the case of an allograft placed i.p. The results are consistent with the ‘asymmetric model’ of memory T-cell development, suggesting that memory T cells are not direct descendents of effector cells, but evolve in parallel, by asymmetric cell division of responding T cells already at the level of antigen presentation at the immune synapse.57
Finally, the data support the following scheme of CTL development during allograft rejection in vivo. Triggered by antigen-laden antigen-presenting cells, in the presence of cytokines, naïve T cells develop into rapidly dividing T lymphoblasts. These in turn migrate to the rejection site, where they continue to proliferate and differentiate into effector CTL of decreasing sizes. In the early stages, the CD8+ CTL population is composed predominantly of FasL-expressing and perforin-expressing lymphoblastoid cells, but as the immune response progresses (and antigen and cytokines become limiting factors), targeted cellular suicide by AICD, probably involving both perforin and Fas, prunes the high perforin-expressing and high Fas-expressing CD8+ cells, resulting in gradual emergence of mature, low Fas-expressing and perforin-deficient CTL, which kill preferentially via FasL (Fig. 10). It has already been shown that apoptosis of activated CD8+ T cells occurs preferentially in cells that have undergone a number of cell divisions.58 Such a scheme of CTL development and perforin expression not only resolves previous contentious data concerning CTL biology, perforin and FasL expression and function in CTL in vivo: it also has implications to monitoring the onset of transplant rejection in a clinical setting which is based at least in part on assessing perforin expression in graft-infiltrating lymphoid cells, shown here to be a rather early event. That is that at later stages graft infiltrating cytocidal lymphoid cells may go undetected when perforin expression is used as a yardstick.
Acknowledgments
These studies were supported by the Israel Science Foundation (ISF) (grant No. 966/05), the Benozio Foundation, and by the Weizmann Institute (Kirk Center)-Tel-Aviv Sourasky Medical Center collaborative grant, 2008. We thank Drs K. Okumora and H. Yagita, Juntendo University, Tokyo, for providing perforin antibody, D. Wallach for recombinant FasL trimmers and Y. Reisner for 1B2 antibody.
Glossary
Abbreviations:
- AICD
activation-induced cell death
- CTL
cytotoxic T lymphocyte
- FITC
fluorescein isothiocyanate
- ICC
immunocytochemistry
- i.p.
intraperitoneal
- LF+ and LF−
high and low Fas-expressing L1210 leukaemia, respectively
- PBS
phosphate-buffered saline
- PEL
peritoneal exudate CD8+ CTL
- PI
propidium iodide
- RT-PCR
reverse transcription–polymerase chain reaction
References
- 1.Cerottini JC, Brunner KT. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- 2.Kägi D, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–32. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 3.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–47. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 4.Henkart PA, Catalfamo M. CD8+ effector cells. Adv Immunol. 2004;83:233–52. doi: 10.1016/S0065-2776(04)83007-4. [DOI] [PubMed] [Google Scholar]
- 5.Berke G, Clark W. Killer lymphocytes. Berlin: Springer; 2005. [Google Scholar]
- 6.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–92. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 7.Kägi D, Odermatt B, Mak TW. Homeostatic regulation of CD8+ T cells by perforin. Eur J Immunol. 1999;29:3262–72. doi: 10.1002/(SICI)1521-4141(199910)29:10<3262::AID-IMMU3262>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 8.Opferman JT, Ober BT, Narayanan R, Ashton-Rickardt PG. Suicide induced by cytolytic activity controls the differentiation of memory CD8+(+) T lymphocytes. Int Immunol. 2001;13:411–19. doi: 10.1093/intimm/13.4.411. [DOI] [PubMed] [Google Scholar]
- 9.Su MW, Pyarajan S, Chang JH, Yu CL, Jin YJ, Stierhof YD, Walden P, Burakoff SJ. Fratricide of CD8+ cytotoxic T lymphocytes is dependent on cellular activation and perforin-mediated killing. Eur J Immunol. 2004;34:2459–70. doi: 10.1002/eji.200425096. [DOI] [PubMed] [Google Scholar]
- 10.Krueger A, Fas SC, Baumann S, Krammer PH. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunol Rev. 2003;193:58–69. doi: 10.1034/j.1600-065x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 11.Podack ER, Hengartner H, Lichtenheld MG. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–57. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- 12.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940–52. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–70. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 14.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annu Rev Cell Dev Biol. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 15.Cullen SP, Martin SJ. Mechanisms of granule-dependent killing. Cell Death Differ. 2008;15:251–62. doi: 10.1038/sj.cdd.4402244. [DOI] [PubMed] [Google Scholar]
- 16.Tirosh R, Berke G. T-Lymphocyte-mediated cytolysis as an excitatory process of the target. I. Evidence that the target cell may be the site of Ca2+ action. Cell Immunol. 1985;95:113–23. doi: 10.1016/0008-8749(85)90300-4. [DOI] [PubMed] [Google Scholar]
- 17.Berke G, Rosen D. Highly lytic in vivo primed cytolytic T lymphocytes devoid of lytic granules and BLT-esterase activity acquire these constituents in the presence of T cell growth factors upon blast transformation in vitro. J Immunol. 1988;141:1429–36. [PubMed] [Google Scholar]
- 18.Berke G. The CTL’s kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 19.Kägi D, Ledermann B, Bürki K, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–7. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 20.Walsh CM, Matloubian M, Liu CC, et al. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–8. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouvier E, Luciani MF, Golstein P. Fas involvement in Ca2+-independent T cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–30. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 23.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 24.Lowin B, Mattman C, Hahne M, Tschopp J. Comparison of Fas(Apo-1/CD95)- and perforin-mediated cytotoxicity in primary T lymphocytes. Int Immunol. 1996;8:57–63. doi: 10.1093/intimm/8.1.57. [DOI] [PubMed] [Google Scholar]
- 25.He JS, Ostergaard HL. CTLs contain and use intracellular stores of FasL distinct from cytolytic granules. J Immunol. 2007;179:2339–48. doi: 10.4049/jimmunol.179.4.2339. [DOI] [PubMed] [Google Scholar]
- 26.Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med. 1999;5:90–6. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- 27.Walsh CM, Glass AA, Chiu V, Clark WR. The role of the Fas lytic pathway in a perforin-less CTL hybridoma. J Immunol. 1994;153:2506–14. [PubMed] [Google Scholar]
- 28.Lee PU, Kranz DM. Allogeneic and syngeneic class I MHC complexes drive the association of CD8+ and TCR on 2C T cells. Mol Immunol. 2002;39:687–95. doi: 10.1016/s0161-5890(02)00259-6. [DOI] [PubMed] [Google Scholar]
- 29.Berke G, Sullivan KA, Amos DB. Rejection of ascites tumor allografts. I. Isolation, characterization and in vitro reactivity of peritoneal lymphoid effector cells from BALB/c mice immune to EL4 leukosis. J Exp Med. 1972;135:1334–50. doi: 10.1084/jem.135.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li HH, Rosen D, Sondel P, Berke G. Immune privilege and FasL: two ways to inactivate effector cytotoxic T lymphocytes by FasL-expressing cells. Immunology. 2002;105:267–77. doi: 10.1046/j.1365-2567.2002.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berke G, Gabison D, Feldman M. The frequency of effector cells in populations containing cytotoxic T lymphocytes. Eur J Immunol. 1975;5:813–18. [Google Scholar]
- 32.Li JH, Rosen D, Ronen D, Behrens CK, Krammer PH, Clark WR, Berke G. The regulation of CD95 ligand expression and function in CTL. J Immunol. 1998;161:3943–9. [PubMed] [Google Scholar]
- 33.Zagury D, Bernard J, Thierness N, Feldman M, Berke G. Isolation and characterization of individual functionally reactive cytotoxic T lymphocytes. Conjugation, killing and recycling at the single cell level. Eur J Immunol. 1975;5:818–22. [Google Scholar]
- 34.Pearce EL, Mullen AC, Martins GA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–3. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 35.Intlekofer AM, Takemoto N, Wherry EJ, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2006;12:1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 36.Clark WR. An antigen-specific component of lectin-mediated cytotoxicity. Cell Immunol. 1975;17:505–16. doi: 10.1016/s0008-8749(75)80054-2. [DOI] [PubMed] [Google Scholar]
- 37.Tsotsiashvilli M, Levi R, Arnon R, Berke G. Activation of influenza-specific memory cytotoxic T lymphocytes by Concanavalin A stimulation. Immunol Lett. 1998;60:89–95. doi: 10.1016/s0165-2478(97)00135-1. [DOI] [PubMed] [Google Scholar]
- 38.Berke G. The binding and lysis of target cells by cytotoxic lymphocytes: molecular and cellular aspects. Annu Rev Immunol. 1994;12:735–73. doi: 10.1146/annurev.iy.12.040194.003511. [DOI] [PubMed] [Google Scholar]
- 39.Berke G, Sullivan KA, Amos DB. Rejection of ascites tumor allograft. II. A pathway for cell-mediated tumor destruction in vitro by peritoneal exudate lymphoid cells. J Exp Med. 1972;136:1594–604. doi: 10.1084/jem.136.6.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berke G, Sullivan KA, Amos DB. Tumor immunity in vitro: destruction of a mouse ascites tumor through a cycling pathway. Science. 1972;177:433–4. doi: 10.1126/science.177.4047.433. [DOI] [PubMed] [Google Scholar]
- 41.Berke G, Amos DB. Cytotoxic lymphocytes in the absence of detectable antibody. Nature New Biol. 1973;242:237–9. doi: 10.1038/newbio242237a0. [DOI] [PubMed] [Google Scholar]
- 42.Todd RF, Stulting RD, Berke G. Mechanism of blocking by hyperimmune serum of lymphocyte-mediated cytolysis of allogeneic tumor cells. Cancer Res. 1973;33:3203–8. [PubMed] [Google Scholar]
- 43.Berke G, Rosen D, Ronen D. Mechanism of lymphocyte-mediated cytolysis: functional cytolytic T cells lacking perforin and granzymes. Immunology. 1993;78:105–12. [PMC free article] [PubMed] [Google Scholar]
- 44.Schick B, Berke G. Competitive inhibition of cytotoxic T lymphocyte-target cell conjugation. A direct evaluation of membrane antigens involved in cell-mediated immunity. Transplantation. 1979;27:365–8. [PubMed] [Google Scholar]
- 45.Peshes-Yaloz N, Rosen D, Sondel PM, Krammer PH, Berke G. Up-regulation of Fas (CD95) expression in tumour cells in vivo. Immunology. 2007;120:502–11. doi: 10.1111/j.1365-2567.2006.02521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berke G, Rosen D. Are lytic granules and perforin 1 involved in lysis induced by in vivo-primed peritoneal exudate cytolytic T lymphocytes? Transplant Proc. 1972;19:412–16. [PubMed] [Google Scholar]
- 47.Dennert G, Anderson CG, Prochazka G. High activity of N-alpha-benzyloxycarbonyl-l-lysine thiobenzyl ester serine esterase and cytolytic perforin in cloned cell lines is not demonstrable in in-vivo-induced cytotoxic effector cells. Proc Natl Acad Sci USA. 1987;84:5004–8. doi: 10.1073/pnas.84.14.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garner R, Helgason CD, Atkinson EA, et al. Characterization of a granule-independent lytic mechanism used by CTL hybridomas. J Immunol. 1994;153:5413–22. [PubMed] [Google Scholar]
- 49.Nagler-Anderson C, Lichtenheld M, Eisen HN, Podack ER. Perforin mRNA in primary peritoneal exudate cytotoxic T ymphocytes. J Immunol. 1989;143:3440–3. [PubMed] [Google Scholar]
- 50.Kelso A, Costelloe EO, Johnson BJ, Groves P, Buttigieg K, Fitzpatrick DR. The genes for perforin, granzymes A-C and IFN-gamma are differentially expressed in single CD8+(+) T cells during primary activation. Int Immunol. 2002;14:605–13. doi: 10.1093/intimm/dxf028. [DOI] [PubMed] [Google Scholar]
- 51.Lehmann C, Zeis M, Schmitz N, Uharek L. Impaired binding of perforin on the surface of tumor cells is a cause of target cell resistance against cytotoxic effector cells. Blood. 2000;96:594–600. [PubMed] [Google Scholar]
- 52.Cartier A, Broberg E, Komai T, Henriksson M, Masucci MG. The herpes simplex virus-1 Us3 protein kinase blocks CD8+ T cell lysis by preventing the cleavage of Bid by granzyme-B. Cell Death Differ. 2003;10:1320–8. doi: 10.1038/sj.cdd.4401308. [DOI] [PubMed] [Google Scholar]
- 53.Curtin JF, Cotter TG. Live and let die: regulatory mechanisms in Fas-mediated apoptosis. Cell Signal. 2003;15:983–92. doi: 10.1016/s0898-6568(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 54.Bidère N, Su HC, Lenardo MJ. Genetic disorders of programmed cell death in the immune system. Annu Rev Immunol. 2006;24:321–52. doi: 10.1146/annurev.immunol.24.021605.090513. [DOI] [PubMed] [Google Scholar]
- 55.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 56.Walker PR, Ohteki T, Lopez JA, MacDonald HR, Maryanski JL. Distinct phenotypes of antigen-selected CD8+ T cells emerge at different stages of an in vivo immune response. J Immunol. 1995;155:3443–52. [PubMed] [Google Scholar]
- 57.Chang JT, Palanivel VR, Kinjyo I, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–91. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 58.Renno T, Attinger A, Locatelli S, Bakker T, Vacheron S, MacDonald HR. Cutting edge: apoptosis of superantigen-activated T cells occurs preferentially after a discrete number of cell divisions in vivo. J Immunol. 1999;162:6312–15. [PubMed] [Google Scholar]