Abstract
The mitotic spindle self-assembles in prometaphase by a combination of centrosomal pathway, in which dynamically unstable microtubules search in space until chromosomes are captured, and a chromosomal pathway, in which microtubules grow from chromosomes and focus to the spindle poles. Quantitative mechanistic understanding of how spindle assembly can be both fast and accurate is lacking. Specifically, it is unclear how, if at all, chromosome movements and combining the centrosomal and chromosomal pathways affect the assembly speed and accuracy. We used computer simulations and high-resolution microscopy to test plausible pathways of spindle assembly in realistic geometry. Our results suggest that an optimal combination of centrosomal and chromosomal pathways, spatially biased microtubule growth, and chromosome movements and rotations is needed to complete prometaphase in 10–20 min while keeping erroneous merotelic attachments down to a few percent. The simulations also provide kinetic constraints for alternative error correction mechanisms, shed light on the dual role of chromosome arm volume, and compare well with experimental data for bipolar and multipolar HT-29 colorectal cancer cells.
Keywords: assembly speed and accuracy, merotelic attachments, microtubules, search and capture
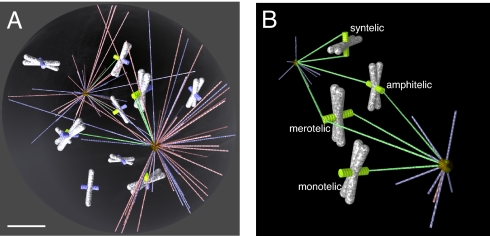
The mitotic spindle is a complex molecular machine segregating chromosomes (1, 2). Molecular inventory and general principles of the spindle dynamics are becoming clear (3), but quantitative understanding of spindle mechanics in general and its self-assembly in particular is lacking. The first hypothesis of how the spindle assembles, elegantly called “search and capture” (Fig. 1A), was put forward in ref. 4 after the discovery of the dynamic instability phenomenon: Microtubules (MTs) grow and shorten rapidly and repeatedly from the centrosomes in random directions “searching” for the kinetochores (KTs), specialized chromosome structures that function as an interface between the chromosomes and the mitotic spindle. Whenever a growing MT plus end runs into a KT, this MT is stabilized; the assembly is complete when all KTs are thus captured transforming two MT asters into a typical bipolar spindle. Capture of a single astral MT by a KT has been visualized directly in newt lung cell cultures (5).
Fig. 1.
Computer model of spindle assembly. (A) MTs (growing in blue, shortening in red, captured in green) searching from two foci (centrosomes) for KTs (captured in green, not captured in blue) on the chromosomes (white/gray). (Scale bar, 2 mm.) (B) Four possible types of chromosome attachments. Amphitelic attachment: The two sister KTs are bound to MTs coming from opposite poles. Monotelic attachment: One sister KT is bound to MTs, whereas the other is unattached. Syntelic attachment: Both sister KTs are bound to MTs from the same spindle pole. Merotelic attachment: One KT is bound to MTs from opposite spindle poles.
How can hundreds of MTs turning over in tens of seconds capture tens of chromosomes within 10–20 min (6) is one of the fundamental questions of mitosis. Mathematical modeling has been instrumental in attempts to answer this question, because it is very hard to experimentally resolve individual MTs, follow their formation, and perturb their dynamics (7). First applications of modeling were the analyses (8, 9) suggesting that the dynamic instability parameters have to be optimized to ensure fast assembly, so that a MT switches from growth to shortening when it is as long as the distance between the centrosome and the chromosome. This analysis was extended (10) to simulate hundreds of MTs searching for tens of KTs in realistic geometry. The simulations demonstrated that even optimally fine-tuned dynamic instability cannot explain the typical observed prometaphase duration of 10–20 min. However, a spatially biased search and capture process, in which the MTs grow without catastrophes within the nuclear sphere (i.e., volume through which chromosomes are distributed upon nuclear envelope breakdown) and catastrophe very fast away from it is predicted to be fast enough (10). The likely mechanisms for such spatial bias are the RanGTP gradient around the chromosomes (11, 12) and motor-dependent mechanisms (13, 14) locally regulating MT dynamics.
Three factors limit predictive power of our previous model (10). First, for technical reasons, the chromosome arms were “transparent” to the searching MTs, which led to overly optimistic predictions: MTs were able to search the whole nuclear space, although in reality, most of it is blocked by the chromosome arms. Second, the search-and-capture cannot explain mitosis in cells lacking centrosomes. In such cells, MTs are nucleated near the chromosomes, and then sorted into arrays with their minus ends extending outward, and finally focused at the minus ends as a result of complex activities of mitotic motors (15) establishing the spindle poles (16). It was thought that the centrosome-, and chromosome-directed pathways operate in different cells, but previously undiscovered data have demonstrated that centrosome-independent pathway occurs in cells that possess centrosomes (7) and that cells adopted both pathways for spindle self-assembly (17). Namely, the astral, centrosome-nucleated MTs capture the bundles of the KT-nucleated MTs, rather than KTs themselves, and then integrate the centrosomal–chromosomal bundles into a spindle-like structure (7). Third, in our previous study (10), we were concerned only with the speed of spindle self-assembly, but the assembly also has to be accurate: Ideally, all chromosomal connections have to be correct amphitelic attachments, in which the two sister KTs on each chromosome are captured from the opposite spindle poles, but monotelic, syntelic, or merotelic attachments are also possible (Fig. 1B). Erroneous attachments exist in early mitosis (18, 19), but later, most of them are corrected (19–22). The questions about how many erroneous attachments would result from the search-and-capture process, what kind of correction mechanisms have to be deployed, and what are the kinetic constraints on such mechanisms were raised qualitatively (23, 24), but never examined quantitatively.
In this study, we explore computationally the simplest “stochastic geometric” hypothesis of erroneous attachment formation (19, 23): Merotelic and syntelic attachments are established as errors inherent to the stochastic nature of the search-and-capture mechanism when one KT is “visible” from both spindle poles, so the MTs from the respective poles reach this KT almost simultaneously (merotely) or when sister KTs are visible from the same pole and, again, are captured from this pole at once (syntely). We estimated the number of such attachments and found it to be tremendous, exceeding by far the numbers observed experimentally. We therefore tested a number of potential error-correction mechanisms including MT turnover and chromosome turning after the first capture, and found stringent constraints on kinetics of these error-correction mechanisms. The simulations revealed that chromosomes also have to move rapidly to ensure timely spindle assembly. The model suggests that the finite chromosome volume plays a dual role, on the one hand hindering the assembly by shielding KTs at the center of the nucleus, but on the other hand accelerating MT cycles by promoting MT catastrophes. The simulations further illustrate that in the hybrid assembly pathway, the longer the chromosomal MT bundles are, the faster, but also less accurate, the assembly is, hinting that the cell has to optimize the MT dynamics to achieve the conflicting goals of efficiency (rapid assembly) and accuracy (minimizing number of erroneous attachments). We calibrated the model by quantifying prometaphase dynamics, timing, and spindle geometry in HT-29 colorectal cancer cells.
Model
We simulated vertebrate cells' spindle assembly in realistic 3D geometry (Fig. 1A). In the model, the chromosomes, KTs, and MTs are dynamic objects behaving according to computational rules inferred from cell biological hypotheses. The model rules and assumptions are: Each of two centrosomes placed at the opposite poles of the nuclear sphere's diameter anchor minus ends of 250 astral MTs. Each MT is a rod of zero thickness undergoing dynamic instability; its plus end grows steadily until a catastrophe occurs with a constant rate, upon which the MT shortens with a constant speed. While growing, the MT does not turn, and the new cycle starts with growth in a random direction. There are no MT rescues: We undertook exhaustive simulations that showed that the fastest capture occurs at zero rescue frequency, because when a MT grows with no KT on the growth path, rescues prolong such futile searches. We simulated both unbiased and biased searches. In the former, the constant catastrophe frequency is equal approximately to the MT growth rate divided by 85% of the nuclear sphere's diameter: At this frequency, a MT on average reaches the length optimal to reach the majority of KTs (10), yet does not waste time on longer cycles. In the latter, MTs are stable in the chromosomes' proximity and do not undergo any catastrophe events inside the nuclear sphere. Once a MT plus end goes beyond the volume of the nuclear sphere, it undergoes a catastrophe event and shrinks all the way back to the centrosome. In both scenarios, MTs start shortening immediately upon a collision with a chromosome arm [see discussion in supporting information (SI) Text]. A MT plus end is instantly stabilized upon encountering a KT, and this KT is said to be captured. Upon such capture, a new dynamic MT replaces the stabilized one at the same pole.
Chromosomes are modeled as solid 3D cylinders that are uniformly randomly distributed within the nuclear sphere and oriented in random directions. In the static regime (Movie S1), the chromosomes stay put, whereas in the dynamic one (Movie S2), they move and rotate randomly. KTs are modeled as cylindrical objects and are placed in pairs on opposite sides of the cylindrical surface of the chromosomes, midway along their length (Fig. 1). To simulate the chromosomal MTs, we assume that they are bundled into cylindrical objects extending from the KTs outward, so that the bundle's radius is equal to that of the KT. Therefore, when we model the hybrid centrosomal–chromosomal pathway, we simply consider longer targets placed exactly like the KTs on the chromosomal surface. When a centrosomal MT reaches the chromosomal bundle, we assume that the capture takes place, upon which, respective MTs get cross-linked by motors and ultimately establish a K-fiber. Chromosomes continue to move when one or both KTs are captured. Each KT (or extended target) has 10 binding sites on it; as soon as 10 MTs attach to a KT, any next MT that encounters such KT undergoes a catastrophe. Below, we describe additional optional model mechanisms of the error correction. The parameters and technical implementation of the computer simulations are described in Materials and Methods and SI Text
Results
Chromosome Arms Both Hinder the Search by Shielding KTs and Accelerate the Search by Shortening Unproductive MT Cycles.
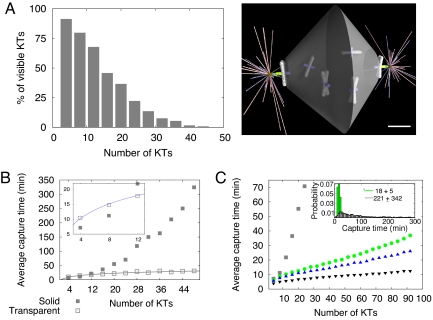
The first problem one encounters when tens of chromosomes of realistic size are uniformly and randomly distributed within the volume of the nuclear sphere is that the chromosome arms crowd the space to the extent that the chromosomes at the periphery completely shield the KTs in the interior from the MTs protruding from the spindle poles (Fig. 2A). We generated thousands of random chromosome configurations and gathered statistics of the number of the visible KTs (such that a projectile from the pole can reach these KTs without encountering a chromosome arm on the way) (Fig. 2A) and observed that <10% of the KTs can be captured at all if their number is >30. Thus, there has to be a special mechanism that makes all tens of KTs available for the centrosome-guided search.
Fig. 2.
Effect of the chromosome volume and movements on the assembly speed. (A) Chromosomes near the poles shield other chromosomes in the interior of the nuclear sphere from the centrosomal MTs. As a result, the number of KTs “visible” from the spindle poles decreases rapidly as the number of the chromosomes grows. In this and all following figures, the chromosome number is equal to half of the KT number. (Scale bar, 2 μm.) (B) Average spindle assembly time (until the last KT is captured) as a function of the KT number with “transparent” (empty squares) and “solid” (shaded squares) chromosome arms. The chromosomes are distributed randomly within the nuclear sphere; only configurations with all KTs at least partially visible were chosen for the searches. The Inset shows this function for small KT numbers. (C) Computed average capture time as a function of the KT number for static chromosomes (squares) and dynamic, moving chromosomes (circles for f = 0.005/sec, upper triangles for f = 0.01/sec, lower triangles for f = 0.1/sec), where f is the characteristic frequency of a chromosome movement across the nuclear sphere. The Inset shows the histograms of the capture times in the unbiased static and dynamic regimes (f = 0.005/sec).
Next, we tested the assembly process for “smartly” arranged chromosomes: In many configurations, chromosomes were randomly distributed within the nuclear sphere but only special configurations were chosen for testing, so that all KTs were either partially or completely visible from at least one of the centrosomes. Then, the assembly was simulated many times for each such configuration. The resulting average spindle assembly time is presented in Fig. 2B as a function of the KT number and compared with the results of our previous model with transparent chromosome arms (10). For >6 chromosomes, the average assembly time with finite chromosome volume is significantly greater compared with the transparent chromosome model. Moreover, this time increases almost linearly with the number of KTs, much faster than the logarithmic increase predicted by the simplified model (10). The simple explanation for this is that more chromosomes shield a greater fraction of the KT area, so the effective target area decreases with the KT number. This rapidly lengthens the assembly time because more MT cycles are necessary before MT growth in the right direction leads to a capture event.
We noticed, however, that when the chromosome number is small, then the average search time, counterintuitively, decreases when the chromosome arms work as a shield (Fig. 2B Inset). The explanation that we gleaned from following the time-lapse movies of the in silico dynamics is that many MTs growing in the wrong direction, not having any KTs in their path, do not waste time on long cycles but, rather, encounter chromosome arms, catastrophe, and get ready to grow in a new direction faster. This observation emphasizes a plausible unexpected role of the chromosome arms in accelerating the assembly by indirectly focusing MTs into the right directions.
Chromosome Movements Accelerate Spindle Assembly.
The number of chromosomes in vertebrate cells is much greater than 10, and it is hard to imagine a mechanism able to arrange completely visible static chromosome configurations. However, chromosomes move around the nuclear sphere in prometaphase (25). Characteristic rates of these movements estimated in refs. 26 and 27, where a few microns-per-minute rates of neighbor-independent chromosome movements with frequent changes in direction were reported, suggest that the chromosomes move across the nuclear sphere within hundreds of seconds. In SIText, we report similar data for HT-29 cells, argue that the chromosomes undergo a random walk in the nuclear sphere with similar rates, and discuss physical mechanisms of these movements.
Thus, we assumed in the model that each chromosome moves (jumps to a random location within the nuclear sphere) with characteristic frequency f ≈0.001/sec to 0.1/sec and simulated such jumps (during the movement, chromosomes also reoriented; see further discussion in SI Text). Fig. 2C shows that the average capture time for dynamic chromosomes moving at frequencies ranging from 0.001/sec to 0.1/sec is order(s) of magnitude shorter than the assembly time in the case of the static chromosomes. Note also that the dynamic assembly time is not very sensitive to the chromosome number: Random displacements periodically expose each KT to multiple searching MTs, so other chromosomes barely interfere with any given KT capture. Finally, faster (greater frequency) movements decrease the assembly time, but there is the saturation effect. To conclude, chromosome movements drastically accelerate the capture, but still the average spindle assembly time at the observed chromosome mobility is ≈40 min, 3-fold longer than that observed (our data below).
Synergy of Centrosomal and Chromosomal MTs Accelerates Spindle Assembly Further.
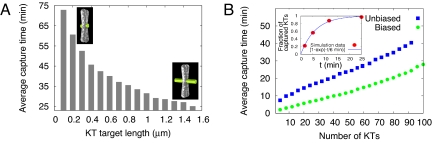
We propose, following refs.17 and 28, that the synergy between centrosomal and chromosomal assembly pathways can accelerate spindle assembly: The MT bundle (K-fiber) minus ends growing from the KTs represent greater targets for the dynamic searching centrosomal MTs. The latter encounter and capture the K-fibers by being integrated with the fibers via cross-linking and/or transport mediated by mitotic motors (15, 29). Geometrically, this means an effective increase of the target lengths. We tested the assembly process for various K-fiber lengths and found that the length increase significantly accelerates the capture (Fig. 3A): The assembly time is inversely proportional to the K-fiber length, so that just 1-μm-long K-fibers accelerate the capture ≈2-fold compared with 0.3-μm-long KTs, from ≈50 to ≈30 min.
Fig. 3.
Effect of chromosomal MTs and biased search on the assembly speed. (A) Average capture time for 92 KTs as a function of the target (either KT or chromosomal MT bundle) length. (B) Average capture time (for the target length 1 μm) in the unbiased (squares) and biased (circles) pathways. (Inset) Fraction of captured KTs as a function of time for 50 chromosomes in the biased search. Circles show the simulation results, and the curve is the exponential fit to the computational data. In A and B, the chromosomes moved with f = 0.005/sec.
Finally, we found that the spatial bias of the MT dynamics resulting from MTs being stabilized in the nuclear sphere leads to further significant (≈2-fold) reduction of the assembly time (Fig. 3B). Together, the spatial bias of the search and synergy of the centrosomal and chromosomal assembly pathways bring the total assembly time down to ≈20 min for ≈100 KTs. We estimated that HT-29 cells with ≈120 KTs take ≈13–14 min from nuclear envelope breakdown to chromosome alignment at the metaphase plate (Movie S3). It is possible that a few chromosomes may not yet be captured when most of them appear aligned at the metaphase plate. When we plotted the fraction of the captured KTs as a function of time in the biased hybrid search with the dynamic chromosomes (Fig. 3B Inset), we observed that the number of captured KTs grows exponentially, so that approximately two-thirds of the chromosomes are captured within just 6 min, and ≈90% are captured in 13 min. Thus, our computational estimate of the assembly time agrees well with the experimental data.
Without Error-Correction Mechanisms, the Majority of Connections in the Spindle Are Erroneous Syntelic and Merotelic Attachments.
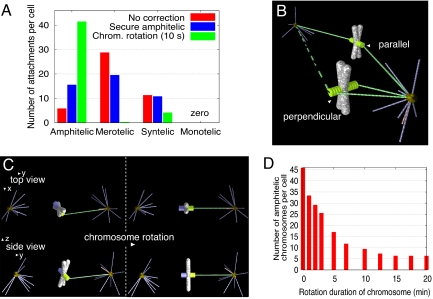
The swiftness achieved through the synergy of centrosomal and chromosomal MTs, however, comes at a price. When we counted and classified the types of MT-KT attachments for 46 chromosomes, it turned out that ≈30 of the attachments were merotelic, ≈10 were syntelic, and only ≈5 were amphitelic (Fig. 4A). This is in contrast with previous observations: Only ≈0.2 chromosomes per cell are syntelically attached in prometaphase PtK1 cells (30), and only ≈30% of prometaphase PtK1 cells possess one or two, very rarely more, merotelically oriented KTs (19).
Fig. 4.
Error generation and correction mechanisms. (A) Numbers of four types of attachments in the biased hybrid searches with 46 dynamic chromosomes (red bars, no correction; blue, amphitelic attachments are secured from further captures; green, chromosomes rotate within 10 sec of the first capture). (B) Geometric configurations conducive of the syntelic and amphitelic attachments. Additional attachment (dashed green line) from the distal pole would turn the syntelic attachment into the merotelic one. (C) Schematic illustration of the chromosome rotation after the first capture. (D) Number of the amphitelic attachments as a function of the rotation duration.
The reasons for the large predicted number of errors are the following. Once an amphitelic attachment is made, the fully captured chromosome is still “waiting” in the system until all other chromosomes are fully captured. During its waiting period, if all binding sites on its KTs are not occupied, it can still form attachments with new MTs. Sometimes the KT captured from one pole turns and gets exposed to MTs from another pole, so this KT can become merotelically attached. Because the captured chromosomes have to wait longer when the total number of chromosomes is large, the number of merotelic attachments increases with the increasing number of chromosomes. Another reason “gleaned from simulation snapshots” is that because of the crowding of the nuclear sphere, mostly the chromosomes at the periphery, close to the centrosomes, get captured. In agreement with this prediction, the attachments were observed to occur mostly between the chromosomes and proximal pole (31). Such chromosomes very often have both sister KTs visible from the centrosome, so many syntelic attachments are established rapidly (Fig. 4B). Then, additional attachments often turn those into merotelic ones.
Chromosome Rotation After Establishment of the First Attachment Is an Effective Error-Correction Mechanism.
We tested a number of plausible error-correction mechanisms. First, we tested the hypothesis that amphitelically attached KTs are secured from any further attachments: Effectively, all binding sites on the captured KT immediately become saturated with MTs from the same pole. The results shown in Fig. 4A illustrate that although merotelic attachments are reduced, this does not improve things much: There are still more merotelic attachments than amphitelic ones. The reason is that, as mentioned above, too many syntelic attachments are created in the first place, and those can only remain syntelic or become merotelic (in the model).
This prompted us to consider the idea, widely discussed in the literature (23, 24, 28), that amphitelic attachments are achieved by a process of trial and error, such that syntelic attachments are initially frequent and are dissolved repeatedly (21) until only correct stable attachments survive. Thus, we assumed that syntelic attachments are dissolved within a few seconds upon the second sister KT capture from the same pole, in addition to all amphitelic attachments being secured from any further captures. We found that this mechanism increases the capture time ≈1.5-fold, and still leaves a significant number of merotelic attachments. When, in addition to dissolving the syntelic attachments, we also implemented rapid dissolving of the merotelic connections [when one KT is being captured from both poles; respective preanaphase correction mechanism is discussed in (19, 22)], we were able to almost wipe out all incorrect attachments but at the price of prolonging the assembly process >5-fold. Thus, simply dissolving syntelic and merotelic attachments and starting the search anew improves the accuracy, but hopelessly delays the assembly.
Finally, we tested the elegant idea (23) that a KT target is shielded from the “wrong” pole by the chromosome arms, if the chromosome is oriented properly, with sister KTs facing opposite poles. Based on this idea, we assumed that, upon capture, the chromosome rotates so that the captured KT faces the pole it is captured from, whereas its sister KT faces away from that pole (Fig. 4C). The observations that the capture is inefficient when KTs point directly away from the source of properly directed MTs (32) and that proper geometry is important for the capture (33) lend indirect support to this hypothesis. Also, a rapid rotation of the centromere was consistently observed just moments before the initiation of chromosome congression (31, 34).
Thus, we propose that after a KT becomes attached to a MT, the chromosome rotates about the point of MT-KT attachment to align the inter-KT axis along the direction of the MT (Fig. 4C). In the simulations, during the finite time of the rotation process, the captured and uncaptured KTs are still capable of forming new attachments with the MTs growing from either pole. After the chromosome is fully rotated, the captured KT is geometrically incapable of making further attachments with the other pole. Fig. 4A shows the simulation results: This error-prevention mechanism leads to both fast and accurate assembly under the condition that the chromosome rotation takes <10–20 sec (Fig. 4D). A few remaining syntelic attachments can be rapidly dissolved, and then the respective chromosomes are likely to be captured accurately without significant delay. We also investigated how the erroneous attachment numbers differ for biased and unbiased searches and for various lengths of the K-fibers and did not see much difference: The growing KT-fibers do not compromise the accuracy because they are shielded from the wrong poles by the chromosome arms, similarly to the KTs. The rotation error-correction mechanism also does not prolong chromosome capture.
Model Correctly Predicts an Increase in Merotelic Attachments in Multipolar Cells.
Both older (35) and recent studies (36, 37) showed that higher numbers of merotelic KTs can be found within multipolar spindles, suggesting that spindle geometry might have an effect on establishment of correct vs. incorrect KT attachment. Thus, we set out to test the hypothesis that spindle assembly in the presence of an increasing number of spindle poles would result in an increasing number of KT misattachments (particularly merotelic), because, as previously suggested (36, 37), a single KT would be more likely to face more than one spindle pole within a multipolar spindle compared with a bipolar one, in which the two spindle poles are positioned 180° from each other (Fig. 5 A and B). Thus, we simulated the search-and-capture process in three-, four- and six-polar spindles. A snapshot of a characteristic tripolar simulation is shown in Fig. 5B. In these simulations, we placed the poles at the North and South poles and equator of the nuclear sphere for the three-polar spindle, one additional pole at the opposite side of the equator for the four-polar spindle, and two more poles at the equator, so that four poles at the equator were equidistant, for the six-polar spindle. We also kept the total number of independently searching MTs constant in all simulations; otherwise, all model parameters were the same as those in the bipolar spindle simulations. These simulations predicted increase of the merotelic attachments per cell from ≈4.2 in the bipolar spindle to ≈4.8, 5.6, and 6.3 in the three-, four-, and six-polar spindles, respectively (Fig. 5C).
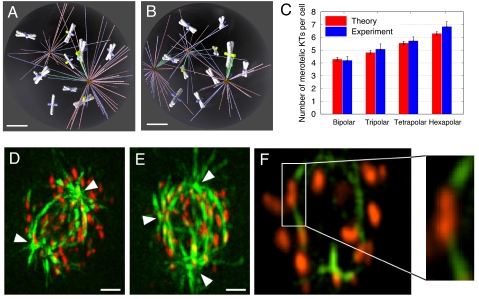
Fig. 5.
Spindle assembly in the presence of an increasing number of spindle poles results in increasing numbers of merotelic KTs. (A and B) Snapshots of the search-and-capture simulation in a bipolar (A) and a tripolar (B) spindle. (Scale bars, 2 μm.) (C) Predicted (red) and observed numbers (blue) of merotelic attachments in prometaphase cells with two, three, four, and six spindle poles. Because cells with more than four spindle poles are rare, the experimental data for cells with five to eight poles were pooled together in the “Hexapolar” category. The data shown represent means and standard errors (bars). (D and E) Bipolar (D) and tripolar (E) prometaphase HT-29 cells immunostained for MTs (green) and KTs (red). Arrowheads point at spindle poles. Images were acquired and processed as described in Methods in SI Text. (Scale bars, 5 μm.) (F) Enlargement of one focal plane from the cell shown in E, in which a merotelic KT (boxed area) is visible. A zoomed view of the boxed area in F is shown on the right.
We next compared the predicted estimates obtained in the simulations with our experimental model of HT-29 cells, which possess ≈60 chromosomes, and in which ≈10% of cells in early prometaphase assemble multipolar spindles (37). We used high-resolution confocal microscopy combined with 3D visualization and image processing (see Methods in SI Text for details) to identify merotelic KTs in prometaphase HT-29 cells immunostained for KTs and MTs (Fig. 5 D–F). We determined the number of merotelic KTs in prometaphases with two, three, four, and five to eight spindle poles (38, 36, 37, and 38 cells, respectively), and found averages of 4.13, 5.08, 5.73, and 6.84 (Fig. 5C), respectively. These frequencies are very close to those determined in the simulations (Fig. 5C), thus demonstrating the predictive power of our model.
Discussion
We reconstituted in silico the process of mitotic spindle self-assembly in prometaphase, in which hundreds of dynamically unstable MTs grow in random directions and shrink repeatedly until their plus ends encounter KTs, and all chromosomes are thus captured. The chromosomes are crowded into the limited volume of the nuclear sphere, so that the chromosome arms at the periphery shield the KTs of the chromosomes in the nuclear interior. Curiously, the chromosome arms not only hinder the search, but also accelerate it by inducing catastrophes of the wrongly oriented MTs and indirectly focusing the MTs in the right directions. The chromosomes are mobile in prometaphase, and we hypothesize that this mobility is crucial for the assembly speed because it steers the chromosomes exposing all KTs to the searching MTs.
The simulations show that the unbiased MT growth cannot ensure the observed 10- to 20-min-long assembly, but that two factors, acting together, can drastically accelerate prometaphase. The first one—RanGTP-mediated spatial bias of the MT growth into the nuclear sphere (11, 12)—has been investigated earlier (10). The second factor is the synergy of the centrosomal and chromosomal pathway. We found that if astral MTs search for the chromosomal MT bundles growing from the KTs, rather than for the KTs themselves, spindle assembly is faster (10–20 min) because of the increased size of the effective search targets. This finding predicts that MT ends growing from the KT should be incorporated within the forming mitotic spindle. Indeed, there is increasing evidence that such a pathway contributes to spindle assembly in a number of cell types (7, 29).
However, although the combined centrosomal and chromosomal pathway can speed up the process, it leads to a high number of erroneous attachments, which is in disagreement with observed frequencies of such misattachments in experimental models (19, 30). We also found that securing amphitelic attachments does not fix the problem. We tested, further, whether the widely discussed schematic mechanism of detecting and dissolving the syntelic (and partially merotelic) attachments works and saw that although such mechanism, indeed, “proofreads” the spindle very effectively, it leads to delays of the assembly, so in this case, accuracy comes at the price of speed. Finally, we found that the spindle assembly can be accurate without compromising its speed if, within ≈10–20 sec after the first capture, chromosomes are rotated so that the captured KT faces the pole from which it was captured, and the sister KT becomes shielded from this pole by the chromosome arms. We further discuss the correction and rotation mechanisms and molecular pathways in the SI Text.
These conclusions provide quantitative constraints and hypotheses for future studies of mitotic spindle assembly. We calibrated the model using observations of bi- and multipolar colorectal cancer (HT-29) cells. The predictive power of the model is confirmed by the correct predictions of the numbers of the merotelic attachments in bipolar and multipolar HT-29 cells. The model is also in qualitative agreement with recent observations (38) that doubling the chromosome number adds ≈10 min to a ≈20-min cell division. Additional suggestions for future experiments to test the model predictions can be found in SI Text.
For clarity, we kept the computational model simple and did not include possible elaborate mechanisms, some reported and other hypothetical, which could significantly accelerate the assembly without compromising the accuracy. For example, we did not consider cooperative chromosome behavior (39, 40). We did not test the possibility of a temporal coordination of the hybrid chromosomal–centrosomal assembly pathway, in which the chromosomal MT bundles growth is delayed relative to the astral MT search (29). More hypothetical mechanisms include clustering of chromosomes or nucleation/branching of nascent MTs off the sides of the K-fibers (41). We discuss relevant issues further in SI Text. In the future, when quantitative data on MT and KT dynamics in prometaphase become available, it will not be hard to add and test an impact of these additional mechanisms on the speed and accuracy of the spindle self-assembly.
Materials and Methods
Model Simulations.
To simulate the spindle assembly model, we implemented the time-dependent, explicit agent-based simulations (42). In the beginning of each simulation, three classes of objects—chromosomes, KTs (or combined KT–chromosomal bundles), and MTs—were constructed, and then their positions and orientations were changed in the 3D space according to the computational rules described in Model, above. Technical details of the simulations and model parameters are described in SI Text.
Experimental Observations.
The model was calibrated and tested by using colorectal cancer HT-29 cells and by using a number of approaches, including high-resolution confocal microscopy and 3D analysis, phase-contrast time-lapse microscopy, and combined phase-contrast/fluorescence live-cell imaging. The detailed methods are described in SIText.
For additional information, see Figs. S1–S5 and Table S1.
Supplementary Material
Acknowledgments.
We acknowledge M. Davidson (Florida State University, Tallahassee) for the generous gift of the pmTagRFP-T-CENPB-N-22 vector. We are grateful to J. R. McIntosh and D. Sharp for useful discussions. This work was supported by National Institutes of Health Grant GM068952 (to A.M.) and partially supported by National Science Foundation Grant MCB-0842551 and Thomas F. and Kate Miller Jeffress Memorial Trust Grant J-828 (to D.C.). I.K.N. was a recipient of a Fralin Institute Summer Undergraduate Research Fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908261106/DCSupplemental.
References
- 1.Karsenti E, Vernos I. The mitotic spindle: A self-made machine. Science. 2001;294:543–547. doi: 10.1126/science.1063488. [DOI] [PubMed] [Google Scholar]
- 2.Mitchison TJ, Salmon ED. Mitosis: A history of division. Nat Cell Biol. 2001;3:E17–E21. doi: 10.1038/35050656. [DOI] [PubMed] [Google Scholar]
- 3.Scholey JM, Brust-Mascher I, Mogilner A. Cell division. Nature. 2003;422:746–752. doi: 10.1038/nature01599. [DOI] [PubMed] [Google Scholar]
- 4.Kirschner M, Mitchison T. Beyond self-assembly: From microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 5.Hayden JH, Bowser SS, Rieder CL. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: Direct visualization in live newt lung cells. J Cell Biol. 1990;111:1039–1045. doi: 10.1083/jcb.111.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rieder CL, Maiato H. Stuck in division or passing through: What happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Maiato H, Rieder CL, Khodjakov A. Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis. J Cell Biol. 2004;167:831–840. doi: 10.1083/jcb.200407090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill TL. Theoretical problems related to the attachment of microtubules to kinetochores. Proc Natl Acad Sci USA. 1985;82:4404–4408. doi: 10.1073/pnas.82.13.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holy TE, Leibler S. Dynamic instability of microtubules as an efficient way to search in space. Proc Natl Acad Sci USA. 1994;91:5682–5685. doi: 10.1073/pnas.91.12.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollman R, et al. Efficient chromosome capture requires a bias in the “search-and-capture” process during mitotic spindle assembly. Curr Biol. 2005;15:828–832. doi: 10.1016/j.cub.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Athale CA, et al. Regulation of microtubule dynamics by reaction cascades around chromosomes. Science. 2008;322:1243–1247. doi: 10.1126/science.1161820. [DOI] [PubMed] [Google Scholar]
- 12.Bastiaens P, Caudron M, Niethammer P, Karsenti E. Gradients in the self-organization of the mitotic spindle. Trends Cell Biol. 2006;16:125–134. doi: 10.1016/j.tcb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Varga V, et al. Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nat Cell Biol. 2006;8:957–962. doi: 10.1038/ncb1462. [DOI] [PubMed] [Google Scholar]
- 14.Gardner MK, et al. Chromosome congression by kinesin-5 motor-mediated disassembly of longer kinetochore microtubules. Cell. 2008;135:894–906. doi: 10.1016/j.cell.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goshima G, Nedelec F, Vale RD. Mechanisms for focusing mitotic spindle poles by minus end-directed motor proteins. J Cell Biol. 2005;171:229–240. doi: 10.1083/jcb.200505107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heald R, et al. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- 17.Wadsworth P, Khodjakov A. E pluribus unum: Towards a universal mechanism for spindle assembly. Trends Cell Biol. 2004;14:413–419. doi: 10.1016/j.tcb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Ault JG, Rieder CL. Chromosome mal-orientation and reorientation during mitosis. Cell Motil Cytoskeleton. 1992;22:155–159. doi: 10.1002/cm.970220302. [DOI] [PubMed] [Google Scholar]
- 19.Cimini D, Moree B, Canman JC, Salmon ED. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J Cell Sci. 2003;116:4213–4225. doi: 10.1242/jcs.00716. [DOI] [PubMed] [Google Scholar]
- 20.Shannon K B, Salmon E. D Chromosome dynamics: New light on Aurora B kinase function. Curr Biol. 2002;12:R458–R460. doi: 10.1016/s0960-9822(02)00945-4. [DOI] [PubMed] [Google Scholar]
- 21.Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM. Correcting improper chromosome-spindle attachments during cell division. Nat Cell Biol. 2004;6:232–237. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- 22.Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Nicklas RB. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- 24.Nicklas RB, Ward SC. Elements of error correction in mitosis: Microtubule capture, release, and tension. J Cell Biol. 1994;126:1241–1253. doi: 10.1083/jcb.126.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostergren G, Mole-Bajer J, Bajer A. An interpretation of transport phenomena at mitosis. Ann NY Acad Sci. 1960;90:381–408. doi: 10.1111/j.1749-6632.1960.tb23258.x. [DOI] [PubMed] [Google Scholar]
- 26.Levesque AA, Compton DA. The chromokinesin Kid is necessary for chromosome arm orientation and oscillation, but not congression, on mitotic spindles. J Cell Biol. 2001;154:1135–1146. doi: 10.1083/jcb.200106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murata-Hori M, Yu-li Wang YL. The kinase activity of Aurora B is required for kinetochore-microtubule interactions during mitosis. Curr Biol. 2002;12:894–899. doi: 10.1016/s0960-9822(02)00848-5. [DOI] [PubMed] [Google Scholar]
- 28.O'Connell CB, Khodjakov AL. Cooperative mechanisms of mitotic spindle formation. J Cell Sci. 2007;120:1717–1722. doi: 10.1242/jcs.03442. [DOI] [PubMed] [Google Scholar]
- 29.Tulu US, Fagerstrom C, Ferenz NP, Wadsworth P. Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr Biol. 2006;16:536–541. doi: 10.1016/j.cub.2006.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauf S, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore–microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roos UP. Light and electron microscopy of rat kangaroo cells in mitosis III. Patterns of chromosome behavior during prometaphase. Chromosoma. 1976;54:363–385. doi: 10.1007/BF00292816. [DOI] [PubMed] [Google Scholar]
- 32.Rieder CL, Salmon ED. Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle. J Cell Biol. 1994;124:223–233. doi: 10.1083/jcb.124.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loncarek J, et al. The centromere geometry essential for keeping mitosis error free is controlled by spindle forces. Nature. 2007;450:745–749. doi: 10.1038/nature06344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skibbens RV, Skeen VP, Salmon ED. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: A push–pull mechanism. J Cell Biol. 1993;122:859–875. doi: 10.1083/jcb.122.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sluder G, Thompson EA, Miller FJ, Hayes J, Rieder CL. The checkpoint control for anaphase onset does not monitor excess numbers of spindle poles or bipolar spindle symmetry. J Cell Sci. 1997;110:421–429. doi: 10.1242/jcs.110.4.421. [DOI] [PubMed] [Google Scholar]
- 36.Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silkworth WT, Nardi IK, Scholl LM, Cimini D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS One. 2009;4(8):e6564. doi: 10.1371/journal.pone.0006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Loncarek J, Khodjakov A, Rieder CL. Extra centrosomes and/or chromosomes prolong mitosis in human cells. Nat Cell Biol. 2008;10:748–751. doi: 10.1038/ncb1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka K, et al. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature. 2005;434:987–994. doi: 10.1038/nature03483. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Yu W, Liang Y, Zhu X. Kinetochore dynein generates a poleward pulling force to facilitate congression and full chromosome alignment. Cell Res. 2007;17:701–712. doi: 10.1038/cr.2007.65. [DOI] [PubMed] [Google Scholar]
- 41.Goshima G, Mayer M, Zhang N, Stuurman N, Vale RD. Augmin: A protein complex required for centrosome-independent microtubule generation within the spindle. J Cell Biol. 2008;181:421–429. doi: 10.1083/jcb.200711053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odell GM, Foe VE. An agent-based model contrasts opposite effects of dynamic and stable microtubules on cleavage furrow positioning. J Cell Biol. 2008;183:471–483. doi: 10.1083/jcb.200807129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.