Abstract
Pancreatic cancer is the fourth leading cause of cancer related death in the United States. Despite numerous efforts in developing new therapies, the prognosis for patients with pancreatic cancer remains poor. Mouse models for spontaneous pancreatic cancer represent an ideal system to develop immunotherapeutic approaches. The aim of this study was to identify new tumour antigens in a murine model that mimics human disease closely, and to verify the results in patients with pancreatic cancer. We analysed a murine pancreatic complementary DNA expression library with serum from tumour-bearing mice, which led to the identification and isolation of several antigens. One of the antigens repeatedly identified in this screening was Tankyrase-2. Here, we show Tankyrase-2 as an antigen eliciting humoral responses not only in mice with established tumours, but also in mice with pre-malignant lesions. Finally, antibody responses to Tankyrase-2 were found in the serum of patients with pancreatic cancer. Reverse transcriptase–polymerase chain reaction analysis showed Tankyrase-2 expression in human pancreatic tumour. These findings show the relevance of spontaneous murine tumour models for the identification of human tumour antigens.
Keywords: antibody, immunotherapy, pancreatic cancer, Tankyrase-2, tumour antigen
Introduction
Pancreatic cancer is a highly aggressive disease and the fourth leading cause of cancer-related mortality in the United Sates.1 Clinical manifestations develop late and in most cases the disease is far advanced at the time of diagnosis with a mean life expectancy of 3–6 months. Surgery is the most effective treatment, but only 15–20% of patients present with surgically resectable tumours.2 In addition, chemotherapy and radiotherapy offer limited benefit.3
Immunotherapy has been suggested as a potential new therapeutic option for patients with pancreatic cancer.4 The identification of tumour-associated antigens recognized by cellular and humoral effectors of the immune system has provided new perspectives for tumour therapy. A variety of tumour-associated antigens have been identified, including: cancer-testis (CT) antigens, differentiation antigens, viral antigens, mutated antigens and the products of over-expressed genes.5 Of all the antigens identified, the CT antigens are the most attractive targets for vaccination in tumour immunotherapy with the potential to eradicate residual tumour cells at multiple sites in the body. In vitro and in vivo experiments have demonstrated that some CT antigens, such as NY-ESO-1, are capable of provoking potent T-cell-mediated immunity to interfere with the growth and propagation of tumour cells.6–8
We have previously shown that C57BL/6 EL-TGF-α × Trp53−/− mice develop spontaneous ductal pancreatic carcinoma with pathomorphological features close to the human disease, while C57BL/6 EL-TGF-α × Trp53+/− mice develop pre-malignant lesions.9,10 We have also demonstrated differences in tumour-specific immune responses in mice with spontaneous tumours and mice upon subcutaneous injection of pancreatic tumour cells derived from these spontaneous tumours.11 Tumour-specific antibody responses were clearly detectable in mice with spontaneous tumours as well as in mice challenged with pancreatic tumour cell lines. Therefore, we decided to determine the specificity of anti-tumour immune responses in these mice and to compare them with tumour-specific immune responses from pancreatic cancer patients. This would further verify the clinical relevance of our murine tumour model.
In this study, we describe the identification of a new tumour antigen for pancreatic adenocarcinoma. Screening of a recombinant complementary DNA (cDNA) library with serum of spontaneous pancreatic tumour-bearing mice revealed an antibody response in these mice against Tankyrase-2, a member of the poly(ADP-ribose) polymerases (PARP) gene family. Further analysis demonstrated that this immune response is not restricted to mice with already established tumours, but could also be detected in mice with pre-malignant lesions. Most importantly, we could demonstrate a significant antibody response to Tankyrase-2 in the serum of patients with pancreatic adenocarcinoma.
Materials and methods
Mice
Transgenic EL-TGF-α × p53−/− and EL-TGF-α × p53+/− mice have been described previously.9,10,12 All experiments were conducted according to institutional guidelines.
RNA extraction and construction of cDNA library
Total RNA was extracted from murine pancreatic tumor cell line (mPAC)11 tumour cells using the RNeasy RNA isolation kit (Qiagen, Hilden, Germany). Poly(A+)-RNA was purified with an Oligotex mRNA kit (Qiagen) and a cDNA library was constructed and cloned into the ZAP Express vector (Uni-ZAP XR Vector) using ZAP-cDNA Gigapack III Cloning kit (Stratagene, La Jolla, CA). The cDNA fragments were packaged into a λ-ZAP express vector and then transfected into Escherichia coli (XL1-Blue MRF’ strain).
Real-time polymerase chain reaction analysis of murine tumour tissue
RNA was isolated from formalin-fixed, paraffin-embedded specimens as previously described.13 To obtain highly enriched fractions of normal and tumour tissue, unstained sections were manually microdissected using a stained serial section as guidance. The cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad, Munich, Germany). The polymerase chain reaction (PCR) was performed with SybrGreen (Bio-Rad) using the LightCycler 480 Real-Time PCR Instrument (Roche Applied Science, Mannheim, Germany). The following primers were used: human Tankyrase2 forward 5′-ACC CAA GGC AGA CAT TCA AC-3′; reverse 5′-CAT TCA CAT CAG CTC CGT GT-3′. Results are shown as relative expression in comparison to cyclophylin A.
Immunoscreening
A total of 2 × 106 recombinant clones were screened by serological analysis of a recombinant cDNA expression library with autologous serum (SEREX) as previously described.14,15 Serum was isolated from mice after injection of irradiated mPAC cells.11 Pre-adsorption of the serum was performed by passing the serum through Escherichia coli lytically infected with λ-phages. The pre-adsorbed serum was then further diluted at a 1 : 100 and used for screening. Briefly, the phage library-infected E. coli was plated on Luria–Bertani agar and expression of recombinant proteins was induced with isopropyl β-d-thiogalactoside. After overnight incubation, proteins were transferred to nitrocellulose membranes, which were washed with Tris-buffered saline plus 0·5% Tween-20 (TBS-T), blocked with 5% low-fat dried milk in TBS and then incubated with pre-adsorbed autologous serum overnight at room temperature. The next day, membranes were incubated with an anti-murine alkaline phosphatase-conjugated immunoglobulin G (IgG-AP) secondary antibody (Sigma, Seelze, Germany) and developed with BCIP/NBT substrate (BIOMOL, Exeter, UK) to identify positive clones. Positive clones were subcloned into pBluescript SK-phagemids by in vivo excision. The cDNA insert fragments were sequenced by T7 and T3 primers (GATC Biotech AG, Konstanz, Germany).
Generation and purification of recombinant Tankyrase-2 protein
Both mouse and human Tankyrase-2 cDNAs were cloned into the prokaryotic expression vector QE (Qiagen) with a histidine tag at their amino termini, expressed in E. coli and purified over a nickel column as previously described.16 The purified proteins were detected in a Western blot with anti-his antibody (Qiagen) and purity was estimated to be > 85% by sodium dodecyl sulphate–polyacrylamide gel electrophoresis.
Patient samples
Written consent was obtained from all of the patients before blood and tissue sampling, and The Ethics Committee of Hannover Medical School approved the study.
Immuno-screening of sera by enzyme-linked immunosorbent assay
Antibody responses were detected in serum from mice and patients with pancreatic cancer by enzyme-linked immunosorbent assay (ELISA) as previously described.16 In brief, Tankyrase-2 recombinant protein was bound to nickel-coated 96-well plates (Qiagen) overnight at 4°. The next day, the plates were washed with phosphate-buffered saline–Tween-20, serum at a variety of dilutions was added to the plates and incubated for 2 hr at room temperature. The plates were then washed and incubated with the secondary antibody (horseradish peroxidase-conjugated anti-mouse IgG and anti-human-IgG-AP; Sigma) for 1 hr at room temperature. The plates were washed and incubated with TMB or pNPP substrate solution (Sigma) and stopped by H2SO4 or NaOH, respectively. All serum samples were tested at a 1 : 50 dilution unless indicated otherwise. An irrelevant recombinant protein, human interleukin-4, was used as the negative control for all samples.
Reverse transcription-PCR analysis for Tankyrase-2 expression
RNA was isolated from tissues or cells using the RNA mini kit (Qiagen). The cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad). The reverse transcription (RT) -PCR reaction was performed with CyberGreen (Bio-Rad,) using the iCycler iQ Real-Time PCR machine (Bio-Rad). The following primers were used: for mouse Tnks2: forward primer 5′-CCC TCT GCT CTG CCT ACG TG-3′; reverse primer 5′-AGA AGC TGC CAG ATA AGT TGT CG-3′.
Statistical analysis
All the statistical analyses were based on Student’s t-test. All P-values were two-tailed and a value < 0·05 was considered to be significant.
Results
SEREX analysis of a murine pancreatic adenocarcinoma cDNA library
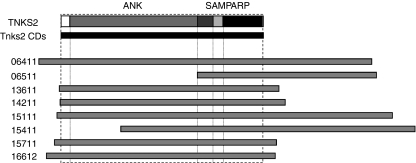
A cDNA expression library of 2 × 106 primary clones was prepared from the murine pancreatic cancer cell line mPAC. This cell line has been established from a C57BL/6 EL-TGF-α × Trp53−/− mouse with spontaneous pancreatic tumours as previously described.11 Phage plaques (1 × 106) were immune-screened and 20 positive clones were identified. The size of the inserts ranged from 1·2 to 5·8 kilobases and sequencing revealed that eight of the 20 clones coded for the same gene, Tankyrase-2. The other clones coded for N-ethylmaleimide sensitive fusion protein (NSF), latent transforming growth factor-β binding protein (LTBP), ornithine-oxe-acid aminotransferase (OAT), PARP-2, and eight unknown genes (Table 1). Next, we compared the eight clones coding for Tankyrase-2. The size of these clones varied from 3·1 to 5·8 kilobases. Six of the clones coded for the entire cDNA sequence, while two coded only for the 3′-end of the sequence, which contains a sterile α-motif (SAM) as well as a PARP catalytic domain (Fig. 1).
Table 1.
List of all antigens identified
| No. | Clone | Size (kilobases) | Product |
|---|---|---|---|
| 1 | 02822 | 1·2 | Unknown |
| 2 | 05311 | 3·7 | NSF (N-ethylmaleimide sensitive fusion protein) |
| 3 | 06411 | 5·7 | Tnks2 (Tankyrase2) |
| 4 | 06511 | 3·1 | Tnks2 (Tankyrase2) |
| 5 | 10721 | 2·8 | Unknown |
| 6 | 10722 | 2·0 | Unknown |
| 7 | 10741 | 4·8 | LTBP (latent transforming growth factor beta binding protein) |
| 8 | 10931 | – | Unknown |
| 9 | 12221 | 2·9 | OAT (ornithine-oxe-acid aminotransferase) |
| 10 | 12311 | – | Unknown |
| 11 | 12711 | 2·7 | Unknown |
| 12 | 12731 | 1·3 | Unknown |
| 13 | 13611 | 3·8 | Tnks2 (Tankyrase2) |
| 14 | 14211 | 3·9 | Tnks2 (Tankyrase2) |
| 15 | 15111 | 5·8 | Tnks2 (Tankyrase2) |
| 16 | 15411 | 5·1 | Tnks2 (Tankyrase2) |
| 17 | 15711 | 3·8 | Tnks2 (Tankyrase2) |
| 18 | 15911 | – | PARP2 poly (ADP-ribose) polymerase family, member 2 |
| 19 | 16611 | – | Unknown |
| 20 | 16612 | 4·1 | Tnks2 (Tankyrase2) |
Figure 1.
Schematic diagram of the Tankyrase-2 clones identified through SEREX. All of the identified Tankyrase-2 clones overlap in the poly(ADP-ribose) polymerase (PARP) domain. The lengths of the clone ranges from 3·1 to 5·7 kilobases. Six of them contain the full-length Tankyrase-2 coding sequence.
Analysis of Tankyrase-2 expression and antibody responses in mice
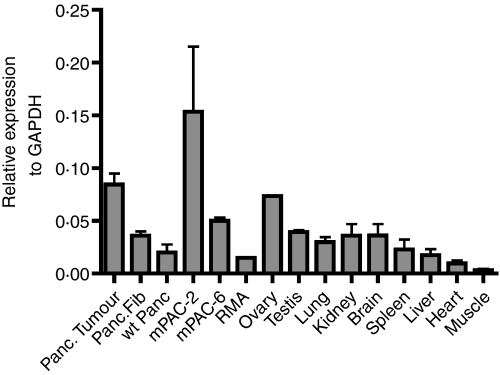
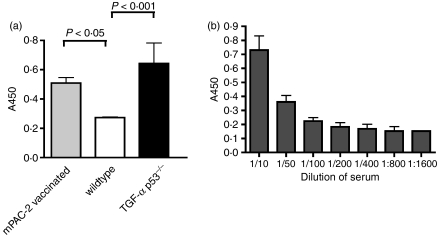
Next, we analysed the expression of Tankyrase-2 in different tissues by RT-PCR. The results are presented in Fig. 2. As expected, the mPAC-2 tumour cell line, as well as spontaneous pancreatic tumours, showed high levels of Tankyrase-2 expression. To prove further the relevance of Tankyrase-2 in our mouse model, we next purified recombinant Tankyrase-2 protein and used it in an ELISA to detect Tankyrase-2-specific antibody responses in mouse serum. As shown in Fig. 3(a), mice with spontaneous tumours and wild-type mice after subcutaneous injection of mPAC-2 tumour cell line displayed clear Tankyrase-2-specific antibody responses. The antibody response in the serum of mice with spontaneous tumours was detectable at serum dilutions of up to 1 : 800 (Fig. 3b).
Figure 2.
Expression of Tankyrase-2 in different mouse tissues and tumour cell lines. Analysis of Tankyrase-2 gene expression by real-time polymerase chain reaction. Mouse GAPDH was used as control. Data are shown as the relative expression level of Tankyrase-2 to GAPDH. Tissue samples were isolated either from tumour-bearing EL-TGF-α × Trp53−/− mice or EL-TGF-α × Trp53+/− mice with pre-malignant lesions. In addition, two tumour cell lines (mPAC-2 and mPAC-6) obtained from EL-TGF-α × Trp53−/− mice were analysed, which were previously described.11
Figure 3.
Antibody responses against the recombinant mouse Tankyrase-2 in tumour-bearing mice. (a) Serum was isolated from wild-type mice, mPAC vaccinated mice and tumour-bearing EL-TGF-α × Trp53−/− mice. Enzyme-linked immunosorbent assay (ELISA) was performed with the 1 : 50 diluted serum against recombinant mouse Tankyrase-2. Data shown represent the difference between recombinant mouse Tankyrase-2 and irrelevant control. (b) Titration of the serum from the tumour-bearing EL-TGF-α × Trp53−/− mice against the recombinant mouse Tankyrase-2. Serum was isolated from tumour-bearing EL-TGF-α × Trp53−/− mice and diluted at 1 : 10 to 1 : 800 and was tested against purified mouse Tankyrase-2 in an ELISA. Data shown represent the difference between recombinant mouse Tankyrase-2 and irrelevant control.
Analysis of Tankyrase-2-specific antibody responses in transgenic mice during tumour development
Transforming growth factor-α (TGF-α) transgenic mice represent a good model not only to analyse immune responses in mice with already established tumours, but also in mice with pre-malignant lesions.9 Therefore, we tested Tankyrase-2-specific antibody responses in EL-TGF-α × Trp53−/−, EL-TGF-α × Trp53+/− and EL-TGF-α × Trp53+/+ mice at different time-points up to 100 days after birth. At this time-point only EL-TGF-α × Trp53−/− mice develop pancreatic carcinomas. As shown in Fig. 4, no significant antibody responses can be detected in any of the mice after birth. Tankyrase-2-specific antibody responses were detected for the first time in 100-day-old mice with pre-malignant lesions (EL-TGF-α × Trp53+/− and EL-TGF-α × Trp53+/+). In contrast, EL-TGF-α × Trp53−/− mice with rapidly developing pancreatic tumours developed Tankyrase-2-specific antibodies 63 days after birth. Antibody responses increased further until mice develop advanced pancreatic tumours.
Figure 4.
Antibody responses against the recombinant mouse Tankyrase-2 during tumour development. Serum was isolated from EL-TGF-α × Trp53−/−, EL-TGF-α × Trp53+/−, EL-TGF-α × Trp53+/+ mice at different time-points. Enzyme-linked immunosorbent assay was performed with the 1 : 50 diluted serum against recombinant mouse Tankyrase-2. Data represent the difference between recombinant mouse Tankyrase-2 and irrelevant control (*P < 0·05).
Analysis of Tankyrase-2-specific antibody responses in patients with pancreatic adenocarcinoma
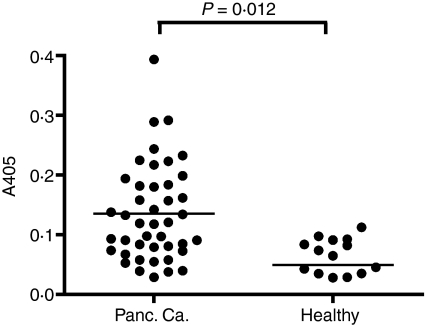
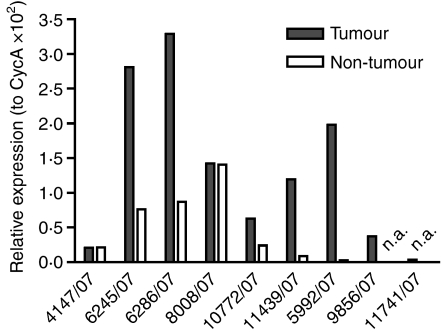
Human Tankyrase-2 shares a 95% identity with its murine counterpart. To investigate the relevance of our findings also for the human disease, we screened serum from patients with pancreatic cancer for the presence of Tankyrase-2-specific antibody responses. Recombinant human Tankyrase protein was purified as described in the Materials and methods and used in an ELISA. Serum samples from a total of 43 patients with advanced pancreatic cancer and 18 healthy controls were tested. As shown in Fig. 5, Tankyrase-2-specific serum responses were detected in patients with pancreatic cancer and not in healthy donors. Twenty-three of the 43 patients (51%) demonstrated significant antibody responses against Tankyrase-2, which demonstrated the relevance of this newly identified tumour antigen for human disease. To verify Tankyrase-2 expression also in human pancreatic tissue, quantitative RNA analysis was performed from microdissected human pancreatic carcinoma tissue and adjacent normal pancreatic tissue. As shown in Fig. 6, Tankyrase-2 expression was observed in eight of the nine tumour samples. In addition, direct comparison of tumour with non-tumour pancreatic tissue demonstrated a higher Tankyrase-2 expression in five of seven patients.
Figure 5.
Antibody responses against human Tankyrase-2 in cancer patients Serum was isolated from cancer patients and enzyme-linked immunosorbent assay was performed with the 1 : 50 diluted serum against recombinant human Tankyrase-2. Data shown represent the difference between recombinant human Tankyrase-2 and irrelevant control.
Figure 6.
Messenger RNA expression analysis of human pancreatic tissue samples. RNA was isolated from nine tumour tissue specimens and normal adjacent tissue and quantitative reverse transcription–polymerase chain reaction was performed. For two cases, adjacent normal pancreatic tissue was not available (n.a.).
Discussion
The identification of new tumour-associated antigens is of utmost importance not only for the development of new cancer vaccines, but also for the analysis of immune responses during conventional and molecular therapy. In contrast to other tumours such as malignant melanoma, only a limited number of pancreatic tumour antigens have been identified.17,18 So far different antigen-based vaccines have been evaluated including vaccines based on telomerase, ras, CEA, MUC and HER2-neu. However, these antigens have not been identified using classical approaches for the identification of tumour antigens, such as the analysis of antigen-specific T-cell responses or antibody responses, so evidence that they can be recognized by the patient’s immune system is lacking.4 Mesothelin has recently been shown to be an immunologically relevant tumour antigen for pancreatic cancer using T cells from a patient vaccinated with a granulocyte–macrphage colony-stimulating factor-expressing tumour cell vaccine.19
However, in all other cases the immunological relevance of pancreatic tumour antigens remains unknown. Therefore, we decided to choose an alternative approach using a murine model of pancreatic adenocarcinoma, which closely resembles the disease in patients with pancreatic cancer. By this approach we could not only demonstrate the relevance of the identified antigen, but could also further prove the advantages of autochthonous murine mouse models, in which tumours arise spontaneously, for immune-related questions.
Tankyrases have been described as potential targets for cancer therapy,20 making these genes interesting targets also for immunotherapy. Maintenance of telomeres in most cancer cells is a critical requirement for continued proliferation so the targeting of telomeres involved in this process is an attractive strategy for cancer treatment. Tankyrase-2 was identified by several groups through two-hybrid screening with insulin-responsive aminopeptidase.21 Interestingly, it has also been identified by serological screening of breast tumour and fetal brain cDNA libraries with breast carcinoma and meningioma patients’ sera respectively.22,23 The domains that comprise Tankyrase-2 are highly homologous with those of Tankyrase-1, with the main exception being that Tankyrase-2 lacks the histidine-proline-serine rich domain. The PARP domain is the most conserved gene motif, with 94% identity. Tankyrase-2 has been shown to have PARP activity similar to that of Tankyrase-1.24 It has been reported that Tankyrase-2 was over-expressed in testis, ovary and skeleton muscle, like Tankyrase-1. Our RT-PCR also confirmed the high expression in testis and ovary, but not in muscle. Until today the exact function of Tankyrase-2 has not been elucidated. In one study it was shown that over-expression of Tankyrase-2 may lead to necrotic cell death in vitro.25 Studies in vivo of Tankyrase-2 knockout mice surprisingly demonstrated that inactivation of this gene does not result in detectable alteration of telomere length, which suggests that either mouse Tankyrases 1 and 2 have redundant functions or mouse Tankyrase-2 differs from human Tankyrase-2 in its role in the maintenance of telomere length.26 Further studies are required to determine the role of Tankyrase-2 in patients with pancreatic cancer.
We have tried to identify Tankyrase-2-specific T-cell responses in our model without success, although the production of Tankyrase-2-specific antibodies probably indicates the involvement of functional CD4+ T-cell help. A number of possible reasons exist as to why we could not detect these T-cell responses, such as different immune suppressor mechanisms, which have been demonstrated to occur in tumour-bearing mice.27 Interestingly, we have been able to detect an increase in the frequency of myeloid-derived suppressor cells also in the mouse model described here (Zhao et al. submitted for publication). Myeloid-derived suppressor cells were already detected in mice with pre-malignant lesions. This observation also explains why tumours develop in these mice despite the presence of tumour-specific immune responses.
Most murine tumour antigens have been identified using transplantable subcutaneous tumours. However, the limitations of these models for immunological studies have been previosuly discussed.11,28 Using TGF-α transgenic mice, which spontaneously develop autochthonous pancreatic tumours within 120 days after birth, we identified a tumour antigen that also elicits immune responses in patients with pancreatic cancer. Therefore, this study demonstrates not only the significance of spontaneous tumour models but also the relevance of murine tumour antigens in human cancer. Further studies will be needed to address the clinical and immunological relevance of this antigen for patients with pancreatic cancer and potentially also other types of tumours.
Acknowledgments
We would like to thank Joseph Mautner for help with the SEREX technology. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) to T.F.G. (Gr1511/3-3).
Disclosures
The authors declare no financial interests.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 3.Merchant N, Berlin J. Past and future of pancreas cancer: are we ready to move forward together? J Clin Oncol. 2008;26:3478–80. doi: 10.1200/JCO.2008.17.0811. [DOI] [PubMed] [Google Scholar]
- 4.Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer – science driving clinical progress. Nat Rev. 2005;5:459–67. doi: 10.1038/nrc1630. [DOI] [PubMed] [Google Scholar]
- 5.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 6.Jäger E, Nagata Y, Gnjatic S, et al. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci USA. 2000;97:4760–5. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jäger E, Jäger D, Karbach J, et al. Identification of NY-ESO-1 epitopes presented by human histocompatibility antigen (HLA)-DRB4*0101-0103 and recognized by CD4(+) T lymphocytes of patients with NY-ESO-1-expressing melanoma. J Exp Med. 2000;191:625–30. doi: 10.1084/jem.191.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jäger E, Gnjatic S, Nagata Y, et al. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc Natl Acad Sci USA. 2000;97:12198–203. doi: 10.1073/pnas.220413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner M, Greten FR, Weber CK, et al. A murine tumor progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev. 2001;15:286–93. doi: 10.1101/gad.184701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greten FR, Weber CK, Greten TF, Schneider G, Wagner M, Adler G, Schmid RM. Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology. 2002;123:2052–63. doi: 10.1053/gast.2002.37075. [DOI] [PubMed] [Google Scholar]
- 11.Garbe AI, Vermeer B, Gamrekelashvili J, et al. Genetically induced pancreatic adenocarcinoma is highly immunogenic and causes spontaneous tumor-specific immune responses. Cancer Res. 2006;66:508–16. doi: 10.1158/0008-5472.CAN-05-2383. [DOI] [PubMed] [Google Scholar]
- 12.Wagner M, Luhrs H, Kloppel G, Adler G, Schmid RM. Malignant transformation of duct-like cells originating from acini in transforming growth factor transgenic mice. Gastroenterology. 1998;115:1254–62. doi: 10.1016/s0016-5085(98)70098-8. [DOI] [PubMed] [Google Scholar]
- 13.Christgen M, Bruchhardt H, Ballmaier M, Krech T, Langer F, Kreipe H, Lehmann U. KAI1/CD82 is a novel target of estrogen receptor-mediated gene repression and downregulated in primary human breast cancer. Int J Cancer. 2008;123:2239–46. doi: 10.1002/ijc.23806. [DOI] [PubMed] [Google Scholar]
- 14.Sahin U, Tureci O, Pfreundschuh M. Serological identification of human tumor antigens. Curr Opin Immunol. 1997;9:709–16. doi: 10.1016/s0952-7915(97)80053-2. [DOI] [PubMed] [Google Scholar]
- 15.Chen YT, Göre AO, Tsang S, Stockert E, Jäger E, Knuth A, Old LJ. Identification of multiple cancer/testis antigens by allogeneic antibody screening of a melanoma cell line library. Proc Natl Acad Sci USA. 1998;95:6919–23. doi: 10.1073/pnas.95.12.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP, Greten TF. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4332–41. doi: 10.1158/1078-0432.CCR-04-0181. [DOI] [PubMed] [Google Scholar]
- 17.Nakatsura T, Senju S, Yamada K, Jotsuka T, Ogawa M, Nishimura Y. Gene cloning of immunogenic antigens overexpressed in pancreatic cancer. Biochem Biophys Res Commun. 2001;281:936–44. doi: 10.1006/bbrc.2001.4377. [DOI] [PubMed] [Google Scholar]
- 18.Wadle A, Kubuschok B, Imig J, et al. Serological immune response to cancer testis antigens in patients with pancreatic cancer. Int J Cancer. 2006;119:117–25. doi: 10.1002/ijc.21744. [DOI] [PubMed] [Google Scholar]
- 19.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seimiya H. The telomeric PARP, tankyrases, as targets for cancer therapy. Br J Cancer. 2006;94:341–5. doi: 10.1038/sj.bjc.6602951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chi NW, Lodish HF. Tankyrase is a golgi-associated mitogen-activated protein kinase substrate that interacts with IRAP in GLUT4 vesicles. J Biol Chem. 2000;275:38437–44. doi: 10.1074/jbc.M007635200. [DOI] [PubMed] [Google Scholar]
- 22.Monz D, Munnia A, Comtesse N, Fischer U, Steudel WI, Feiden W, Glass B, Meese EU. Novel tankyrase-related gene detected with meningioma-specific sera. Clin Cancer Res. 2001;7:113–9. [PubMed] [Google Scholar]
- 23.Kuimov AN, Kuprash DV, Petrov VN, Vdovichenko KK, Scanlan MJ, Jongeneel CV, Lagarkova MA, Nedospasov SA. Cloning and characterization of TNKL, a member of tankyrase gene family. Genes Immun. 2001;2:52–5. doi: 10.1038/sj.gene.6363722. [DOI] [PubMed] [Google Scholar]
- 24.Cook BD, Dynek JN, Chang W, Shostak G, Smith S. Role for the related poly(ADP-ribose) polymerases tankyrase 1 and 2 at human telomeres. Mol Cell Biol. 2002;22:332–42. doi: 10.1128/MCB.22.1.332-342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaminker PG, Kim SH, Taylor RD, Zebarjadian Y, Funk WD, Morin GB, Yaswen P, Campisi J. TANK2, a new TRF1-associated poly(ADP-ribose) polymerase, causes rapid induction of cell death upon overexpression. J Biol Chem. 2001;276:35891–9. doi: 10.1074/jbc.M105968200. [DOI] [PubMed] [Google Scholar]
- 26.Chiang YJ, Nguyen ML, Gurunathan S, Kaminker P, Tessarollo L, Campisi J, Hodes RJ. Generation and characterization of telomere length maintenance in tankyrase 2-deficient mice. Mol Cell Biol. 2006;26:2037–43. doi: 10.1128/MCB.26.6.2037-2043.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 28.Ostrand-Rosenberg S. Animal models of tumor immunity, immunotherapy and cancer vaccines. Curr Opin Immunol. 2004;16:143–50. doi: 10.1016/j.coi.2004.01.003. [DOI] [PubMed] [Google Scholar]