Abstract
Migration of cells is important for tissue maintenance, immune response, and often altered in disease. While biochemical aspects, including cell adhesion, have been studied in detail, much less is known about the role of the mechanical properties of cells. Previous measurement methods rely on contact with artificial surfaces, which can convolute the results. Here, we used a non-contact, microfluidic optical stretcher to study cell mechanics, isolated from other parameters, in the context of tissue infiltration by acute promyelocytic leukemia (APL) cells, which occurs during differentiation therapy with retinoic acid. Compliance measurements of APL cells reveal a significant softening during differentiation, with the mechanical properties of differentiated cells resembling those of normal neutrophils. To interfere with the migratory ability acquired with the softening, differentiated APL cells were exposed to paclitaxel, which stabilizes microtubules. This treatment does not alter compliance but reduces cell relaxation after cessation of mechanical stress six-fold, congruent with a significant reduction of motility. Our observations imply that the dynamical remodeling of cell shape required for tissue infiltration can be frustrated by stiffening the microtubular system. This link between the cytokeleton, cell mechanics, and motility suggests treatment options for pathologies relying on migration of cells, notably cancer metastasis.
Keywords: acute promyelocytic leukemia, cytoskeleton, microtubules, optical stretcher, paclitaxel
Migration of cells is essential for the development and survival of multicellular organisms and the homeostasis of tissues (1). Moreover, the prognosis of numerous pathologies is determined by the motility of the cell populations involved, notably in malignant tumors (2). While cell adhesion as a regulator of cell motility has been studied in great detail, the mechanisms governing the actual movement of cells through surrounding three-dimensional tissue structures are not yet fully understood (3). This movement was observed to require secretion of proteolytic enzymes, which, however, appears to be dispensable, and changes of cell shape (4). The latter process is particularly important for amoeboid cell migration, the predominant mode of leukocyte migration, which proceeds with weakly adhesive to non-adhesive interactions (5, 6). A sufficient ability of the cell body to dynamically remodel its shape appears to be pivotal for cell motility (7). On the contrary, a rigid cell body would frustrate any attempt of the cell to squeeze through tissue gaps and channels. The major determinant of cell rigidity is the filamentous cytoskeleton (8). While actin is generally considered most important for elastic resistance to deformation, microtubules have been implicated in the polarization of cell shape and migration (9, 10). Microtubules, therefore, constitute a potential target for modifying cell mechanics and, consequently, interfering with cell motility.
These aspects can be exemplified in the development of blood cells, where immature myeloid precursor cells lack the ability to migrate, while mature neutrophils must be highly motile, reflecting their role in the immune response (11, 12). Acute promyelocytic leukemia (APL) has been studied not only as a malignant disease but also as a model for myeloid differentiation along the neutrophil pathway, which can be induced by all-trans retinoic acid (ATRA) (13). ATRA-induced differentiation of the NB4 cell line, which serves as an in vitro model for APL (14), was shown to increase cell motility (15), in parallel with cytoskeletal remodeling. ATRA is also used in the treatment of APL patients; however, its use can lead to a potentially lethal syndrome associated with a massive infiltration of organs by maturing myeloid cells, which gain the motile characteristics of neutrophils after being exposed to ATRA (16). Any insight into the specific cytoskeletal mechanisms regulating APL cell migration might also lead to management strategies for this and other infiltrative disorders.
In the search of such points for pharmacological intervention, how could one assess the global mechanical properties of cells relevant for their migratory ability? A popular approach for investigating cell mechanics is atomic force microscopy (AFM), which was recently applied to study leukemia (17) and cancer cell elasticity (18). Also, intracellular microrheology (ICM) has been used to study cell mechanics, even in the context of cell migration (9). Both AFM and ICM require the localization of cells on artificial 2D surfaces and probe local mechanical properties of cell compartments with high spatial resolution. However, it is the assessment of cells acting as a physical entity in a three-dimensional environment that is critical for understanding their behavior during migration through endothelial gaps and tissue channels. Microplates (19) or micropipettes (8, 20) allow the study of global cell mechanical properties, but still induce alterations of subcellular structures, notably of the cytoskeleton (21), due to cell-probe contact. Thus, an ideal approach for investigating the mechanical properties of cells, especially of those migrating without significant adhesive contacts, would apply non-contact measurement techniques and integrate local variations of cell mechanics into a single parameter, that is, deformability of the whole cell. For this purpose, we have recently developed a microfluidic optical stretcher (μOS). This technology was designed to trap and deform suspended cells by optically induced surface forces from two counterpropagating non-focused laser beams (22) (Fig. 1).
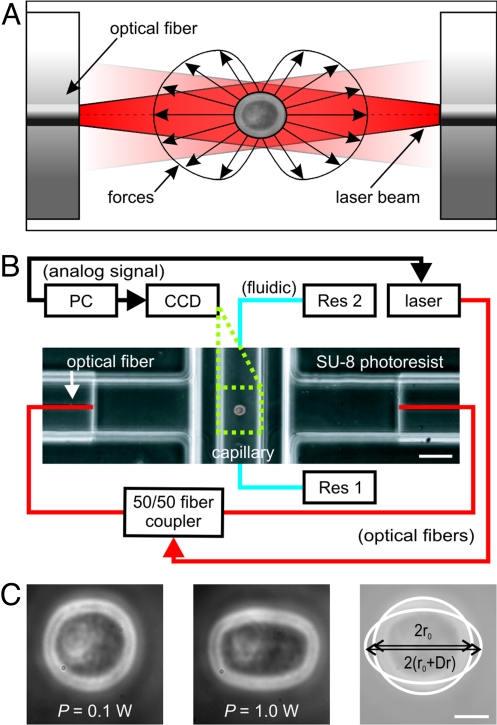
Fig. 1.
Principle and setup of a microfluidic optical stretcher (μOS). (A) Two-counter propagating NIR laser beams (λ = 1,064 nm) emanating from the cores of single-mode optical fibers are used to trap (P = 0.1 W each beam) and deform (P = 1 W each beam) single cells. Cells are deformed by the forces arising from the momentum transfer of light to the surface of the cell due to the change in refractive index. (B) Experimental setup. The flow of the cell suspension between two reservoirs (Res 1 and Res 2) is controlled by a hydrostatic pressure differential. The phase contrast image shows the microfluidic flowchamber with a cell trapped inside the glass microcapillary. (C) Phase-contrast images of APL cell being optically trapped (left) and stretched (middle) in the μOS. Optically induced surface stress causes a deformation of the cell along the optical axis. (Right) overlay of the two other images and definition of the strain, γ(t) = Δr(t)/r0. (Scale bar, 5 μm.)
The aim of this study was to investigate the global biomechanical properties of APL cells during ATRA-induced differentiation using the μOS technology. We find that the compliance of these cells acquired during myeloid maturation resembles that of normal neutrophils. Further, their ability to relax after deformation can be attenuated by modulating the cytoskeletal architecture with microtubule-stabilizing drugs. The consequence of this treatment is a reduced ability of differentiated APL cells to migrate through small pores, which is the rate-limiting step in neutrophil extravasation.
Results
Deformability Measurements of Nucleated Blood Cells with the μOS.
Mechanical properties of APL cells were characterized by creep compliance measurements using the μOS technique (see Methods for details). This approach is model-independent and, thus, can be used directly to compare cells with different mechanical properties. The creep compliance (synonymously referred to as ‘deformability’ to enable a more intuitive understanding), D(t) = γ(t)/(σoFG), is the time-dependent relative deformation, or strain, γ(t), of cells (Fig. 1C) normalized by the constant peak stress, σo, that is causing the deformation (Fig. 1A). The geometric factor FG accounts for different sizes or refractive indices of cells to be compared (23). The sizes (2RAPL = 18.1 ± 1.1 μm, 2Rneutrophils = 11.11 ± 0.24 μm, 2RAPL+ATRA = 18.7 ± 1.3 μm), where statistics follow the pattern of mean ± SEM at all points in the text, were directly available from image analysis. The refractive indices were measured to be n = 1.3654 ± 0.0019 and n = 1.3657 ± 0.0029 for APL cells and neutrophils, respectively, using immersion refractometry (see Methods for details). The reproducibility of creep compliance measurements with a μOS is demonstrated in Fig. S1. Notably, the strain response γ(t) of the APL cells to increasing levels of laser power shows a clear nonlinear behavior with an indication of a yield stress (Fig. S2).
ATRA-Induced Differentiation Increases Deformability of APL Cells.
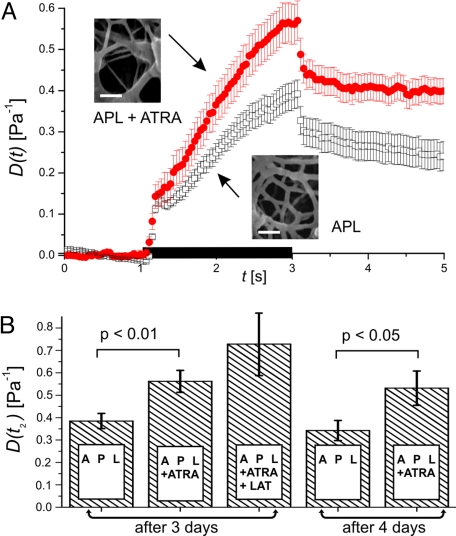
To assess changes of cell deformability associated with differentiation, APL cells treated with ATRA for 3 days were analyzed with the μOS and compared to untreated cells. Differentiated APL cells were found to have an increased compliance (Fig. 2). At the end of the stretching process the differentiated cells were 45% more compliant than controls. Differentiation of APL cells in response to ATRA had previously been found to proceed for up to 5 days (14). A measurement of APL cell compliance after 4 days of treatment with ATRA revealed no significant differences to the data obtained after 3 days (Fig. 2B).
Fig. 2.
Change of APL cell compliance in response to ATRA. (A) ATRA differentiated APL cells (filled circles; n = 44) are significantly more compliant than controls (open squares; n = 42), mean ± SEM. The black bar on the time axis indicates the application of stress. Insets depict electron microscopy images of the subcortical actin filament network. (Scale bars, 100 nm.) (B) Deformability at the end of stress application, mean ± SEM. There was no significant difference between APL cells exposed to ATRA for 3 and 4 days. A higher deformability could be measured after depolymerizing F-actin with latrunculin A (LAT).
Remodeling of the Actin Filament Network During Differentiation.
Elastic deformability of cells is regulated by the actin cytoskeleton (23). Visualization of the subcortical actin network in APL cells by electron microscopy revealed that the mesh size of the actin cytoskeleton, which is inversely related to the elastic shear modulus of biopolymer networks (24), is increased during differentiation (Fig. 2A, inset). This finding is in accordance with the increased compliance of ATRA differentiated APL cells. Depolymerization of F-actin by latrunculin A was found to further increase the deformability of differentiated APL cells (Fig. 2B), thus confirming the existence of a functional actin filament network in these cells. Differentiation of APL cells proceeds along the pathway of neutrophil maturation. The deformability of normal neutrophils from healthy donors was measured with the μOS (Fig. S3). In comparison to untreated APL cells, neutrophils were found to be more compliant. After differentiation, APL cells treated with ATRA presented a compliance similar to neutrophils within the first second of stretching. Subsequently, there was a difference in the viscoelastic behavior, with differentiated APL cells being more compliant than neutrophils.
Stabilizing Microtubules Prevents Relaxation of Differentiated APL Cells.
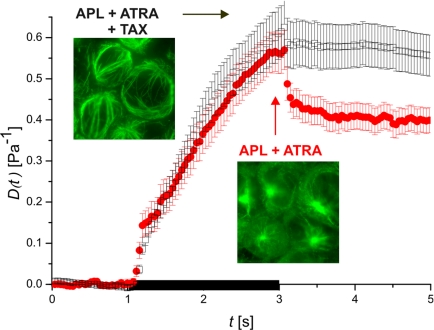
Treatment of differentiated APL cells with paclitaxel results in an increased bundling and polarization of microtubules as revealed by fluorescence microscopy (Fig. 3, inset). The large bending stiffness of microtubules is unique among the cytoskeletal filament systems (25). Thus, stabilizing microtubules with paclitaxel could, in principle, modulate the deformability of differentiated APL cells. Measurements of ATRA-differentiated APL cells treated with 5 μM paclitaxel for 1 h showed otherwise (Fig. 3). During stress application the extension behavior of these cells was indistinguishable from untreated controls. However, after cessation of stress significant differences in the relaxation behavior between differentiated APL cells with and without paclitaxel treatment could be observed. Whereas the former group of cells relaxed within fractions of seconds by about 27% from their maximum deformation, APL cells exposed to paclitaxel only relaxed by about 4.5%. After that the shape remained largely unaltered in both cases for the duration of the experiment. Repeating deformation with 60-s intervals, a relevant time scale for shape changes during migration, did not change this behavior for both groups of cells (Fig. S4). These observations are consistent with a stabilization of cells in a deformed state.
Fig. 3.
Relaxation behavior of ATRA differentiated APL cells before and after exposure to paclitaxel. The relaxation of differentiated APL cells after cessation of stress is decreased when treated with paclitaxel (TAX). (filled circles) APL cells treated with ATRA (n = 44), (open squares) APL cells treated with ATRA and paclitaxel (n = 56). The duration of stress application is indicated by the black bar on the time axis. Data show mean ± SEM. Insets show fluorescence images of the microtubule organization in both groups of cells.
Stabilizing Microtubules Decreases Motility of Differentiated APL Cells.
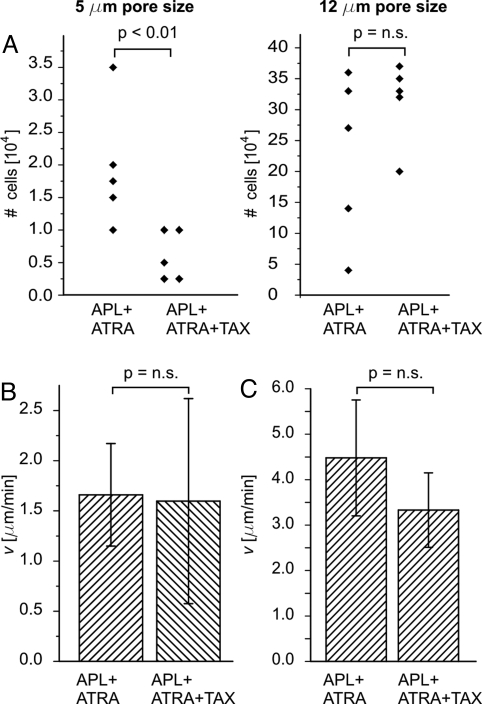
To investigate whether the lack of shape recovery of differentiated APL cells after deformation induced by paclitaxel influences cell motility, we performed chemotactic migration assays using Transwell filters with pore sizes of 5 μm, a typical size for endothelial gaps (26), or 12 μm, a size much closer to the diameter of the cells. The differences in the number of differentiated APL cells that migrated through these filters after 3 h are depicted in Fig. 4A. For the 5-μm pores, there was a significant reduction of the number of migrated APL cells in response to treatment with paclitaxel. For the larger pores there was no statistically significant difference. Undifferentiated APL cells were not able to migrate through the 5-μm pore filters at all (data not shown). Primary human neutrophils showed the same significant reduction as differentiated APL cells in migrated cell number in response to paclitaxel (Fig. S5). Here 3-μm pores were used to account for the smaller size of normal neutrophils, which is due to the smaller nucleus. Stabilizing microtubules could modulate actin-myosin-interactions during cell migration (27). However, motility of differentiated APL cells with myosin II activity being abolished by blebbistatin was still significantly reduced by the addition of paclitaxel (Fig. S6).
Fig. 4.
Migration of differentiated APL cells. (A) Chemotaxis through small pores. Paclitaxel significantly impedes the migration within 3 h of differentiated APL cells through 5-μm large pores (left). There is no statistically significant difference for 12-μm pores (right). Both experiments were performed five times. No change in chemotactic speed of differentiated APL cells could be observed after treatment with paclitaxel on 2D surfaces (B) or in 3D microchannels of 5–7-μm width (C). Data show mean ± SEM.
Pore Entry Is the Rate-Limiting Step in Diapedesis.
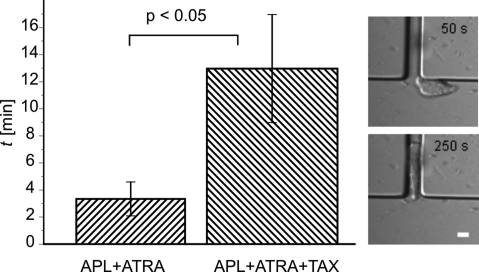
To identify whether the migration through, or the migration toward the pores was responsible for the reduction in migrated cell numbers in response to paclitaxel treatment, we also measured the cells' migration speed on 2D surfaces and in 5–7-μm wide 3D channels made from PDMS. There was no difference in the chemotactic speed between untreated cells and those treated with paclitaxel in either case (Fig. 4 B and C). The use of PDMS channels also allowed us to determine the time for entry into the PDMS channels directly (Fig. 5 and Movies S1 and S2). The results show unambiguously that paclitaxel increases the time for entry about four times, clearly identifying pore entry as the rate-limiting step in this migration assay for neutrophil diapedesis. Moreover, video-microscopy also documented the deformations a cell has to undergo as an entity to pass into the pore.
Fig. 5.
Pore entry as rate-limiting step. Paclitaxel significantly increases the entry time of differentiated APL cells into 3D channels (ntotal = 11). Data are mean ± SEM. Insets show a differentiated APL cell before and after entering the microfluidic channel (7-μm width). (Scale bar, 10 μm.)
Discussion
Cell Compliance and Differentiation.
We measured whole-cell mechanical properties of myeloid cells with a microfluidic optical stretcher (μOS). Since blood cells usually reside in suspension, this technique allows the investigation of their mechanical properties in a physiological state, thus avoiding unwanted adhesion-mediated alterations of the cytoskeleton and consequently of their mechanical properties.
Our findings demonstrate that ATRA-induced differentiation of APL cells is associated with a significant increase in compliance. This softening does not appear to be associated with apoptosis since cell compliance has been found to decrease in daunorubicin-induced apoptosis of acute myeloid leukemia (17) and other cells. Our measurements also emphasize that maturation of APL cells follows the neutrophil lineage because APL cell mechanics becomes more similar to neutrophils upon differentiation. The residual difference in deformability between differentiated APL cells and neutrophils could be due to the properties of the cell nucleus of APL cells, which differs in shape and DNA content from normal neutrophils (28). In this context it might be interesting to point out that measuring changes of cellular deformability can monitor the differentiation of precursor cells into mature neutrophils on the single cell level. This finding is in line with recent reports of cell mechanical changes during the osteogenic differentiation of mesenchymal stem cells (29). The generalization of this pattern for the characterization and sorting of stem cells from heterogeneous populations warrants further studies.
Cell Compliance and the Cytoskeleton.
The mechanical properties of cells are mainly governed by the cytoskeleton, an intracellular polymeric network. We have previously shown that the differentiation of APL cells is associated with a remodeling of the vimentin network (15). However, it was later demonstrated that mice lacking the intermediate filament vimentin showed a normal inflammatory response (30) so that a significant role of intermediate filaments for neutrophil motility appears to be unlikely. Moreover, contributions of intermediate filaments to cell mechanical response should only be visible at very large strains (31), which are not usually achieved by optically induced forces. On the other hand, the subcortical actin filament network is a significant determinant of cell elasticity, especially at small deformations (8, 32). In the present study, enhanced depolymerization of the actin network by latrunculin was shown to further increase deformability of differentiated APL cells. This finding confirms the important role of actin for cell elasticity found in neutrophils (8) and other cell types (33). Moreover, it indicates that, although being significantly more compliant than undifferentiated cells, APL cells differentiated with ATRA still have a subcortical actin network modulating cell mechanics, which was independently confirmed by electron microscopy. The observed changes of the actin network during differentiation of APL cells might explain the softening of these cells in response to ATRA. The indistinguishable refractive indices of the cells exclude an increased optical stress on the cells as a potential cause for the resulting larger deformability of the mature neutrophils.
Cell Compliance and Motility.
The connection between the mechanical properties of cells and their ability to migrate has received only limited attention compared to biochemical aspects, with some early experiments focusing mostly on blood cells (11, 12). The movement of myeloid cells seems to require the dynamic and fast remodeling of cell shape usually thought to be controlled by an elastic cytoskeleton. In a physiological context, undifferentiated precursor cells, which normally reside in the bone marrow, can be stiffer than their differentiated counterparts because there is no physiological need for migration. A lack of migration of undifferentiated myeloid cells has been implicated previously with their relative stiffness (12). This correlation has now been confirmed with our compliance measurements and migration assays.
An uncontrolled infiltration of cells into tissues, which occurs in certain disorders, could be frustrated by an artificial stiffening of the cytoskeleton. As a potential molecular target for inducing this effect, microtubules in vivo can bear significant compressive loads due to lateral reinforcement by the surrounding cytoplasm (34). Consequently, the enhancement of microtubule assembly by paclitaxel could increase cell stiffness and decrease compliance. The findings in this study do not support this hypothesis, since APL cells treated with paclitaxel exhibited the same extension behavior as untreated cells. This is in agreement with micropipette (8) and AFM data (33) showing that paclitaxel does not change the elasticity of neutrophils or fibroblasts, respectively. Importantly, however, our data demonstrate that paclitaxel alters the relaxation timescale of deformed differentiated APL cells after the release of stress. APL cells exposed to paclitaxel seemed to be temporarily frozen into the shape they were left in by the application of optical stress. The observed bundling and polarization of microtubules induced by paclitaxel could directly prevent the cell from relaxing back to their original shape. A similar bundling has been reported after treating another myeloid cell line (HL60) with pacitaxel (35). Bundled microtubules could also impact the freedom of the nucleus for rearrangement within the cytosol, which is relevant because nuclear size and properties have also been implicated in modifying shape recovery in leukocytes (12). This reduced inherent ability for shape recovery, induced by paclitaxel, arises from our migration studies as an alternative mechanism besides elastic stiffening for inhibiting extravasation of neutrophils through endothelial gaps. While the effect of stabilizing microtubules on cell migration could in principle be indirect, by modulating the actin cytoskeleton (27), previous experiments demonstrated that stabilization of microtubules does not have an effect on actin in neutrophils (8). Moreover, neither interference with the actin network nor inhibition of actomyosin contraction affect relaxation of leukocytes after deformation for pore migration (37). These reports, together with our finding that paclitaxel impairs cell migration independently of myosin II, show that microtubules exert a role for cell mechanics that is independent of actin dynamics.
Cell Mechanics and Infiltrative Disorders.
The differentiation of APL cells with ATRA is also used in the treatment of this specific type of leukemia; however, ATRA treatment is associated with potentially life-threatening complications affecting up to 26% of patients (38). This retinoic acid syndrome (RAS) is characterized by pulmonary infiltrates, pleural and epicardial effusions, preceded by the appearance of large numbers of neutrophils or their precursors in the peripheral blood (16). Importantly, tissue infiltration in RAS patients occurs days after induction of differentiation therapy. This time is sufficient for large-scale modifications of the cytoskeleton in APL cells. Consequently, we were able to detect changes of the mechanical properties and an enhanced motility of APL cells by 3 days after exposure to ATRA.
Previous experiments with APL cells indicate a role of cell adhesion molecules and the secretion of proteolytic enzymes in cell motility (39), but blocking adhesion molecules on the surface of APL cells or inhibiting matrix metalloproteinases can only partially impair their motility pointing at cell mechanics as potential additional parameter for intervention (39). Dexamethasone is the current mainstay of the management of patients with RAS (40). Interestingly, Lam et al. (17) demonstrated that this drug induces stiffening of acute lymphatic leukemia cells. Although dexamethasone is effective in APL patients there is still a substantial morbidity and mortality by RAS, and additional approaches to attenuate motility of differentiating cells are needed (41). A further increase of cell stiffening could be one such approach, which might be achieved by enhancing polymerization or cross-linking of actin filaments. However, no drug that targets the actin cytoskeleton has been approved for clinical use and such drugs would probably induce a variety of adverse effects.
In this study, we have shown that strengthening the microtubule cytoskeleton by paclitaxel is associated with a reduced ability to dynamically alter cell shape. This leads to a decreased ability of cells to enter size-limited pores, reflecting the process of neutrophil extravasation, which is the hallmark of RAS. The impairment of neutrophil motility by paclitaxel was also shown in an animal model of pneumonia (42). However, the experimental setup in this study did not allow dissection of the mechanisms of action since paclitaxel also acts on endothelial cells and can block secretion of proteolytic enzymes (43). In contrast, our assay system provided the opportunity to focus on neutrophil mechanics and to analyze the different steps of neutrophil extravasation—approach, entry into gaps, and interstitial migration in channels. Our data clearly demonstrate that paclitaxel specifically impairs the entry of differentiated APL cells into pores with a diameter similar to that of endothelial gaps (26). These findings could be reproduced in neutrophils from healthy donors. Importantly, videomicroscopy showed that the entry into small pores as the rate-limiting step in diapedesis requires a directional change and a relaxation of the whole cell body, thus linking prolonged relaxation after deformation to delayed passage through endothelial gaps. Of note, adhesion of neutrophils to endothelial cells is not required for this step (44). If the pores, however, are larger than physiological gaps in the endothelium, the bending of the cell that is required to fully enter the pore is reduced so that a defective relaxation can be better tolerated and motility is not significantly impaired as shown in our transmigration experiments. Migration either on 2D surfaces or through 3D channels, where the shape does not have to change, was not impacted by paclitaxel.
Even if paclitaxel is not the ideal drug for clinical use, there are already several reports on the development of less toxic microtubule stabilizers (45). Thus, the balance between benefits and risks of mechanomanipulation of cells as a therapeutic approach is likely to further improve in the future. In this way, the concept of cell shape stabilization to inhibit cell migration will most likely become part of the pharmacological armamentarium in clinical medicine.
Conclusion
We have shown that differentiation of APL cells following the neutrophil lineage is associated with an increase of cell deformability, which appears to be regulated by the actin cytoskeleton. This softening of cells facilitates cell mobility, which is crucial for physiological neutrophil function. On the other hand, pharmacological stabilization of microtubules interferes with the dynamical shape remodeling of differentiated cells leading to a significant reduction of cell motility through size-limited pores. Both aspects demonstrate the important link between cell mechanics and migration. Impairment of cell shape changes has the potential to further extend the treatment options for diseases relying on an increased migratory activity of cells, notably cancer metastasis.
Methods
Cell Culture.
The APL cell line NB4 (14) was kept in RPMI medium 1640 supplemented with 10% FCS, 2 mM L-glutamine, and 100 U/mL penicillin and streptomycin (Gibco). For differentiation, APL cells were incubated with 5 μM all-trans retinoic acid (ATRA; Sigma) dissolved in dimethyl sulfoxide (DMSO) for up to 4 days (46). Neutrophils were separated from whole blood samples with the density gradient Polymorphprep (AxisShield) according to the instructions of the manufacturer. Cells were incubated with 5 μM paclitaxel (T7402) and 100 μM blebbistatin (B0560) for 1 h or with 1 μM latrunculin A (L5163) for 30 min. All chemicals were obtained from Sigma Aldrich, unless stated otherwise.
Microfluidic Optical Stretcher (μOS) Setup and Experiments.
The setup and handling of the μOS has been described previously (47). Mechanical properties of the cells were characterized with μOS by creep compliance measurements (48). Cells at a concentration of 5 × 105 cells/mL were introduced into the microfluidic system and serially trapped and measured. The cell size and the relative deformation during the experiment were recorded by video-microscopy (Fig. 1C). The refractive index of cells, which is required for the calculation of the applied stress, was determined by immersion refractometry using BSA solutions as described previously (32). From the power applied, the refractive indices measured, the known laser beam parameters, and the size of the cell in the trap, the stress magnitude, σo, and distribution was calculated as described elsewhere (22). Details of the stress distribution were used to determine the geometric factor FGas described in Ananthakrishnan et al. (23). The relative deformation of the cells was then normalized by the stress magnitude and the geometric factor to result in the creep compliance, or deformability, D(t) of each cell. In addition to the creep experiments, we always performed a relaxation experiment. The experiments were done at room temperature. All statistical analysis to determine significant differences between two populations was performed with an independent t test at the 95% confidence level.
Migration Assays.
Migration assays were performed in three different ways. In a 2D experiment a drop of cell suspension was observed on a human fibronectin coated glass slide. A drop of 100 nM fMLP was added at one corner of the glass slide to induce chemotaxis. A second assay used 5- and 12-μm pore-Transwell filters (Costar). Both sides of the polycarbonate membrane were coated with human fibronectin at a concentration of 5 μg/mL. Cells were placed in the upper chamber and allowed to migrate for 3 h into the lower chamber, which contained 100 nM fMLP. The third migration experiment used the setup described in ref. 36. 3D microfluidic chambers made from PDMS were fabricated by photolithography. Two reservoirs respectively containing cell suspension and 100 nM fMLP in cell medium were connected by 5–7-μm wide, 8-μm high and 100-μm long channels. Cells were observed migrating into and along these channels. The entry time of a cell migrating into a channel was defined as the time span from first contact of the cell with the channel to the moment when the whole cell body was completely inside the channel. Statistical comparisons of migration speeds and entry times were done with a Mann-Whitney test.
Electron Microscopy.
Cells were centrifuged on slides, washed with PBS, and an extraction solution (0.5% Triton X-100 in PBS) was added for 10 min. Cells were then fixed with 2% glutaraldehyde and 2% formaldehyde (Polysciences) in 0.1 M cacodylate buffer (pH 7.3) for 10 min. Slides were subjected to critical-point drying, coated with 3 nm platinum-carbon by electron beam evaporation, and imaged with an in-lens scanning electron microscope (S-5200, Hitachi).
Fluorescence Microscopy.
Cells were centrifuged onto slides, fixed with 4% formaldehyde, and permeabilized with 0.1% Triton X-100. Primary antibodies against α-tubulin (Sigma) and, subsequently, Cy-3 coupled secondary antibodies (Dianova) were added for 1 h each. Imaging was performed with a fluorescence microscope (Leica TCS 4D) equipped with a 100× objective.
Supplementary Material
Acknowledgments.
We thank Susanne Ebert, Brian Lincoln, Elke Wolff-Hieber, Iris Repple, Paul Walther, Falk Wottawah, Kristian Franze, Kevin Chalut, and Josef Käs for technical assistance, advice, and helpful discussions and Pietro Cicuta for the cell speed analysis in 2D. This work was supported by European Fund for Regional Development 2000–2006 Sächsische Aufbau-Bank Project R&D Grant 9889/1519 and the state of Saxony (to J.G.) and by the German Research Association Grants SFB 518 and BE2339/2-1 (to M.B.).
Footnotes
Conflict of interest statement: J.G. holds a patent on the optical stretcher technique and consults on its potential applications.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811261106/DCSupplemental.
References
- 1.Dormann D, Weijer CJ. Imaging of cell migration. EMBO J. 2006;25:3480–3493. doi: 10.1038/sj.emboj.7601227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffy MJ, McGowan PM, Gallagher WM. Cancer invasion and metastasis: Changing views. J Pathol. 2008;214:283–293. doi: 10.1002/path.2282. [DOI] [PubMed] [Google Scholar]
- 3.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17:524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Wolf K, et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Bio. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nature Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 6.Lammermann T, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 7.Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3:413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai MA, Waugh RE, Keng PC. Passive mechanical behavior of human neutrophils: effects of colchicine and paclitaxel. Biophys J. 1998;74:3282–3291. doi: 10.1016/S0006-3495(98)78035-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kole TP, Tseng Y, Jiang I, Katz JL, Wirtz D. Intracellular mechanics of migrating fibroblasts. Mol Biol Cell. 2005;16:328–338. doi: 10.1091/mbc.E04-06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olins AL, Herrmann H, Lichter P, Olins DE. Retinoic acid differentiation of HL-60 cells promotes cytoskeletal polarization. Exp Cell Res. 2000;254:130–142. doi: 10.1006/excr.1999.4727. [DOI] [PubMed] [Google Scholar]
- 11.Lichtman MA. Cellular deformability during maturation of myeloblast - Possible role in marrow egress. N Engl J Med. 1970;283:943–948. doi: 10.1056/NEJM197010292831801. [DOI] [PubMed] [Google Scholar]
- 12.Lichtman MA. Rheology of leukocytes, leukocyte suspensions, and blood in leukemia - Possible relationship to clinical manifestations. J Clin Invest. 1973;52:350–358. doi: 10.1172/JCI107191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avvisati G, Tallman MS. All-trans retinoic acid in acute promyelocytic leukaemia. Best Pract Res Clin Haematol. 2003;16:419–432. doi: 10.1016/s1521-6926(03)00057-4. [DOI] [PubMed] [Google Scholar]
- 14.Lanotte M, Martin-Thouvenin V, Najman S, Balerini P, Valensi F, Berger R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3) Blood. 1991;77:1080–1086. [PubMed] [Google Scholar]
- 15.Bruel A, et al. Remodeling of vimentin cytoskeleton correlates with enhanced motility of promyelocytic leukemia cells during differentiation induced by retinoic acid. Anticancer Res. 2001;21:3973–3980. [PubMed] [Google Scholar]
- 16.Frankel S, Eardley A, Lauwers G, Weiss M, Warrell R. The retinoic acid syndrome in acute promyelocytic leukemia. Ann Intern Med. 1992;117:292–296. doi: 10.7326/0003-4819-117-4-292. [DOI] [PubMed] [Google Scholar]
- 17.Lam WA, Rosenbluth M J, Fletcher DA. Chemotherapy exposure increases leukemia cell stiffness. Blood. 2007;109:3505–3508. doi: 10.1182/blood-2006-08-043570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol. 2007;2:780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- 19.Thoumine O, Ott A. Time scale dependent viscoelastic and contractile regimes in fibroblasts probed by microplate manipulation. J Cell Sci. 1997;110:2109–2116. doi: 10.1242/jcs.110.17.2109. [DOI] [PubMed] [Google Scholar]
- 20.Hochmuth RM. Micropipette aspiration of living cells. J Biomech. 2000;33:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- 21.Dubin-Thaler BJ, Giannone G, Dobereiner HG, Sheetz MP. Nanometer analysis of cell spreading on matrix-coated surfaces reveals two distinct cell states and STEPs. Biophys J. 2004;86:1794–1806. doi: 10.1016/S0006-3495(04)74246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guck J, Ananthakrishnan R, Mahmood H, Moon TJ, Cunningham CC, Käs J. The optical stretcher: A novel laser tool to micromanipulate cells. Biophys J. 2001;81:767–784. doi: 10.1016/S0006-3495(01)75740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ananthakrishnan R, et al. Quantifying the contribution of actin networks to the elastic strength of fibroblasts. J Theor Biol. 2006;242:502–516. doi: 10.1016/j.jtbi.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 24.MacKintosh FC, Käs J, Janmey PA. Elasticity of semiflexible biopolymer networks. Phys Rev Lett. 1995;75:4425–4428. doi: 10.1103/PhysRevLett.75.4425. [DOI] [PubMed] [Google Scholar]
- 25.Gittes F, Mickey B, Nettleton J, Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J Cell Biol. 1993;120:923–934. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- 28.Beil M, et al. Spatial distribution patterns of interphase centromeres during retinoic acid-induced differentiation of promyelocytic leukemia cells. Cytometry. 2002;47:217–225. doi: 10.1002/cyto.10077. [DOI] [PubMed] [Google Scholar]
- 29.Titushkin I, Cho M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys J. 2007;93:3693–3702. doi: 10.1529/biophysj.107.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moisan , Chiasson S, Girard D. The intriguing normal acute inflammatory response in mice lacking vimentin. Clin Exp Immunol. 2007;150:158–168. doi: 10.1111/j.1365-2249.2007.03460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang N, Stamenovic D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am J Physiol Cell Physiol. 2000;279:C188–194. doi: 10.1152/ajpcell.2000.279.1.C188. [DOI] [PubMed] [Google Scholar]
- 32.Guck J, et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J. 2005;88:3689–3698. doi: 10.1529/biophysj.104.045476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rotsch C, Radmacher M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: An atomic force microscopy study. Biophys J. 2000;78:520–535. doi: 10.1016/S0006-3495(00)76614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brangwynne CP, et al. Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J Cell Biol. 2006;173:733–741. doi: 10.1083/jcb.200601060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olins AL, Olins DE. Cytoskeletal influences on nuclear shape in granulocytic HL-60 cells. BMC Cell Biol. 2004;5:30. doi: 10.1186/1471-2121-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faure-Andre G, et al. Regulation of dendritic cell migration by CD74, the MHC class II-associated invariant chain. Science. 2008;322:1705–1710. doi: 10.1126/science.1159894. [DOI] [PubMed] [Google Scholar]
- 37.Gabriele S, Benoliel AM, Bongrand P, Theodoly O. Microfluidic investigation reveals distinct roles for actin cytoskeleton and myosin II activity in capillary leukocyte trafficking. Biophys J. 2009;96:4308–4318. doi: 10.1016/j.bpj.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tallman MS, et al. Clinical description of 44 patients with acute promyelocytic leukemia who developed the retinoic acid syndrome. Blood. 2000;95:90–95. [PubMed] [Google Scholar]
- 39.Zang CB, Liu HY, Ries C, Ismair MG, Petrides PE. Enhanced migration of the acute promyelocytic leukemia cell line NB4 under in vitro conditions during short-term all-trans-retinoic acid treatment. J Cancer Res Clin Oncol. 2000;126:33–40. doi: 10.1007/PL00008462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanz MA, Tallman MS, Lo-Coco F. Tricks of the trade for the appropriate management of newly diagnosed acute promyelocytic leukemia. Blood. 2005;105:3019–3025. doi: 10.1182/blood-2004-09-3475. [DOI] [PubMed] [Google Scholar]
- 41.de la Serna J, et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;111:3395–3402. doi: 10.1182/blood-2007-07-100669. [DOI] [PubMed] [Google Scholar]
- 42.Mirzapoiazova T, Kolosova IA, Moreno L, Sammani S, Garcia JG, Verin AD. Suppression of endotoxin-induced inflammation by taxol. Eur Respir J. 2007;30:429–435. doi: 10.1183/09031936.00154206. [DOI] [PubMed] [Google Scholar]
- 43.Schnaeker EM, et al. Microtubule-dependent matrix metalloproteinase-2/matrix metalloproteinase-9 exocytosis: Prerequisite in human melanoma cell invasion. Cancer Res. 2004;64:8924–8931. doi: 10.1158/0008-5472.CAN-04-0324. [DOI] [PubMed] [Google Scholar]
- 44.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nature Rev. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 45.Mooberry S. Strategies for the development of novel Taxol-like agents. Methods Mol Med. 2007;137:289–302. doi: 10.1007/978-1-59745-442-1_20. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, et al. Retinoic acid induces leukemia cell G1 arrest and transition into differentiation by inhibiting cyclin-dependent kinase-activating kinase binding and phosphorylation of PML/RAR{alpha} FASEB J. 2006;20:2142–2144. doi: 10.1096/fj.06-5900fje. [DOI] [PubMed] [Google Scholar]
- 47.Lincoln B, Wottawah F, Schinkinger S, Ebert S, Guck J. High-throughput rheological measurements with an optical stretcher. Methods Cell Biol. 2007;83:397–423. doi: 10.1016/S0091-679X(07)83017-2. [DOI] [PubMed] [Google Scholar]
- 48.Wottawah F, et al. Characterizing single suspended cells by optorheology. Acta Biomater. 2005;1:263–271. doi: 10.1016/j.actbio.2005.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.