Abstract
Emergence of the neural crest (NC) is considered an essential asset in the evolution of the chordate phylum, as specific vertebrate traits such as peripheral nervous system, cephalic skeletal tissues, and head development are linked to the NC and its derivatives. It has been proposed that the emergence of the NC was responsible for the formation of a “new head” characterized by the spectacular development of the forebrain and associated sense organs. It was previously shown that removal of the cephalic NC (CNC) prevents the formation of the facial structures but also results in anencephaly. This article reports on the molecular mechanisms whereby the CNC controls cephalic neurulation and brain morphogenesis. This study demonstrates that molecular variations of Gremlin and Noggin level in CNC account for morphological changes in brain size and development. CNC cells act in these processes through a multi-step control and exert cumulative effects counteracting bone morphogenetic protein signaling produced by the neighboring tissues (e.g., adjacent neuroepithelium, ventro-medial mesoderm, superficial ectoderm). These data provide an explanation for the fact that acquisition of the NC during the protochordate-to-vertebrate transition has coincided with a large increase of brain vesicles.
Keywords: anencephaly, anterior neural ridge, encephalic vesicles, pre-cordal plate
Vertebrates belong, together with cephalochordates and urochordates (or tunicates), to the group of chordates. They are generally considered to have evolved from ancestors very similar to the extant cephalochordate, the amphioxus. Like vertebrates, the amphioxus has a dorsal neural tube with ventrally a notochord and a digestive tract. However, amphioxus has a very rudimentary cephalic vesicle and sense organs. The transition between protochordates and vertebrates has been characterized by the development of sense organs (i.e., eyes and smell and auditory apparatus) and of a much larger brain, endowed with neuro-associative structures, which have developed into cognitive capacities that are particularly sophisticated in humans. The protochordate/vertebrate transition has coincided with a change in lifestyle from passive filter feeder to predator and with the appearance of a new embryonic structure, the vertebrate neural crest (NC) (1). The NC has been proposed to be responsible for the formation of a “new head” (2) on the basis of embryological data showing that the cephalic NC is at the origin of the entire facial skeleton, part of the skull vault, and most of the connective components of the vertebrate head (3, 4).
The NC originates from a fold bulging at the margins of the neural epithelium (the so-called neural folds) (3, 4). While the neural epithelium engages its dorsal closure to form the neural tube (NT), NC cells detach, become mesenchymal, and migrate throughout the embryo to yield a broad range of derivatives. In the chicken embryo, the surgical extirpation of the cephalic NC (CNC) at the early neurulation stage [5- to 6-somites stage (ss)], responsible for building up the craniofacial skeleton, results in the absence of facial skeleton together with severe defects in the mesencephalon and prosencephalon leading to anencephaly (5, 6). We have previously shown that the NT defects are accompanied by the down-regulation of several genes that are normally expressed in the telencephalon, diencephalon, and mesencephalon. Such is the case for Fgf8, the expression of which is strongly down-regulated in the anterior neural ridge (ANR), considered as the prosencephalic organizer (7, 8). Structural defects in the development of the telencephalic vesicles ensue, together with agenesis of the thalamic and pretectal nuclei (6). The CNC and Fgf8 have been shown to be critical for growth and patterning of the prosencephalic and mesencephalic alar plate, as well as for formation of the pre-otic roof plate (6). The present work deciphers the mechanisms whereby the CNC controls the morphogenesis of the forebrain and midbrain and reveals the nature of signals involved in this process. This regulation results from the action of bone morphogenetic protein (Bmp) antagonists of CNC origin, and depends on multi-step interactions between the CNC cells and the neighboring tissues, involving the cephalic neuroepithelium, the pre-cordal plate (Pcp), and the superficial ectoderm.
At embryonic day 2.5 (E2.5), while Fgf8 expression is maximal in the ANR, Bmp4 transcripts are detected in the neighboring ectoderm (9). Changes in Bmp activity have been shown to influence Fgf8 expression in a variety of morphogenetic processes (10, 11). Moreover, mis-expression of Bmp antagonists in the node and ventral mesoderm are detrimental to head development (12, 13). As the CNC is a source of Bmp antagonists (i.e., Gremlin and Noggin) (14, 15), it may be hypothesized that the NC controls the expression of Fgf8 in the ANR by down-regulating the activity of Bmp through the production of Gremlin and Noggin. If so, removal of the CNC would then result in an increase of Bmp4 signaling, which in turn could decrease the production of Fgf8. To test this hypothesis, the expression of the signaling molecules in the ANR and CNC were selectively modulated in chick embryos at neurula stages.
Results and Discussion
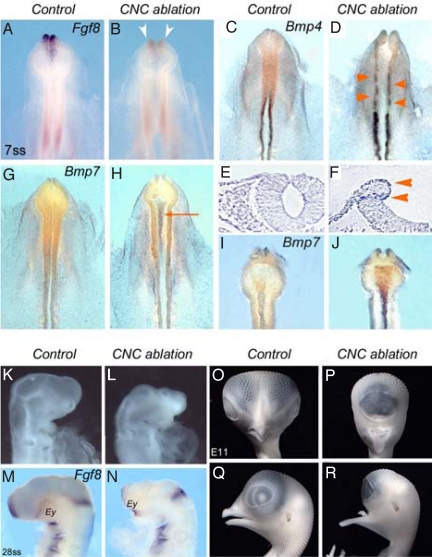
In a first attempt to influence Bmp expression in the developing head, the source of noggin and gremlin, i.e., the CNC itself [supporting information (SI) Fig. S1], was surgically removed at 5 to 6 ss. This operation was previously shown to abolish facial skeleton development and to result in anencephaly (6). As soon as 90 min after the operation, Fgf8 expression was already down-regulated in the presumptive territory of the ANR (n = 18; Fig. 1 A and B). The observation of Bmp expression in the embryo only 90 min after CNC excision revealed changes in the expression pattern of both Bmp4 and Bmp7 in 2 different sites. Bmp4 expression was activated in the dorsal NT, at the level of the ablation (n = 16; Fig. 1 C and D). Bmp4 transcripts were present at the level of the “healing” point, where the superficial ectoderm and the neuro-epithelium recovered their continuity along the edges of the excision, as well as at the level of the “hinge” point where the latero-dorsal neuroepithelium folded (Fig. 1 E and F). Moreover, Bmp4 was up-regulated in the neural folds posterior to the level of the ablation. Concomitantly, Bmp7 transcripts that are normally present in very small amounts in the Pcp and in the floor plate-notocord complex at this stage (16) were abundantly accumulated in these structures in CNC-deprived embryos (n = 17; Fig. 1 G-J). In contrast, gene expression patterns of other morphogens such as Bmp2 or Shh (in notocord and Pcp), responsible for neurulation defects in the spinal cord (17) were not modified in CNC-deprived embryos at 7 ss (Fig. S2 A–D).
Fig. 1.
Expression Fgf8 and Bmps at 7 ss following the ablation of CNC at 5 to 6 ss. (A and B) Fgf8 activation in ANR of 7-ss control embryo (A) is lost in CNC-ablated embryo (B; arrowheads). (C-F) Bmp4, which is normally no longer expressed in the anterior CNC at 7 ss (C), is activated in the neuroepithelium underlying the excised CNC and up-regulated in the more caudal rhombencephalic CNC (D; arrowheads). (E and F) On a transverse section at the mesencephalic level, Bmp4 transcripts are present at the healing point where the ectoderm and neuroepithelium abut each other and at the hinge point where the neuroepithelium folds (F; arrowheads; compare with control in E). (G-J) At 7 ss, Bmp7 transcripts are slightly accumulated in the Pcp in control (G and I), whereas in CNC-ablated embryos (H; arrow), Bmp7 expression is considerably increased in Pcp and notochord, as evidenced on the ventral side after dissection (J). (K-R) Effect of Bmp7 on brain development. (K) Normal head development at E2.5. Injection of Bmp7 results in the inhibition of pre-otic brain development (L), the down-regulation of Fgf8 in ANR (N), and the convergence of eye fields (Ey; compare with control in M). (O-R) Gross anatomy of control (O and Q) and Bmp7-supplemented (P and R) embryos at E11. (M and N) These alterations result in long-term reduction of forebrain morphogenesis, cyclopia, and agenesis of naso-frontal structures, whereas the mandibular ones are not affected (Q and R).
These observations indicated that the activation of Bmp7 in Pcp (at least partly) relied on an interaction with the CNC cells, and raised the hypothesis that elimination of the source of Bmp antagonists (i.e., the CNC) resulted in the increase of Bmp7 expression in Pcp. To explore this supposition, dsRNA molecules (18) designed against Noggin and Gremlin were bilaterally transfected into the cephalic neural folds at 5 to 6 ss, before the onset of CNC cell migration (Fig. S2 E–H). When analyzed 90 min later, silencing Noggin and Gremlin in CNC cells brought about remodeling of Bmp7 activity in Pcp. It resulted in expanding Bmp7 expression but to a lesser extent than observed in CNC-deprived embryos (Fig. S2 F–J). This indicated that migrating CNC cells likely early interact with the Bmp synthesis in Pcp through a signaling pathway that has not been identified so far, and that partly depends on the level of Bmp inhibitors.
To document the effect of the CNC on forebrain development through the possible down-regulation of Bmp7 in the Pcp, exogenous Bmp7 protein was injected within the Pcp area at 5 to 6 ss in normal chick embryos. Supplying Pcp with Bmp7 strongly affected fore- and midbrain development and resulted in a nearly complete loss of Fgf8 expression in the ANR at E2 (Fig. 1 K-N). The long-term effect of Bmp7 supplementation was spectacular. At E11, the nasofrontal and maxillary buds had not developed, and operated embryos had a cyclopean morphology. Whereas the formation of the upper face was totally inhibited, the more caudal structures (i.e., mandible and entoglossum, which are derived from the first branchial arch) were unaffected (n = 7; Fig. 1 O-R). Here, cyclopia resulted from the total failure of telencephalic growth and fusion of 2 optic vesicles, a phenotype reminiscent of that obtained in CNC ablation performed at earlier stages (at 3 ss) (19).
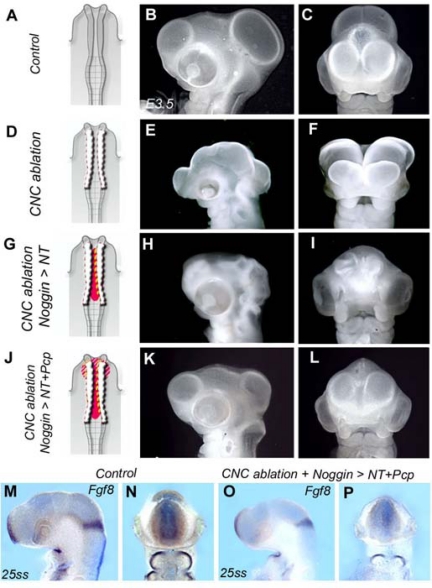
Experiments were designed to test whether the sources of Bmp4 and Bmp7 (i.e., the Pcp and the NT) could interfere with brain morphogenesis (Fig. 2). In a first series, Noggin protein was injected at the concentration of 0.1 μg/μL (5–6 ng per injection) within the neural groove following CNC ablation in 5- to 6-ss chick embryos (Fig. 2 A, D, and G). The choice of this low concentration was to restrict the effect of Bmp inhibitor to the neural epithelium. In the treated embryos, the neural folds fused and the NT remained closed up to E4 (Fig. 2 G-I; compare with anencephaly in Fig. 2 E and F). However, the cephalic vesicles failed to develop normally and remained collapsed (n = 11).
Fig. 2.
Graduated and cumulative effects of CNC on NT closure and cephalic vesicle growth. Morphology of control (A-C), CNC-ablated (D-F), CNC-ablated Noggin-injected in NT (G-I), and CNC-ablated noggin-injected in NT and Pcp (J-L) embryos at E4. Side (B, E, H, and K) and frontal (C, F, I, and L) views are shown. (D) CNC ablation (dotted lines) results in anencephaly (E and F). (G) When noggin protein (in red) is injected in the NT after CNC ablation, the brain closes but prosencephalon and mesencephalon remain collapsed (H and I). (J) Simultaneous injections of noggin in NT and Pcp in CNC-deprived embryos result in maintenance of NT closure and expansion of cephalic vesicles (K and L). However, even in this context, the brain remains under-developed (compare with B). Local injections of Noggin are not sufficient to restore Fgf8 in the ANR and the isthmus (O and P; compare with control in M and N).
In another series, in addition to the neural groove, the same amount of Noggin was also injected into the Pcp of CNC-ablated embryos (Fig. 2J). In this series, when Noggin was injected in both places (Fig. 2J), the development of the brain was significantly improved (Fig. 2 K and L). The NT was not only closed, but the cerebral vesicles were clearly formed (n = 13), meaning the effects of Bmp4 and Bmp7 had been counteracted by the injection of Noggin in the NT (for Bmp4) and the Pcp (for Bmp7). However, Fgf8 expression in the ANR at E2.5 (26 ss) was still significantly reduced and normal brain morphogenesis was not completely restored: the cephalic vesicles remained under-developed compared with those in control embryos (compare Fig. 2 K and L with Fig. 2 B and C). Therefore, inhibition of Bmp signaling in both the neural groove and the Pcp was not sufficient to properly restore Fgf8 expression in the ANR, which was nearly completely abolished by CNC excision (Fig. 2 M–P). In these series, embryos were lacking a local positive regulation of Fgf8 expression in the ANR. Repression of Bmp signaling from the neuroepithelium and Pcp in early neurula was responsible for the induction of Fgf8 activity in the ANR but did not account for the prolonged expression of Fgf8 in ANR up to E2.5. The mechanisms involved in such a regulation were explored by the following experiments.
To test whether an additional control of Bmp signaling restricted to the level of the ANR could operate later in development, a retroviral construct (RCAS) driving the expression of Noggin was selectively transfected in the ANR following the removal of the CNC (Fig. S3 A–C). Twenty-four hours after the operation (E2.5), head development of CNC-deprived Noggin-RCAS-transfected embryos was restored to a large extent compared with that of embryos subjected merely to CNC ablation (Fig. S3 D–F). Hybridization for Fgf8 showed that the uptake of Bmp inhibitor in ANR was able to stimulate Fgf8 activity. In Noggin-RCAS-treated embryo, Fgf8 expression tended to normalize (Fig. S3F; compare with control in Fig. S3D), whereas in ablated embryos, its expression was nearly abolished (Fig. S3E). Restoration of Fgf8 expression was accompanied by a massive outflow of migrating CNC cells that emanated from the edge of the resected territory as shown by HNK1 Ab immunolabeling (Fig. S3 G–I). The migration of CNC cells in these embryos resulted in replenishing the first branchial arch and the naso-frontal bud with mesenchymal cells and concurred in maintaining NT closure (Fig. S3 G–H). At E6, despite midline defects, a significant restoration of brain morphogenesis ensued (Fig. S3 J and K). These observations suggested that, aside from the early and short-term repression of Bmp signals from the Pcp and the adjacent NT, an additional focal and prolonged regulation of Fgf8 in ANR was required for the proper patterning of brain morphogenesis.
In normal development, when the domain of Fgf8 expression is maximal in ANR at 24 to 26 ss (i.e., 50 h of incubation), Bmp4 transcripts are detected in the neighboring naso-frontal ectoderm (Fig. 3 A–D). Later on, at 30 ss (i.e., 60 h of incubation), while Bmp4 expression is initiated in the ANR, Fgf8 expression declines (9). To figure out how the morphogenetic regulations take place at the level of the ANR selectively, the following experiments were undertaken.
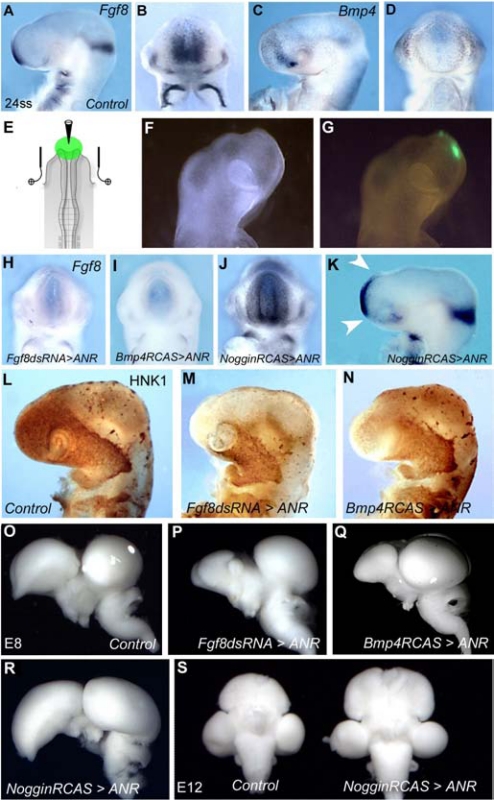
Fig. 3.
Regulation Fgf8 expression in the ANR. Side (A) and frontal (B) views of 24-ss chick (E2.5) embryo showing Fgf8 expression at the level of the ANR. (C and D) Concomitantly, Bmp4 transcripts accumulate in the superficial ectoderm flanking the site of Fgf8 expression. (E) Electroporation in the ANR: a triangular electrical field created with a triple electrode system set around the developing head of a chicken embryo at 5 ss forces the bilateral transfection of exogenous sequences into the ANR anlage. (F) Profile view of an operated embryo shows the GFP labeling (G) of the targeted ANR at 18 ss (i.e., 20 h after electroporation). Following the electroporation of Fgf8-dsRNA (H) or Bmp4-RCAS (I) in the ANR in 5- to 6-ss chick embryos, Fgf8 expression is similarly abolished in the ANR in both situations. In contrast, electroporation of Noggin in the ANR entails the production of Fgf8 transcripts in the neighboring territories, both laterally (J) and dorsally (K, arrowheads). Whole-mount HNK1 immunolabeling of E2.5 control (L), Fgf8-dsRNA (M), and Bmp4-RCAS transfected embryos (N). Knocking down Fgf8 in ANR hampers the progression of NCC in forehead territory. (O-Q) Whole-mount brain preparations isolated from control (O) and ANR-transfected (P-R) embryos at E8. Mis-expression of Fgf8 expression in the ANR following Fgf8-dsRNA (P) and Bmp4-RCAS (Q) entails a dramatic reduction of the telencephalon. (R and S) Transfection of Noggin-RCAS in ANR embryos causes the hypertrophic development of the telencephalic hemispheres and optic tectum at E8. (S) Ventral view of control (Left) and Noggin-RCAS-transfected (Right) brains at E12.
To selectively inhibit Fgf8 production in the ANR, dsRNA (18) designed against Fgf8 (Fgf8-dsRNA) was electroporated into the presumptive territory of the ANR of 5- to 6-ss chick embryos, along with pCAGGS-GFP vector (20) (Fig. 3 E–G). In these series, CNC cells were left un-perturbed. According to the same paradigm, the second strategy consisted in increasing Bmp4 expression by electroporating a retroviral construct driving the expression of Bmp4 (Bmp4-RCAS) in the ANR area. The results of these 2 approaches were strikingly similar: 30 h later, at 24 ss, Fgf8 expression was strongly down-regulated in the ANR (Fig. 3 H and I) and the rostral migration of the CNC cells that normally cover the forebrain was totally inhibited (Fig. 3 L–M). These observations were in accordance with the previously described role of Fgf8 in (i) directing the directionality of CNC cell migration in the developing head and (ii) creating a permissive environment for their proliferation (4). Following Fgf8 depletion in ANR, the phenotype of the brain at E8 showed a strong reduction of forebrain development (n = 12; Fig. 3 O–Q). This was accompanied by the disorganization of inter-hemispheric structures, the absence of olfactory bulb and nerve, and the atrophy of choroid plexuses.
The problem was thus raised how CNC cells locally interfere with the production of Fgf8 in the ANR (Fig. S1 A–C). As CNC cells produce the BMP antagonists Gremlin and Noggin (Fig. S1 D–G), the effects on Fgf8 expression and brain development of gain or loss of function of these genes were analyzed in the ANR and CNC, respectively. When the ANR was transfected with either Gremlin- or Noggin-RCAS, an increase of the volume of the whole pre-otic brain was observed. The more spectacular effect was obtained with Noggin gain of function, with the telencephalon and the optic tectum being the most affected parts (n = 11; Fig. 3R). This resulted in a long-term striking hypertrophy of the entire brain at E12 (Fig. 3S).
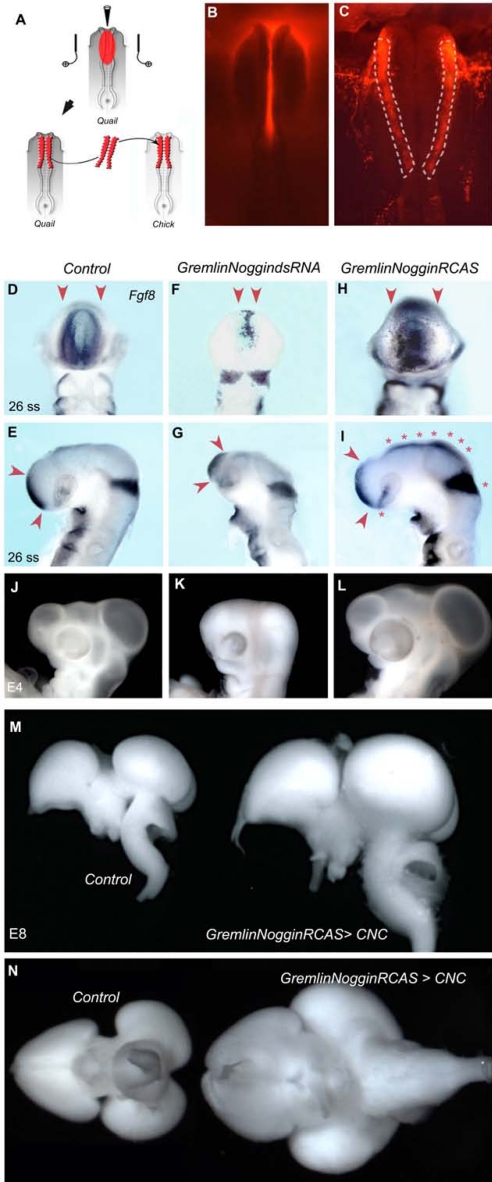
In a second step, Gremlin and Noggin were together down- or up-regulated in the CNC selectively (Fig. 4 A–C). The transfection of Gremlin- and Noggin-dsRNA in the CNC resulted in the loss of Fgf8 expression in the ANR at E2 (Fig. 4 D–G) and ruined the dorso-lateral growth of the cephalic vesicles at E4 (Fig. 4 J and K); these embryos no longer developed beyond E4 (n = 16). In contrast, co-electroporation of Gremlin- and Noggin-RCAS increased considerably the amount of Fgf8 transcripts in the ANR (Fig. 4 H and I), as well as in the isthmus (Fig. 4I). Moreover, an ectopic zone of Fgf8 expression appeared along the dorsal midline of the diencephalon and mesencephalon (Fig. 4I). At E4, the cephalic and optic vesicles were greatly enlarged when both Gremlin and Noggin cDNA were transfected in CNC cells (Fig. 4L). Gain-of-function experiments in CNC resulted in the hypertrophic growth of the entire pre-otic brain at E8 (n = 10; Fig. 4 M and N). If transfected separately to CNC cells, Bmp antagonists had a rather modest trophic effect on brain development, compared with the outcome of the joint overexpression (Fig. S4). Morphometric analysis revealed that the trophic effects exerted by the CNC subjected to Gremlin and Noggin gain of function were primarily borne on cephalic vesicles, whereas facial structures were slightly modified (Fig. S5). In contrast, it was shown that delivering Noggin in older embryos had pronounced and opposite effects on facial prominence development (21, 22).
Fig. 4.
Influence of the activity of Bmp antagonists in CNC on Fgf8 expression and brain development. (A) Two-step procedure for CNC transfection. Nucleic acids, injected in the neural groove of quail neurula, are bilaterally transfected into CNC by electroporation before CNC bilateral transplantation into un-transfected chicken embryo. (B) Co-injection and co-electroporation (C) with rhodamine dextran (Invitrogen) enables visualization of the transfected CNC (C; dotted lines) before transplantation. Fgf8 expression at E2 (D-I) and E4 morphology (J-L) of control (D, E, and J), Gremlin Noggin-dsRNA-treated (F, G, and K), and Gremlin Noggin-RCAS-treated (H, I, and L) embryos. Loss of function of Bmp antagonists in CNC alters Fgf8 expression in ANR (F and G; arrowheads). Inversely, Gremlin Noggin over-expression in CNC increases the FgF8 expression in the ANR (H and I; arrowheads) and isthmus, and induces transcript accumulation along the dorsal midline (I; stars). Normal morphology of E4 control embryo (J). (K) The loss of function results in the underdevelopment of cephalic vesicles and eyes. (L) Dorsal expansion of cephalic vesicles resulting from the overexpression of Gremlin and Noggin in CNC (M and N). Whole-mount E8 pre-otic brains: lateral (M) and ventral (N) views show a normal brain (Left) and the hypertrophied morphology (Right) induced by Gremlin Noggin overexpression in CNC.
Taken together, these results document the early mechanisms whereby the CNC participates in the shaping of the developing pre-otic brain while also regulating its size (Fig. S6). Positive changes in brain size are triggered when up-regulation of Gremlin and Noggin is selectively induced in CNC cells. Reverse effects leading to microcephaly are obtained if Bmp antagonists are knocked down in the CNC cells themselves. Hence, the level of Gremlin and Noggin expression in CNC cells influences the growth of the prosencephalon and mesencephalon. By acting on the amount of these signaling molecules, it is possible to increase the size of the fore- and midbrain of the embryo as much as 25% more than its normal size (Fig. S5). The structure and further evolution of these hypertrophied brains (as well as the development of the skeletal structures) is now under scrutiny. Moreover, the uptake of Bmp antagonists in CNC cells early induces the broadening of Fgf8 expression in the ANR and in the isthmus. Hence, the morphogenetic activities of the secondary brain organizers—the ANR, the isthmus, and the dorsal midline—are regulated by the CNC cells. This regulation is exerted by the modulation or induction of Fgf signals emanating from these territories. In addition to the correlation between variations in Gremlin and Noggin levels and cephalic vesicle growth, cumulative and multiple interactions take place between the CNC and the neighboring tissues to promote brain development. From neurula stage on, CNC counteracts the deleterious effects of Bmp production from the NT and Pcp to stimulate the cephalic neurulation and the lateral expansion of pre-otic vesicles. Altogether, these experiments argue in favor of the contention that the emergence of the NC was critical for the process of cephalization in chordates (2).
Materials and Methods
Microsurgery in Avian Embryos.
Chick and quail embryos were operated at 5 to 6 ss according to techniques previously described (23). The fate maps of the cephalic NF (24–26) have served as reference for the operations throughout this study. The fragment of the cephalic NF subjected for transfection, microsurgery, and transplantation extends from the mid-diencephalon down to, and including, r2.
dsRNA Synthesis.
dsRNA (18) were synthesized from cDNA encoding for the targeted genes. Sense and antisense strands of RNA were subsequently annealed for 5 min at 95 °C. Single strands of sense or antisense RNA were synthesized for control series. Delivery of RNAi molecules was achieved by in ovo electroporation into the 5- to 6-ss avian embryos.
In Ovo Electroporation.
Exogenous nucleic sequences (RCAS or dsRNA), mixed in a solution of Fast Green FCF 0.01% (Sigma), were blown in the presumptive anterior neuropore or the cephalic neural groove to transfect the ANR of the CNC, respectively. Triple stainless steel electrodes were placed on the vitelline membrane flanking the developing head with a gap of 5 mm, the anode facing the anterior neuropore (27). Retroviral constructs and dsRNA molecules were headed for the targeted tissues by a series of 5 × 25-V square pulses (T830 BTX; Genetronics). Co-electroporation of pCAGGS-GFP construct with the exogenous sequences (Fgf8-dsRNA or Bmp4-RCAS) was performed to monitor that the transfection was restricted to the ANR and that the telencephalic primordium was left un-transfected. Retrovirus constructs exempted of any insert were used as control vectors in knock-in series. Single strands of sense or antisense RNA were used for control series in knock-down experiments.
Recombinant Protein Injection.
Solutions of 0.5 μg/μL Bmp7 or 0.1 μg/μL Noggin recombinant proteins (R&D Systems) were injected in the Pcp, under the ventral side of the diencephalon, or in the neural groove depending on experimental series. As for plasmid injection, the protein solution was contrasted with Fast Green FCF (Sigma) to visualize the injection site.
Embryo Processing.
Whole-mount in situ hybridizations, immuno-cytochemistry, and brain dissection were performed as described (6).
Morphometric Analyses of Cephalic and Facial Variation.
Cephalic vesicles and facial structures were measured as shown in Fig. S5. The Student t test was used to compare the variation between control (n = 7) and GremlinNoggin-treated (n = 10) embryos.
Supplementary Material
Acknowledgments.
The author thanks N.M. Le Douarin for her help in preparing the manuscript, Drs. P. Brickell, M. Marx, A.-H. Monsoro-Burq, and L. Niswander for the gift of plasmids, and J.Y. Tiercelin for technical help with triple electrode design. This work was supported by the Centre National de la Recherche Scientifique, Fondation Bettencourt-Schueller, and grant 3929 from the Association de la Recherche sur le Cancer.
Footnotes
The author declares no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906072106/DCSupplemental.
References
- 1.Donoghue PC, Graham A, Kelsh RN. The origin and evolution of the neural crest. Bioessays. 2008;30:530–541. doi: 10.1002/bies.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gans C, Northcutt RG. Neural crest and the origin of vertebrates: a new head. Science. 1983;220:268–273. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- 3.Le Douarin N. The Neural Crest. Cambridge, UK: Cambridge Univ Press; 1982. [Google Scholar]
- 4.Le Douarin NM, Kalcheim C. The Neural Crest. 2nd ed. Cambridge, UK: Cambridge Univ Press; 1999. [Google Scholar]
- 5.Creuzet S, Schuler B, Couly G, Le Douarin NM. Reciprocal relationships between Fgf8 and neural crest cells in facial and forebrain development. Proc Natl Acad Sci USA. 2004;101:4843–4847. doi: 10.1073/pnas.0400869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creuzet S, Martinez S, Le Douarin NM. The cephalic neural crest exerts a critical effect on forebrain and midbrain development. Proc Natl Acad Sci USA. 2006;103:14033–14038. doi: 10.1073/pnas.0605899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- 8.Houart C, Westerfield M, Wilson SW. A small population of anterior cells patterns the forebrain during zebrafish gastrulation. Nature. 1998;391:788–792. doi: 10.1038/35853. [DOI] [PubMed] [Google Scholar]
- 9.Crossley PH, Martinez S, Ohkubo Y, Rubenstein JL. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108:183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- 10.Ohkubo Y, Chiang C, Rubenstein JL. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2001;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- 11.Aoto K, Nishimura T, Eto K, Motoyama J. Mouse GLI3 regulates Fgf8 expression and apoptosis in the developing neural tube, face, and limb bud. Dev Biol. 2002;251:320–332. doi: 10.1006/dbio.2002.0811. [DOI] [PubMed] [Google Scholar]
- 12.Bachiller D, et al. The organizer factors chordin and noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. doi: 10.1242/dev.129.21.4975. [DOI] [PubMed] [Google Scholar]
- 14.Bardot B, Lecoin L, Huillard E, Calothy G, Marx M. Expression pattern of the drm/gremlin gene during chicken embryonic development. Mech Dev. 2001;101:263–265. doi: 10.1016/s0925-4773(00)00571-2. [DOI] [PubMed] [Google Scholar]
- 15.Tzahor E, et al. Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev. 2003;17:3087–3099. doi: 10.1101/gad.1154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale JK, et al. Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal mesoderm. Cell. 1997;90:257–269. doi: 10.1016/s0092-8674(00)80334-7. [DOI] [PubMed] [Google Scholar]
- 17.Ybot-Gonzalez P, et al. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development. 2007;134:3203–3211. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
- 18.Pekarik V, et al. Screening for gene function in chicken embryo using RNAi and electroporation. Nature Biotech. 2003;21:93–96. doi: 10.1038/nbt770. [DOI] [PubMed] [Google Scholar]
- 19.Etchevers HC, Couly G, Vincent C, Le Douarin NM. Anterior cephalic neural crest is required for forebrain viability. Development. 1999;126:3533–3543. doi: 10.1242/dev.126.16.3533. [DOI] [PubMed] [Google Scholar]
- 20.Momose T, et al. Efficient targeting of gene expression in chick embryos by microelectroporation. Dev Growth Differ. 1999;41:335–344. doi: 10.1046/j.1440-169x.1999.413437.x. [DOI] [PubMed] [Google Scholar]
- 21.Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin's finches. Science. 2004;305:1462–1465. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- 22.Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Science. 2004;305:1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Douarin N, Dieterlen-Lièvre F, Creuzet S, Teillet MA. Quail-chick transplantations. Methods Cell Biol. 2008;87:19–58. doi: 10.1016/S0091-679X(08)00202-1. [DOI] [PubMed] [Google Scholar]
- 24.Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev Biol. 1985;110:422–439. doi: 10.1016/0012-1606(85)90101-0. [DOI] [PubMed] [Google Scholar]
- 25.Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev Biol. 1987;120:198–214. doi: 10.1016/0012-1606(87)90118-7. [DOI] [PubMed] [Google Scholar]
- 26.Grapin-Botton A, Bonnin MA, McNaughton LA, Krumlauf R, Le Douarin NM. Plasticity of transposed rhombomeres: Hox gene induction is correlated with phenotypic modifications. Development. 1995;121:2707–2721. doi: 10.1242/dev.121.9.2707. [DOI] [PubMed] [Google Scholar]
- 27.Creuzet S, Couly G, Vincent C, Le Douarin NM. Negative effect of Hox gene expression on the development of the neural crest-derived facial skeleton. Development. 2002;131:4301–4313. doi: 10.1242/dev.129.18.4301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.