Abstract
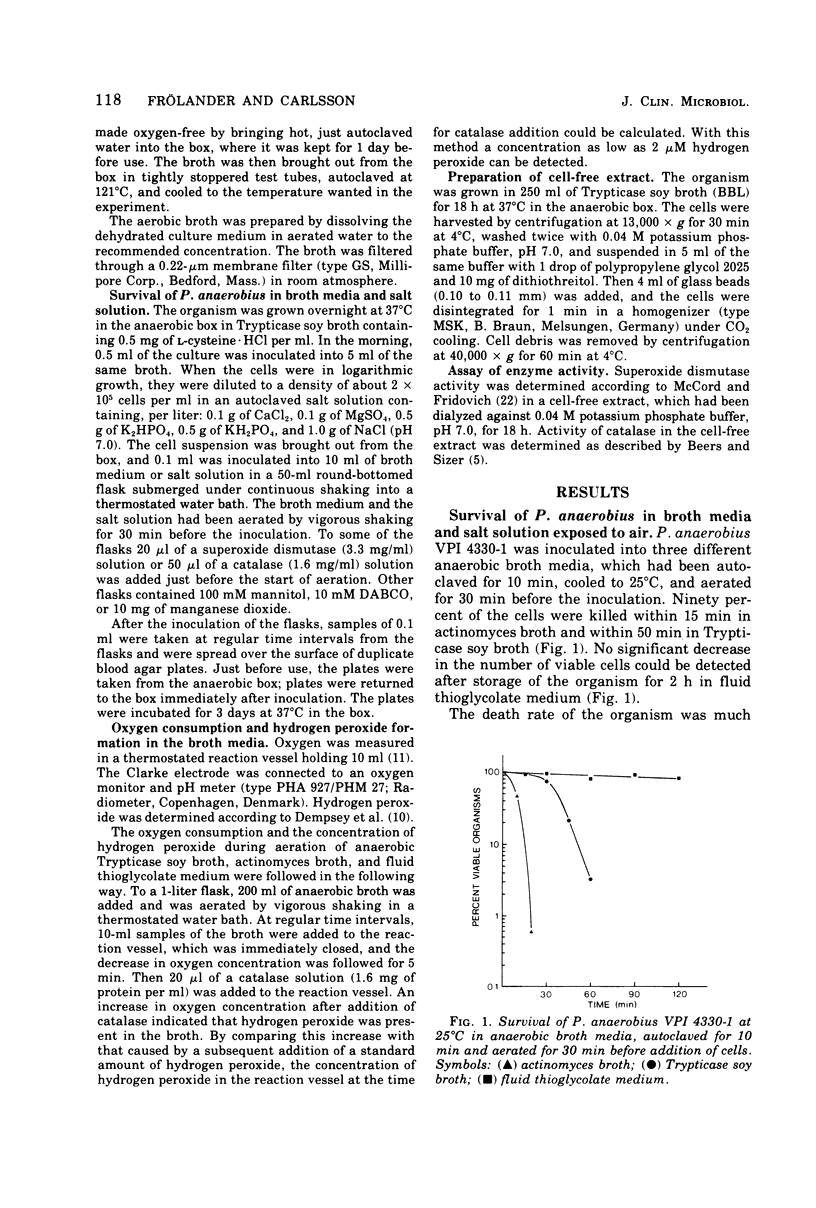
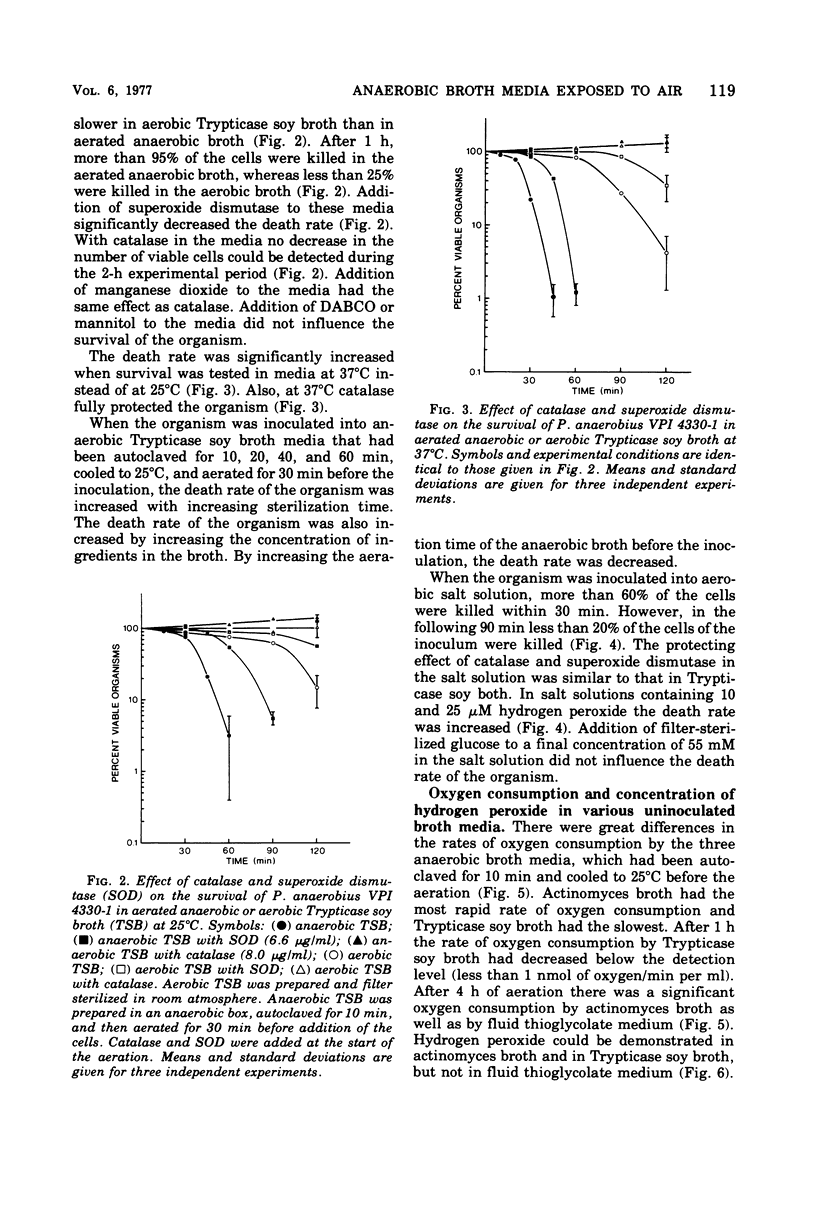
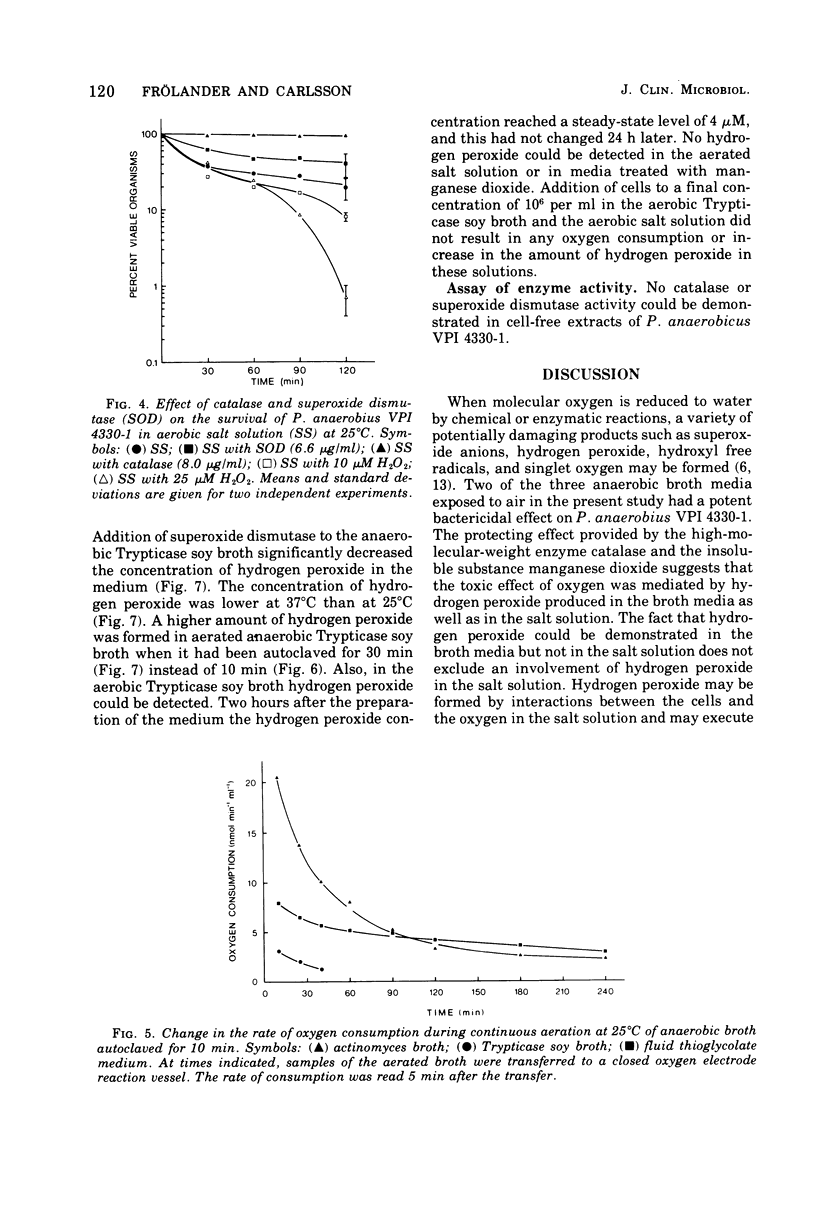
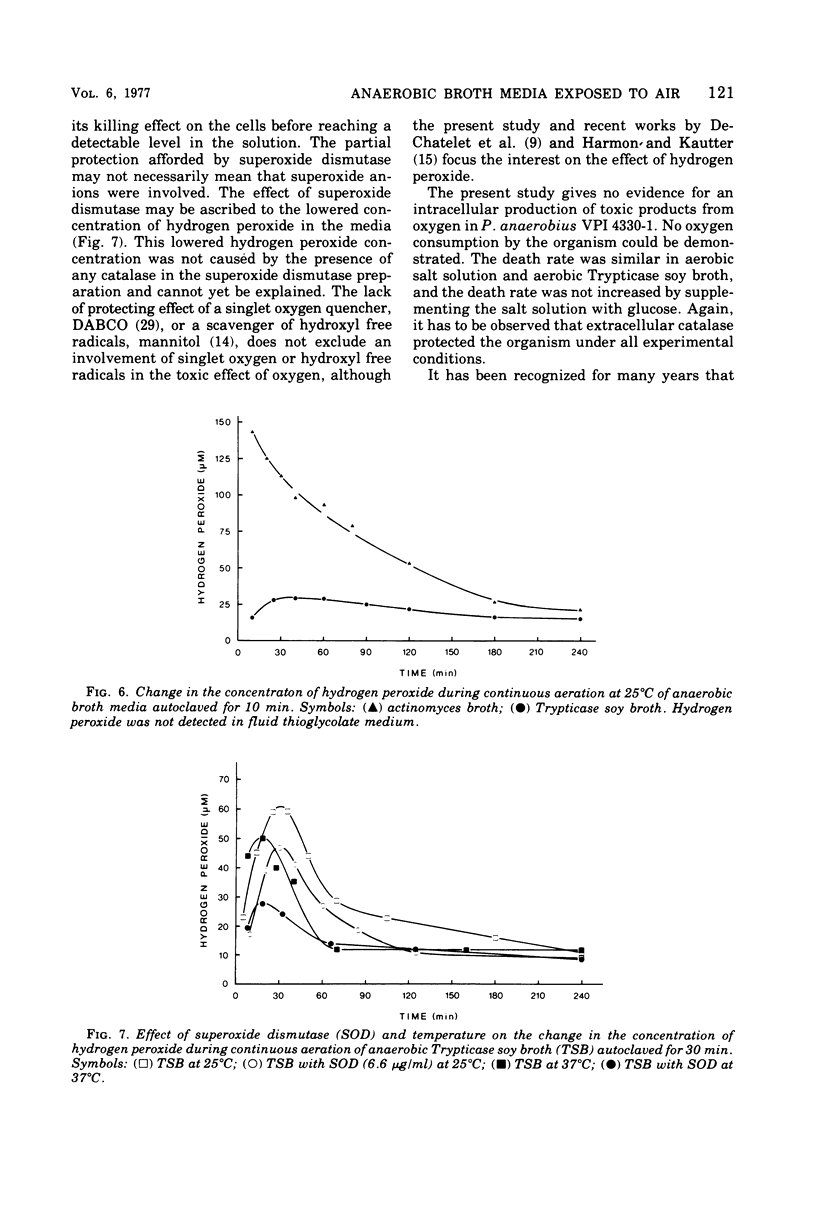
Peptostreptococcus anaerobius strain VPI 4330-1 was used as the test organism in an evaluation of the bactericidal effect of anaerobic broth exposed to air. The test organism, grown under anaerobic conditions in Trypticase soy broth, was diluted in buffered salt solution, and about 2 × 104 cells were suspended in 10 ml of an aerated broth. Ninety percent of the cells were killed within 15 min in actinomyces broth and within 50 min in Trypticase soy broth. All cells survived for 2 h in fluid thioglycolate medium. Addition of DABCO [1,4-diazabicyclo (2.2.2) octane] or mannitol to Trypticase soy broth did not influence the death rate of the organism, whereas superoxide dismutase decreased the death rate. Addition of catalase or manganese dioxide to the broth kept all the cells viable for 2 h. Of the three broth media tested, actinomyces broth reduced oxygen at the highest rate and Trypticase soy broth reduced it at the slowest rate. Hydrogen peroxide could be demonstrated in actinomyces broth and in Trypticase soy broth but not in fluid thioglycolate medium. In addition to catalase, manganese dioxide also removed all hydrogen peroxide from Trypticase soy broth, and superoxide dismutase significantly decreased the concentration of hydrogen peroxide in the broth. The results suggest that hydrogen peroxide mediated the toxic effect of atmospheric oxygen in these broth media.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. E., Goodman D. B., Besarab A., Rasmussen H. Studies on the biochemical basis of oxygen toxicity. Biochim Biophys Acta. 1973 Oct 5;320(3):708–728. doi: 10.1016/0304-4165(73)90151-7. [DOI] [PubMed] [Google Scholar]
- BARRY V. C., CONALTY M. L., DENNENY J. M., WINDER F. Peroxide formation in bacteriological media. Nature. 1956 Sep 15;178(4533):596–597. doi: 10.1038/178596a0. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Bartlett J. G., Sullivan-Sigler N., Louie T. J., Gorbach S. L. Anaerobes survive in clinical specimens despite delayed processing. J Clin Microbiol. 1976 Feb;3(2):133–136. doi: 10.1128/jcm.3.2.133-136.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bors W., Saran M., Lengfelder E., Spöttl R., Michel C. The relevance of the superoxide anion radical in biological systems. Curr Top Radiat Res Q. 1974 May;9(3):247–309. [PubMed] [Google Scholar]
- Carlsson J., Frölander F., Sundquist G. Oxygen tolerance of anaerobic bacteria isolated from necrotic dental pulps. Acta Odontol Scand. 1977;35(3):139–145. doi: 10.3109/00016357709056002. [DOI] [PubMed] [Google Scholar]
- Chow A. W., Cunningham P. J., Guze L. B. Survival of anaerobic and aerobic bacteria in a nonsupportive gassed transport system. J Clin Microbiol. 1976 Feb;3(2):128–132. doi: 10.1128/jcm.3.2.128-132.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., Goodson P. R., McCall C. E. Bactericidal activity of superoxide anion and of hydrogen peroxide: investigations employing dialuric acid, a superoxide-generating drug. Antimicrob Agents Chemother. 1975 Aug;8(2):146–153. doi: 10.1128/aac.8.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey P. M., O'Leary J., Condon S. Polarographic assay of hydrogen peroxide accumulation in microbial cultures. Appl Microbiol. 1975 Feb;29(2):170–174. doi: 10.1128/am.29.2.170-174.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L., Keele B. B., Misra H. Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem. 1973 Nov 25;248(22):7792–7797. [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen metabolism in Lactobacillus plantarum. J Bacteriol. 1974 Jan;117(1):166–169. doi: 10.1128/jb.117.1.166-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon S. M., Kautter D. A. Beneficial effect of catalase treatment on growth of Clostridium perfringens. Appl Environ Microbiol. 1976 Sep;32(3):409–416. doi: 10.1128/aem.32.3.409-416.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill G. B., Osterhout S. Experimental effects of hyperbaric oxgen on selected clostridial species. I. In-vitro studies. J Infect Dis. 1972 Jan;125(1):17–25. doi: 10.1093/infdis/125.1.17. [DOI] [PubMed] [Google Scholar]
- Israeli E., Kohn A., Gitelman J. The molecular nature of damage by oxygen to freeze-dried Escherichia coli. Cryobiology. 1975 Feb;12(1):15–25. doi: 10.1016/0011-2240(75)90037-1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969 Nov;18(5):723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCormick J. P., Fischer J. R., Pachlatko J. P., Eisenstark A. Characterization of a cell-lethal product from the photooxidation of tryptophan: hydrogen peroxide. Science. 1976 Feb 6;191(4226):468–469. doi: 10.1126/science.1108203. [DOI] [PubMed] [Google Scholar]
- McMinn M. T., Crawford J. J. Recovery of anaerobic microorganisms from clinical specimens in prereduced media versus recovery by routine clinical laboratory methods. Appl Microbiol. 1970 Feb;19(2):207–213. doi: 10.1128/am.19.2.207-213.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROOM H., WOIWOD A. J., BARNES J. M., ORBELL W. G. A growth-inhibitory effect on Shigella dysenteriae which occurs with some batches of nutrient agar and is associated with the production of peroxide. J Gen Microbiol. 1950 May;4(2):270–276. doi: 10.1099/00221287-4-2-270. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Stewart P. R., Sutter V. L., Rosenblatt J. E. Oxygen tolerance of fresh clinical anaerobic bacteria. J Clin Microbiol. 1975 Feb;1(2):161–164. doi: 10.1128/jcm.1.2.161-164.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. L. Uptake and incorporation of exogenous leucine in bacterial cells under high oxygen tension. Nature. 1968 Sep 7;219(5158):1068–1069. doi: 10.1038/2191068a0. [DOI] [PubMed] [Google Scholar]



