Abstract
CD100 participates in adaptive immune responses and is important in neural cell migration. To determine the role of endogenous CD100 in severe glomerular inflammation, we induced experimental crescentic glomerulonephritis by planting a foreign antigen in glomeruli of sensitized normal and CD100-deficient (CD100−/−) mice. Fewer CD100−/− glomeruli exhibited crescent formation or severe histological changes. Antigen-specific immune responses were reduced in CD100−/− mice. There was less interferon (IFN)-γ and interleukin (IL)-4 production by splenocytes and fewer activated T and B cells were present in lymph nodes of immunized CD100−/− mice. Serum antigen-specific immunoglobulin (IgG) levels were also decreased. Glomerular macrophage and CD4+ cell infiltration, and IgG and C3 deposition were attenuated. Normal kidneys expressed mRNA for CD100 and plexin-B1 (the tissue receptor of CD100). Direct immunofluorescence showed that renal-CD100 protein was predominantly in tubules, while plexin-B1 was present in both glomeruli and tubules. To determine whether glomerular plexin-B1 mediates leucocyte recruitment via leucocyte CD100, recruitment was studied after passive transfer of heterologous antibody (attracting neutrophils) or isologous antibody (attracting macrophages). Glomerular macrophages were reduced in CD100−/− mice, but neutrophil recruitment was equivalent, consistent with CD100 expression on macrophages, but not neutrophils. CD100 promotes severe nephritogenic immune responses and leucocyte CD100–glomerular plexin-B1 interactions enhance macrophage recruitment to glomeruli.
Keywords: antibodies, CD100, cytokines, glomerulonephritis, plexin-B1, transgenic/knockout mice
Introduction
Members of the semaphorin (Sema) family were originally identified as phylogenetically conserved proteins in the central nervous system. However, accumulating evidence indicates that several semaphorins, known as ‘immune semaphorins’, are important in immune responses, both in the induction of T-cell and B-cell responses and in the effector phase of tissue injury.1 There are eight classes of semaphorins involved in the immune system and in other biological processes, including axonal guidance, angiogenesis, cardiac development and osteogenesis.2
CD100, also known as Sema4D, was the first semaphorin characterized in the immune system.3,4 Increasing evidence demonstrates an important role of CD100 in both humoral and cellular immunity.1,5,6 CD100 regulates immune responses via interactions with CD72, the predominant receptor for CD100 in lymphoid tissues. CD100 provides a positive costimulatory signal in an unusual manner by switching off tonic negative signalling by CD72, resulting in enhancement of B-cell and dendritic cell (DC) responses.1,7 T-cell-dependent B-cell responses are decreased in CD100−/− mice, and T-cell responses are also impaired. Splenic DCs isolated from CD100−/− mice are unable to efficiently initiate T-cell proliferation and cytokine production.8,9 Similar events occur in both humans and mice.10 In addition to its presence as a membrane-bound protein, CD100 can also be detected in a soluble form, which correlates with autoantibody levels in lupus-prone mice.11 Mice transgenic for soluble CD100 have enhanced T- and B-cell responses.12 In non-lymphoid tissues, CD100 utilizes plexin-B1 as a receptor,7,13 plexin-B1 being highly expressed by epithelial cells in the kidney.13,14 While many studies examining ‘immune’ semaphorins have been focused on the adaptive immune system, looking at interactions between CD100-CD72, CD100–plexin-B1 interactions also influence cell migration (reviewed in Reference 2).
Crescentic glomerulonephritis (GN) is the most severe and rapidly progressive subset of GN. While CD100 has been shown to have a pathogenic role in mild experimental GN induced by circulating immune complexes,9 the role of CD100 in severe forms of immune renal injury, such as crescentic GN, is not known. In the current study, we have defined the involvement of endogenous CD100 in tissue-specific inflammatory responses in murine crescentic GN, where renal injury is induced by a planted foreign antigen [known as ‘autologous phase anti-glomerular basement membrane (GBM) glomerulonephritis’].
Materials and methods
Mice, immunization and induction of GN
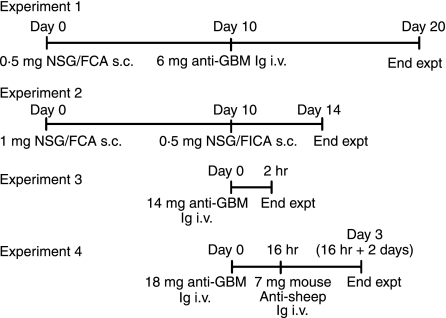
BALB/c (CD100+/+) mice were obtained from Monash University Centre Animal Services (Clayton, Victoria, Australia). CD100−/− mice (BALB/c, backcrossed for more than eight generations onto a BALB/c background),5,8 obtained from Osaka University (Osaka, Japan) were bred and maintained at Monash Medical Centre (Clayton, Victoria, Australia) in specific pathogen-free conditions. Figure 1 illustrates the four experimental protocols used in these series of studies. In the first, accelerated autologous phase ‘anti-GBM’ GN was induced in 8- to 10-week-old male CD100+/+ (n = 9) and CD100−/− mice (n= 12). Mice were immunized by subcutaneous injection of 500 μg of normal sheep globulin in complete Freund’s adjuvant (Sigma, Castle Hill, New South Wales, Australia). After 10 days, 6 mg/mouse of sheep anti-mouse GBM globulin was intravenously injected. Immune responses and renal injury were assessed after a further 10 days, the standard time-point in this model. Two further groups of mice, CD100+/+ (n = 4) and CD100−/− mice (n = 4), were immunized and challenged in a similar way, except that they were injected with 6 mg of normal sheep globulin intravenously. As normal sheep globulin has no specificity for the kidney, these groups served as negative controls for renal endpoints. In the second experiment, further studies of immune responses used mice immunized with 1 mg of sheep globulin in complete Freund’s adjuvant in each flank subcutaneously (day 0), boosted after 10 days with 500 μg of sheep globulin in Freund’s incomplete adjuvant (Sigma), and sera and draining lymph nodes were collected on day 14 (n = 6 per group). The third and fourth series of experiments used passive transfer of antibodies. In experiment 3, 14 mg of sheep anti-mouse GBM antibodies was injected intravenously into CD100+/+ mice and CD100−/− mice (n = 8 per group), neutrophil infiltration being assessed after 2 hr (maximal infiltration). In the fourth experiment, a passive accelerated model (previously published in rats15) to attract macrophages by intravenous injection of 18 mg of sheep anti-mouse anti-GBM antibodies, then after 16 hr intravenous injection of 7 mg of polyclonal mouse-anti-sheep IgG (prepared by protein G purification of pooled sera from previous experiments in mice with accelerated autologous phase GN), was used. Glomerular macrophage accumulation was assessed after a further 2 days (n = 7 per group). Studies were approved by the Monash University (Monash Medical Centre) Animal Ethics Committee.
Figure 1.
Outline of the experimental design for the four series of in vivo experiments performed. For full details, please see the Materials and methods section. NSG, normal sheep globulin; FCA, Freund’s complete adjuvant; FIA, Freund’s incomplete adjuvant.
Statistical analyses
Results are expressed as mean ± standard error of the mean (SEM) and differences between groups were determined by unpaired t-test (Graph Pad Prism, San Diego, CA). For urinary nitrite, where data could not be assumed to be normally distributed, the Mann–Whitney test for non-parametric data was used. A P-value of < 0·05 was used for statistical significance.
Assessment of glomerular injury, leucocyte infiltration and humoral mediators
Histological injury was assessed on periodic acid-Schiff’s reagent (PAS)-stained, coded slides by determining crescent formation (≥ 2 layers of cells in Bowman’s space; ≥ 50 glomeruli assessed) and the proportion of severely abnormal glomeruli (in all glomeruli within the section), as previously described.16 Criteria for defining a severely affected glomerulus were crescent formation, accumulation of cells in Bowman’s space that did not satisfy the criteria for crescent formation, > 50% of glomerular tuft affected by necrosis or severe proliferative changes. The deposition of PAS-positive material was scored 0 to 3+ based on the degree of deposition per glomerulus (1, > 25%; 2, 25–50%; 3, > 50% of the glomerular tuft involved). Mice were housed individually in cages for collection of urine over the final 24 hr of experiments and urinary protein excretion was determined using a modified Bradford assay. Macrophages, neutrophils and CD4+ cells in glomeruli were demonstrated by immunoperoxidase staining [≥ 40 glomeruli per mouse, expressed as cells per glomerular cross-section (c/gcs)] as described previously,17 the primary antibodies being FA/11 (anti-CD68, macrophages), GK1·5 (CD4+ cells; ATCC, Manassas, VA) and RB6-8C5 (Gr-1, neutrophils; Schering Plough, Palo Alto, CA). Isotype control antibodies and splenic sections served as negative and positive controls. Immunofluorescence for glomerular deposition of mouse immunoglobulin and C3 was evaluated by means of 0 to 3+ scale scoring based on intensity (≥ 20 glomeruli per mouse, with a normal mouse as a baseline of ‘0’), using fluorescein isothiocyanate (FITC)-sheep-anti-mouse immunoglobulin (1 : 100; Silenus, Victoria, Australia) or FITC-goat anti-mouse C3 (1 : 100; Cappel, Durham, NC).18 Urinary nitrite [as a marker of nitric oxide (NO)] concentrations were determined by Griess assay.19
Assessment of immune responses
Systemic antigen-specific antibody responses were assessed by measuring levels of IgG, IgG1 and IgG2a by enzyme-linked immunosorbent assay (ELISA) using serum collected at the end of experiments,20 with horseradish peroxidase (HRP)-conjugated sheep anti-mouse IgG (1 : 2000; Amersham, Little Chalfont, UK), HRP-goat anti-mouse IgG1 (1 : 4000; Southern Biotechnology Associates, Birmingham, AL) and, for IgG2a, a biotinylated rat anti-mouse IgG2a antibody (BD Pharmingen, San Diego, CA), followed by streptavidin-HRP (Chemicon, Boronia, Victoria, Australia). Antibody concentrations were expressed in arbitrary units (AU) compared to a reference pool of immunized mouse serum. For cytokine production, single-cell suspensions from spleens and lymph nodes (draining site of immunization) from mice with GN were removed, erythrocytes were lysed and cells were incubated at 4 × 106 cells/ml (splenocytes) or 2 × 106 cells/ml (lymph node cells) in 10% fetal calf serum (FCS) RPMI complete culture media (72 hr) stimulated with sterile protein G-purified normal sheep IgG (10 μg/ml). IFN-γ and IL-4 in culture supernatant were measured by ELISA, using rat anti-mouse IFN-γ (R46A2; BD Pharmingen); biotinylated rat anti-mouse IFN-γ (XMG1·2; BD Pharmingen); rat anti-mouse IL-4 (11B11; ATCC), biotinylated rat anti-mouse IL-4 (BVD6; Schering Plough), rat anti-mouse IL-17A (eBio17CK15A5; eBioscience, San Diego, CA) and biotinylated rat anti-mouse IL-17A (eBio17B7; eBioscience).
Immune cell CD100 expression and T- and B-cell activation
To elicit cells by peritoneal lavage (neutrophils, 4 hr; macrophages, 72 hr), mice were injected with 0·5 ml of sterile 3·85% thioglycollate intraperitoneally. For peripheral blood, heparinized blood was diluted in media and overlaid onto Ficoll-Paque (1·077 Ficoll-Paque™ Plus; Amersham Pharmacia, Uppsala, Sweden). Cells were centrifuged and then peripheral mononuclear cells were collected and washed, and 106 cells/sample were used for fluorescence-activated cell sorting (FACS) analyses. Propidium iodide (1 μg/ml; Calbiochem, La Jolla, CA) was added to exclude dead cells. Single-cell suspensions were prepared from draining lymph nodes. The following monoclonal antibodies were used (BD Pharmingen unless otherwise noted): R-phycoerythrin (PE)-rat anti-mouse CD4 (GK1·5), FITC-rat anti-mouse CD45R/B220 (RA3-6B2), allophycocyanin (APC)-rat anti-mouse CD44 (IM7), FITC-rat anti-mouse CD25 (7D4), PE-hamster anti-mouse CD69 (H1·2F3), PE-rat anti-mouse Gr-1 (RB6-8C5) and APC-rat-anti-mouse CD11b (M1/70). Rat anti-mouse CD100 (BMA12)7 was labelled with Alexa Fluor-488 (Molecular Probes, Eugene, OR). Three-colour flow cytometry was performed on FACScan® (Dako Cytomation, Fort Collins, CO).
Real-time polymerase chain reaction (PCR) and intrarenal CD100 and plexin B1
For reverse transcription (RT)-PCR, total kidney RNA was extracted and reverse transcribed to produce cDNA as previously described. Gene-specific primers for mouse CD100, plexin B1, tumour necrosis factor (TNF), IL-1β and β-actin are listed in Table 1. Real-time PCR was performed on a Rotor Gene RG-3000 (Corbett Research, Mortlake, New South Wales, Australia) using Power SYBR Green PCR master mix (Applied Biosystems, Foster City, CA). Quantification of products was performed using serial dilutions of an exogenous standard, and levels were normalized to β-actin and expressed as a percentage. PCR products were confirmed by melt curve analysis. For CD100 and plexin B1 immunofluorescent staining, 6-μm frozen kidney sections from non-diseased mice and mice with anti-GBM GN were fixed with acetone and blocked with biotin blocking system (Dako), followed by 10% normal rabbit serum or 10% normal swine serum in 5% bovine serum albumin (BSA)–phosphate-buffered saline (PBS). Slides were incubated with 10 μg/ml BMA-12 (rat anti-mouse CD100 mAb) or 20 μg/ml rabbit anti-mouse plexin B1 antibody (overnight at 4°; H-300; Santa Cruz Biotechnology, Santa Cruz, CA). Biotinylated rabbit anti-rat immunoglobulin (1 : 100; Dako) and biotinylated swine anti-rabbit immunoglobulin (1 : 100; Dako) were used as secondary antibodies, and then FITC-conjugated streptavidin (1 : 100; Dako) was applied. As negative controls, sections from CD100−/− mice were used for CD100, and for plexin-B1, non-immune rabbit IgG was used.
Table 1.
Primers used for real-time reverse transcription–polymerase chain reaction (RT-PCR) analyses
| Forward | Reverse | Product (bp) | |
|---|---|---|---|
| CD100 | ACCACCTGAACTTGACATCCTT | ACCATGACTGATGTGTAGCTGTG | 100 |
| Plexin-B1 | CTTCTCCTTGGGTCATGTGCAGTAC | TCTTATAGTCCCTCAGGGCCTGCT | 164 |
| TNF | GCCTCTTCTCATTCCTGCTT | CACTTGGTGGTTTGCTACGA | 203 |
| IL-1β | GGGCCTCAAAGGAAAGAATC | TACCAGTTGGGGAACTCTGC | 183 |
| β-actin | AGGCTGTGCTGTCCCTGTAT | AAGGAAGGCTGGAAAAGAGC | 388 |
IL-1β, interleukin-1β; TNF, tumour necrosis factor.
Results
Endogenous CD100 promotes injury in experimental anti-GBM GN
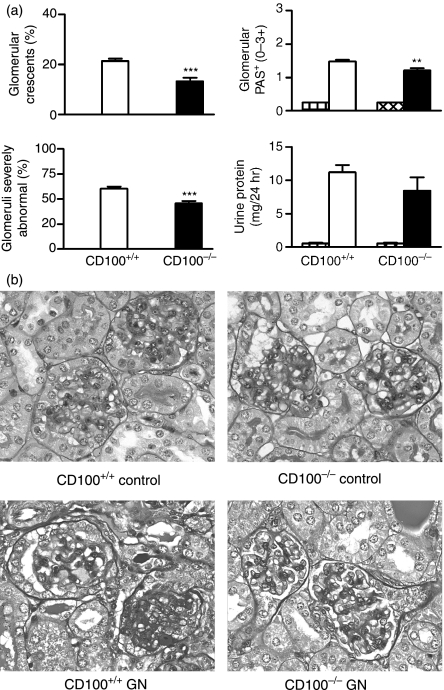
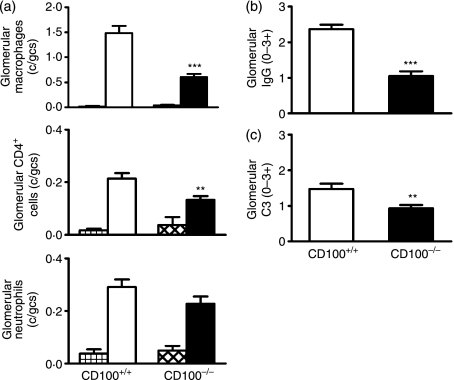
Using an accelerated autologous phase ‘anti-GBM’ GN model that plants a foreign antigen in glomeruli of sensitized mice, the role of CD100 in the development of severe immune glomerular injury was determined. These experiments correspond to experiment 1 in Fig. 1. Ten days after challenge with sheep anti-mouse GBM globulin, CD100+/+ mice demonstrated significant glomerular abnormalities including crescent formation, glomerular tuft necrosis, significant crescent formation and proteinuria (Fig. 2a,b). Control mice (CD100+/+ or CD100−/−) immunized with sheep globulin, then injected with normal (non-immune) sheep globulin intravenously, did not develop significant abnormalities. In the absence of endogenous CD100, fewer glomeruli were severely affected by either crescent formation or other severe histological changes (Fig. 2a,b). Proteinuria was not significantly reduced in CD100−/− mice. Leucocyte accumulation in glomeruli was examined (Fig. 3a). In the absence of endogenous CD100, there were significantly fewer macrophages and CD4+ in glomeruli [results expressed as cells per glomerular cross-section (c/gcs)], but no significant decrease in glomerular neutrophils. Humoral mediators of injury, glomerular antibody (Fig. 3b) and glomerular C3 deposition (Fig. 3c) were decreased in the absence of CD100. Markers of cellular activation and injury, intrarenal IL-1β mRNA, TNF mRNA and urinary nitrite (a marker of NO; normal values in this assay 6 μmol/24 hr), were assessed (Fig. 4). In the absence of CD100, intrarenal IL-1β mRNA expression and urinary nitrite were reduced, but intrarenal TNF mRNA expression was unchanged.
Figure 2.
Attenuated glomerular injury in CD100−/− mice with glomerulonephritis (experiment 1). (a) Compared with CD100+/+ mice with glomerulonephritis, CD100−/− mice exhibited lower levels of crescent formation, a lower proportion of severely affected glomeruli and less accumulation of periodic acid-Schiff’s reagent (PAS)-positive material. There was no significant reduction of proteinuria in CD100−/− mice. CD100+/+ and CD100−/− mice immunized with and injected with normal (non-immune) sheep globulin did not develop histological abnormalities or proteinuria (hatched bars). (b) Representative glomeruli from affected mice. Severe glomerular injury with significant glomerular crescent formation was observed in CD100+/+ mice with glomerulonephritis and was less significant in the absence of CD100. **P< 0·005; ***P< 0·001, unpaired t-test.
Figure 3.
Immune mediators in glomeruli of mice with glomerulonephritis. (a) There were fewer macrophages and CD4+ cells in glomeruli of CD100−/− mice and (b, c) reduced glomerular deposition of both (b) immunoglobulin G (IgG) and (c) C3 in CD100−/− mice. CD100+/+ and CD100−/− mice immunized with and injected with normal sheep globulin did not develop significant glomerular leucocyte infiltrates (hatched bars). **P< 0·005; ***P< 0·0005, unpaired t-test. c/gcs, cells per glomerular cross-section.
Figure 4.
Molecular mediators of injury in mice with glomerulonephritis. CD100-deficient mice had lower urinary nitrite excretion and lower intrarenal interleukin (IL)-1β mRNA expression, but intrarenal tumour necrosis factor (TNF) mRNA expression was unchanged. The hatched bars represent values for CD100+/+ and CD100−/− mice immunized with and injected with normal sheep globulin. *P< 0·05, unpaired t-test for IL-1β and TNF; Mann–Whitney test for urinary nitrite.
Both cellular and humoral systemic immune responses in CD100−/− mice were suppressed
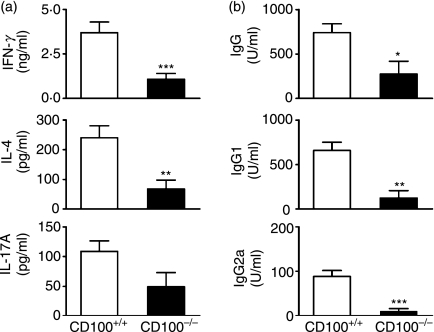
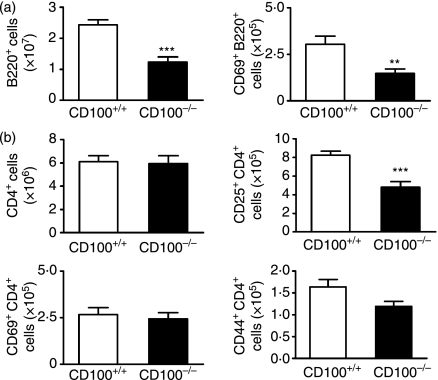
To determine the influence of CD100 in pathogenic immune responses in this model, we examined T helper (Th) cell cytokine production and serum antigen-specific IgG responses in both CD100−/− and CD100+/+ recipients. IFN-γ (Th1) and IL-4 (Th2) levels in culture supernatant from CD100−/− cells were reduced (Fig. 5a) compared with CD100+/+ mice. There was a trend towards reduced IL-17A levels (Th17; P= 0·078). Serum sheep globulin-specific IgG, IgG1 and IgG2a were decreased in CD100−/− mice (Fig. 5b). In experiment 2, additional assays were performed in mice that had been immunized and boosted with sheep globulin but that did not have GN. There were fewer B220+ cells (predominantly B cells) in the draining lymph nodes of CD100−/− mice and fewer B220+ cells expressing CD69, an activation marker (Fig. 6a). CD4+ T-cell numbers were not affected, nor were numbers of CD4+ CD69+ cells (Fig. 6b). However, there were fewer CD4+ cells expressing CD25, another activation marker, and a trend towards fewer CD4+ CD44+ T cells in the absence of CD100. These studies also showed similar reductions in serum antigen-specific IgG, IgG1, IgG2a and antigen-stimulated cytokine levels (Table 2), which confirmed observations in CD100−/− mice with GN.
Figure 5.
Reduced systemic antigen-stimulated cytokine production and serum antigen-specific antibody production in CD100−/− mice. (a) Both interferon (IFN)-γ and interleukin (IL)-4 produced in the culture supernatant of CD100−/− cells were decreased compared with CD100+/+ mice. There was a trend towards reduced IL-17A levels (P= 0·078). (b) Serum antigen-specific immunoglobulin G (IgG), IgG1 and IgG2a levels in CD100-deficient mice were lower than those in CD100-intact mice. *P= 0·03; **P< 0·005; ***P< 0·001, unpaired t-test.
Figure 6.
Cell numbers and cellular activation markers expressed on draining lymph node cells in mice immunized with sheep globulin (experiment 2). (a) Compared with CD100+/+ mice, CD100−/− mice had fewer B220+ cells and B220+ CD69+ cells. (b) Although the numbers of CD4+ and CD69+ CD4+ cells were similar in wild-type and CD100−/− splenocytes, CD4+ CD25+ cells were reduced in CD100−/− mice and a reduction in CD4+ CD44+ cells approached statistical significance (P =0·051). **P< 0·01; ***P< 0·0001, unpaired t-test.
Table 2.
Splenocyte-derived cytokines and serum immunoglobulin G (IgG) production in sheep globulin-immunized mice
| CD100+/+ mice | CD100−/− mice | |
|---|---|---|
| Splenocyte IFN-γ (pg/ml) | 42 ± 8 | 9 ± 31 |
| Splenocyte IL-4 (pg/ml) | 264 ± 29 | 17 ± 82 |
| Serum IgG (units/ml) | 1199 ± 65 | 139 ± 762 |
| Serum IgG1 (units/ml) | 1206 ± 134 | 126 ± 702 |
| Serum IgG2a (units/ml) | 2981 ± 692 | 174 ± 671 |
IFN-γ, interferon-γ; IL-4, interleukin-4.
1P< 0·005; 2P< 0·0001, unpaired t-test.
Intrarenal expression of CD100 and its receptor plexin-B1
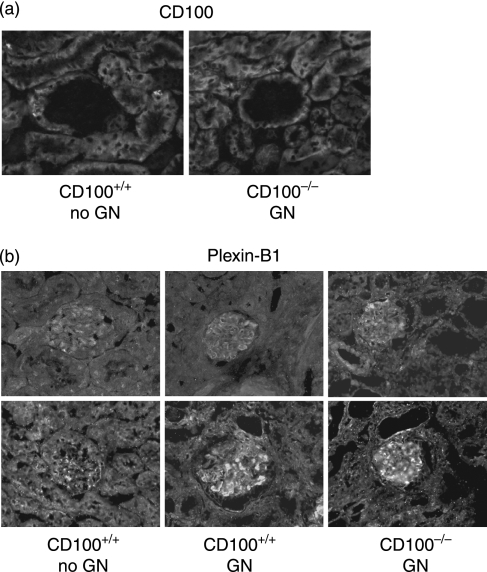
By immunofluorescent staining of mice in experiment 1, intrarenal CD100 was demonstrated on tubular cells, but not in glomeruli of normal mice without GN (Fig. 7a). In GN, the predominantly tubular pattern of staining was not altered, although in mice with GN, occasional CD100-positive cells, probably infiltrating leucocytes, were present in glomeruli of CD100+/+ mice (data not shown). CD100 mRNA was present in kidneys of normal mice and mice with GN, at similar levels [mice with GN: 0·041 ± 0·003 AU; normal mice without GN: 0·037 ± 0·003 AU]. The distribution of plexin-B1 (the tissue receptor for CD100) in the kidney is somewhat different. Plexin-B1 was observed on both tubular cells and glomerular cells (Fig. 7b). Enhanced expression of plexin-B1 was observed in some, but not all glomeruli of mice with GN (Fig. 7b). RT-PCR analysis did not demonstrate significant differences in intrarenal plexin-B1 mRNA expression between CD100+/+ mice without GN (0·025 ± 0·003 AU) and CD100+/+ mice with GN (0·033 ± 0·003 AU; P> 0·05). In mice with GN, plexin-B1 expression was similar in the presence and absence of CD100 (CD100−/− mice: 0·031 ± 0·004 AU).
Figure 7.
CD100 and plexin-B1 in kidneys. (a) Expression of CD100 was observed on tubular cells but rarely in glomeruli; (b) plexin-B1 was expressed within the tubulointerstitium and the glomerulus with increased expression in some but not all glomeruli. Magnification, ×400.
Intraglomerular plexin-B1/leucocyte CD100 interactions promote glomerular macrophage recruitment
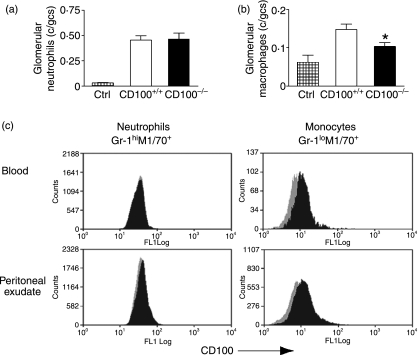
As plexin-B1 is expressed in glomeruli, and as leucocytes express CD100, it was hypothesized that interactions between intraglomerular plexin-B1 and leucocyte CD100 would facilitate glomerular leucocyte recruitment (as shown in Fig 3a). This hypothesis cannot be tested in a model of active immunity, as CD100 deficiency results in an impaired systemic immune response that would confound results. To avoid the influence of active immunity, leucocyte recruitment was assessed in passive transfer systems, as outlined in Fig. 1, experiments 3 and 4. Neutrophils are recruited in heterologous phase anti-GBM GN in naïve mice, induced by injection of sheep anti-mouse GBM antibodies (maximally at 2 hr). We have previously shown that transfer of normal sheep globulin into mice does not induce neutrophil recruitment.21 Two hours after injection of sheep anti-mouse GBM antibodies (experiment 3), there was no difference in neutrophil accumulation in glomeruli in the presence or absence of endogenous CD100 (Fig. 8a). The passive transfer of isologous mouse anti-sheep globulin antibodies (to naïve mice that have been injected with sheep anti-mouse GBM globulin) results in the recruitment of macrophages to glomeruli, a function of the different characteristics of the Fc regions of heterologous and isologous antibodies.22 After passive transfer of mouse anti-sheep globulin antibodies (experiment 4), macrophage accumulation in glomeruli of CD100−/− mice was reduced (Fig. 8b). To elucidate why macrophage, but not neutrophil recruitment to glomeruli was impaired in CD100−/− mice, CD100 expression was assessed on neutrophils and monocytes/macrophages from both peripheral blood and peritoneal exudate (4 hr after thioglycollate for neutrophils; 72 hr for macrophages). Neutrophils do not express CD100. However, blood monocytes and peritoneal macrophages do express CD100 (Fig. 8c).
Figure 8.
Passive antibody transfer studies. (a) Experiment 3: neutrophil accumulation in glomeruli was unaffected by CD100 deficiency after passive transfer of heterologous sheep anti-mouse glomerular basement membrane (GBM) globulin. (b) Experiment 4: after passive transfer of autologous mouse anti-sheep globulin antiserum, macrophages were recruited to glomeruli, and macrophage accumulation was reduced in the absence of CD100. (c) CD100 was not expressed on neutrophils (Gr1hiM1/70+ cells), but CD100 was expressed on macrophages (Gr1loM1/70+ cells) elicited from peripheral blood or the peritoneum. Grey shading represents CD100−/− cells (negative controls), and black shading cells from CD100+/+ mice. *P= 0·02, unpaired t-test. c/gcs, cells per glomerular cross-section.
Discussion
This study investigated the role of CD100 in immune responses leading to renal injury in foreign antigen-induced severe GN (also known as ‘accelerated autologous phase anti-GBM GN’). It tested the hypothesis that CD100 would enhance nephritogenic immune responses and severe crescentic renal disease. We found that, compared with CD100-intact mice, CD100−/− mice developed less severe GN, with diminished antigen-specific immune responses and glomerular inflammatory responses. There was no particular skewing of the nephritogenic immune responses away from (or towards) a Th1, Th2 or Th17 phenotype. Splenocyte production of IFN-γ and IL-4 was significantly reduced, but IL-17A was only mildly decreased in CD100−/− mice. When antigen-specific antibody production was measured, titres of both the Th1-associated IgG subclass IgG2a and the Th2-associated subclass IgG1 were found to be reduced. Therefore, in nephritogenic immune responses leading to severe crescentic GN, CD100 acts as an immunostimulatory molecule that does not selectively stimulate T helper cell differentiation down one particular pathway.
While the current studies do not further dissect the mechanism behind this immunostimulatory effect on T helper cells, our previous studies have shown that CD100 acts as a costimulatory molecule to help activate DCs which in turn stimulate T-cell proliferation and differentiation.8,9 The reduction in antigen-specific antibody responses is consistent both with effects on the T cells and with a more direct costimulatory effect of T-cell CD100 on B cells, as has been described.5 Overall, the current findings are consistent with our studies in apoferritin-induced circulating immune complex GN, where CD100 deficiency attenuated T-cell activation and T-cell-dependent antibody responses.9
Macrophage accumulation is a feature of aggressive forms of human and experimental GN. Macrophages themselves are key inflammatory effectors of injury in these conditions.23–25 In autologous phase anti-GBM GN, this recruitment can be mediated either by antibody-FcR interactions15,22,26 or by effector helper T cells.27 In the current studies, macrophages accumulated in glomeruli of CD100+/+ mice with GN. In the absence of CD100, macrophage infiltration in glomeruli was decreased. There are several potential mechanisms underpinning this reduced infiltrate. Fewer CD4+ cells infiltrated CD100-deficient glomeruli, and less IgG was deposited in glomeruli of CD100−/− mice. Both these factors are likely to have contributed to the decreased glomerular macrophage numbers observed in the absence of CD100.
The current studies also examined intrarenal expression of CD100 and plexin-B1 in normal mice and in mice with GN. In normal kidneys, CD100 was present mainly in tubular cells and not in glomeruli. In disease, CD100 mRNA expression levels did not change. Plexin-B1 was also found in the kidney, predominantly in tubules but also in glomeruli of normal and diseased mice. Given the potential for plexin-B1/leucocyte–CD100 interactions to influence cell migration and localization,2 as well as the presence of plexin-B1 in glomeruli, we tested the hypothesis that the absence of CD100 on macrophages contributed to the reduction in macrophages in glomeruli of CD100−/− mice with GN. Neutrophils do not express CD100, whereas macrophages and monocytes are CD100 positive. To remove the effects of endogenous CD100 on the induction of adaptive immune responses, we used two different transfer models where antibody was passively transferred into CD100-intact and CD100-deficient hosts. In one experiment, sheep anti-mouse GBM antibodies were transferred into mice. The presence of sheep globulin in glomeruli induces transient neutrophil recruitment,21,28 which was not altered in the absence of CD100 (neutrophils do not express CD100). When mouse IgG directed against sheep globulin is transferred after the injection of sheep anti-mouse GBM antibodies, the autologous IgG Fc region recruits macrophages. In the absence of CD100, present on macrophages, macrophage recruitment was reduced. Therefore, independent of its effects on the adaptive immune response, CD100 plays a direct role in macrophage recruitment to glomeruli.
It remains to be seen whether inhibition of CD100 is a potential therapeutic strategy in immune renal disease, especially as the role of CD100 on tubular epithelial cells remains unclear. Recent evidence suggests that interactions between CD100 and plexin-B1 may be important in branching morphogenesis in the kidney29 as well as tumour angiogenesis.30 Whether inhibition of CD100 might impair reparative processes in the tubulointerstitium or the renal microvasculature following injury remains to be determined. Measurements of soluble CD100 in sera of lupus-prone mice correlate well with autoantibody titre, raising the possibility that measuring soluble CD100 might help in monitoring the activity of inflammatory diseases.11
In summary, the current studies demonstrate that (i) endogenous CD100 promotes nephritogenic immune responses leading to severe GN, (ii) CD100 does not involve the selective enhancement of Th1, Th2 or Th17 subset differentiation, and (iii) in addition to enhancing T- and B-cell responses to induce glomerular macrophage recruitment, CD100 is involved in macrophage infiltration to glomeruli. Leucocyte semaphorins have the potential to be significant contributors to immune renal injury.
Acknowledgments
The authors thank Ms Alice Wright and Gabrielle Wilson for technical assistance with histology and Dr Paul Hutchinson for assistance with flow cytometry. Parts of this work were previously published in abstract form [Nephrology 12 (suppl. 2) A36, 2007; J Am Soc Nephrol 18:191A, 2007]. These studies were supported by a National Health and Medical Research Council of Australia Program Grant.
Disclosures
None of the authors have any conflict of interest with any of the content of this manuscript.
References
- 1.Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- 2.Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- 3.Bougeret C, Mansur IG, Dastot H, Schmid M, Mahouy G, Bensussan A, Boumsell L. Increased surface expression of a newly identified 150-kDa dimer early after human T lymphocyte activation. J Immunol. 1992;148:318–23. [PubMed] [Google Scholar]
- 4.Hall KT, Boumsell L, Schultze JL, et al. Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc Natl Acad Sci USA. 1996;93:11780–5. doi: 10.1073/pnas.93.21.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi W, Kumanogoh A, Watanabe C, et al. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity. 2000;13:633–42. doi: 10.1016/s1074-7613(00)00063-7. [DOI] [PubMed] [Google Scholar]
- 6.Kikutani H, Kumanogoh A. Semaphorins in interactions between T cells and antigen-presenting cells. Nat Rev Immunol. 2003;3:159–67. doi: 10.1038/nri1003. [DOI] [PubMed] [Google Scholar]
- 7.Kumanogoh A, Watanabe C, Lee I, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13:621–31. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- 8.Kumanogoh A, Suzuki K, Ch’ng E, et al. Requirement for the lymphocyte semaphorin, CD100, in the induction of antigen-specific T cells and the maturation of dendritic cells. J Immunol. 2002;169:1175–81. doi: 10.4049/jimmunol.169.3.1175. [DOI] [PubMed] [Google Scholar]
- 9.Li M, O’Sullivan KM, Jones LK, Semple T, Kumanogoh A, Kikutani H, Holdsworth SR, Kitching AR. CD100 enhances dendritic cell and CD4+ cell activation leading to pathogenetic humoral responses and immune complex glomerulonephritis. J Immunol. 2006;177:3406–12. doi: 10.4049/jimmunol.177.5.3406. [DOI] [PubMed] [Google Scholar]
- 10.Ishida I, Kumanogoh A, Suzuki K, Akahani S, Noda K, Kikutani H. Involvement of CD100, a lymphocyte semaphorin, in the activation of the human immune system via CD72: implications for the regulation of immune and inflammatory responses. Int Immunol. 2003;15:1027–34. doi: 10.1093/intimm/dxg098. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Kumanogoh A, Watanabe C, Shi W, Yoshida K, Kikutani H. Functional soluble CD100/Sema4D released from activated lymphocytes: possible role in normal and pathologic immune responses. Blood. 2001;97:3498–504. doi: 10.1182/blood.v97.11.3498. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe C, Kumanogoh A, Shi W, Suzuki K, Yamada S, Okabe M, Yoshida K, Kikutani H. Enhanced immune responses in transgenic mice expressing a truncated form of the lymphocyte semaphorin CD100. J Immunol. 2001;167:4321–8. doi: 10.4049/jimmunol.167.8.4321. [DOI] [PubMed] [Google Scholar]
- 13.Tamagnone L, Artigiani S, Chen H, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 14.Maestrini E, Tamagnone L, Longati P, et al. A family of transmembrane proteins with homology to the MET-hepatocyte growth factor receptor. Proc Natl Acad Sci USA. 1996;93:674–8. doi: 10.1073/pnas.93.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang XR, Kitching AR, Tipping PG, Holdsworth SR. Interleukin-10 inhibits macrophage-induced glomerular injury. J Am Soc Nephrol. 2000;11:262–9. doi: 10.1681/ASN.V112262. [DOI] [PubMed] [Google Scholar]
- 16.Kitching AR, Turner AL, Wilson GR, et al. IL-12p40 and IL-18 in crescentic glomerulonephritis: IL-12p40 is the key Th1-defining cytokine chain, whereas IL-18 promotes local inflammation and leukocyte recruitment. J Am Soc Nephrol. 2005;16:2023–33. doi: 10.1681/ASN.2004121075. [DOI] [PubMed] [Google Scholar]
- 17.Kitching AR, Turner AL, Semple T, et al. Experimental autoimmune anti-glomerular basement membrane glomerulonephritis: a protective role for IFN-gamma. J Am Soc Nephrol. 2004;15:1764–74. doi: 10.1097/01.asn.0000128968.27705.5e. [DOI] [PubMed] [Google Scholar]
- 18.Kitching AR, Tipping PG, Kurimoto M, Holdsworth SR. IL-18 has IL-12-independent effects in delayed-type hypersensitivity: studies in cell-mediated crescentic glomerulonephritis. J Immunol. 2000;165:4649–57. doi: 10.4049/jimmunol.165.8.4649. [DOI] [PubMed] [Google Scholar]
- 19.Ruth AJ, Kitching AR, Li M, Semple TJ, Timoshanko JR, Tipping PG, Holdsworth SR. An IL-12-independent role for CD40-CD154 in mediating effector responses: studies in cell-mediated glomerulonephritis and dermal delayed-type hypersensitivity. J Immunol. 2004;173:136–44. doi: 10.4049/jimmunol.173.1.136. [DOI] [PubMed] [Google Scholar]
- 20.Kitching AR, Holdsworth SR, Tipping PG. IFN-gamma mediates crescent formation and cell-mediated immune injury in murine glomerulonephritis. J Am Soc Nephrol. 1999;10:752–9. doi: 10.1681/ASN.V104752. [DOI] [PubMed] [Google Scholar]
- 21.Kuligowski MP, Kitching AR, Hickey MJ. Leukocyte recruitment to the inflamed glomerulus: a critical role for platelet-derived P-selectin in the absence of rolling. J Immunol. 2006;176:6991–9. doi: 10.4049/jimmunol.176.11.6991. [DOI] [PubMed] [Google Scholar]
- 22.Boyce NW, Holdsworth SR. Macrophage-Fc-receptor affinity: role in cellular mediation of antibody initiated glomerulonephritis. Kidney Int. 1989;36:537–44. doi: 10.1038/ki.1989.228. [DOI] [PubMed] [Google Scholar]
- 23.Huang XR, Tipping PG, Apostolopoulos J, Oettinger C, D’Souza M, Milton G, Holdsworth SR. Mechanisms of T cell-induced glomerular injury in anti-glomerular basement membrane (GBM) glomerulonephritis in rats. Clin Exp Immunol. 1997;109:134–42. doi: 10.1046/j.1365-2249.1997.4091307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikezumi Y, Hurst LA, Masaki T, Atkins RC, Nikolic-Paterson DJ. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int. 2003;63:83–95. doi: 10.1046/j.1523-1755.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- 25.Duffield JS, Tipping PG, Kipari T, Cailhier JF, Clay S, Lang R, Bonventre JV, Hughes J. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–19. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarzi RM, Davies KA, Robson MG, Fossati-Jimack L, Saito T, Walport MJ, Cook HT. Nephrotoxic nephritis is mediated by Fcgamma receptors on circulating leukocytes and not intrinsic renal cells. Kidney Int. 2002;62:2087–96. doi: 10.1046/j.1523-1755.2002.00687.x. [DOI] [PubMed] [Google Scholar]
- 27.Huang XR, Tipping PG, Shuo L, Holdsworth SR. Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int. 1997;51:94–103. doi: 10.1038/ki.1997.12. [DOI] [PubMed] [Google Scholar]
- 28.Schrijver G, Bogman MJ, Assmann KJ, de Waal RM, Robben HC, van Gasteren H, Koene RA. Anti-GBM nephritis in the mouse: role of granulocytes in the heterologous phase. Kidney Int. 1990;38:86–95. doi: 10.1038/ki.1990.171. [DOI] [PubMed] [Google Scholar]
- 29.Korostylev A, Worzfeld T, Deng S, et al. A functional role for semaphorin 4D/plexin B1 interactions in epithelial branching morphogenesis during organogenesis. Development. 2008;135:3333–43. doi: 10.1242/dev.019760. [DOI] [PubMed] [Google Scholar]
- 30.Sierra JR, Corso S, Caione L, et al. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med. 2008;205:1673–85. doi: 10.1084/jem.20072602. [DOI] [PMC free article] [PubMed] [Google Scholar]