Abstract
An estimated 3% of the global population are infected with hepatitis C virus (HCV), and the majority of these individuals will develop chronic liver disease. As with other chronic viruses, establishment of persistent infection requires that HCV-infected cells must be refractory to a range of pro-apoptotic stimuli. In response to oxidative stress, amplification of an outward K+ current mediated by the Kv2.1 channel, precedes the onset of apoptosis. We show here that in human hepatoma cells either infected with HCV or harboring an HCV subgenomic replicon, oxidative stress failed to initiate apoptosis via Kv2.1. The HCV NS5A protein mediated this effect by inhibiting oxidative stress-induced p38 MAPK phosphorylation of Kv2.1. The inhibition of a host cell K+ channel by a viral protein is a hitherto undescribed viral anti-apoptotic mechanism and represents a potential target for antiviral therapy.
Keywords: apoptosis, Kv2.1, NS5A, oxidative stress
Hepatitis C virus (HCV) is a major human pathogen, infecting ≈3% of the global population (123 million individuals) (1). The virus has a positive sense RNA genome of only 9.5 kb with a single ORF translated into a polyprotein that is subsequently processed to yield only 10 mature protein products (2). Despite this simplicity, HCV establishes a persistent infection leading to chronic liver disease in 85% of infected individuals. In common with other chronic viruses, maintenance of the viability of HCV-infected cells is vital for viral persistence and pathogenesis, yet the molecular mechanisms of this remain largely uncharacterized. In this context, the five nonstructural proteins NS3–5B comprising the C-terminal two-thirds of the polyprotein (2) in addition to replicating the viral genome, modulate host cell physiology to provide an optimal environment for viral replication and persistence.
Ion channels play a key role in the regulation of cell physiology in both excitable and nonexcitable cells. Given the importance of these channels, it is surprising that there is a paucity of published data pertaining to their dysregulation by virus infection. Here, we demonstrate that HCV inhibits the function of a specific K+ channel; Kv2.1. In response to oxidative stress, p38 MAPK is activated and phosphorylates Kv2.1. The latter is then trafficked to the plasma membrane where it mediates an outward K+ current resulting in the induction of apoptosis (Fig. S1) (3). Here, we combine biochemical and electrophysiological approaches to demonstrate that in cells infected with HCV or harboring a subgenomic HCV replicon, oxidant treatment does not induce p38 MAPK activation, Kv2.1 channel phosphorylation, or apoptosis. The HCV nonstructural NS5A protein was necessary and sufficient for this effect as, not only was suppression of Kv2.1 activity abrogated by a mutation within an SH3-binding polyproline motif in NS5A (4), but also NS5A expressed in the absence of the other viral proteins was able to mediate Kv2.1 inhibition. This study demonstrates that a viral protein can specifically target a host cell K+ channel and establishes host cell ion channel function as a potential target for the development of much needed chemotherapeutics.
Results
Expression of the HCV Nonstructural Proteins Suppresses an Outward K+ Current.
We used whole-cell patch clamp recordings to characterize ionic currents in the human hepatoma cell line, Huh-7, as this is the only cell line that supports robust HCV replication. In these experiments, we compared the current profile in parental Huh-7 cells with those stably harboring an autonomously replicating HCV subgenomic replicon RNA expressing the viral nonstructural proteins NS3-NS5B of genotype 1b (5). Recordings revealed a voltage-gated K+ current present in parental Huh-7 cells (Fig. 1A) that was absent in replicon-expressing cells (Fig. 1B). In addition, cells from which the replicon had been eradicated by prolonged treatment with IFN-α (termed “cured” cells) (Fig. 1C) showed similar K+ currents to parental Huh-7 cells demonstrating that the presence of the HCV subgenomic replicon inhibited host cell K+ channel activity.
Fig. 1.
HCV NS5A suppresses an outwardly rectifying K+ current. I/V relationships for: Parental Huh-7 (A), repliconWT (B), IFN-cured replicon (C), and repliconPA2 (D) cells (n = 6–8 cells). Insets show representative traces of outward K+ currents evoked by step depolarizations. (E) I/V relationship for cells transfected with either pEGFP (▲) or pNS5A-GFP (■), compared with mock transfected Huh-7 cells (▼). (F) I/V relationship for JFH-1ΔE1/E2 transfected cells (△) compared with mock transfected Huh-7 cells (■).
We reasoned that the NS5A protein was a likely candidate for a specific viral protein responsible for inhibition of the K+ current, as NS5A has been shown to interact with a range of cellular signaling proteins with potential to modulate host cell physiology (6). In particular, NS5A contains a C-terminal polyproline motif (PxxPxR) that binds to the SH3 domains of a number of cellular proteins including Src-family kinases, Grb2, and amphiphysin II (4, 7, 8) and regulates signaling via the Ras-Erk MAPK pathway (9). This motif is absolutely conserved throughout all HCV genotypes, yet is dispensable for viral replication in cell culture (8, 10). The conservation of this motif implies a potential importance in the HCV lifecycle. which is most likely only manifest in vivo, for example in the context of HCV persistence. Consistent with this hypothesis, Huh-7 cells harboring a subgenomic replicon in which the proline motif had been disrupted by alanine substitution (repliconPA2) (10) displayed K+ current densities that were similar to the parental Huh-7 cells (Fig. 1D). To further prove that NS5A was both necessary and sufficient for K+ current inhibition, we transfected Huh-7 cells with a NS5A-GFP fusion protein expression vector, selected GFP positive cells by FACS, and subjected the resulting population of cells to patch-clamp analysis. Compared with control cells (expressing GFP alone), K+ current densities were reduced in the NS5A-GFP-expressing cells (Fig. 1E). These observations are consistent with the notion that the inhibition of K+ channel activity by HCV is mediated by NS5A and required the interaction with one or more host cellular SH3 domain-containing proteins.
Since subgenomic HCV replicons only express the HCV nonstructural proteins NS3-NS5B, it was important to determine whether the effect on K+ efflux was also observed in the context of virus-infected cells. For electrophysiological studies, necessarily conducted outside a category 3 containment facility, we used a construct derived from the genotype 2a cell culture infectious clone, JFH-1, which lacked the E1 and E2 envelope glycoproteins (JFH-1ΔE1/E2). This construct expresses core, p7 and NS2 in addition to NS3–5B, but does not produce infectious viral particles (11). The outward K+ current was almost abrogated in cells transfected with in vitro-transcribed JFH-1ΔE1/E2 RNA compared with control cells (Fig. 1F), confirming that the blockade to K+ channel activity by NS5A is conserved in a complete infectious HCV system and in addition is not restricted to genotype 1b HCV.
HCV Nonstructural Protein NS5A Inhibits Kv2.1 Function.
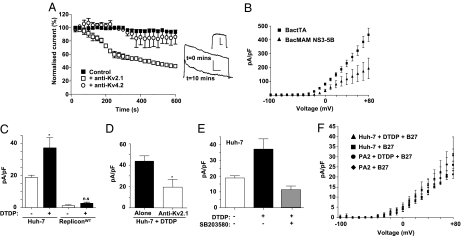
Previous studies have indicated the presence of several distinct ionic conductances within hepatocytes (12, 13), although their molecular nature remains unresolved. Therefore, we sought to determine which ion channel(s) contributed to the outward current in Huh-7 cells by intracellular dialysis with antibodies to specific channel subunits (14). Inclusion of an anti-Kv2.1 antibody in the patch pipet led to a time-dependent reduction of the outward K+ current as has previously been reported in neuronal cells (15) (Fig. 2A). In contrast to this, intracellular dialysis of an antibody targeted to another Kv channel (Kv4.2) had no significant effect on the amplitude of the outward current (Fig. 2A). These observations suggested that Kv2.1 is the major functional Kv channel in Huh-7 cells and was therefore a likely target of NS5A inhibition. In support of this, expression of Kv2.1 in Huh-7 was confirmed by both Western blot (Fig. 3B) and immunofluorescence (Fig. S2C)—these data showed that levels of Kv2.1 expression were unaffected by the presence of the HCV subgenomic replicon.
Fig. 2.
Identification of Kv2.1 as the target of NS5A. (A) Intracellular dialysis of an anti-Kv2.1 antibody reduces the whole cell K+ current (evoked at +50 mV step) in a Huh-7 cell over a 10-min time course. Also shown are example currents evoked at t = 0 min and t = 10 min. Inset shows difference current. (B) Transient replicon expression from a baculovirus vector reduces the Kv2.1 current (evoked at +50 mV step) in HEK293 Kv2.1 expressing cells (n = 7). Where error bars are shown, results are expressed as mean and SEM. All scale bars, 100 pA (vertical), 50 ms (horizontal). *, P < 0.05. (C) Pooled data (n = 8) showing the K+ current density at +50 mV in Huh-7 (Left) and repliconWT cells (Right), without or with DTDP treatment. (D) Intracellular dialysis of an anti-Kv2.1 antibody significantly decreased the DTDP-induced upsurge in K+ current density at +50 mV. (E) Pooled data showing the effect of p38 inhibition on K+ current density increase after DTDP treatment in Huh-7 cells at +50 mV (n = 7). Results are expressed as mean and SEM. (F) I/V relationships for parental Huh-7 (▲, ■), or repliconPA2 cells (●, ♦), either treated with DTDP alone, or preincubated with the antioxidant mixture B27 before DTDP treatment (n = 6–8 cells).
Fig. 3.
Kv2.1 suppression is mediated by perturbation of p38 MAPK signaling. (A and B) Naive Huh-7 cells or replicon-harboring cells were either untreated, or treated with DTDP (100 μM for 10 min), and lysed. (C) Cells transfected with full length HCV JFH-1 RNA or a PA2 mutant derivative were treated with DTDP 48 h after transfection and lysed. (D) Parental Huh-7 cells were treated with DTDP, with or without prior incubation with the antioxidant mixture B27, and lysed. Lysates (50 μg total protein) were resolved by SDS-PAGE and probed with antibodies specific to the indicated proteins.
To further confirm that Kv2.1 was the target of NS5A, we asked whether our observations could be recapitulated in a heterologous system. To this end, we used a baculovirus delivery system (16) to introduce a genotype 1b NS3–5B replicon into HEK293 cells that stably expressed Kv2.1 (17). Cells were transduced with two baculovirus vectors, one expressing a tTA tetracycline transactivator protein (BACtTA) and a second containing the HCV subgenomic replicon under the control of a tet-off promoter. In these cells, a reduction in the Kv2.1 current was observed compared with cells transduced with BactTA alone, confirming that the HCV replicon was able to inhibit Kv2.1 activity and that this effect was not restricted to Huh-7 cells (Fig. 2B). The presence of the replicon had no effect on the expression levels of Kv2.1 in either HEK293 (Fig. S2A) or Huh-7 cells (Fig. 3B and Fig. S2C). Importantly, the genotype 1b subgenomic replicon is not able to undergo RNA replication in 293 cells, indicating that inhibition of Kv2.1 was not dependent on active RNA replication.
HCV NS5A Blocks p38 MAPK-Induced Kv2.1 Current Upsurge.
We next addressed the mechanism by which NS5A mediated inhibition of Kv2.1 activity. In previous studies, Kv2.1 currents have been shown to be induced by oxidative stress via a mechanism that involves activation of p38 MAPK (3, 18). Phosphorylation of Kv2.1 by p38 MAPK at a serine residue in the cytoplasmic C terminus (S800) results in the insertion of additional channels into the plasma membrane, subsequently resulting in increased Kv2.1-mediated K+ efflux (3). We therefore asked if HCV might suppress Kv2.1 activity by inhibiting p38 MAPK-dependent phosphorylation of Kv2.1 following induction of oxidative stress. Following treatment with 2,2′-dithiodipyridine (DTDP), a sulfhydryl oxidizing agent that induces intracellular zinc release resulting in p38 activation, oxidative stress, and apoptosis (19), a robust K+ current surge in parental Huh-7 cells was observed that was absent in repliconWT cells (Fig. 2C) and was reduced by the application of a Kv2.1 antibody (Fig. 2D). We then confirmed that Kv2.1 activity was regulated by p38 MAPK in Huh-7 cells by treatment with a highly specific p38 MAPK inhibitor (SB203580)—as anticipated this prevented the stimulation of Kv2.1 activity by DTDP treatment (Fig. 2E). To further confirm that the activation of Kv2.1 by DTDP was because of oxidative stress, we preincubated either parental Huh-7 or repliconPA2 harboring cells with an antioxidant mixture (B27) (19) before DTDP treatment. Under these conditions, DTDP was unable to effect an increase in Kv2.1 activity (Fig. 2F). Interestingly, the basal level of Kv2.1 activity was unaffected by antioxidant treatment, suggesting that this was not a response to constitutive low levels of oxidative stress in Huh-7 cells.
We then performed a biochemical analysis of the p38 MAPK pathway following DTDP treatment. Western blot analysis of Huh-7 cells revealed an increase in phosphorylation of both p38 MAPK and MAPKAP-2 (a p38 MAPK substrate) following DTDP treatment (Fig. 3A), confirming that p38 MAPK was activated by oxidative stress in these cells. Phosphorylation of both kinases was markedly reduced in repliconWT cells but not in repliconPA2 cells following DTDP treatment (Fig. 3A), demonstrating that not only was p38 MAPK phosphorylation reduced, but its activity was also abrogated. Using an antibody specific for S800-phosphorylated Kv2.1 (3), we observed a significant increase in the level of channel phosphorylation in both parental Huh-7 and repliconPA2 cells following DTDP treatment (Fig. 3B). In the repliconWT cells, however, Kv2.1 S800 phosphorylation was not stimulated by DTDP treatment, consistent with the inhibition of p38 MAPK induction (Fig. 3B). These data support the notion that NS5A inhibits activation of p38 MAPK by oxidative stress, one consequence of which is to block Kv2.1 phosphorylation and thereby prevent the elevation of Kv2.1 channel activity.
We also determined whether phosphorylation of Kv2.1 by p38 MAPK was perturbed in the context of virus-infected cells. Huh-7 cells were transfected with in vitro-transcribed full-length RNA of the cell culture infectious genotype 2a JFH-1 isolate (11) or a mutant in which the conserved PxxPxR motif in NS5A described in the context of the replicon (Figs. 1D and 2D) had been disrupted by alanine substitution (JFH-1PA2). In wild-type JFH-1 transfected cells, DTDP stimulated Kv2.1 S800 phosphorylation was abrogated (Fig. 3C), whereas levels of phosphorylation were unaffected by the JFH-1PA2 mutant. To confirm that Kv2.1 S800 phosphorylation was directly responsive to oxidative stress, we preincubated parental Huh-7 cells with the antioxidant mixture, B27. This analysis showed that the DTDP-induced elevation of both p38 MAPK and Kv2.1 phosphorylation was abrogated by antioxidant treatment (Fig. 3D).
Induction of Apoptosis by Oxidative Stress Is Perturbed by HCV NS5A.
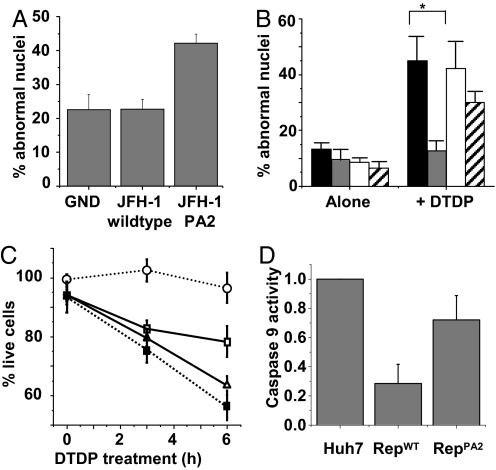
Recently, S800 phosphorylation of Kv2.1 was shown to be required for induction of apoptosis in oxidant-treated CHO cells transiently overexpressing the channel (3). This was assessed by the increased viability of cells expressing a nonphosphorylatable mutant of Kv2.1 (S800A) following DTDP treatment. Activation of Kv2.1 by oxidative stress also induces K+ efflux and apoptosis in neuronal cells (20) (Fig. S1). Having identified Kv2.1 as a target for HCV NS5A, we therefore investigated the physiological consequences of such channel inhibition. It has been well documented that HCV induces oxidative stress (21, 22), and it is implicit that to persist, the virus would need to possess a mechanism to avoid the apoptotic consequences of this induction. To test this, we therefore measured the levels of apoptosis in Huh-7 cells transfected with full-length JFH-1 RNA—either wild-type, PA2 mutant, or GND (a mutation in the NS5B polymerase that abrogates viral genome replication). Seventy-two hours posttransfection, we quantified cells exhibiting nuclear fragmentation as a late marker of apoptosis (for example, see images Fig. S2D). In the cells transfected with the PA2 mutant RNA, 42% of cells were apoptotic compared with 22% of those transfected with either the GND mutant or wild-type JFH-1 RNA (Fig. 4A). We conclude that JFH-1 infection induces apoptosis and that this can be blocked by NS5A, via the PP2 motif, presumably by inhibiting p38 MAPK activation of Kv2.1.
Fig. 4.
Induction of apoptosis by viral replication or exogenously applied oxidative stress is blocked by HCV NS5A. (A and B) Cells were assessed for nuclear fragmentation by staining untreated (A) or DTDP-treated cells (B) with DAPI and an anti-NS5A serum. Wide field images (40×) were taken from each population, and the number of apoptotic cells from each wide field image was then counted and expressed as a percentage of the total cell number (n = 5). The cells in B are Huh-7 (black), repliconWT (gray), repliconPA2 (white), and cured replicon (stripes). (C) Cell viability following DTDP treatment was assessed using the MTT CellTiter96 assay (Promega). Values are the mean normalized absorbance at 590 nm (n = 12). Huh-7 (□), repliconWT (○), repliconPA2 (△), and cured replicon cells (■). (D) Caspase 9 activity was measured in cells following DTDP treatment using a commercial kit (Promega). For all experiments, where error bars are shown, results are expressed as mean and SEM. *, P < 0.05.
We also asked whether the blockade of Kv2.1 activity in replicon cells observed previously (Fig. 1) would also manifest in an anti-apoptotic phenotype. Contrary to the situation in JFH-1 infected cells, we observed no significant difference in the numbers of apoptotic cells in populations of parental Huh-7, IFN-cured Huh-7, repliconWT, or repliconPA2 lines (Fig. 4B). This was expected as the replicon-harboring cells had been selected over a long period (months) and stably maintained the replicon. This process would presumably have resulted in the selection of a subpopulation of cells with resistance to the oxidative, and other, stresses induced by replicon replication. It could also reflect the fact that the replicon does not express the structural proteins that are likely to accumulate in the ER, inducing both ER and additional oxidative stress. However, when we provided an exogenous oxidative stress challenge to the cells by treatment with DTDP, parental Huh-7 cells exhibited elevated levels of apoptosis, as measured by nuclear fragmentation (Fig. 4B and Fig. S2D), absolute numbers of viable cells (Fig. 4C), and a specific biochemical marker of the early stages of intrinsic apoptosis—caspase 9 activity (Fig. 4D). This elevation was suppressed in repliconWT cells, while both cured and repliconPA2 cells exhibited apoptotic responses to DTDP treatment that were equivalent to parental Huh-7 cells (Fig. 4 B–D). Collectively, these data support the hypothesis that blockade of Kv2.1 activation by NS5A mediates resistance to the induction of apoptosis and thereby facilitates HCV persistence.
Discussion
The current study provides evidence for a previously undocumented physiological interplay between a virus and host cell ion channel activity. Specifically, the voltage-gated outwardly rectifying K+ channel Kv2.1 is blocked in cells harboring an HCV subgenomic replicon or infected with cell culture infectious HCV, by the NS5A protein, leading to an inhibition of proapoptotic K+ efflux. This is mediated by preventing the p38 MAPK-mediated phosphorylation of S800 in the C-terminal cytoplasmic domain of Kv2.1 that is required for Kv2.1 membrane insertion, current upsurge, and induction of cellular apoptosis (3). Our data also reveal a role for a highly conserved proline motif (PxxPxR) within NS5A in this modulation of Kv2.1 function.
A key question raised by this study is: What is the role of Kv2.1 inhibition in the HCV infection process? Clinically, chronic HCV infection is associated with elevated levels of reactive oxygen species (ROS) leading to oxidative stress within the liver of patients (23). Additionally, although the literature is somewhat controversial, it is generally accepted that expression of Core, NS3, or NS5A induces oxidative stress in established cell lines via interactions with ER and/or mitochondrial membranes (22, 23). Viral persistence would necessitate a parallel strategy by which HCV could circumvent the ROS-mediated induction of apoptosis. Our data demonstrate that one facet of this strategy may be to inhibit both oxidative stress-induced activation of p38 MAPK and consequent Kv2.1-mediated apoptosis.
Our data have demonstrated that Kv2.1 activity in Huh-7 or HEK293 cells is modulated by NS5A. A critical question that attests to the physiological validity of these data are whether Kv2.1 is expressed in primary, untransformed human hepatocytes? We have demonstrated Kv2.1 expression in primary human hepatocytes using both immunofluorescence and Western blot (Fig. S3 A and B). In addition, whole cell patch clamp recordings demonstrated that they exhibited an outward K+ current, which was strongly enhanced by DTDP (Fig. S3C) and was sensitive to the K+ channel blocker tetraethylammonium (TEA) (Fig. S3D). Although we have not been able to confirm the effects of HCV on Kv2.1 activity in this system, because of technical difficulties with transfection, we believe that the activity of Kv2.1 in these cells strongly supports the physiological validity and relevance of our findings.
Somewhat surprisingly, given the critical importance of ion channels for cell function, there are few examples of viral modulation of host cell ion channel activity. Of note, the HIV-1 Nef protein, which like NS5A also possesses polyproline motifs known to interact with cellular SH3 domains (24), has been demonstrated to affect cellular K+ levels (25, 26) and inactivate an undefined large-conductance K+ channel in U251 glioma cells (27), although these effects have not been shown to have any role in the virus lifecycle. It is pertinent to note that Nef has been demonstrated to bind to and inhibit an upstream p38 MAPK kinase, apoptosis signal-regulating kinase-1 (ASK-1) (28), which has also been shown to play a role in the apoptotic function of Kv2.1 (29). It is thus tempting to speculate that HIV and other viruses, through perturbation of cell signaling pathways, may use similar control of ion channel homeostasis to promote viral persistence. We propose that HCV NS5A is thus a viral protein that has been described to target a defined host ion channel to inhibit apoptosis. Intriguingly, the SARS coronavirus 3a protein has recently been shown to function as a K+ channel, and this activity was required for its proapoptotic function (30). Viruses such as SARS, which cause acute infection, frequently induce apoptosis late in the infectious cycle to facilitate virus particle release without inducing necrosis and an inflammatory response. Contrary to the situation with HCV, SARS has therefore exploited the link between K+ efflux and apoptosis to induce cell death, rather than promote cell viability.
In addition to a role in pathogenesis and persistence, viral perturbation of ion channel function would be expected to alter normal cellular channels and responses to intracellular stimuli in infected cells. Interestingly, HCV infection is often associated with cognitive dysfunction, depression, insulin resistance, and cardiac myopathies; disorders associated with aberrant cellular ion channel function in a diverse range of central and peripheral tissues. The ability of HCV to display tropism beyond the liver has recently been demonstrated by detection in brain microglia/macrophages and, to a lesser extent, astrocytes (31). Modulation of channel function in these cells could contribute to the basis of neurocognitive abnormalities in HCV infection.
In conclusion, these studies reveal a mechanism by which HCV NS5A can inhibit apoptosis in infected cells by inhibiting oxidative stress-induced p38 MAPK activation and subsequent K+ efflux. At the present time, a number of candidate anti-NS5A compounds are in clinical trials. Although these compounds were initially selected based on activity directed against subgenomic replicon replication, it will be intriguing to determine if additional effects on functions of NS5A, such as perturbation of p38 MAPK activation, may contribute to the reported potency of these compounds. This would be reminiscent of the HCV NS3 protease inhibitors, which also block the cleavage of the innate immune proteins, TRIF and MAVS, by NS3 (32, 33). Targeting of viral proteins that modulate host cell ion channel activity may therefore offer potential prospects for future antiviral drug development.
Materials and Methods
Cell Culture.
Huh-7 (human hepatoma cells) and primary human hepatocytes were cultured in Dulbecco's MEM (DMEM) supplemented with 10% FCS, 1% nonessential amino acids, 2 mM l-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin. Primary human hepatocytes were obtained from UK Human Tissue Bank (UKHTB) and were originally obtained from nontransplantable sources under appropriate ethical approval. Replicon-harboring cell lines were generated as previously described (10) and maintained as a polyclonal cell population in medium supplemented with 250 μg/mL G418. The subgenomic replicon was the FK5.1 genotype 1b culture-adapted replicon that contains seven coding mutations compared with the parental Con1 replicon sequence (E1202G and T1280I in NS3, L1757I in NS4B, and N2109D, S2197P, P2327S, and K2350E in NS5A) that enhance RNA replication (5). The PA2 mutant derivative of FK5.1 has no effect on RNA replication and was described in ref. 10. HEK293 Kv2.1 cells (18) were maintained in DMEM supplemented with 10% FCS, 1% nonessential amino acids, 2 mM l-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin supplemented with glutamax and 250 μg/mL G418. All cells were incubated at 37 °C in a humidified 5% CO2 incubator.
DNA Constructs.
NS5A was amplified from the J4 genotype 1b infectious clone (34) (primers available upon request) and cloned into pEGFP-N1 to generate a construct expressing a fusion protein in which GFP was fused to the C terminus of NS5A. Plasmid DNA was transfected using polyethyleneimine (PEI) as described in ref. 35.
Infection of Huh-7 Cells with Cell Culture-Derived HCV (JFH-1).
Huh-7 cells were electroporated with in vitro transcribed JFH-1 genomic RNA, as described in ref. 36, and seeded onto glass coverslips in six-well plates at a concentration of 2 × 105 per well. Electrophysiological recordings were performed on JFH-1ΔE1/E2 infected cells 48 h after electroporation.
Baculovirus-Mediated Replicon Delivery.
HEK293 Kv2.1 cells were seeded at 2.5 × 104 cm−2 in six-well plates, and 24 h later were transduced either with BACtTA or with BACtTA and BACrepFK5.1 (16) for 4 h (final concentration of 1 × 107 plaque-forming U/mL). Expression was confirmed by immunofluorescence labeling for the NS5A protein.
Electrophysiology.
Fragments of coverslip with attached cells were transferred to a continuously perfused (3–5 mL/min) recording chamber mounted on the stage of an Olympus CK40 inverted microscope. Patch pipets had resistances 4–6 MΩ, and tight seals were obtained before proceeding to whole cell configuration. Series resistance was monitored after breaking into the whole cell configuration throughout the duration of experiments. If a significant increase occurred (>20%), the experiment was terminated. To examine K+ currents, two protocols were adopted: First, a series of depolarizing steps from −100 to +80 mV in 10-mV increments for 500 ms, and second, a single step to +50 mV from −70 mV for 100 ms. For some experiments, anti-Kv2.1 antibody (NeuroMab) or an anti-Kv4.2 antibody (Sigma) was added to the intracellular solution to a final concentration of 0.5 μg/mL. Signals were sampled at 10 kHz and low pass filtered at 2 kHz. Voltage-clamp recordings were performed with the use of an Axopatch 200A amplifier/Digidata 1200 interface controlled by Clampex 9.0 software (Molecular Devices). Offline analysis was performed using the data analysis package Clampfit 9.0 (Molecular Devices). Results are presented as means ± SEM, and statistical analysis was performed using unpaired Student's t tests, where P < 0.05 was considered statistically significant. Whole cell patch clamp recordings were performed using a patch pipet solution containing 140 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM CaCl2, 10 mM HEPES-KOH, pH 7.2, 10 mM glucose. The standard perfusate contained 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, 10 mM HEPES-NaOH, pH 7.2, 2 mM CaCl2, 10 mM glucose.
Western Blot Analysis.
To analyze protein expression, cells were lysed in GLB buffer (10 mM Pipes-KOH, pH 7.2, 120 mM KCl, 30 mM NaCl, 5 mM MgCl2, 1% Triton X-100, 10% glycerol) plus protease inhibitors (Complete; Roche) and phosphatase inhibitors (2 mM Na3VO4, 5 mM NaF, 5 mM Na4P2O7). Cell lysates (50 μg protein) were normalized by BCA assay and resolved by SDS-PAGE, transferred to a PVDF membrane (Millipore) using a Bio-Rad Laboratories semidry transfer apparatus, and probed with the indicated antibodies. All Western blots were visualized using an in-house ECL system.
Antibodies.
A polyclonal sheep anti-NS5A serum was used for detection of NS5A expression as previously described (9). p38 MAPK-phosphorylated Kv2.1 was probed using an S800 phosphospecific Kv2.1 antibody as previously described (3), while total Kv2.1 was probed with a commercial antibody that was not targeted to the p38 site (NeuroMab). Antibodies to phosphorylated p38 MAPK and MAPKAP2, a phosphorylation status-independent p38 antibody (all from Cell Signaling Technologies) and GAPDH (Abcam) were used according to manufacturers' instructions.
Immunofluorescence.
Huh-7 cells were stained as previously described (35). Briefly, cells grown on glass coverslips were fixed with 3% PFA for 10 min, permeabilized in ice-cold methanol/acetone for 10 min, and blocked in PBS/1% BSA for 30 min. Cells were then labeled with a polyclonal sheep anti-NS5A serum before staining with Alexa Fluor 488 nm conjugated anti-sheep secondary antibody (Invitrogen-Molecular Probes) in PBS/1% BSA. Endogenous Kv2.1 was probed using a commercial mouse anti-Kv2.1 antibody and stained using Alexa Fluor 594 nm conjugated anti-mouse secondary antibody. Cells were washed and mounted onto microscope slides using Citifluor (Agar Scientific). Labeled cells were viewed on a Zeiss 510-META laser-scanning confocal microscope under an oil-immersion 63× objective lens (NA = 1.40). Alexa Fluor 488 nm (494 nm excitation, 519 nm emission) was excited using an argon laser fitted with 488-nm filters, and Alexa Fluor 594 nm (550 nm excitation, 570 nm emission) was excited using a helium/neon laser fitted with 543-nm filters. Images displayed are representative and displayed as single optical sections of 50 μM thickness.
Drug Treatment.
The stress stimulus for all experiments consisted of a 10-min treatment with 100 μM DTDP at 37 °C, 5% CO2. The DTDP-containing solution was then removed and replaced with fresh medium. Where indicated, cells were preincubated with 10 μM SB203580 for 20 min, or B27 media supplement (GIBCO) (19) for 30 min, before DTDP treatment. For electrophysiological recording, the caspase inhibitor Boc-D-FMK (10 μM) was included in the media to maintain viability of cells for electrophysiological recordings as the naive Huh-7 cells were susceptible to DTDP-induced apoptosis. Electrophysiological recordings were performed 1–3 h and apoptosis assays 6 h after induction of oxidative stress.
Apoptosis Assays.
The number of cells undergoing nuclear fragmentation was quantified after staining with DAPI (Molecular Probes). Briefly, cells were seeded into 12-well plates containing glass coverslips and allowed to settle for 24 h. To induce apoptosis, cells were treated with DTDP, fixed in 3% paraformaldehyde, and permeabilized in methanol/acetone before staining with DAPI and sheep NS5A antibody. Randomly chosen fields were observed under fluorescence microscopy for cells with fragmented nuclei, which were considered to be apoptotic. Data were expressed as a percentage of apoptotic cells. Huh-7 cell viability was evaluated using the 3-(4,5-dimethylthylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Cells were seeded into 96-well plates (103 cells/well) and allowed 24 h to settle following cellular stress with DTDP. Cells were then subjected to MTT assays. Caspase 9 activity was determined using the Caspase-Glo 9 luminescent assay (Promega). Briefly, Huh-7 cells grown in a 96-well plate were stimulated with DTDP and incubated with a single Caspase-Glo 9 reagent resulting in cell lysis, caspase cleavage of the substrate, and generation of a luminescent signal. The signal generated is proportional to the amount of caspase activity present and was normalized to the untreated control samples. Samples were read using a FLUOstar OPTIMA microplate reader (BMG LABTECH).
Supplementary Material
Acknowledgments.
We thank T. Wakita (Tokyo) for the JFH-1 construct, R. Bartenschlager (Heidelberg) for the JFH-1ΔE1/E2 construct, E. Aizenman (Pittsburgh) for the antibody to S800 phosphorylated Kv2.1, J.S. Trimmer (Davis) for the HEK293 cells stably expressing Kv2.1, and D. Rowlands and N. Stonehouse for helpful discussions and critical reading of this manuscript. This work was supported by a Medical Research Council Grant G0401577 (to M.H.), and Medical Research Council New Investigator Award G0700124 (to S.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906798106/DCSupplemental.
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- 3.Redman PT, et al. Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1. Proc Natl Acad Sci USA. 2007;104:3568–3573. doi: 10.1073/pnas.0610159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macdonald A, Crowder K, Street A, McCormick C, Harris M. The hepatitis C virus NS5A protein binds to members of the Src family of tyrosine kinases and regulates kinase activity. J Gen Virol. 2004;85:721–729. doi: 10.1099/vir.0.19691-0. [DOI] [PubMed] [Google Scholar]
- 5.Krieger N, Lohmann V, Bartenschlager R. Enhancement of hepatitis C virus RNA replication by cell culture-adaptive mutations. J Virol. 2001;75:4614–4624. doi: 10.1128/JVI.75.10.4614-4624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macdonald A, Harris M. Hepatitis C virus NS5A: Tales of a promiscuous protein. J Gen Virol. 2004;85:2485–2502. doi: 10.1099/vir.0.80204-0. [DOI] [PubMed] [Google Scholar]
- 7.Tan SL, et al. NS5A, a nonstructural protein of hepatitis C virus, binds growth factor receptor-bound protein 2 adaptor protein in a Src homology 3 domain/ligand-dependent manner and perturbs mitogenic signaling. Proc Natl Acad Sci USA. 1999;96:5533–5538. doi: 10.1073/pnas.96.10.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zech B, et al. Identification and characterization of amphiphysin II as a novel cellular interaction partner of the hepatitis C virus NS5A protein. J Gen Virol. 2003;84:555–560. doi: 10.1099/vir.0.18801-0. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald A, et al. The hepatitis C virus NS5A protein inhibits activating protein-1 (AP1) function by perturbing Ras-ERK pathway signalling. J Biol Chem. 2003;278:17775–17784. doi: 10.1074/jbc.M210900200. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald A, et al. Further studies on hepatitis C virus NS5A-SH3 domain interactions: Identification of residues critical for binding and implications for viral RNA replication and modulation of cell signalling. J Gen Virol. 2005;86:1035–1044. doi: 10.1099/vir.0.80734-0. [DOI] [PubMed] [Google Scholar]
- 11.Wakita T, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddad P, Graf J. Volume-regulatory K+ fluxes in the isolated perfused rat liver: Characterization by ion transport inhibitors. Am J Physiol. 1989;257:G357–G363. doi: 10.1152/ajpgi.1989.257.3.G357. [DOI] [PubMed] [Google Scholar]
- 13.Nietsch HH, Roe MW, Fiekers JF, Moore AL, Lidofsky SD. Activation of potassium and chloride channels by tumor necrosis factor alpha. Role in liver cell death. J Biol Chem. 2000;275:20556–20561. doi: 10.1074/jbc.M002535200. [DOI] [PubMed] [Google Scholar]
- 14.Dallas ML, Deuchars SA, Deuchars J. Modulation of potassium ion channel proteins utilising antibodies. Methods Mol Biol. 2008;491:247–255. doi: 10.1007/978-1-59745-526-8_19. [DOI] [PubMed] [Google Scholar]
- 15.Murakoshi H, Trimmer JS. Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J Neurosci. 1999;19:1728–1735. doi: 10.1523/JNEUROSCI.19-05-01728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormick CJ, Challinor L, Macdonald A, Rowlands DJ, Harris M. Introduction of replication-competent hepatitis C virus transcripts using a tetracycline-regulable baculovirus delivery system. J Gen Virol. 2004;85:429–439. doi: 10.1099/vir.0.19676-0. [DOI] [PubMed] [Google Scholar]
- 17.Mohapatra DP, Park KS, Trimmer JS. Dynamic regulation of the voltage-gated Kv2.1 potassium channel by multisite phosphorylation. Biochem Soc Trans. 2007;35:1064–1068. doi: 10.1042/BST0351064. [DOI] [PubMed] [Google Scholar]
- 18.Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313:976–979. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin B, et al. p38 activation is required upstream of potassium current enhancement and caspase cleavage in thiol oxidant-induced neuronal apoptosis. J Neurosci. 2001;21:3303–3311. doi: 10.1523/JNEUROSCI.21-10-03303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J Neurosci. 2003;23:4798–4802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joyce MA, et al. HCV induces oxidative and ER stress, and sensitizes infected cells to apoptosis in SCID/Alb-uPA mice. PLoS Pathog. 2009;5:e1000291. doi: 10.1371/journal.ppat.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T, Weinman SA. Causes and consequences of mitochondrial reactive oxygen species generation in hepatitis C. J Gastroenterol Hepatol. 2006;21(Suppl 3):S34–S37. doi: 10.1111/j.1440-1746.2006.04591.x. [DOI] [PubMed] [Google Scholar]
- 23.Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127–2133. doi: 10.1002/hep.22269. [DOI] [PubMed] [Google Scholar]
- 24.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zegarra-Moran O, et al. HIV-1 Nef expression inhibits the activity of a Ca2+-dependent K+ channel involved in the control of the resting potential in CEM lymphocytes. J Immunol. 1999;162:5359–5366. [PubMed] [Google Scholar]
- 26.Choi B, et al. Alterations in intracellular potassium concentration by HIV-1 and SIV Nef. Virol J. 2008;5:60. doi: 10.1186/1743-422X-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kort JJ, Jalonen TO. The nef protein of the human immunodeficiency virus type 1 (HIV-1) inhibits a large-conductance potassium channel in human glial cells. Neurosci Lett. 1998;251:1–4. doi: 10.1016/s0304-3940(98)00495-9. [DOI] [PubMed] [Google Scholar]
- 28.Geleziunas R, Xu WD, Takeda K, Ichijo H, Greene WC. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature. 2001;410:834–838. doi: 10.1038/35071111. [DOI] [PubMed] [Google Scholar]
- 29.Aras MA, Aizenman E. Obligatory role of ASK1 in the apoptotic surge of K+ currents. Neurosci Lett. 2005;387:136–140. doi: 10.1016/j.neulet.2005.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan CM, et al. The ion channel activity of the SARS-coronavirus 3a protein is linked to its pro-apoptotic function. Int J Biochem Cell Biol. 2009 May 3; doi: 10.1016/j.biocel.2009.04.019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkinson J, Radkowski M, Laskus T. Hepatitis C virus neuroinvasion: Identification of infected cells. J Virol. 2009;83:1312–1319. doi: 10.1128/JVI.01890-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li K, et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci USA. 2005;102:2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meylan E, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 34.Yanagi M, et al. Transcripts of a chimeric cDNA clone of hepatitis C virus genotype 1b are infectious in vivo. Virology. 1998;244:161–172. doi: 10.1006/viro.1998.9092. [DOI] [PubMed] [Google Scholar]
- 35.Mankouri J, Griffin S, Harris M. The hepatitis C virus non-structural protein NS5A alters the trafficking profile of the epidermal growth factor receptor. Traffic. 2008;9:1497–1509. doi: 10.1111/j.1600-0854.2008.00779.x. [DOI] [PubMed] [Google Scholar]
- 36.Griffin S, et al. Genotype-dependent sensitivity of hepatitis C virus to inhibitors of the p7 ion channel. Hepatology. 2008;48:1779–1790. doi: 10.1002/hep.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.