Abstract
Methanogens use an unusual energy-conserving electron transport chain that involves reduction of a limited number of electron acceptors to methane gas. Previous biochemical studies suggested that the proton-pumping F420H2 dehydrogenase (Fpo) plays a crucial role in this process during growth on methanol. However, Methanosarcina barkeri Δfpo mutants constructed in this study display no measurable phenotype on this substrate, indicating that Fpo plays a minor role, if any. In contrast, Δfrh mutants lacking the cytoplasmic F420-reducing hydrogenase (Frh) are severely affected in their ability to grow and make methane from methanol, and double Δfpo/Δfrh mutants are completely unable to use this substrate. These data suggest that the preferred electron transport chain involves production of hydrogen gas in the cytoplasm, which then diffuses out of the cell, where it is reoxidized with transfer of electrons into the energy-conserving electron transport chain. This hydrogen-cycling metabolism leads directly to production of a proton motive force that can be used by the cell for ATP synthesis. Nevertheless, M. barkeri does have the flexibility to use the Fpo-dependent electron transport chain when needed, as shown by the poor growth of the Δfrh mutant. Our data suggest that the rapid enzymatic turnover of hydrogenases may allow a competitive advantage via faster growth rates in this freshwater organism. The mutant analysis also confirms the proposed role of Frh in growth on hydrogen/carbon dioxide and suggests that either Frh or Fpo is needed for aceticlastic growth of M. barkeri.
Keywords: hydrogen electron transport, F420, H2 cycling, methanogenesis
Methanogenesis is the terminal step in biomass degradation in many anaerobic environments and plays a central role in the global carbon cycle. Although most of the methane (CH4) produced is oxidized to carbon dioxide (CO2) by methane-consuming organisms, substantial quantities (ca. 1014 g/year) escape into the atmosphere where it acts as a potent greenhouse gas (1). Most methanogens produce CH4 by reducing CO2 with hydrogen gas (H2) (2). However, some Methanosarcina species such as M. barkeri and M. mazei also are capable of using a variety of other substrates, including acetate, which accounts for ca. 2/3 of global CH4 production (3), and C1 compounds such as methanol, methylsulfides, and methylamines (4).
Methanogenic organisms produce CH4 as a byproduct of anaerobic respiration involving a unique energy-conserving electron transport chain found only in Archaea. At least 2 distinct types of methanogenic respiration exist: 1 found in methanogens, including Methanosarcina species that synthesize cytochromes, and the other in those that lack cytochromes (1). The penultimate step of both respiratory pathways involves the reduction of methyl-coenzyme M (CoM-SH, mercaptoethanesulfonic acid) to CH4 using coenzyme B (CoB-SH, N-7-mercaptoheptanoyl-O-phospho-L-threonine) and CoM-SH as electron donors. The other product of this reaction is the heterodisulfide of CoM-SH and CoB-SH (CoM-S-S-CoB), which serves as the terminal electron acceptor in the energy-conserving electron transport chain. In methanogenic Archaea that lack cytochromes, the means by which energy is conserved is poorly understood but probably involves a cytoplasmic electron bifurcation pathway similar to that recently characterized in Clostridium (1, 5). In contrast, the energy-conserving electron transport chain of cytochrome-containing methanogens, exemplified by Methanosarcina species, has been studied in detail, including the reconstitution of 2 distinct proton-translocating electron transport systems in vitro (6–9).
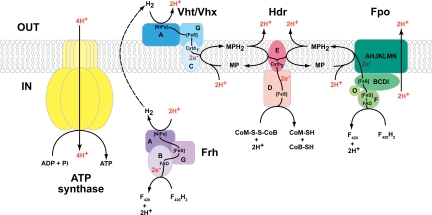
In Methanosarcina species, a membrane-bound electron transport chain that terminates with the reduction of the CoM-S-S-CoB heterodisulfide generates ion-motive force that can be used by ATP synthase to form ATP (Fig. 1). Either H2 or reduced coenzyme F420 (F420H2) can donate electrons for reduction of CoM-S-S-CoB (2, 4). In the H2:heterodisulfide oxidoreductase system, a methanophenazine-dependent hydrogenase (Vht or Vhx) (10) oxidizes H2 in the periplasm with transfer of 2 electrons to the membrane-soluble electron carrier methanophenazine. The reduced methanophenazine (MPH2) subsequently is oxidized by the enzyme heterodisulfide reductase (Hdr) with concomitant reduction of CoM-S-S-CoB. In the F420H2:heterodisulfide oxidoreductase system, the F420H2 dehydrogenase (Fpo) catalyzes electron transfer from F420H2 to methanophenazine, concomitantly pumping 2 protons out of the cell. As in the H2:heterodisulfide oxidoreductase system, MPH2 passes electrons to CoM-S-S-CoB, leading to translocation of 2 additional protons. In vitro measurements suggest that the magnitude of proton motive force generated is the same for both oxidoreductase systems, probably 4H+/2e− (9, 11).
Fig. 1.
The electron transport chain of M. barkeri has been proposed to comprise 2 energy-conserving systems, the F420H2:heterodisulfide oxidoreductase and the H2:heterodisulfide oxidoreductase. In the former system, F420H2 is oxidized by FpoF releasing 2 electrons that are transferred through FpoBCDI and then FpoAHJKLMN to membrane-soluble methanophenazine. This reaction is coupled to the pumping of 2 protons outside the cell. Reduction of methanophenazine consumes 2 protons from the cytoplasm, which subsequently are released outside the cell upon oxidation of MPH2. The electrons then are transferred through HdrED to reduce CoM-S-S-CoB with 2 protons from the cytoplasm. Alternatively, in the H2:heterodisulfide oxidoreductase, H2 is oxidized by Vht/Vhx to produce 2 protons outside the cell and 2 electrons that are transferred to MPH2, which then is used to reduce CoM-S-S-CoB. The dashed arrow represents a third possible energy-conserving mechanism that is proposed in this study. In this pathway, F420H2 is oxidized by the cytoplasmic hydrogenase Frh to generate H2. The H2 then diffuses outside the cell to the active site of membrane-bound hydrogenase Vht/Vhx, where it is reoxidized, resulting in the translocation of 2 protons via a H2-cycling mechanism. The electrons are passed through methanophenazine to CoM-S-S-CoB, as in the other 2 systems. In all 3 systems, the entire electron transport process leads to the net translocation of 4 protons (highlighted in red) outside the cell per 2 electrons transferred from F420H2 or H2 to the CoM-S-S-CoB. The electrochemical gradient generated is coupled to ATP synthesis via an A-type ATPase. Abbreviations: CoB-SH, coenzyme B; CoM-SH, coenzyme M; CoM-S-S-CoB, mixed disulfide of CoM-SH and CoB-SH; Cytb2, cytochrome b2; F420/F420H2, oxidized and reduced Factor 420; FAD, flavin adenine dinucleotide; [FeS], iron-sulfur cluster; Fpo, F420H2:phenazine oxidoreductase; Frh, F420-reducing hydrogenase; Hdr, heterodisulfide reductase; MP/MPH2, oxidized and reduced methanophenazine; [NiFe], bimetallic catalytic center; Vht/Vhx, methanophenazine-dependent hydrogenase.
The F420H2:heterodisulfide oxidoreductase system shares features with the aerobic electron transport chain found in many bacteria. Fpo was purified from M. mazei Gö1 as a 5-subunit complex (FpoBCDIF) capable of oxidizing F420H2 with a variety of artificial electron acceptors (12). Most of these proteins were shown later to be encoded by a 13-gene operon, fpoABCDHIJJKLMNO (fpo), with fpoF being located elsewhere on the genome (supporting information (SI) Fig. S1) (11). Fpo shares significant homology with NADH dehydrogenase I (Nuo) of Escherichia coli, and the nomenclature reflects that similarity (Table S1). Accordingly, both Fpo and Nuo are composed of similar membrane-integral modules (the A, H, J, K, L, M, and N subunits) and membrane-associated modules (the B, C, D, and I subunits). However, the enzymes differ in their substrates and hence in their input modules, so that NuoEFG oxidizes NADH, whereas FpoF oxidizes F420H2, which is a hydride carrier analogous to NADH (2).
The source of electrons for the 2 disparate Methanosarcina electron transport chains varies with the growth substrate (Fig. S2). During growth on H2/CO2 (the hydrogenotrophic pathway), or on H2 in combination with a C1 electron acceptor (the methyl-respiration pathway), electrons are derived from H2 oxidation and are used via the H2:heterodisulfide oxidoreductase system (2). Genetic and biochemical experiments in M. barkeri suggest that use of acetate (the aceticlastic pathway) also involves the obligate intermediacy of H2 and the H2:heterodisulfide oxidoreductase system (13, 14). In contrast, metabolism of compounds such as methanol or methylamines (the methylotrophic pathway) produces 2 equivalents of F420H2 per C1 molecule oxidized. Thus, it has been suggested that F420H2:heterodisulfide oxidoreductase system plays a central, perhaps essential, role in the energy-conserving electron transport chain of the methylotrophic pathway in all Methanosarcina species (reviewed in refs. 2, 4, 15–18).
Surprisingly, the in vivo genetic data presented here tell a different story. Contrary to expectations, Fpo is not required for growth under any condition tested, including during growth on methanol. Instead, reducing equivalents from methanol oxidation seem to be preferentially passed to molecular H2 by the cytoplasmic F420-reducing hydrogenase (Frh). Subsequently, H2 may enter the electron transport chain via the H2:heterodisulfide oxidoreductase system (Fig. 1). These data are reminiscent of the hydrogen-cycling model for electron transport first proposed by Odom and Peck for sulfate-reducing bacteria (19) and suggest that a reanalysis of energy-conservation mechanisms in freshwater Methanosarcina species is warranted.
Results
The F420H2:Heterodisulfide Oxidoreductase Is Conserved in All Sequenced Methanosarcina Genomes.
The genomes of M. barkeri Fusaro (20) and M. acetivorans C2A (21) encode fpoABCDHIJJKLMNO and fpoF operons that are nearly identical to those found in M. mazei (Fig. S1 and Table S1) (22). All known structural and catalytic amino acid residues are conserved in the predicted protein sequences of the Fpo subunits from all 3 Methanosarcina species (Fig. S3) (2, 4, 11). Further, each sequenced genome carries homologues of the hdrDE operon, which encodes the methanophenazine-linked Hdr (Fig. S1) (20–23). Thus, although biochemical activity has been examined only in M. mazei, functional F420H2:heterodisulfide oxidoreductase systems probably are present in each of the Methanosarcina species examined to date.
F420H2 Dehydrogenase Is Not Required for Methanogenesis or Growth in M. barkeri.
As described in earlier sections, in vitro biochemical experiments led to the suggestion that the F420H2:heterodisulfide oxidoreductase system should be essential for growth on C1 compounds via the methylotrophic pathway. To test this hypothesis in vivo, we constructed M. barkeri mutants lacking F420H2 dehydrogenase by deleting the fpoA-O operon or the fpoF gene (Table S2).
The ΔfpoA-O and ΔfpoF mutants were tested for their ability to grow on various methanogenic substrates (Table 1). As expected, both mutants grow on H2/CO2, on methanol plus H2/CO2, and on acetate with growth rates and yields similar to the isogenic parental strain. However, we were surprised to discover that growth of the mutants on methanol also was unaffected. Further, CH4 and CO2 were produced by the ΔfpoA-O and ΔfpoF mutants in the expected 3:1 stoichiometry in amounts and at rates similar to the parent on all substrates (Tables 2 and 3). Thus, loss of F420H2 dehydrogenase, and therefore of the F420H2:heterodisulfide oxidoreductase system, does not measurably affect methanogenesis or growth in M. barkeri.
Table 1.
| Strain | Substrates |
|||
|---|---|---|---|---|
| CH3OH | CH3OH/H2/CO2 | H2/CO2 | CH3COOH | |
| Δhpt‡ | 7.3 ± 0.3 (100%) | 5.7 ± 0.1 (100%) | 13.7 ± 2.5 (100%) | 37 ± 3.4 (100%) |
| Δfpo | 7.3 ± 0.2 (101%) | 6.1 ± 0.5 (100%) | 11.9 ± 0.9 (100%) | 36 ± 2.6 (100%) |
| ΔfpoF | 7.3 ± 0.2 (96%) | 5.8 ± 0.5 (96%) | 12.4 ± 1.2 (90%) | 39 ± 1.9 (84%) |
| Δfre | 7.7 ± 0.3 (116%) | 5.5 ± 0.4 (125%) | 8.8 ± 0.8 (95%) | 41 ± 3.1 (84%) |
| Δfrh | 13.7 ± 0.6 (52%) | 6.5 ± 0.3 (80%) | NG (NA) | 55 ± 7.0 (84%) |
| Δfpo Δfrh | NG (NA) | 5.0 ± 0.3 (80%) | NG (NA) | NG (NA) |
| ΔfpoF Δfrh | NG (NA) | 6.4 ± 0.4 (73%) | NG (NA) | 76 ± 0.8 (66%) |
*Growth rate and yield were measured as described in Materials and Methods; growth yield is relative to the parental strain on the same substrate. Values represent the average and standard deviation of at least triplicate measurements.
†Strains used were WWM85 (Δhpt), WWM71 (Δfpo), WWM123 (ΔfpoF), WWM122 (Δfrh), WWM108 (Δfpo Δfrh), and WWM145 (ΔfpoF Δfrh).
‡M. barkeri Fusaro parent strain in which all deletions were constructed.
NG, no growth for at least 6 months of incubation; NA, not applicable.
Table 2.
Methane (μmoL) and carbon dioxide (μmoL) production* from resting cell suspensions of M. barkeri Fusaro strains†
| Strain | Substrates‡ |
|||||||
|---|---|---|---|---|---|---|---|---|
| N2 |
CH3OH |
CH3OH/H2 |
H2/CO2 |
|||||
| CH4 | CO2 | CH4 | CO2 | CH4 | CO2 | CH4 | CO2 | |
| Δhpt§ | <1 | 2 ± 0.1 | 343 ± 4 | 108 ± 2 | 465 ± 17 | <1 | 298 ± 21 | NA |
| Δfpo | <1 | 1 ± 0.1 | 365 ± 3 | 115 ± 1 | 494 ± 11 | <1 | 356 ± 6 | NA |
| ΔfpoF | <1 | 1 ± 0.1 | 332 ± 7 | 106 ± 2 | 439 ± 27 | <1 | 359 ± 9 | NA |
| Δfre | <1 | 1 ± 0.1 | 399 ± 14 | 133 ± 4 | 508 ± 66 | <1 | 381 ± 21 | NA |
| Δfrh | <1 | 2 ± 0.9 | 149 ± 3 | 42 ± 1 | 481 ± 14 | <1 | 7 ± 2 | NA |
| Δfpo Δfrh | <1 | 2 ± 0.7 | 47 ± 2 | 9 ± 1 | 480 ± 59 | <1 | 10 ± 1 | NA |
| ΔfpoF Δfrh | <1 | 4 ± 0.1 | 54 ± 3 | 16 ± 1 | 465 ± 9 | <1 | 11 ± 5 | NA |
*Values are the average and standard deviation of at least 3 trials.
†Strains used were WWM85 (Δhpt), WWM71 (Δfpo), WWM123 (ΔfpoF), WWM122 (Δfrh), WWM108 (Δfpo Δfrh), and WWM145 (ΔfpoF Δfrh).
‡ Assays were conducted as described in Supplementary Information.
§M. barkeri Fusaro parent strain in which all deletions were constructed.
NA, not applicable (CO2 produced could not be measured because it was added to headspace).
Table 3.
Rate (nmol min−1 mg−1) of methane production* from resting cell suspensions of M. barkeri Fusaro strains†
| Strain | Substrate‡ |
||
|---|---|---|---|
| N2 | CH3OH | CH3OH/H2 | |
| Δhpt§ | <1 | 70 ± 6 | 121 ± 35 |
| Δfpo | <1 | 93 ± 13 | 127 ± 29 |
| ΔfpoF | <1 | 82 ± 2 | 143 ± 7 |
| Δfre | <1 | 95 ± 13 | 134 ± 32 |
| Δfrh | <1 | 14 ± 0.5 | 97 ± 3 |
| Δfpo Δfrh | <1 | 4 ± .5 | 151 ± 3 |
| ΔfpoF Δfrh | <1 | 4 ± 0.1 | 135 ± 15 |
*Values are the average and standard deviation of at least three trials.
†Strains used were WWM85 (Δhpt), WWM71 (Δfpo), WWM123 (ΔfpoF), WWM122 (Δfrh), WWM108 (Δfpo Δfrh), and WWM145 (ΔfpoF Δfrh).
‡Assays were conducted as described in SI Text.
§M. barkeri Fusaro parent strain in which all deletions were constructed.
Frh Is Essential for Growth of M. barkeri on H2/CO2 and Plays an Important Role in the Methylotrophic Pathway.
The dispensability of Fpo raises the question of which enzyme(s) transfers electrons from F420H2 to methanophenazine in the methylotrophic pathway. Because M. barkeri possesses a highly active F420-reducing hydrogenase (24), we considered the possibility of electrons being channeled from F420H2 into the H2:heterodisulfide oxidoreductase system via production of H2 by Frh (Fig. 1).
The F420-reducing hydrogenase couples oxidation of H2 to F420 reduction in vitro and is fully reversible (24–26). M. barkeri has 2 operons, frhADGB and freAEGB, with the potential to encode F420-reducing hydrogenases (Fig. S1) (27). It is unclear whether freAEGB encodes an active hydrogenase, because it lacks the required maturation protease encoded by frhD in the homologous operon. Nevertheless, the putative fre-encoded hydrogenase shares all important catalytic and structural amino acid residues with the frh-encoded enzyme. Thus, freAEGB could encode a functional F420-reducing hydrogenase if the FrhD protease can function in trans (10). The F420-reducing hydrogenase is proposed to provide F420H2, which is needed for CO2 reduction in the hydrogenotrophic pathway (Fig. S2) (24); however, frhADGB and freAEGB are expressed during growth on both H2/CO2 and methanol (10, 27). Thus, it seems possible that these genes play a role in both the hydrogenotrophic and methylotrophic pathways.
To test this possibility, the freAEGB or frhADGB operons were deleted from the chromosome of M. barkeri (Table S2), and the resulting mutants were characterized. The Δfre mutant is indistinguishable from its parent with respect to growth rate and yield on all substrates tested (Table 1). Further, deletion of fre from M. barkeri does not affect the amount and rate of CH4 produced in resting cell suspensions from the various substrates, nor does the mutation change the expected 3:1 ratio of CH4 to CO2 on methanol (Tables 2 and 3). These data indicate that Fre is not required for growth of M. barkeri on any of the substrates tested.
In contrast, the Δfrh mutation has severe phenotypic consequences on several of the growth media examined (Tables 1–3). Although the Δfrh mutant is indistinguishable from its parent when grown on methanol plus H2/CO2 and acetate, it is unable to grow on H2/CO2. Moreover, the Δfrh mutant has a 2-fold slower growth rate and a 50% reduction in growth yield when methanol alone is used as a substrate. The Δfrh mutant exhibits a very long lag phase of 911 ± 21 h, as compared with 31 ± 0.4 h for wild-type M. barkeri on methanol. The growth defects of the Δfrh mutant are reflected further in CH4 production. Resting cell suspensions of the Δfrh mutant produce negligible amounts of CH4 from H2/CO2 and although they disproportionate methanol in the expected 3:1 ratio, they consistently produce only half as much CH4 and CO2 as the parent. Finally, the Δfrh mutation lowers the rate of CH4 production from methanol in resting cells by ca. 4-fold relative to the parent.
Taken together, these data indicate that Frh is essential for growth by the hydrogenotrophic pathway and plays an important but dispensable role in the methylotrophic pathway, the latter potentially being the delivery of electrons from F420H2 into the H2:heterodisulfide oxidoreductase system. Importantly, Fre is not able to substitute for the role of Frh under the conditions tested, suggesting that it does not encode a functional F420-reducing hydrogenase in the absence of frhADGB.
M. barkeri Possesses Two Functional Pathways for Electron Transfer from F420H2 to Methanophenazine.
The fact that the Δfrh mutant retains the ability to grow and produce CH4 from methanol, although at reduced rates, suggests that the cell has an alternative, less efficient route to deliver electrons from F420H2 to the CoM-S-S-CoB heterodisulfide. The most obvious candidate for this electron transfer pathway is the F420H2:heterodisulfide oxidoreductase system. To test this hypothesis, we constructed and characterized double mutants lacking frhADGB and either fpoA-O or fpoF (Tables S2 and Tables 1, 2, and 3).
Like the single Δfrh mutant, the ΔfpoA-O/Δfrh and ΔfpoF/Δfrh double mutants are unable to grow on H2/CO2 and produce negligible amounts of CH4 in resting cell suspensions from this substrate. Both mutants are able to grow and produce CH4 at levels and rates comparable to the parent on methanol plus H2/CO2. However, in contrast to the Δfrh, ΔfpoA-O, and ΔfpoF single mutants, the double mutants are incapable of growth on methanol. Thus, Frh and Fpo fulfill a similar role during growth on this substrate that is lost in the absence of both enzymes. Quantitative RT-PCR experiments show that although fpoA-O transcripts levels are slightly higher in the Δfrh mutant (3.9 ± 1.5-fold), they are easily detectable in the parental strain. Therefore, Fpo is expressed and has the potential to contribute to the methylotrophic electron transport chain in wild-type M. barkeri. Interestingly, the ΔfpoA-O/Δfrh and ΔfpoF/Δfrh mutants still produce small amounts of CH4 and CO2 from methanol despite their inability to grow on this substrate. Hence, an additional minor electron transport pathway(s) exists in M. barkeri that is incapable of supporting growth.
Surprisingly, the double mutants display different phenotypes when grown on acetate. The ΔfpoF/Δfrh mutant grows slowly on acetate, whereas the ΔfpoA-O/Δfrh mutant is unable to use acetate. It is unclear how the absence of both Fpo and Frh affects growth on acetate, because neither the ΔfpoA-O nor the Δfrh single mutant exhibits a growth defect on this substrate. These data seem to suggest that the input module of Fpo (FpoF) can be dispensable under conditions in which the proton-pumping methanophenazine oxidoreductase (FpoABCDHIJKLMNO) is not.
Discussion
Methanosarcina species have proven to be exceptional model organisms for genetic analysis of methanogenesis (14, 28–30), an approach that modified our concept of energy conservation in M. barkeri. The data presented here indicate that M. barkeri has 2 distinct energy-conserving electron transport pathways during growth via the methylotrophic methanogenic pathway (Fig. 1). Contrary to expectations, M. barkeri apparently prefers to transfer electrons obtained from C1 compound oxidation to the H2:heterodisulfide oxidoreductase system via H2 rather than to transfer them directly into the F420H2:heterodisulfide oxidoreductase system. Our results suggest that the cytoplasmic F420-reducing hydrogenase mediates electron transfer to H2 via oxidation of F420H2, which, along with reduced ferredoxin (Fdred), is the direct product of C1 compound oxidation (24, 26). When the cells lose the ability to produce H2 via this route, growth on methanol is severely affected, with reduction in the rates of growth and CH4 production, in total growth yield, and in the amount of CH4 produced. We suggest that the H2 diffuses out of the cell and enters the H2:heterodisulfide oxidoreductase system via methanophenazine-dependent hydrogenase (Vht or Vhx) (10), whose active site is known to be in the periplasm (4). Because production of H2 by Frh consumes protons within the cytoplasm, whereas oxidation of H2 by Vht/Vhx releases protons outside the cell, this electron transport chain is capable of establishing a proton gradient across the membrane that can be used to generate ATP by the ATP synthase (31), thus conserving energy via a H2-cycling mechanism (19). M. barkeri genome harbors 2 operons, vhtGACD and vhxGAC, that potentially can encode methanophenazine-dependent hydrogenases. Analogous to the fre operon, the vhx operon lacks gene D that encodes the hydrogenase maturation protease and therefore may not encode a functional hydrogenase. However, Vhx shares all important catalytic and structural amino acid residues with Vht and could encode an active methanophenazine-dependent hydrogenase if the VhtD protease can function in trans (10).
H2 cycling, as a mechanism of energy conservation, was first proposed in sulfate-reducing bacteria (19). Later, it also was proposed in other anaerobic organisms such as Acetobacterium woodii (32) and Geobacter sulfurreducens (33). Based on the production of H2 during growth of Methanosarcina species on methylated substrates such as methanol, trimethylamine, and acetate, H2 cycling also was suggested to occur in Methanosarcina (34). Experimental support for H2 cycling has been provided in Desulfovibrio vulgaris, wherein simultaneous production and consumption of H2 were detected during metabolism of pyruvate and sulfate (35). Also, suppression of a D. vulgaris H2-evolving hydrogenase led to reduced growth rates on lactate and sulfate, suggesting the importance of H2 production in growth (36). Nevertheless, the H2-cycling theory is not accepted universally, and several lines of evidence have been used to argue that this mechanism is unlikely (reviewed in ref. 37). For example, high concentrations of H2 do not inhibit lactate oxidation in sulfate-reducing bacteria (38), nor does a mutation that blocks use of H2 prevent growth on lactate (39). Further, the idea that the cell would transfer electrons preferentially to a molecule that can diffuse freely away from the cell seems highly problematic.
The data presented here suggest a way to reconcile these conflicting views. Although M. barkeri apparently prefers to transfer electrons via H2, they remain capable of using the F420H2:heterodisulfide oxidoreductase system when the ability to produce cytoplasmic H2 is lost. Thus, M. barkeri employs a branched electron transport chain with most electrons flowing from F420H2 into the H2:heterodisulfide oxidoreductase system via H2 but with some fraction flowing directly into the F420H2:heterodisulfide oxidoreductase system. A similar branched electron transport chain in sulfate reducers would explain the results cited as arguing against H2 cycling without invalidating the model. It should be noted that a recently proposed metabolic model for D. vulgaris suggests just such a branched electron transport chain (37).
Why, then, is the H2-cycling pathway the preferred electron transport chain in M. barkeri? Because the initial electron donor (F420H2) and final electron acceptor (methanophenazine) are the same, the amount of energy available from the 2 electron transport schemes must be identical. Experimental measurements suggest that electron transfer from F420H2 to CoM-S-S-CoB via Fpo and Hdr is accompanied by translocation of 4 protons across the membrane (11). Similarly, the flow of electrons from F420H2 to methanophenazine via Frh and Vht/Vhx leads to the translocation of 2 protons across the membrane by virtue of the H2-cycling mechanism, whereas electron transfer from MPH2 to CoM-S-S-CoB translocates another 2 protons (9). Thus, the magnitude of proton motive force generated should be identical via either route (Fig. 1). In contrast, the rate of CH4 production from methanol in resting cell suspensions of Δfrh mutant is ca. 4 times slower than that of ΔfpoA-O and ΔfpoF mutants, indicating that H2 cycling is a much faster mechanism of energy conservation and, as observed in our mutant strains, allows correspondingly faster growth rates. The biochemical properties of the enzymes involved are remarkably consistent with our in vivo data. Thus, FpoBCDIF [molecular weight (MW) = 135.1 kDa] (11) purified from M. mazei catalyzes F420H2 oxidation with a turnover number (Kcat) of 38 s−1 and catalytic efficiency [Kcat/Michaelis constant (Km)] of 5.4 × 106 M−1s−1 (12); FrhAGB (MW = 194.4 kDa) (27) purified from M. barkeri catalyzes H2 oxidation using F420 as the electron acceptor with a Kcat of 134 s−1 and Kcat/Km of 4.4 × 107 M−1s−1 (24). Because methanol-metabolizing M. barkeri cells have been shown to maintain ratios of F420H2 and F420 in thermodynamic equilibrium with the H2 partial pressure (25), it is reasonable to assume that Frh catalyzes the reverse reaction (F420H2 oxidation) with similar efficiency as the forward reaction (H2 oxidation). Therefore, the Kcat of Frh is ca. 4 times higher than that of Fpo for F420H2 oxidation, suggesting that Frh is faster than Fpo in catalyzing this reaction.
A variety of data indicate that H2 cycling is important to many, but not all, Methanosarcina species. Growth of M. barkeri on acetate involves the obligate production of H2 (14). Thus, in combination with the data presented here, it seems likely that this species prefers to use H2 cycling for all soluble substrates. The situation probably is similar in M. mazei because it has functional Frh, Vht, and Fpo enzymes as well (11). However, methylotrophic species such as M. acetivorans (10, 21, 28), Methanolobus tindarius (40, 41), and Methanococcoides burtonii (42) do not encode functional hydro-genases (22). Hence, these organisms probably rely exclusively on the F420H2:heterodisulfide oxidoreductase system for energy conservation. Moreover, we made an intensive effort to delete the fpo genes from M. acetivorans without success, suggesting that F420H2 dehydrogenase is essential in this organism. It has been suggested previously that these organisms forego H2-dependent electron transport pathways because of their high-salt marine habitat, wherein they exist as disaggregated single cells and would be prone to lose the freely diffusible H2 gas to competing organisms. In contrast, freshwater organisms such as M. barkeri that exist as large multicellular aggregates have a higher chance of retaining H2 gas within the aggregates, enabling them to use it as an electron carrier (28).
Finally, the phenotypes of the mutants constructed here also provide insight into the metabolism of H2/CO2 and acetate by M. barkeri. Because of its ability to catalyze F420 reduction with H2, F420-reducing hydrogenase was proposed to provide F420H2 for reduction of methenyl-tetrahydrosarcinapterin (H4SPT) and methylene-H4SPT in the hydrogenotrophic pathway (Fig. S2) (24, 43). The inability of the frh mutants to grow on H2/CO2 provides direct experimental support for this proposal. Interestingly, the putative fre-encoded hydrogenase cannot substitute for the frh-encoded hydrogenase. This inability may be caused by low expression of fre, absence of posttranslational processing, mutations in structural or catalytic residues, or some combination of these factors (10). Nevertheless, the dispensability of fre in M. barkeri is not surprising because M. mazei lacks the fre operon and is able to grow via all 4 methanogenic pathways (4, 10). Thus, the role of Fre in M. barkeri remains mysterious.
Methanogenesis from acetate does not require either Frh or Fpo; however, our results show that 1 of the 2 enzymes, but not both, is needed for growth on this substrate. We previously showed that mutations in the C1 oxidation pathway prevent growth on acetate, presumably by blocking the production of reducing equivalents needed for biosynthetic reactions (30). The lack of growth of the Δfrh/Δfpo double mutant on acetate medium suggests that these reducing equivalents must flow through either Frh or Fpo to allow growth. Interestingly, the Δfrh/ΔfpoF double mutant is able to grow on acetate, clearly suggesting that the membrane-bound proton-pumping module has the ability to accept electrons from input modules other than FpoF. In this regard, FpoF is homologous to the β-subunit (B) of F420-reducing hydrogenases and shares a common substrate, coenzyme F420 (11, 27). It is conceivable that in the absence of FpoF and FrhB, FreB serves as the input module, thus channeling electrons from F420H2 to Fpo and allowing growth of Δfrh/ΔfpoF double mutant on acetate; however, this conjecture remains to be tested.
Materials and Methods
Sequence Analysis.
All sequence data are from publicly available genomes (11, 16, 20, 21). The Integrated Microbial Genome (IMG) system was used to identify orthologs and assess the genomic context of genes (22).
Strains, Media, and Growth Conditions.
The construction and genotype of all Methanosarcina strains is presented in Table S2. Methanosarcina strains were grown as single cells at 37 °C in high-salt broth medium (44) or on agar-solidified medium as described (45). Standard conditions were used for growth of E. coli strains DH5α/λ-pir and DH10B (Stratagene) (46), which were used as hosts for plasmid constructions.
DNA Methods, Plasmid, and Strain Construction.
Standard methods were used for plasmid DNA isolation and manipulation in E. coli (47). Liposome-mediated transformation was used for Methanosarcina as described (48). Genomic DNA isolation and DNA hybridization were as described (44, 45, 49). DNA sequences were determined from double-stranded templates by the W.M. Keck Center for Comparative and Functional Genomics, University of Illinois. Plasmid constructions are described in the SI Text (Tables S3 and S4).
Characterization of Mutants in Terms of Growth Characteristics and CH4 and CO2 Production in Cell Suspensions.
Growth was quantified by measuring OD600. Generation times were calculated during exponential growth phase, and growth yield was determined by measuring the maximal OD600 of the culture. Growth curve and cell suspension experiments were performed as described in SI Text.
Quantitative RT-PCR.
Gene-specific primers (Table S3) were designed using Primer Express Software v2.0 (Applied Biosystems). A 1-step qRT-PCR was performed using SuperScript III Platinum SYBR Green One-Step qRT-PCR Kit with ROX (Invitrogen) in the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). The relative standard curve method was used to quantify expression of fpo (ABI PRISM 7700 Sequence Detection System User Bulletin #2) using rpoA1 as the reference gene (described in Supporting Information).
Supplementary Material
Acknowledgments.
We thank Nicole Buan and Rina Opulencia for critical review of the manuscript. This work was supported in part by Department of Energy Grant DE-FG02–02ER15296 and by National Science Foundation Grant MCB0517419. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation or the Department of Energy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905914106/DCSupplemental.
References
- 1.Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat Rev Microbiol. 2008;6:579–591. doi: 10.1038/nrmicro1931. [DOI] [PubMed] [Google Scholar]
- 2.Deppenmeier U. Redox-driven proton translocation in methanogenic Archaea. Cell Mol Life Sci. 2002;59:1513–1533. doi: 10.1007/s00018-002-8526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferry JG. Methane from acetate. J Bacteriol. 1992;174:5489–5495. doi: 10.1128/jb.174.17.5489-5495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deppenmeier U. The membrane-bound electron transport system of Methanosarcina species. J Bionenerg Biomembr. 2004;36:55–64. doi: 10.1023/b:jobb.0000019598.64642.97. [DOI] [PubMed] [Google Scholar]
- 5.Li F, et al. Coupled ferredoxin and crotonyl coenzyme A (CoA) reduction with NADH catalyzed by the butyryl-CoA dehydrogenase/Etf complex from Clostridium kluyveri. J Bacteriol. 2008;190:843–850. doi: 10.1128/JB.01417-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumer S, Lentes S, Gottschalk G, Deppenmeier U. Identification and analysis of proton-translocating pyrophosphatases in the methanogenic archaeon Methansarcina mazei. Archaea. 2002;1:1–7. doi: 10.1155/2002/371325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumer S, et al. The F420H2:heterodisulfide oxidoreductase system from Methanosarcina species. 2-Hydroxyphenazine mediates electron transfer from F420H2 dehydrogenase to heterodisulfide reductase. FEBS Lett. 1998;428:295–298. doi: 10.1016/s0014-5793(98)00555-9. [DOI] [PubMed] [Google Scholar]
- 8.Deppenmeier U, Blaut M, Mahlmann A, Gottschalk G. Reduced coenzyme F420: Heterodisulfide oxidoreductase, a proton- translocating redox system in methanogenic bacteria. Proc Natl Acad Sci USA. 1990;87:9449–9453. doi: 10.1073/pnas.87.23.9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ide T, Baumer S, Deppenmeier U. Energy conservation by the H2:heterodisulfide oxidoreductase from Methanosarcina mazei Gö1: Identification of two proton-translocating segments. J Bacteriol. 1999;181:4076–4080. doi: 10.1128/jb.181.13.4076-4080.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guss AM, Kulkarni G, Metcalf WW. Differences in hydrogenase gene expression between Methanosarcina acetivorans and Methanosarcina barkeri. J Bacteriol. 2009;191:2826–2833. doi: 10.1128/JB.00563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumer S, et al. The F420H2 dehydrogenase from Methanosarcina mazei is a redox-driven proton pump closely related to NADH dehydrogenases. J Biol Chem. 2000;275:17968–17973. doi: 10.1074/jbc.M000650200. [DOI] [PubMed] [Google Scholar]
- 12.Abken HJ, Deppenmeier U. Purification and properties of an F420H2 dehydrogenase from Methanosarcina mazei Gö1. FEMS Microbiol Lett. 1997;154:231–237. [Google Scholar]
- 13.Meuer J, Bartoschek S, Koch J, Kunkel A, Hedderich R. Purification and catalytic properties of Ech hydrogenase from Methanosarcina barkeri. Eur J Biochem. 1999;265:325–335. doi: 10.1046/j.1432-1327.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- 14.Meuer J, Kuettner HC, Zhang JK, Hedderich R, Metcalf WW. Genetic analysis of the archaeon Methanosarcina barkeri Fusaro reveals a central role for Ech hydrogenase and ferredoxin in methanogenesis and carbon fixation. Proc Natl Acad Sci USA. 2002;99:5632–5637. doi: 10.1073/pnas.072615499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deppenmeier U, Muller V, Gottschalk G. Pathways of energy conservation in methanogenic archaea. Arch Microbiol. 1996;165:149–163. [Google Scholar]
- 16.Deppenmeier U. The unique biochemistry of methanogenesis. Prog Nucleic Acid Res Mol Biol. 2002;71:223–283. doi: 10.1016/s0079-6603(02)71045-3. [DOI] [PubMed] [Google Scholar]
- 17.Deppenmeier U, Lienard T, Gottschalk G. Novel reactions involved in energy conservation by methanogenic archaea. FEBS Lett. 1999;457:291–297. doi: 10.1016/s0014-5793(99)01026-1. [DOI] [PubMed] [Google Scholar]
- 18.Deppenmeier U, Muller V. Life close to the thermodynamic limit: How methanogenic archaea conserve energy. Results Probl Cell Differ. 2008;45:123–152. doi: 10.1007/400_2006_026. [DOI] [PubMed] [Google Scholar]
- 19.Odom JM, Peck HD., Jr Hydrogen cycling as a general mechanism for energy coupling in the sulfate-reducing bacteria, Desulfovibrio sp. FEMS Microbiol Lett. 1981;12:47–50. [Google Scholar]
- 20.Maeder DL, et al. The Methanosarcina barkeri genome: Comparative analysis with Methanosarcina acetivorans and Methanosarcina mazei reveals extensive rearrangement within methanosarcinal genomes. J Bacteriol. 2006;188:7922–7931. doi: 10.1128/JB.00810-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galagan JE, et al. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 2002;12:532–542. doi: 10.1101/gr.223902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markowitz VM, et al. The integrated microbial genomes (IMG) system in 2007: Data content and analysis tool extensions. Nucleic Acids Res. 2008;36:D528–533. doi: 10.1093/nar/gkm846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deppenmeier U, et al. The genome of Methanosarcina mazei: Evidence for lateral gene transfer between bacteria and archaea. J Mol Microbiol Biotechnol. 2002;4:453–461. [PubMed] [Google Scholar]
- 24.Michel R, Massanz C, Kostka S, Richter M, Fiebig K. Biochemical characterization of the 8-hydroxy-5-deazaflavin-reactive hydrogenase from Methanosarcina barkeri Fusaro. Eur J Biochem. 1995;233:727–735. doi: 10.1111/j.1432-1033.1995.727_3.x. [DOI] [PubMed] [Google Scholar]
- 25.de Poorter LM, Geerts WJ, Keltjens JT. Hydrogen concentrations in methane-forming cells probed by the ratios of reduced and oxidized coenzyme F420. Microbiology. 2005;151:1697–1705. doi: 10.1099/mic.0.27679-0. [DOI] [PubMed] [Google Scholar]
- 26.Vignais PM, Billoud B, Meyer J. Classification and phylogeny of hydrogenases. FEMS Microbiol Rev. 2001;25:455–501. doi: 10.1111/j.1574-6976.2001.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 27.Vaupel M, Thauer RK. Two F420-reducing hydrogenases in Methanosarcina barkeri. Arch Microbiol. 1998;169:201–205. doi: 10.1007/s002030050561. [DOI] [PubMed] [Google Scholar]
- 28.Guss AM, Mukhopadhyay B, Zhang JK, Metcalf WW. Genetic analysis of mch mutants in two Methanosarcina species demonstrates multiple roles for the methanopterin-dependent C-1 oxidation/reduction pathway and differences in H(2) metabolism between closely related species. Mol Microbiol. 2005;55:1671–1680. doi: 10.1111/j.1365-2958.2005.04514.x. [DOI] [PubMed] [Google Scholar]
- 29.Welander PV, Metcalf WW. Loss of the mtr operon in Methanosarcina blocks growth on methanol, but not methanogenesis, and reveals an unknown methanogenic pathway. Proc Natl Acad Sci USA. 2005;102:10664–10669. doi: 10.1073/pnas.0502623102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welander PV, Metcalf WW. Mutagenesis of the C1 oxidation pathway in Methanosarcina barkeri: New insights into the Mtr/Mer bypass pathway. J Bacteriol. 2008;190:1928–1936. doi: 10.1128/JB.01424-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller V. An exceptional variability in the motor of archael A1A0 ATPases: From multimeric to monomeric rotors comprising 6–13 ion binding sites. J Bionenerg Biomembr. 2004;36:115–125. doi: 10.1023/b:jobb.0000019603.68282.04. [DOI] [PubMed] [Google Scholar]
- 32.Odom JM, Peck HD., Jr. Hydrogenase, electron-transfer proteins, and energy coupling in the sulfate-reducing bacteria Desulfovibrio. Annu Rev Microbiol. 1984;38:551–592. doi: 10.1146/annurev.mi.38.100184.003003. [DOI] [PubMed] [Google Scholar]
- 33.Coppi MV. The hydrogenases of Geobacter sulfurreducens: A comparative genomic perspective. Microbiology. 2005;151:1239–1254. doi: 10.1099/mic.0.27535-0. [DOI] [PubMed] [Google Scholar]
- 34.Lovley DR, Ferry JG. Production and consumption of H(2) during growth of Methanosarcina spp. on acetate. Appl Environ Microbiol. 1985;49:247–249. doi: 10.1128/aem.49.1.247-249.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peck HDJ, LeGall J, Lespinat PA, Berlier Y, Fauque G. A direct demonstration of hydrogen cycling by Desulfovibrio vulgaris employing membrane-inlet mass spectrometry. FEMS Microbiol Lett. 1987;40:295–299. [Google Scholar]
- 36.van den Berg WA, van Dongen WM, Veeger C. Reduction of the amount of periplasmic hydrogenase in Desulfovibrio vulgaris (Hildenborough) with antisense RNA: Direct evidence for an important role of this hydrogenase in lactate metabolism. J Bacteriol. 1991;173:3688–3694. doi: 10.1128/jb.173.12.3688-3694.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noguera DR, Brusseau GA, Rittmann BE, Stahl DA. A unified model describing the role of hydrogen in the growth of Desulfovibrio vulgaris under different environmental conditions. Biotechnol Bioeng. 1998;59:732–746. doi: 10.1002/(sici)1097-0290(19980920)59:6<732::aid-bit10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Pankhania IP, Gow LA, Hamilton WA. The effect of hydrogen on the growth of Desulfovibrio vulgaris (Hildenborough) on lactate. J Gen Microbiol. 1986;132:3349–3356. [Google Scholar]
- 39.Odom JM, Wall JD. Properties of a hydrogen-inhibited mutant of Desulfovibrio desulfuricans ATCC 27774. J Bacteriol. 1987;169:1335–1337. doi: 10.1128/jb.169.3.1335-1337.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haase P, Deppenmeier U, Blaut M, Gottschalk G. Purification and characterization of F420H2-dehydrogenase from Methanolobus tindarius. Eur J Biochem. 1992;203:527–531. doi: 10.1111/j.1432-1033.1992.tb16579.x. [DOI] [PubMed] [Google Scholar]
- 41.Scheel E, Schafer G. Chemiosmotic energy conversion and the membrane ATPase of Methanolobus tindarius. Eur J Biochem. 1990;187:727–735. doi: 10.1111/j.1432-1033.1990.tb15360.x. [DOI] [PubMed] [Google Scholar]
- 42.Saunders NF, et al. Mechanisms of thermal adaptation revealed from the genomes of the Antarctic archaea Methanogenium frigidum and Methanococcoides burtonii. Genome Res. 2003;13:1580–1588. doi: 10.1101/gr.1180903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thauer RK, Hedderich R, Fischer R. Methanogenesis. New York: Chapman and Hall; 1993. pp. 209–252. [Google Scholar]
- 44.Metcalf WW, Zhang JK, Shi X, Wolfe RS. Molecular, genetic, and biochemical characterization of the serC gene of Methanosarcina barkeri Fusaro. J Bacteriol. 1996;178:5797–5802. doi: 10.1128/jb.178.19.5797-5802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boccazzi P, Zhang JK, Metcalf WW. Generation of dominant selectable markers for resistance to pseudomonic acid by cloning and mutagenesis of the ileS gene from the archaeon Methanosarcina barkeri Fusaro. J Bacteriol. 2000;182:2611–2618. doi: 10.1128/jb.182.9.2611-2618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wanner BL. Novel regulatory mutants of the phosphate regulon in Escherichia coli K12. J Mol Biol. 1986;191:39–58. doi: 10.1016/0022-2836(86)90421-3. [DOI] [PubMed] [Google Scholar]
- 47.Ausubel FM, et al. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1992. [Google Scholar]
- 48.Metcalf WW, Zhang JK, Apolinario E, Sowers KR, Wolfe RS. A genetic system for archaea of the genus Methanosarcina: Liposome-mediated transformation and construction of shuttle vectors. Proc Natl Acad Sci USA. 1997;94:2626–2631. doi: 10.1073/pnas.94.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang JK, White AK, Kuettner HC, Boccazzi P, Metcalf WW. Directed mutagenesis and plasmid-based complementation in the methanogenic archaeon Methanosarcina acetivorans C2A demonstrated by genetic analysis of proline biosynthesis. J Bacteriol. 2002;184:1449–1454. doi: 10.1128/JB.184.5.1449-1454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.