Abstract
After encounter with Mycobacterium tuberculosis, a series of non-uniform immune responses are triggered that define the course of the infection. Eight M. tuberculosis strains were selected from a prospective population-based study of pulmonary tuberculosis patients (1995–2003) based on relevant clinical/epidemiological patterns and tested in a well-characterized BALB/c mouse model of progressive pulmonary tuberculosis. In addition, a new mouse model of transmissibility consisting of prolonged cohousing (up to 60 days) of infected and naïve animals was tested. Four phenotypes were defined based on strain virulence (mouse survival, lung bacillary load and tissue damage), immunology response (cytokine expression determined by real-time polymerase chain reaction) and transmissibility (lung bacillary loads and cutaneous delayed-type hypersensitivity in naïve animals).We identified four clearly defined strain phenotypes: (1) hypervirulent strain with non-protective immune response and highly transmissible; (2) virulent strain, associated with high expression of proinflammatory cytokines (tumour necrosis factor and interferon) and very low anti-inflammatory cytokine expression (interleukins 4 and 10), which induced accelerated death by immunopathology; (3) strain inducing efficient protective immunity with lower virulence, and (4) strain demonstrating strong and early macrophage activation (innate immunity) with delayed participation of acquired immunity (interferon expression). We were able to correlate virulent and transmissible phenotypes in the mouse model and markers of community transmission such as tuberculin reactivity among contacts, rapid progression to disease and cluster status. However, we were not able to find correlation with the other two phenotypes. Our new transmission model supported the hypothesis that among these strains increased virulence was linked to increased transmission.
Keywords: immunogenicity, immunopathology, molecular epidemiology, murine model, transmission, tuberculosis
Introduction
Tuberculosis (TB) remains a major public health problem, with almost two million deaths annually, and a third of the global population latently infected. In the developed world, TB is a prototypical re-emerging infectious disease, while in the developing nations TB is a devastating health problem.1
After encounter with Mycobacterium tuberculosis, a series of immune responses are triggered that define the course of the infection. However, this host response is not uniform in exposed people. Indeed, the vast majority of humans never develop overt disease despite being infected for many years.2 Furthermore, an array of clinical manifestations may occur at any stage of life in those patients who cannot control the infection. Although considerable information is already available about host determinants and the environmental factors that influence a particular outcome,2–4 there is a lack of systematic information on the influence of the genetic diversity of the pathogen itself that may provide important clues for basic and applied research on TB and its relation with the human host.
To explain this variability, it has been proposed that the course of the infection and its epidemiological consequences depend upon a complex interplay of factors. It is increasingly thought that the virulence and load of the infecting strain, together with host genetic factors, contribute to such differences.3,5,6 In particular, several reports have demonstrated that the severity and clinical manifestations of TB depend on differences in the immunogenicity and pathogenicity of the infecting strains of M. tuberculosis.6–10 Some of these results are derived from animal models that take advantage of a uniform immune response within the host, allowing the genetic diversity among strains to be analysed.5–9 One such model has been used extensively by our group to demonstrate major differences in the progress of infection after exposure to selected genotypes using the intratracheal route of administration.11–14 This model offers the following benefits: first, it is based on airway infection, which is the usual route in humans. Second, the rate of bacterial multiplication in the lungs correlates with the extent of tissue damage (pneumonia) and mortality. Third, the infection is controlled successfully as long as a strong T helper type 1 (Th1) cell response is sustained, which is endorsed by previous evidence on the protective role of Th1 cytokines against mycobacterial infection.11–14 Consequently, in the light of the high degree of genetic diversity observed among M. tuberculosis isolates worldwide in the last decade, it seems worthwhile to investigate differences in the pathogenicity and immunogenicity of a spectrum of bacterial genotypes from an area that provides a unique opportunity to study well-characterized mycobacterial isolates.
Another important concern in TB is the relationship between transmissibility and bacterial genotype. In fact, the relationship between the transmission dynamics of infectious diseases and the evolution of pathogenic organisms has been a matter of controversy for many years.15 The question of how the virulence of any particular pathogen is linked to its transmission is important because transmission determines the evolutionary success of pathogenic organisms. This important matter has not been studied in TB in a well-controlled system because of the lack of an experimental transmission model. Differences in transmissibility could be related to differences in bacterial virulence and the immune responses evoked by different mycobacterial strains.
We have conducted a population-based prospective study of pulmonary TB in Southern Mexico since 1995. All cases of pulmonary TB within the area have been detected and their clinical isolates have been well characterized. We have also obtained in-depth analysis of household contacts, as well as comprehensive epidemiological and clinical data, including follow-up outcomes of TB patients and their contacts. Moreover, the majority of these isolates have been fingerprinted using a number of genotyping tools.16–19
In this study, we used the BALB/c mouse model of progressive pulmonary TB by the intratracheal route to examine the course of infection in terms of strain virulence (mouse survival, lung bacillary load, histopathology) and immune responses [cytokine expression determined by real-time polymerase chain reaction (PCR)] produced by different M. tuberculosis strains selected from our clinical/epidemiological study in Orizaba, Mexico. In a second part of the study, we designed a new mouse model of transmissibility, which consists of prolonged cohousing (up to 60 days) of infected and naïve animals. The ability of these strains to be transmitted was measured using lung bacillus loads of the naïve animal and cutaneous delayed type hypersensitivity (DTH) against mycobacterial antigens as markers of disease and transmission, respectively.
We selected a panel of isolates representing clinical/epidemiological diversity in the population of M. tuberculosis strains in Mexico and tested them in both animal models. We were able to define a series of distinct bacterial phenotypes in the mouse models based on time to death, bacterial load, immunology kinetics and transmission to naïve animals. We then tried to correlate these bacterial phenotypes with simple clinical/epidemiology markers.
Materials and methods
Selection of M. tuberculosis clinical isolates
The study site, enrolment, follow-up and laboratory procedures have been described previously.16–19 Briefly, between March 1995 and May 2003, 8202 individuals with more than 2 weeks of productive cough were screened for acid-fast bacilli or M. tuberculosis in sputa; 667 pulmonary TB patients underwent epidemiological, clinical [standardized questionnaire, physical examination, chest radiograph, human immunodeficiency virus (HIV) test], mycobacteriological and molecular evaluations. Two hundred and ninety-six patients (44·3%) belonged to 75 1S6110-restriction fragment length polymorphism and spoligotype clusters: the size of clusters ranged from 2 to 20 members. Fifty per cent of clustered isolates belonged to small clusters (three or fewer isolates). Treatment was provided using the official norms of Mexico’s National Tuberculosis Control Programme in accordance with the World Health Organization (WHO) guidelines for the period in which the cases were detected. Results of treatment were classified as described previously,20 and follow-up visits were made 6 months later and then on an annual basis to monitor the results of treatment, the incidence of retreatments and patients’ vital status during the duration of the study. All deaths were confirmed by death certificates. The homes of deceased patients were visited to interview the person who had attended the person during the terminal stage and to collect data on their symptoms. The surveys were reviewed by two physicians, who determined if death was attributable to TB. Causes of death were established as reported previously.21 Of the 667 patients, 42 (6·2%) died from TB. For analysis, we only considered one TB isolate per patient. A summary of the main epidemiological and clinical characteristics of the patients with TB is shown in Table 1. More than half of patients were male, HIV infection was infrequent, one-third of patients had more severe clinical manifestations such as weight loss, haemoptysis or cavitations, approximately one-fifth of patients harboured drug resistance. Treatment was administered under the WHO recommended control strategy DOTS (Directly-Observed Treatment, Short-course), including its five components: government commitment; case detection by predominantly passive case-finding; standardized short-course chemotherapy to, at least, all confirmed sputum smear-positive cases, provided under proper case management conditions; a system of regular drug supply; and a monitoring system for programme supervision and evaluation. More than 80% of patients were cured, 6% died from TB.
Table 1.
Summary of main characteristics of patients and contacts
| Characteristic | n/total | % |
|---|---|---|
| Patients | ||
| Males | 398/667 | 59·7 |
| Age in years (mean, SD) | 44·5 | 17·7 |
| Completed elementary school | 350/664 | 52·7 |
| HIV infection | 16/632 | 2·5 |
| Fever | 435/667 | 65·2 |
| Weight loss | 497/666 | 74·6 |
| Haemoptysis | 218/662 | 32·9 |
| Cavitation in chest X-ray | 201/602 | 33·4 |
| Drug sensitivity | ||
| Pan-sensitive | 527/665 | 79·2 |
| Joint resistant to isoniazid and rifampin | 50/665 | 7·5 |
| Other resistance | 88/665 | 13·2 |
| Clinical outcomes | ||
| Cure | 472/586 | 80·6 |
| Failure | 27/586 | 4·7 |
| Drop out | 58/586 | 9·9 |
| Death | 20/586 | 3·4 |
| Transfer | 9/586 | 1·5 |
| Follow up | ||
| Death due to any cause | 65/667 | 9·7 |
| Death due to tuberculosis | 42/667 | 6·3 |
| Cluster status | ||
| Cluster | 150/667 | 22·5 |
| Unique | 502/667 | 75·3 |
| Unknown | 15/667 | 2·2 |
| Contacts | ||
| Males | 322/777 | 41·4 |
| Age (mean, SD) | 26·2 | 20·6 |
| Tuberculin skin test positive | 351/779 | 45·1 |
| Tuberculin skin positive among individuals with family relatedness with index case | 208/438 | 47·5 |
| Active tuberculosis during follow up | 6/779 | 0·8 |
SD, standard deviation.
Contact investigations
After May 2003, we also collected information from 779 household contacts with a standardized questionnaire and tuberculin skin test (TST) (Tuberculin PPD RT 23 SSI, 2 T.U./0.1 ml, Statens Serum Institut, Copenhagen, Denmark). Individuals with a negative baseline TST were retested at least once a year (minimum of 3 months after the initial test to avoid boosting) for up to 2 years after diagnosis of the index case, to detect TST conversion. Individuals were considered TST positive if they presented an induration of at least 10 mm.22 In compliance with international recommendations, a TST conversion was defined as an increase of > 10 mm in the diameter of the TST, and was considered a true biological phenomenon representing a newly acquired infection.23 Of the 779 contacts, 300 (38·5%) were tuberculin reactive. Of the 384 contacts who were initially tuberculin non-reactive and were retested at 3-monthly intervals, 51 (13·2%) converted. A summary of the main epidemiological and clinical characteristics of the household contacts is shown in Table 1. There were a larger proportion of females among this group and they were younger than the patients. The proportion of individuals who were family related to the index case and tuberculin reactive (208/438, 47·4%) was similar to the proportion of individuals who were not family related and tuberculin reactive (143/341, 41·9%), P = NS. Therefore, it is unlikely that tuberculin reactivity was attributable to genetic predisposition to TB. Six individuals developed active disease.
Selection of study strains
For the purpose of this study, we defined as follows four major groups of isolates depending on clinical and epidemiologic characteristics, molecular fingerprinting and TST results among their household contacts:
(1) Tuberculosis cases in which more than 20% of their household contacts either were TST positive at the moment of recruitment for contact investigation studies or, although their initial TST was negative, they converted during follow up. Thirty-two patients met this condition, 23 shared their M. tuberculosis strain genotype with another isolate in the collection (totalling 19 clones) and nine were unique fingerprints. Among the 23 strains, we selected one (09005186) that belonged to a cluster of recently transmitted infection overrepresented in this community (17 isolates shared the same genotype), and for comparison, we selected one strain (3004245) among the nine non-clustered strains with a high percentage of purified protein derivative reactivity in their contacts; this strain had a unique genotype and therefore was considered the result of reactivated disease. There were no deaths attributed to TB in any of these groups. We considered these cases and their isolates as ‘highly transmissible’.
(2) Tuberculosis cases in which their household contacts were always TST negative, at detection of the TB case or during follow-up. There were 122 patients that met this condition, 65 of their isolates (53·3%) were clustered with 37 different fingerprint patterns. We tested two strains: 2008920 which belonged to a cluster (with three members) and 1036226 which had a unique fingerprint. We considered these cases and their isolates as ‘poorly transmissible’.
(3) Cases with a ‘short’ period of latency: individuals living in the same household as the pulmonary TB index case who were diagnosed with active pulmonary TB within 2 years from the diagnosis of the first case, and whose M. tuberculosis isolates shared the same genotype. We found 10 cases meeting these criteria, from which we selected strains 1015312 and 1020319 from two different clusters.
(4) Cases in which we suspected reactivated disease after a ‘long’ period of latency: to meet the criteria, we chose clinical isolates that were monoresistant to streptomycin. This is a rare phenotype in this region and in other regions of the country. We found this characteristic in 14 of 779 (2%) of all isolates. We selected strain 09985449, which was the index case of a cluster of 11 members, and strain 2013094, which had a unique pattern.
The research protocols were approved by the appropriate institutional review boards of participating institutions and all patients and their contacts signed informed consent before enrolment.
Experimental model of pulmonary TB in BALB/c mice
Virulence (as determined by survival, lung pathology and bacterial load) and immune response induced by each isolate were evaluated in 6- to 8-week-old male BALB/c mice as previously described.7,11–14,24 Briefly, to induce progressive pulmonary TB, mice were anaesthetized with sevoflurane and inoculated intratracheally with 2·5 × 105 bacilli in 100 μl phosphate-buffered saline (PBS).12,13 Infected mice were kept in a vertical position until the effect of anaesthesia had passed. Bacteria were grown in Middlebrook 7H9 broth (Difco, Detroit, MI) enriched with glycerol and albumin, catalase and dextrose (Becton Dickinson. Cockysville, MD), and incubated with constant agitation at 37° and 5% CO2 for 21 days. Growth was monitored by densitometry. As soon as the culture reached stationary phase (optical density at 600 nm = 1), the bacilli were harvested, and the concentration was adjusted to 2·5 × 105 viable bacilli per millilitre of PBS as determined by diacetate of fluorescein (DAF) incorporation,24 and 100-μl were aliquots frozen at −70° until use.
Two experiments were performed: in each experiment nine groups of 70 mice were infected with the eight different M. tuberculosis clinical isolates plus H37Rv controls; 20 mice from each group were left undisturbed to record survival from day 8 up to day 120 after infection. Six animals from each group were killed by exsanguination at 1, 3, 7, 14, 21, 28, 60 and 120 days after infection. One lung lobe, right or left, was perfused with 10% formaldehyde dissolved in PBS and prepared for histopathological studies. The other lobe was snap-frozen in liquid nitrogen then stored at −70° for microbiological and immunological analysis.12,13 All procedures were performed in a class III cabinet in a biosafety level III facility. Infected mice were kept in cages fitted with microisolators connected to negative pressure.
Progression of disease in our model has been previously described.12 Briefly, after inoculation with the laboratory strain H37Rv, an initial immune response dominated by high production of Th1 cell cytokines and tumour necrosis factor-α (TNF-α) is induced and temporarily controls the infection. Granulomas develop during this phase. After the third week of infection, there is a drop in interferon-γ (IFN-γ) expression with high expression of Th2 cytokines. Gradually, pneumonic areas prevail over granulomas. Pneumonia, in coexistence with a high burden of bacteria, causes death. Animal work was performed in accordance with the National Regulations on Animal Care and Experimentation.
Preparation of lung tissue for histology and automated morphometry
One lobe of the lung was fixed by intratracheal perfusion with 10% formaldehyde for 24 hr, then sectioned through the hilus and embedded in paraffin. Sections, 5 μm thick, were stained with haematoxylin & eosin for the histological/morphometric analysis. The percentage of the pulmonary area affected by pneumonia was determinate using an automated image analyser (Q Win Leica, Milton Keynes, UK).12,13
Determination of colony-forming units in infected lungs
Right or left lungs from four mice at each time-point, in two different experiments, were used for colony counting. Lungs were homogenized with a Polytron (Kinematica, Luzern, Switzerland) in sterile 50-ml tubes containing 3 ml isotonic saline. Four dilutions of each homogenate were spread onto duplicate plates containing Bacto Middlebrook 7H10 agar (Difco Lab code 0627-17-4; Difco) enriched with oleic acid, albumin, catalase and dextrose. The time for incubation and colony counting was 21 days.14
Real-time PCR analysis of cytokines in lung homogenates
Left or right lung lobes from three different mice per group in two different experiments were used to isolate messenger RNA (mRNA) using the RNeasy Mini Kit (QIAGEN Mexico, Colima, México), according to the recommendations of the manufacturer. Quality and quantity of RNA were evaluated through spectrophotometry (260/280) and on agarose gels. Reverse transcription of the mRNA was performed using 5 μg RNA, oligo-dT and the Omniscript kit (QIAGEN Mexico). Real-time PCR was performed using the 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA) and Quantitect SYBR Green Mastermix kit (Qiagen). Standard curves of quantified and diluted PCR product, as well as negative controls, were included in each PCR run. Specific primers were designed using the program Primer Express (Applied Biosystems) for the following targets: glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 5′-CATTGTGGAAGGGCTCATGA-3′, 5′-GGAAGGCCATGCCAGTGAGC-3′; TNF-α: 5′-TGTGGCTTCGACCTCTACCTC-3′, 5′-GCCGAGAAAGGCTGCTTG-3′; IFN-γ: 5′-GGTGACATGAAAATCCTGCAG-3′, 5′-CCTCAAACTTGGCAATACTCATGA-3′; and interleukin-4 (IL-4): 5′-CGTCCTCACAGCAACGGAGA-3′, 5′-GCAGCTTATCGATGAATCCAGG-3′. Cycling conditions used were: initial denaturation at 95° for 15 min, followed by 40 cycles at 95° for 20 seconds, 60° for 20 seconds, 72° for 34 seconds. Quantities of the specific mRNA in the sample were measured according to the corresponding gene-specific standard. The mRNA copy number of each cytokine was related to one million copies of mRNA encoding the GAPDH gene.14 H37Rv was used as a control for all experiments in our BALB/c model.
Experimental model of M. tuberculosis transmissibility in BALB/c mice
To evaluate the transmissibility of each selected clinical isolate, we tried to reproduce the close-contact living conditions between TB patients and their household contacts. Five BALB/c mice, all infected with one of the selected strains, were kept in the same microisolator cage from the first day of infection with another five healthy non-infected BALB/c mice (healthy contacts). To determine if these animals were infected by their close and long-term contact with the tuberculous mice, we killed them after 1 and 2 months of cohousing with the infected mice and determined lung colony-forming units (CFU) as described above, as well as DTH against whole mycobacterial antigens from culture filtrate harvested from M. tuberculosis H37Rv grown as described above for 4–5 weeks. Culture filtrate protein antigens were obtained by precipitation with 45% (w/v) ammonium sulphate, washed and redissolved in PBS. For DTH measurement, each mouse received an injection of 20 μg antigen in 40 μl PBS into the hind footpad. The footpad was measured with a precision micrometer before and 24 hr after the antigen injection, as previously described.14
Statistical analysis
Statistical analysis of survival curves was performed using Kaplan–Meier plots and Long Rank tests. Student’s t-test was used to determine statistical significance of CFU, histopathology and cytokine expression. P< 0·05 was considered significant.
Results
The eight strains of M. tuberculosis representing the groups defined in the Materials and methods section were used to infect mice by the intratracheal route in both virulence and transmissibility models. Based on the results from these models, we were able to classify these clinical isolates into four groups based on immunopathology.
Phenotype 1: strain that does not induce a protective immune response and shows increased virulence and transmission
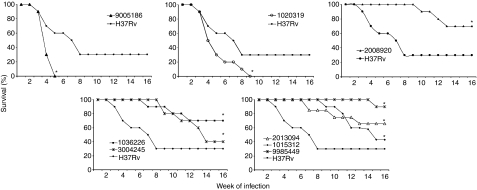
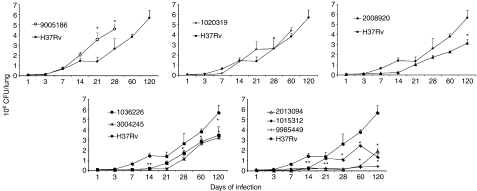
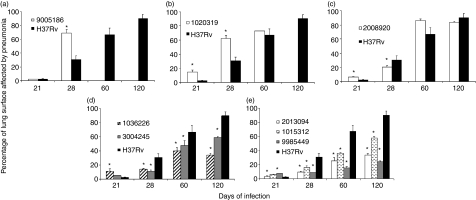
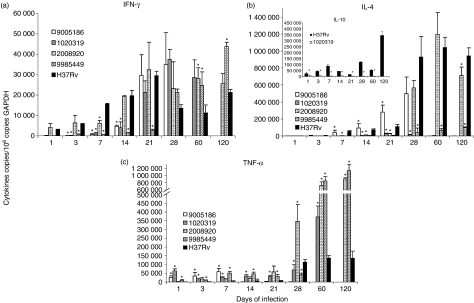
The strain which clearly represents this category is 9005186. BALB/c mice infected with this clinical isolate started to die after 3 weeks, and by the 5th week all the animals had died (Fig. 1a). This strain grew rapidly in the lung, peaking at day 21 with a CFU double that of the control strain H37Rv (Fig. 2a), and produced significantly more pneumonic areas at day 28 than H37Rv (Fig. 3a). In comparison with mice infected with H37Rv, in mice infected with strain 9005186, the induction of IFN-γ expression was delayed, but there was rapid and high IL-4 expression (Fig. 4a,b). Interestingly, this strain also induced high but transient TNF-α expression (Fig. 4c). Overall, these results indicate that this strain is more virulent and does not induce a protective immune response in our animal model.
Figure 1.
Survival rates produced by selected Mycobacterium tuberculosis clinical isolates. Twenty mice were infected with the indicated M. tuberculosis strain by the intratracheal route, and spontaneous death was recorded along the infection comparing with control animals infected with the laboratory strain H37Rv. *Statistically significant (P< 0·05), when compared with mice infected with H37Rv strain analysed by Kaplan–Meier test.
Figure 2.
Kinetics of lung bacillary loads along the infection. BALB/c mice were infected through the intratracheal route with the indicated Mycobacterium tuberculosis clinical isolate and groups of four animals were killed at different time-points after infection, their lungs were removed and used to determine bacillary loads by counting colony forming units (CFU), animals infected with laboratory strain H37Rv were used as controls. Data are presented as mean and standard deviation at each time-point. *Statistically significant (P< 0·05) when compared with mice infected with H37Rv strain.
Figure 3.
Percentage of lung surface affected by pneumonia. Lung histology sections from four animals per time-point infected with the indicated strain were used to determine the percentage of lung surface affected by pneumonia using automated morphometry. Data are presented as mean and standard deviation. *Statistically significant (P< 0·05) when compared with mice infected with H37Rv strain.
Figure 4.
Gene expression of cytokines determined by real-time reverse transcription–polymerase chain reaction in the infected lungs. BALB/c mice were infected with the indicated Mycobacterium tuberculosis strain and killed at different time-points. The lungs from four different animals at each time-point were used to determine the gene expression of interferon-γ (IFN-γ), interleukin-4 (IL-4) and tumour necrosis factor-α (TNF-α). In comparison with control strain H37Rv (black bars), clinical isolate 9005186 (white bars) induced delayed IFN-γ expression (a), rapid and higher IL-4 expression (b), and higher but ephemeral TNF-α expression (c). After the first month of infection, clinical isolate 1020319 (diagonal lines) induced higher expression of IFN-γ (a), with very low IL-4 and IL-10 expression (b) and constantly higher TNF-α (c). Strain 2008920 (white squares) induced higher IFN-γ expression during the first week and later infection (a), lower IL-4 (b) and higher TNF-α expression (c) during late disease. Strain 9985449 (hatched bars) induced lower IFN-γ expression during early disease, followed by very high and sustained IFN-γ; lower IL-4 during late disease and higher TNF-α expression particularly during the first month of infection. Data are the means and standard deviation. *Statistically significant (P < 0·05) when compared with mice infected with H37Rv strain.
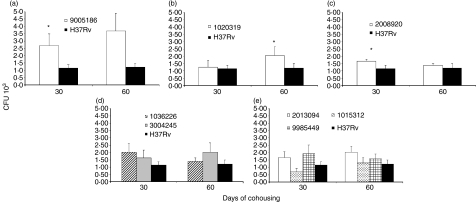
Our transmissibility study based on cohousing of infected and healthy mice showed that this strain was highly transmissible, as demonstrated by the fact that the lungs from contact mice showed threefold more CFU than the contacts of H37Rv-infected animals after 1 month, and fourfold more CFU after 2 months (Fig. 5a). There were also significantly higher DTH responses at 30 days (data not shown). These results were in agreement with the clinical/epidemiological information, which showed that this isolate was part of a big cluster that was over-represented in the community and that was highly transmissible as indicated by a high percentage of purified protein derivative conversion in household contacts.
Figure 5.
Pulmonary bacillary loads in animals with natural infection after cohousing with infected mice (transmissibility model). Groups of five infected mice with the indicated strain were cohoused with five naïve mice for 1 or 2 months, after which they were killed and their lungs were cultured to determine bacterial colony forming units (CFU). Data are means and standard deviation. *Statistically significant (P < 0·05) when compared with the control group.
Phenotype 2: strain that induces a low anti-inflammatory cytokine response and shows increased virulence
Mice infected with strain 1020319 showed 100% mortality after 9 weeks postinfection (Fig. 1b), more pneumonia than H37Rv-infected mice (Fig. 3b), but similar CFUs (Fig. 2b). In comparison with animals infected with H37Rv, this strain induced higher expression of IFN-γ during late stages of disease, higher TNF-α levels throughout, and very low IL-4 and IL-10 expression (Fig. 4a–c).
Healthy contact animals cohoused with mice infected with this 1020319 strain showed similar lung CFU (Fig. 5b), but a significantly higher DTH response after 1 month of cohabitation, when compared with contact mice that lived together with H37Rv-infected animals, although the difference in DTH was gone by 2 months (data not shown). By our clinical/epidemiological criteria, strain 1020319 was one of the short latency strains, which was part of a cluster and provoked active disease in a household contact within the first 2 years after diagnosis of the index case.
Phenotype 3: strain that induces a strong protective immune response early during infection and shows less virulence
Animals infected with strain 2008920 survived better than H37Rv-infected animals, with 70% survival compared with 30% survival at 16 weeks postinfection (Fig. 1c). This was coincident with lower CFUs and less lung surface affected by pneumonia at 4 months postinfection (Figs 2c and 3c). The study of cytokine expression in infected lungs showed that this strain induced higher expression of IFN-γ during the first week in comparison with H37Rv, and this was maintained during progressive disease (Fig. 4a). Interleukin-4 was expressed at low levels (Fig. 4b), whereas expression of TNF-α was significantly higher during early and late disease (Fig. 4c). It would seem that strain 2008920 is less virulent and stimulates an effective, protective cellular immune response. The bacillus loads in the lungs of contact animals were similar to those produced by H37Rv-infected mice (Fig. 5c), but the induced DTH was significantly higher after 1 month (data not shown). The clinical/epidemiological picture of these strains was one of ‘poorly transmissible’, with most of the household contacts of the TB patient who harboured strain 2008920 remaining skin test negative, although the strain belonged to a small cluster of three members.
Phenotype 4: strains that eventually induced a protective acquired immune response, and were less virulent
Several strains showed this pattern. For example, 9985449, which was classified as a ‘long’ latency isolate from the clinical/epidemiological data. Animals infected with this strain showed an increased survival rate of 90% after 16 weeks of infection (Fig. 1e), with lung bacterial loads and pneumonic areas less than those seen in H37Rv-infected mice at all time-points (Figs 2e and 3e). During early infection (first month) and in comparison with H37Rv, this strain induced lower levels of IFN-γ, followed by very high and sustained IFN-γ. However, there was very high TNF-α expression from the first day of infection with significantly lower IL-4 expression (Fig. 4). Strains 1015312, 1036226, 3004245 and 2013094 produced a similar mouse phenotype (Figs 1–3).
In the transmission experiments, healthy contact animals cohoused with mice infected with these strains showed similar lung CFU to contacts of H37Rv-infected animals (Fig. 5d,e). Similar DTH responses were also seen after 1 month of cohousing (data not shown). Of the five strains that demonstrated this phenotype, strains 1036226 and 2013094 were recovered from patients whose household contacts were mostly tuberculin negative; both strains had unique fingerprints. Therefore, the clinical/epidemiological picture for these strains fitted well with the murine phenotype. The other three strains did not match because they produced rapid skin test conversion among household contacts (1015312), monoresistance to streptomycin but belonged to a cluster of 11 members (09985449) and high frequency of tuberculin reactivity among household contacts (3004245).
Discussion
In this study, we took advantage of our population-based study,17,19,25 to select a panel of isolates representing clinical/epidemiological diversity, and tested them in two animal models. The first model has been previously characterized by our group,7,11–14,24 and is based on time to death, bacterial load, tissue damage and immunology kinetics. The second model is new and additionally considers bacterial pulmonary load and delayed tuberculin reactivity among healthy mice that are cohoused with infected animals. Based upon these markers, we were able to identify four clearly defined strain phenotypes in the mouse models. However, there was no absolute correlation with the clinical/epidemiological criteria. We found that rapid death, higher bacterial loads, more tissue damage, immunological responses consistent with a Th2 response and transmission of infection to contact animals (phenotypes 1 and 2) correlated with indicators of transmission in the community, such as size of cluster and tuberculin reactivity (strain 09005186) or rapid progression to disease (1020319) among household contacts. However, when we analysed the mouse-model phenotypes (3 and 4) characterized by slower time to death, lower bacterial loads, less tissue damage, cytokines indicating a Th1 response and absence or very little transmission to contacts, only two of six tested strains showed consistent patterns, These strains showed reduced transmission in the community (very few tuberculin-positive household contacts and unique fingerprint patterns, strains 1036226 and 2013094).
Our transmissibility model tries to reproduce the close and prolonged contact between patients with TB and household contacts using microisolator cages where infected animals and naive mice lived together during 2 months. Lung CFUs demonstrated that this close contact efficiently permitted the natural infection of naïve animals, and also induced activation of cellular immunity, as was demonstrated by the DTH responses. Linkage between high transmissibility and high virulence governs the evolution of a wide range of infectious organisms,26 particularly when the virulence is associated with pathogen rate of replication and transmission.27 Animals that were exposed to the more virulent strain 09005186 developed the hallmark of progressive disease (pneumonic patches) after 2 months of cohousing, whereas the other low or intermediate virulence strains only induced small granulomas and chronic inflammatory infiltrates (data not shown). Transmission of bacilli from infected to naïve contact mice probably resulted from coprophagia because only faeces produced positive cultures.
The first pattern was represented by a more virulent, highly transmissible organism (09005186) with lower and delayed expression of IFN-γ and more rapid and higher IL-4 expression and ephemeral TNF-α production. Both IFN-γ, leading to macrophage activation,28 and the emergence of a Th2 response counteracting the protective Th1 response through IL-4 expression correlate with the severity of human and murine disease.29,30 Interestingly, 30 days after infection there were high bacillary loads despite high IFN-γ expression, suggesting involvement of other protective mechanisms. The clinical/epidemiological profile of strain 09005186 fits these criteria well because it formed a large clone overrepresented in the community, and produced higher tuberculin reactivity among household contacts.
The second pattern was represented by strain 1020319, which was a more virulent strain with higher IFN-γ and TNF-α expression associated with very low IL-4 and IL-10 expression during the progressive phase of the disease (after 1 month of infection) resulting in significantly higher lung inflammation and death. High expression of IFN-γ has been observed in human TB, while experimental lymphocytic lung infiltration, associated with specific mycobacterial genes,27 has been correlated with immunopathology rather than protection. Consequently, very low production of immunomodulatory cytokines together with high production of proinflammatory cytokines induced death by immunopathology. This clinical isolate showed similar lung bacillus counts to H37Rv in the transmissibility model but higher DTH, indicating strong inflammatory responses. From the epidemiological point of view, strain 102319 was classified as a member of the ‘short latency’ group; it was part of a cluster and produced active disease in a household contact suggesting that this strain was efficient in producing active disease.
The third pattern was related to a less virulent strain (2008920) which induced an early and vigorous protective immune response resulting in lower mortality, decreased bacillus loads and less tissue damage. This strain also induced rapid and higher IFN-γ expression with lower IL-4 production, whereas during late disease IFN-γ expression was maintained at high levels with significantly higher expression of TNF-γ, and mild expression of IL-4 resulting in efficient and rapid protective cellular immune response, without excessive lung consolidation. Transmissibility experiments showed higher DTH after 1 month. This strain is similar to isolate CDC 1551, which also induced strong Th1 responses in mice and is not hypervirulent.5 We were unable to correlate this mouse phenotype with the clinical epidemiological data.
The fourth pattern was related to less virulent strains with delayed acquired protective immune responses. Five clinical isolates showed these characteristics (strains 2013094, 1015312, 3004245, 09985449 and 1036226). These clinical isolates induced higher IFN-γ expression after 1 month of infection, contrasting with progressive disease and the decline of this Th1 cytokine in controls. Delayed activation of a Th1 response together with early higher TNF-α expression suggested that innate immunity could be a significant factor for efficient control of early infection. Contrasting with previous observations,31 beta-defensins were not involved in the early efficient control of bacillus growth (data not shown). These clinical isolates showed transmissibility that was similar to (strains 3004245, 2013094, 1015312) or lower than (strain 2013094) that of H37Rv in the murine model. When the clinical and epidemiological characteristics were reviewed only strains 1036226 and 2013094 showed reduced transmission in the community.
It is important to emphasize that all of these strains had similar in vitro phenotypes (cord formation, growth curves, response to exposure to hydrogen peroxide, data not shown). Moreover, our data suggest that the current use of H37Rv as the standard for animal models may be flawed because there were important differences in pathology caused by H37Rv.
Our results warrant further studies using subtractive genomics, transcriptome hybridization and comparative proteomics to better identify the extent to which the genetic and genomic diversity of M. tuberculosis is correlated with the quality of the host immune response.
Acknowledgments
We thank the population, patients and health-care workers of the Orizaba Health Jurisdiction, Mexico, for their generous support and cooperation; Dr Carmen Soler for performing the HIV tests, Drs Manuel Tielve, Ruben Acevedo and Luis Felipe Alva for chest radiograph interpretation, and the personnel of the Orizaba health jurisdiction who supported the study among patients. This study was supported by the Mexican Secretariat of Health, the National Institutes of Health of the United States (A135969 and K01TW000001), Wellcome Trust (176W009), Howard Hughes Medical Institute (55000632) and by the Mexican Council of Science and Technology (G26264M, 30987-M, SALUD-2003-C01-132-A-1). Ms Brenda Marquina received a doctoral scholarship from the Posgrado en Ciencias Biomédicas, Fac. Medicina, Universidad Nacional Autonoma de Mexico. Funding agencies did not participate in the study design, nor did they participate in the decision to submit the paper for publication.
References
- 1.Bloom BR, Murray CJ. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–64. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 2.Vynnycky E, Fine PE. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;119:183–201. doi: 10.1017/s0950268897007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutacker MM, Smoot JC, Migliaccio CA, et al. Genome-wide analysis of synonymous single nucleotide polymorphisms in Mycobacterium tuberculosis complex organisms: resolution of genetic relationships among closely related microbial strains. Genetics. 2002;162:1533–43. doi: 10.1093/genetics/162.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nardell EA. Environmental control of tuberculosis. Med Clin North Am. 1993;77:1315–34. doi: 10.1016/s0025-7125(16)30196-1. [DOI] [PubMed] [Google Scholar]
- 5.Manca C, Tsenova L, Barry CE, 3rd, et al. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J Immunol. 1999;162:6740–6. [PubMed] [Google Scholar]
- 6.Manca C, Tsenova L, Bergtold A, et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proc Natl Acad Sci USA. 2001;98:5752–7. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dormans J, Burger M, Aguilar D, Hernandez-Pando R, Kremer K, Roholl P, Arend SM, Van Soolingen D. Correlation of virulence, lung pathology, bacterial load and delayed type hypersensitivity responses after infection with different Mycobacterium tuberculosis genotypes in a BALB/c mouse model. Clin Exp Immunol. 2004;137:460–8. doi: 10.1111/j.1365-2249.2004.02551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleischmann RD, Alland D, Eisen JA, et al. Whole-genome comparison of Mycobacterium tuberculosis clinical and laboratory strains. J Bacteriol. 2002;184:5479–90. doi: 10.1128/JB.184.19.5479-5490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez B, Aguilar D, Orozco H, et al. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol. 2003;133:30–7. doi: 10.1046/j.1365-2249.2003.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malik AN, Godfrey-Faussett P. Effects of genetic variability of Mycobacterium tuberculosis strains on the presentation of disease. Lancet Infect Dis. 2005;5:174–83. doi: 10.1016/S1473-3099(05)01310-1. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez-Pando R, Orozco H, Arriaga K, Sampieri A, Larriva-Sahd J, Madrid-Marina V. Analysis of the local kinetics and localization of interleukin-1 alpha, tumour necrosis factor-alpha and transforming growth factor-beta, during the course of experimental pulmonary tuberculosis. Immunology. 1997;90:607–17. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez-Pando R, Orozcoe H, Sampieri A, Pavon L, Velasquillo C, Larriva-Sahd J, Alcocer JM, Madrid MV. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Pando R, Pavon L, Arriaga K, Orozco H, Madrid-Marina V, Rook G. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect Immun. 1997;65:3317–27. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Infante E, Aguilar LD, Gicquel B, Pando RH. Immunogenicity and protective efficacy of the Mycobacterium tuberculosis fadD26 mutant. Clin Exp Immunol. 2005;141:21–8. doi: 10.1111/j.1365-2249.2005.02832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipsitch M, Moxon ER. Virulence and transmissibility of pathogens: what is the relationship? Trends Microbiol. 1997;5:31–7. doi: 10.1016/S0966-842X(97)81772-6. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Garcia M, Palacios-Martinez M, Ponce-de-Leon A, et al. The role of core groups in transmitting Mycobacterium tuberculosis in a high prevalence community in Southern Mexico. Int J Tuberc Lung Dis. 2000;4:12–7. [PubMed] [Google Scholar]
- 17.Garcia-Garcia ML, Jimenez-Corona ME, Ponce-de-Leon A, et al. Mycobacterium tuberculosis drug resistance in a suburban community in southern Mexico. Int J Tuberc Lung Dis. 2000;4:S168–70. [PubMed] [Google Scholar]
- 18.Garcia-Garcia ML, Small PM, Garcia-Sancho C, et al. Tuberculosis epidemiology and control in Veracruz, Mexico. Int J Epidemiol. 1999;28:135–40. doi: 10.1093/ije/28.1.135. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez-Corona ME, Garcia-Garcia L, DeRiemer K, et al. Gender differentials of pulmonary tuberculosis transmission and reactivation in an endemic area. Thorax. 2006;61:348–53. doi: 10.1136/thx.2005.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeRiemer K, Garcia-Garcia L, Bobadilla-del-Valle M, Palacios-Martinez M, Martinez-Gamboa A, Small PM, Sifuentes-Osornio J, Ponce-de-Leon A. Does DOTS work in populations with drug-resistant tuberculosis? Lancet. 2005;365:1239–45. doi: 10.1016/S0140-6736(05)74812-1. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Garcia Mde L, Ponce-De-Leon A, Garcia-Sancho MC, et al. Tuberculosis-related deaths within a well-functioning DOTS control program. Emerg Infect Dis. 2002;8:1327–33. doi: 10.3201/eid0811.020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Secretaria de Salud . Norma Oficial Mexicana NOM-006-ssa2-1993, para la prevención y control de la tuberculosis en la atención primaria a la salud. Río Amazonas, México: Diario Oficial de la Federación; 1995. pp. 20–9. [Google Scholar]
- 23.Anonymous Screening for tuberculosis and tuberculosis infection in high-risk populations. Recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995;44:19–34. [PubMed] [Google Scholar]
- 24.Jarnagin JL, Luchsinger DW. The use of fluorescein diacetate and ethidium bromide as a stain for evaluating viability of mycobacteria. Stain Technol. 1980;55:253–8. doi: 10.3109/10520298009067249. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Garcia ML, Ponce de Leon A, Jimenez-Corona ME, et al. Clinical consequences and transmissibility of drug-resistant tuberculosis in southern Mexico. Arch Intern Med. 2000;160:630–6. doi: 10.1001/archinte.160.5.630. [DOI] [PubMed] [Google Scholar]
- 26.Ewald P. Evolution of Infectious Disease. New York: Oxford University Press; 1994. [Google Scholar]
- 27.Kaushal D, Schroeder BG, Tyagi S, et al. Reduced immunopathology and mortality despite tissue persistence in a Mycobacterium tuberculosis mutant lacking alternative sigma factor, SigH. Proc Natl Acad Sci USA. 2002;99:8330–5. doi: 10.1073/pnas.102055799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ottenhoff TH, Verreck FA, Hoeve MA, Van de Vosse E. Control of human host immunity to mycobacteria. Tuberculosis (Edinb) 2005;85:53–64. doi: 10.1016/j.tube.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Seah GT, Scott GM, Rook GA. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis. 2000;181:385–9. doi: 10.1086/315200. [DOI] [PubMed] [Google Scholar]
- 30.van Crevel R, Karyadi E, Preyers F, Leenders M, Kullberg BJ, Nelwan RH, van der Meer JW. Increased production of interleukin 4 by CD4+ and CD8+ T cells from patients with tuberculosis is related to the presence of pulmonary cavities. J Infect Dis. 2000;181:1194–7. doi: 10.1086/315325. [DOI] [PubMed] [Google Scholar]
- 31.Rivas-Santiago B, Sada E, Tsutsumi V, Aguilar-Leon D, Contreras JL, Hernandez-Pando R. Beta-defensin gene expression during the course of experimental tuberculosis infection. J Infect Dis. 2006;194:697–701. doi: 10.1086/506454. [DOI] [PubMed] [Google Scholar]