Abstract
In female mammals including rodents and humans, feeding decreases during the periovulatory period of the ovarian cycle, which coincides with a surge in circulating estrogen levels. Ovariectomy increases food intake, which can be normalized by estrogen treatment at a dose and frequency mimicking those during the estrous cycle. Furthermore, administration of estrogen to rodents potently inhibits food intake. Despite these well-known effects of estrogen, neuronal subtypes that mediate estrogen's anorexigenic effects have not been identified. In this study, we show that changes in hypothalamic expression of agouti-related protein (Agrp) and neuropeptide Y (Npy) coincide with the cyclic changes in feeding across the estrous cycle. These cyclic changes in feeding are abolished in mice with degenerated AgRP neurons even though these mice cycle normally. Central administration of 17β-estradiol (E2) decreases food intake in controls but not in mice lacking the AgRP neurons. Furthermore, E2 treatment suppresses fasting-induced c-Fos activation in AgRP and NPY neurons and blunts the refeeding response. Surprisingly, although estrogen receptor alpha (ERα) is the key mediator of estrogen's anorexigenic effects, we find that expression of ERα is completely excluded from AgRP and NPY neurons in the mouse hypothalamus, suggesting that estrogen may regulate these neurons indirectly via presynaptic neurons that express ERα. This study indicates that neurons coexpressing AgRP and NPY are functionally required for the cyclic changes in feeding across estrous cycle and that AgRP and NPY neurons are essential mediators of estrogen's anorexigenic function.
Keywords: estrogen, feeding
Proper regulation of energy homeostasis and reproduction is fundamental for fitness and survival. Reproduction is an energy intensive process, and precise interaction of regulators for energy balance and reproduction allows coordinated regulation of these two processes. Leptin, a hormone secreted from adipose tissue, plays a critical role in both energy balance and reproduction. Leptin is produced proportional to body fat mass and it conveys the abundance of the body's energy stores to the brain, where it acts to regulate feeding and energy expenditure (1). A decline in leptin level signals a state of negative energy balance, which triggers robust counterregulatory mechanisms to increase feeding. One consequence of negative energy balance is induction of hypogonadonism and inhibition of reproductive function (2). Consistent with this notion, leptin deficiency results in profound hyperphagia and infertility in rodents and humans (1, 3–5).
Estrogen, a hormone essential for sexual reproduction, plays a role in feeding and energy balance regulation. Serum levels of estrogen decline during negative energy balance (6) and estrogen deficiency or loss of function of estrogen receptor (ER) results in increased feeding and adiposity in rodents and humans (7–10). Feeding and body weight increase in ovariectomized females and estrogen replacement reverses such effects (11, 12). Furthermore, acute ablation of ER alpha (ERα) in the brain results in severe obesity and metabolic syndrome (13). Thus, decreased estrogen signaling also activates regulatory mechanisms to increase body adiposity. Consistent with the notion that estrogen acts in the brain to regulate energy balance, central administration of estrogen has been shown to decrease food intake in ovarian-intact rodents (14). Food intake has also been shown to exhibit cyclic changes across ovarian cycle. Estrogen levels rise right before estrus, during which time food intake is at its nadir (11, 12). Similar observations have been made in humans: women tend to eat less during the 4-day periovulatory phase of the ovarian cycle, which coincides with a surge in estrogen levels (15). These cyclic changes in feeding are absent during anovulatory cycles (16).
Leptin's effect on energy balance is to a large degree mediated by its regulation of hypothalamic neurons (1). Within the arcuate nucleus (ARC) of the hypothalamus, neurons expressing proopiomelanocortin (POMC) and neurons coexpressing agouti-related protein (AgRP) and neuropeptide Y (NPY) are direct leptin targets. Both AgRP and NPY are potent orexigens, and due to their coexpression in neurons within the ARC, these neurons are termed AgRP/NPY neurons. While AgRP/NPY neurons promote positive energy balance, POMC neurons promote negative energy balance. During food deprivation, leptin levels decline precipitously, leading to dramatic upregulation of Agrp and Npy expression and modest downregulation of Pomc. This reciprocal change in Agrp, Npy and Pomc expression leads to a robust hyperphagic response upon refeeding, which ensures rapid replenishment of energy stores and restoration of energy balance. Importantly, leptin's effect on reproduction is mediated, at least in part, by its negative regulation on the Npy gene, since deletion of Npy gene restores fertility in leptin deficient mice (17). Much of this effect is mediated by NPY Y4 receptor as deletion of Y4 receptor in leptin deficient mice rescues fertility without affecting feeding and body weight (18). Thus, leptin regulates both energy balance and reproduction by negatively regulating Npy expression.
In contrast to leptin, the underlying mechanism by which estrogen regulates feeding is still largely unknown. Previous studies have shown that estrogen influences the feeding efficacy of cholecystokinin (CCK) and ghrelin (12). Recently, estrogen has been shown to exert leptin-like effects by modulating synaptic densities on the POMC neurons, although the identity of these presynaptic estrogen-responsive neurons is not known (14). Despite these findings, the functional requirement of specific neuronal subgroups in mediating estrogen's anorexigenic effect has not been established. In this study, we use a transgenic mouse model in which AgRP/NPY neurons are degenerated and show that AgRP/NPY neurons are functionally required for the cyclic changes in feeding across the estrous cycle and that these neurons are essential targets for estrogen's anorexigenic effects.
Results
Changes in Agrp and Npy Expression Across the Estrous Cycle Coincide with Cyclic Changes in Food Intake and Body Weight.
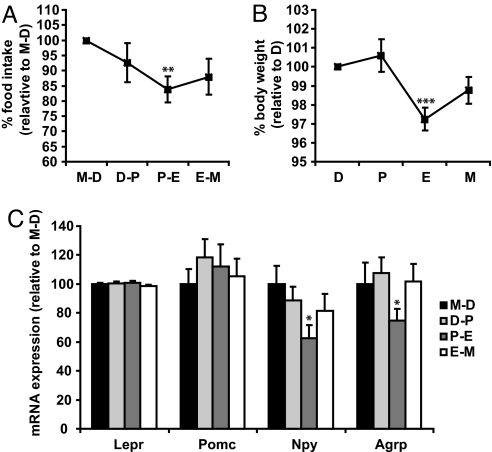
Female rodents exhibit cyclic changes in feeding across the estrous cycle (11, 12). We sought to confirm this phenomenon in 12-week-old C57BL/6J female mice. Phases of the estrous cycle were determined by cytological analysis of vaginal smears. Food intake, body weight, and phases of estrous cycle were monitored daily. Consistent with previous studies, food intake was highest between metestrus and diestrus, declined during proestrus, and reached its nadir between proestrus and estrus (Fig. 1A). On average, food intake in proestrus-estrus phase was 16.3% lower than in the metestrus-diestrus (2.5 ± 0.16 g and 3.0 ± 0.10 g for 24-h food intake, respectively). Changes in body weight across the estrous cycle mirrored changes in food intake (Fig. 1B). Since POMC, AgRP, and NPY are key neuropeptides in feeding regulation, we next examined whether their expression in the hypothalamus also follows cyclic changes. Mice were killed at different phases of the estrous cycle, and hypothalamic gene expression was analyzed by semiquantitative real time RT-PCR. While expression of leptin receptor (Lepr) and Pomc remained constant throughout the estrous cycle, Npy and Agrp expression were significantly reduced in proestrus-estrus (Fig. 1C). This temporal decrease in Npy and Agrp expression coincides with the decline in feeding and body weight, as described above. Thus, hypothalamic expression of Npy and Agrp is dynamically regulated in different phases of the estrous cycle and this change coincides with the cyclic change in food intake and body weight.
Fig. 1.
Agrp and Npy expression undergoes cyclic changes during the estrous cycle and such changes coincide with cyclic changes in food intake. (A, B) Phase of estrous cycle, food intake and body weight were determined daily in 12-week-old female mice (n = 12). Food intake and body weight measurements were normalized to measurements obtained in M-D for each mouse. **, P < 0.01 comparing food intake in M-D and P–E. ***, P < 0.001 comparing body weight in D and E using Student's paired t test. (C) Female mice were killed at 4 PM in different phases of the estrous cycle, and hypothalamic gene expression of Pomc, Lepr, Npy, and Agrp was analyzed by semiquantitative real time RT-PCR. β-actin was used as internal control. *, P < 0.05. Npy expression in M-D and P-E and Agrp expression in D-P and P-E were compared. n = 7 (M-D), 13 (D-P), 7 (P-E) and 9 (E-M). D, diestrus; P, proestrus; E, estrus; M, metestrus.
Estrous Cycle Dependent Regulation of Food Intake and Body Weight Is Abolished in Mice Lacking AgRP/NPY Neurons.
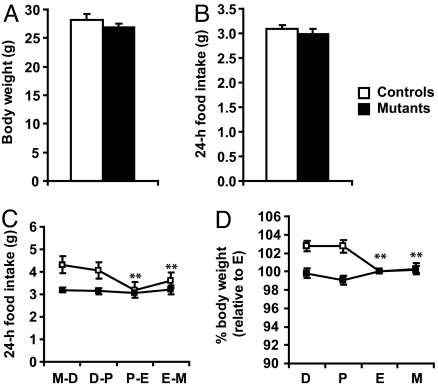
The precise temporal correlation of Agrp and Npy downregulation and the decrease in feeding and body weight suggests that cyclic modulation of AgRP/NPY neurons plays a causal role in estrous cycle dependent changes in feeding and body weight. To test this hypothesis, we used transgenic mice in which AgRP neurons are degenerated due to deletion of the mitochondrial transcription factor A gene, specifically in the AgRP neurons (Agrp-Tfam). We have previously reported that 85% of AgRP neurons are degenerated in the Agrp-Tfam mice by 6–7 months of age and that these mice exhibit normal food intake and body weight (19). Consistent with the previous report, Agrp-Tfam control and mutant mice used in this study displayed similar body weight and food intake when measured in all female mice regardless of their cycling status (Fig. 2 A and B). The Agrp-Tfam mutant mice are also fertile, consistent with a previous report showing that ablation of AgRP neurons in neonatal mice does not interfere with pregnancy, parturition or lactation (20). The cycling female Agrp-Tfam mutants exhibited typical morphologic change of vaginal smears in different phases of estrous cycle and the length of their estrous cycle did not differ from that of controls (Fig. S1). A similar percentage of the control and mutant mice did not cycle regularly and were excluded from the experiment described below. As expected, food intake and body weight decreased significantly between proestrus and estrus in the control females. However, food intake and body weight of Agrp-Tfam mutant animals remains unchanged throughout the estrous cycle, and are similar to values from the proestrus-estrus of the control animals (Fig. 2 C and D). These results suggest that AgRP/NPY neurons are functionally required for estrous cycle dependent regulation of food intake and body weight. They support the notion that AgRP/NPY neurons are functionally downstream of estrogen's anorexigenic effects. Thus, in the absence of AgRP/NPY neurons, food intake, and body weight in all phases of the estrous cycles resemble those in the proestrus-estrus of normal mice, suggesting that the decrease in feeding and body weight during proestrus-estrus in normal mice is caused by estrogen-mediated suppression of AgRP/NPY neuronal function.
Fig. 2.
Estrous cycle dependent regulation of food intake and body weight is abolished in mice lacking AgRP/NPY neurons. (A, B) Body weight and 24-h food intake were measured continuously for 20 days in all female Agrp-Tfam control and mutant mice regardless of their cycling status. n = 15 for controls, n = 14 for mutants. (C, D) Twenty-four–hour food intake and body weight were measured daily in cycling female Agrp-Tfam controls and mutants and the data are presented in panel C and D, respectively. For each mouse, body weight measurements were normalized to measurements obtained in estrus. n = 8 controls, n = 11 mutants. D, diestrus; P, proestrus; E, estrus; M, metestrus. **, P < 0.01 comparing food intake/body weight in M-D/D with P-E/E or E-M/M in controls as analyzed by Student's paired t test.
E2 Inhibits Agrp and Npy Expression in Hypothalamic Explants.
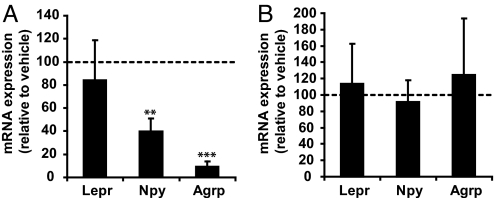
We next investigated whether Agrp and Npy expression are regulated by sex hormones, such as estrogen or progesterone. Coronal hypothalamic slices were prepared from adult females. Such slice preparation preserves the architecture of neuronal networks and neuronal communication. Each slice was cut into two identical halves along the 3rd ventricle and cultured. One half was treated with E2 (10 nM) for 24 h, and the other identical half was treated with vehicle. Similarly, slices were treated with progesterone (100 nM) or its vehicle for 24 h. RNA was then extracted and semiquantitative RT-PCR was performed. Treatment with E2 for 24 h significantly inhibited Npy and Agrp expression by 60.5% and 91.2%, respectively, compared with vehicle treatment (Fig. 3A). In contrast, E2 did not affect expression of Lepr. No significant changes in Npy, Agrp or Lepr expression were detected in hypothalamic explants treated with progesterone (Fig. 3B). These results suggest that E2, but not progesterone, inhibits Npy and Agrp expression in the hypothalamus.
Fig. 3.
E2 decreases Npy and Agrp expression in cultured hypothalamic explants. Coronal hypothalamic slices were prepared from adult C57BL/6 female mice killed 3–4 PM during the day of diestrus-proestrus. Each slice were cut into two identical halves and cultured. One half was treated with either 10 nM E2 (A) or 100 nM progesterone (B) and the other half was treated with the corresponding vehicle (water). After 24 h of treatment, RNA was extracted and semiquantitative RT-PCR was performed. Lepr, Npy, and Agrp expression was analyzed using β-actin as internal control. Gene expression in hormone treated explants was normalized to the vehicle values (broken line). **, P < 0.01 and ***, P < 0.001 by pair-wise comparison between hormone and vehicle treated samples. n = 5 for each treatment group.
Central Administration of E2 Inhibits Food Intake in Controls but Not in Mice Lacking AgRP/NPY Neurons.
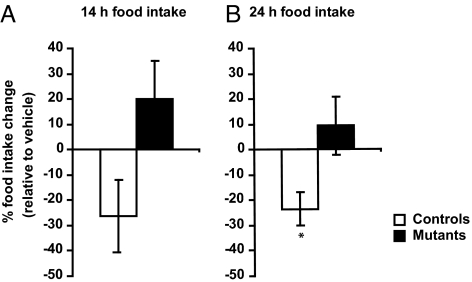
It has been shown that central administration of E2 inhibits food intake in ovarian-intact rodents (14). We thus sought to determine whether E2 exerts its anorexigenic effects by acting on AgRP/NPY neurons. Agrp-Tfam controls and mutants were icv injected with vehicle (aCSF) via a 3rd ventricle cannula for at least 6 days, during which time vaginal smears and food intake data were collected daily. After these first 6 days, mice were continuously injected with aCSF until they reached metestrus/diestrus. In this particular experiment, the mice were synchronous in cycling and most of them reached metestrus/diestrus on the 7th day. When the mice reached metestrus/diestrus, they were injected with 2 μg water soluble E2. Fourteen-hour and 24-h food intake after E2 treatment was compared with values obtained in the same phase (metestrus/diestrus) during vehicle treatment, such that each mouse served as its own control. While E2 inhibited 24-h food intake in the controls by 23.6%, it failed to alter food intake in the Agrp-Tfam mutants (Fig. 4 A and B). This result indicates that AgRP/NPY neurons are necessary for estrogen's anorexigenic effects.
Fig. 4.
Central administration of E2 decreases food intake in controls but not in mice lacking AgRP/NPY neurons. Agrp-Tfam controls and mutants were infused with aCSF for at least 6 days until they reached metestrus/diestrus. In this particular experiment, the mice were synchronous in cycling and most of them reached metestrus/diestrus on the seventh day, at which point the mice were infused icv with water-soluble E2 (2 μg in aCSF per mouse). Fourteen- and twenty-four–hour food intake after E2 treatment was compared with values obtained in the same phase (metestrus/diestrus) during vehicle treatment, such that each mouse served as its own control. All injections were made at 6 PM and at the same time vaginal smears were collected. Food intake was measured at 8 AM and 6 PM *, P < 0.05. n = 6 controls, n = 6 mutants.
Expression of ERα Is Completely Excluded from AgRP/NPY Neurons Within the Hypothalamus.
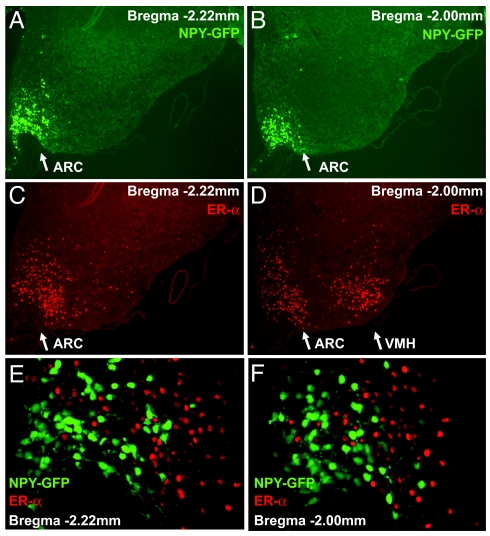
Although multiple estrogen receptors have been identified, evidence indicates that ERα is the main mediator of estrogen's effects on energy balance (21). ERα-deficient mice are obese, whereas ERβ-deficient mice are normal (7, 22). ERα is abundantly expressed in ARC and ventromedial hypothalamus (VMH) (23). Thus, we sought to determine whether AgRP/NPY neurons express ERα in vivo by immunofluorescence analysis. Since AgRP and NPY are coexpressed by the same neurons within the ARC, we used transgenic mice, in which humanized Renilla Green Fluorescent Protein (hrGFP) was specifically expressed under the control of the mouse Npy regulatory sequence such that AgRP/NPY neurons can be readily identified by GFP expression. The specificity of GFP expression has been demonstrated in that GFP expression is completely colocalized with NPY immunoreactivity in hypothalamus and other brain regions in colchicine-injected animals (24). We also confirmed complete colocalization of NPY and GFP in cortical neurons (Fig. S2 A-C). Intense GFP signal was detected in ARC (Fig. 5 A and B), while ERα positive cells were detected in both ARC and VMH (Fig. 5 C and D). Surprisingly, none of the 2,449 GFP positive neurons were positive for ERα immunoreactivity (Fig. 5 E and F). The specificity of the ERα antibody was confirmed by lack of immunoreactivity in ERα-deficient mice. Thus, ERα expression was completely excluded from AgRP/NPY neurons in the mouse hypothalamus. This result suggests that estrogen may regulate AgRP/NPY neurons indirectly via presynaptic neurons that express ERα.
Fig. 5.
Expression of ERα is abundant in the ARC of the hypothalamus, but completely excluded from AgRP/NPY neurons. Hypothalamic sections were prepared from transgenic mice expressing GFP in NPY neurons. (A and B) The GFP signal was strong in the ARC (white arrows) and showed an expression pattern characteristic for NPY in the hypothalamus. ERα positive cells were found in the ARC and the VMH in the hypothalamus indicated by white arrows (C and D). However, zero out of 2,449 GFP positive neurons was found to be positive for ERα immunoreactivity (E-F). A total of 24 sections (bregma −2.46 mm to bregma −1.06) from four female and two male mice were analyzed. The specificity of the ERα antibody was validated as no signal was detected in ERα-deficient mice.
E2 Inhibits Fasting-Induced c-Fos Activation in AgRP/NPY-Neurons and Refeeding.
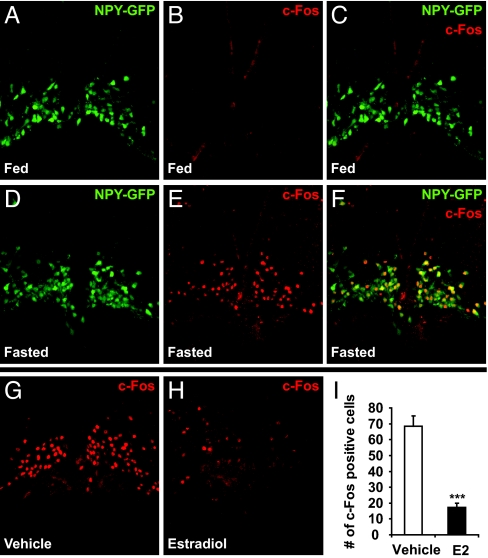
The indirect nature of AgRP/NPY regulation via ERα prompted us to investigate whether estrogen would suppress AgRP/NPY function by inhibiting their neuronal activities. It is known that AgRP/NPY neurons are activated upon fasting, which is associated with dramatic induction of c-Fos expression in these neurons (25). By using Tg.NPY-hrGFP mice, we found that c-Fos expression was barely detectable in AgRP/NPY neurons in fed females (Fig. 6 A–C). However, a marked increase in c-Fos expression in AgRP/NPY neurons was observed after a 25-h fast (Fig. 6 D–F). In fasted females, 97.0% of c-Fos positive cells (800 of 825 cells) in basomedial ARC are positive for GFP. Together with previous findings (25), our results indicate that a majority of the c-Fos positive cells in the basomedial ARC of fasted animals are AgRP/NPY neurons. To investigate the role of estrogen on AgRP/NPY neuronal activation, female mice were fasted for 25 h, during which the mice were injected three times with either E2 or vehicle (saline) and killed 1 h after the last injection. As expected, high c-Fos expression was found in neurons in the basomedial ARC in saline injected mice. However, in E2 treated mice, c-Fos expression was significantly diminished (Fig. 6 G and H). Quantification of c-Fos positive cells in basomedial ARC showed that the number of c-Fos positive cells in E2 treated mice was about 25% of control values (Fig. 6I). These results suggest that E2 inhibits AgRP/NPY neuronal activities. To examine if E2 treatment during fasting affects refeeding, a separate cohort of mice were allowed to refeed after hormone treatment during the 25-h fast, as described above. While E2 treatment did not affect the extent of body weight decrease upon fasting, it significantly reduced food intake during the first 2 h of refeeding by 38% but had less effect on later time points (Fig. S3). The reduced refeeding is consistent with the inhibitory effect of estrogen on AgRP/NPY neuronal activation.
Fig. 6.
E2 inhibits fasting-induced c-Fos activation in AgRP/NPY-neurons. (A–F) Fed and 25-h fasted female NPY-hrGFP transgenic mice were perfused. Immunofluorescence was performed to examine c-Fos expression in the basomedial ARC (Bregma –2.06 to −2.46 mm). NPY neurons were identified by expression of GFP in both nucleus and cytoplasm. c-Fos immunoreactivity was nuclear. In fasted mice, 97.0% of the c-Fos positive cells (800 out of 825 cells) within this region were AgRP/NPY neurons. Seven sections from four fed mice and 14 sections from four fasted mice were used. (G–I) Female mice were fasted for 25 h (9 AM to 10 AM) and injected with either vehicle (saline) or E2 (150 μg water soluble E2) at three time points during this period (9 AM, 6 PM on day 1 and 9 AM on day 2). Mice were perfused 1 h after the last injection and immunofluorescence was performed to examine c-Fos expression in the basomedial ARC (bregma −2.06 to −2.46 mm). Number of c-Fos positive cells were quantified in panel I. ***, P < 0.001. Thirteen to sixteen sections from four saline- and four E2-injected mice were used.
Discussion
It has been well-documented that estrogen exerts an inhibitory effect on feeding. In female mammals including rodents and humans, feeding decreases during the periovulatory period of the ovarian cycle, a period that coincides with a surge in serum estrogen levels (11). Administration of estrogen, both peripherally and centrally, potently inhibits food intake and decreases body weight (11, 12, 14). Furthermore, ovariectomy results in increased food intake and body weight compared with sham-operated females, and estrogen replacement at a dose and frequency mimicking those during estrous cycle inhibits weight gain in ovariectomized animals (11, 12). However, despite these well-known effects, neuronal subtypes that are functionally required for estrogen's anorexigenic effects have not yet been identified. In this study, we show that Npy and Agrp expression, but not Pomc expression, coincides with the cyclic changes in feeding, and that estrous cycle dependent changes in feeding and body weight are abolished in mice lacking AgRP neurons. Furthermore, central administration of E2 decreases food intake in control but not in mice with ablated AgRP neurons. These results establish that AgRP/NPY neurons are essential mediators of estrogen's anorexigenic function.
It has been reported that estrogen influences the potency of some peripheral hormones on feeding. Ghrelin, a gut derived hormone, has been shown to stimulate feeding more effectively in males and ovariectomized females than in intact female rats, and that E2 inhibits ghrelin's orexigenic effects (26). Interesting, ghrelin has been shown to exert its orexigenic effect through AgRP/NPY neurons as this effect is lost in mice lacking the AgRP neurons (27). Thus, our current results suggest that estrogen may affect the efficacy of ghrelin by inhibiting AgRP/NPY neurons, the ghrelin target neurons. In addition, E2 has been shown to modulate CCK's effects on feeding (12). While estrogen may act on neurons within the hindbrain to influence CCK's anorexigenic effect, the ARC has recently been shown to regulate CCK's efficacy on feeding. In particular, leptin, a long-term adiposity signal, has been shown to act on neurons in the ARC to regulate CCK's satiety effects (28, 29). Thus, it is conceivable that estrogen influences CCK's satiety effects by regulating AgRP/NPY neurons.
Several ERs have been identified to date, most notably ERα and ERβ. It has been shown that estrogen's effects on feeding and body weight require ERα, and that ERα-deficient mice, both males and females, have increased body weight and adiposity (7). Inhibition of ERα by RNA interference in the VMH of hypothalamus results in severe obesity and metabolic syndrome (13). ERα is abundantly expressed in the ARC and the VMH. In contrast, ERβ expression is barely detectable in the ARC, while it is abundantly expressed in other regions of the brain (30). Surprisingly, our results showed that ERα expression is completely excluded from AgRP/NPY neurons in the hypothalamus of adult female and male mice. These results are in contrast to in vitro studies in which ERα, AgRP and NPY were coexpressed in immortalized hypothalamic neuronal cell lines derived from embryonic mice (31). It is currently unclear whether this discrepancy represents a difference in expression of ERα in adult and embryonic neurons, or if it represents alteration of their expression profiles during prolonged in vitro cultures. Although our study suggests that estrogen does not directly regulate AgRP/NPY neurons via ERα, we cannot rule out the possibility that a novel estrogen receptor may be present in these neurons. Indeed, a membrane bound estrogen receptor has been recently reported (21, 32). However, given the importance of ERα in mediating estrogen's anorexigenic effect, our data suggest that estrogen may regulate AgRP/NPY neurons indirectly via an ERα-dependent mechanism. It has been shown that neurons within the ARC receive neuronal innervations from the VMH (33), so it is possible that ERα expressing neurons within the VMH project to the AgRP/NPY neurons in the ARC. Alternatively, ERα positive neurons within the ARC could project to neighboring AgRP/NPY neurons (34). One such candidate neuronal subtype is the Kiss1 neurons, which are abundant in the ARC. These neurons express both ERα and leptin receptor and are essential for regulating GnRH secretion (35, 36). It would be interesting to investigate whether Kiss1 neurons exert inhibitory input onto the AgRP/NPY neurons. Consistent with the notion that estrogen indirectly regulates neuronal activities, estrogen has been shown to regulate presynaptic inputs on POMC neurons (14). It is also possible that these estrogen responsive inputs to the POMC neurons may originate from the same cohort of ERα positive neurons that project to the AgRP/NPY neurons.
Although our results demonstrate the functional requirement of AgRP/NPY neurons for estrogen's anorexigenic effects, it is currently unclear whether AgRP, NPY, or neurotransmitter γ-aminobutyric acid (GABA) mediates such effects. Leptin is known to regulate AgRP/NPY neuronal function by transcriptional regulation of Agrp and Npy and also by modulation of their neuronal activities (1). Food restriction or leptin deficiency induces dramatic upregulation of Agrp and Npy expression, and leptin replacement reverses this effect. In addition, leptin inhibits AgRP/NPY neuronal activity by reducing GABA release onto the POMC neurons (37, 38). Therefore, like leptin, estrogen may regulate AgRP/NPY neuronal function by multiple mechanisms.
The ability to regulate energy homeostasis and reproduction in a coordinated fashion is of evolutionary advantage, since reproductive success depends on adequate energy reserve in females. Leptin and estrogen, two seemingly very different hormones are critical regulators of energy balance and reproduction, although leptin may take on a more specialized role in energy balance and estrogen is more specialized in reproduction. However, both leptin and estrogen possess very similar roles in the regulation of food intake and body weight. Deficiency of either hormone results in decreased fertility and increased feeding (1, 3–5, 7–9, 39). Thus, a decline in circulating levels of either hormone signals an undesirable physiologic state for reproduction. Increased feeding, as a result of either leptin or estrogen deficiency, may serve as a counterregulatory mechanism to increase energy reserve, which is a prerequisite for reproductive success. While it is well established that leptin regulates energy balance and reproduction by suppression of AgRP/NPY neurons, our study indicates that estrogen also regulates feeding by antagonizing these neurons. Indeed, prolonged hyperactivation of AgRP/NPY neurons, often associated with negative energy balance, causes not only robust hyperphagia to restore adiposity stores but also suppresses reproductive function. Although NPY has been shown to influence GnRH and gonadotrophin secretion, chronic administration of NPY delays sexual maturity and impairs reproductive function (40). Furthermore, deletion of NPY or Y4 receptor from leptin-deficient mice restores fertility (17). On the other hand, ablation of AgRP neurons in the neonates does not affect fertility or lactation-induced hyperphagia, suggesting that mice can cope with the loss the AgRP/NPY neurons by developing compensatory mechanisms (20). Thus, AgRP/NPY neurons integrate both leptin and estrogen action to regulate energy balance and reproduction.
Materials and Methods
Mice.
C57BL/6 mice were purchased from the Jackson Laboratories and subsequently bred in house. Mice with deletion of mitochondrial transcription factor A (Tfam) gene specifically in the AgRP neurons (Agrp-Tfam mutants) have previously been developed and characterized in details (19). About 85% of AgRP neurons undergo progressive neurodegeneration by 7 months of age in the mutant animals. To generate Agrp-Tfam control and mutant mice, male homozygous for the floxed Tfam allele and heterozygous for the Agrp-Cre transgene were mated to females that were homozygous for the floxed Tfam allele. Eight month old female Agrp-Tfam mutants and littermate controls were used in this study. Mice expressing hrGFP under the control of the mouse Npy promoter were purchased from the Jackson Laboratory [B6.FVB-Tg(NPY-hrGFP)1Lowl/J] and the specificity of GFP expression has been validated (24). All mice were housed in the University of California, San Francisco's mouse barrier facility in a room with a 7 AM–7 PM light/dark cycle. All experiments were carried out according to a protocol approved by the UCSF Institutional Animal Care and Use Committee.
Determination of Estrous Cycle.
Vaginal smears were collected in the morning (9 AM) and at the time the mice were killed unless otherwise stated. Vaginal smears were then stained with Giemsa stain (Sigma). Cytological changes in different phases of the estrous cycle are illustrated in Fig. S1.
Gene Expression Analysis.
RNA was isolated using TRIzol reagent (Invitrogen) and RNeasy mini kit (QIAGEN). RNA (1 μg) was reverse transcribed to cDNA using reagents from Invitrogen. mRNA levels were then analyzed using a 7900HT Fast Real-Time PCR System (Applied Biosystems). Npy, Agrp, Pomc and Lepr expression were analyzed using TaqMan Gene Expression Assays. β-actin was used as internal reference.
Hypothalamic Explant Culture.
Three 0.5-mm thick coronal brain sections through the hypothalamus were prepared in dissection media (50% DMEM, 50% HBSS, 25 mM HEPES, 10 mM Tris-HCl, pH 7.4, 100 μg/mL Penicillin-Streptomycin), cut in half along the third ventricle, placed in Millicell CM 0.4 μM culture plate inserts (Millipore) and cultured at 37 °C in culture media (50% DMEM, 25% HBSS, 25% heat-inactivated horse serum, 2 mM Glutamine, and 100 μg/mL Penicillin-Streptomycin) in a 5% CO2 incubator overnight. The next morning, medium was changed and hormone or vehicle (water) was added to the medium. One half of each slice was treated for 24 h with either 10 nM E2 (β-Estradiol water-soluble, Sigma) or 100 nM progesterone (water-soluble progesterone, Sigma), and the other identical half was treated with vehicle (water).
Intracerebral Ventricular Injection.
For implantation of the guide cannula, mice were anesthetized with 100 mg/kg Ketamine and 5 mg/kg Xylazine. 0.5% Isofluorane was used as needed to maintain surgical plane anesthesia. Custom 5.7 mm guide cannulas (Plastics One) were implanted using a stereotaxic apparatus (David Kopf Instruments) into the third ventricle (x: 0.0, y: bregma −2.0, z: −5.7). Buprenorphine (0.1 mg/kg) was used immediately after surgery and as needed. The correct placement of the guides was verified by drinking response to 100 μg/mL Angiotensin II (Sigma) in aCSF (150 mM NaCl, 3.0 mM KCl, 1.4 mM CaCl2, 0.8 mM MgCl2, 1 mM NaH2PO4, pH 7.4), and also by postmortem histochemical examination. For icv injection, 1 μL of vehicle (aCSF) or water-soluble E2 (2 μg in aCSF) was infused at a rate of 10 nL/s using a micropump (World Precision Instruments) and a custom 5.9 mm injector (Plastics One).
Immunohistochemistry.
Mice were perfused with 4% paraformaldehyde, cryoprotected in 30% sucrose overnight at 4 °C, and sectioned using a cryostat as described in (19). For NPY staining, coronal brain sections (10 μm) were boiled in a 10 mM citrate solution. For c-Fos staining, sections were incubated sequentially for 10 min each in base solution (1% NaOH, 1% H2O2), 0.3% glycine and 0.3% SDS. Sections were incubated with primary antibody against ERα (1:10,000; Upstate), NPY (1:250; Bachem Peninsula Laboratories) or c-Fos (1:500; Calbiochem) overnight at 4 °C, washed and incubated 1 h at room temperature with a secondary goat anti-rabbit IgG antibody (1:200; Invitrogen).
Statistical Analysis.
All comparisons were done using the Student's t test using either paired samples or two-samples with unequal variance. Mean values in the text and figures are expressed as mean ± SEM.
Supplementary Material
Acknowledgments.
We thank Dr. Nirao Shah (University of California, San Francisco) for providing ERα-deficient mice to confirm the specificity of the ERα antibody. This work was supported in part by the Swedish Research Council to L.E.O. and, in part, by the Hurlbut Johnson Foundation to A.W.X. This work was also supported in part by University of California San Francisco core facilities funded by the National Institutes of Health DERC P30 DK063720.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904747106/DCSupplemental.
References
- 1.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 2.Judd SJ. Disturbance of the reproductive axis induced by negative energy balance. Reprod Fertil Dev. 1998;10:65–72. doi: 10.1071/r98024. [DOI] [PubMed] [Google Scholar]
- 3.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 4.Montague CT, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 5.Farooqi IS. Leptin and the onset of puberty: Insights from rodent and human genetics. Semin Reprod Med. 2002;20:139–144. doi: 10.1055/s-2002-32505. [DOI] [PubMed] [Google Scholar]
- 6.Bronson FH. Food-restricted, prepubertal, female rats: Rapid recovery of luteinizing hormone pulsing with excess food, and full recovery of pubertal development with gonadotropin-releasing hormone. Endocrinology. 1986;118:2483–2487. doi: 10.1210/endo-118-6-2483. [DOI] [PubMed] [Google Scholar]
- 7.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wade GN, Gray JM. Gonadal effects on food intake and adiposity: A metabolic hypothesis. Physiol Behav. 1979;22:583–593. doi: 10.1016/0031-9384(79)90028-3. [DOI] [PubMed] [Google Scholar]
- 9.Tchernof A, Calles-Escandon J, Sites CK, Poehlman ET. Menopause, central body fatness, and insulin resistance: Effects of hormone-replacement therapy. Coron Artery Dis. 1998;9:503–511. doi: 10.1097/00019501-199809080-00006. [DOI] [PubMed] [Google Scholar]
- 10.Smith EP, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 11.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 12.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musatov S, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Q, et al. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 15.Lissner L, Stevens J, Levitsky DA, Rasmussen KM, Strupp BJ. Variation in energy intake during the menstrual cycle: Implications for food-intake research. Am J Clin Nutr. 1988;48:956–962. doi: 10.1093/ajcn/48.4.956. [DOI] [PubMed] [Google Scholar]
- 16.Barr SI, Janelle KC, Prior JC. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am J Clin Nutr. 1995;61:39–43. doi: 10.1093/ajcn/61.1.39. [DOI] [PubMed] [Google Scholar]
- 17.Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- 18.Sainsbury A, et al. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 2002;16:1077–1088. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu AW, et al. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol. 2005;3:e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips CT, Palmiter RD. Role of agouti-related protein-expressing neurons in lactation. Endocrinology. 2008;149:544–550. doi: 10.1210/en.2007-1153. [DOI] [PubMed] [Google Scholar]
- 21.Ropero AB, Alonso-Magdalena P, Quesada I, Nadal A. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids. 2008;73:874–879. doi: 10.1016/j.steroids.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Ohlsson C, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. 2000;278:640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 23.Mori H, Matsuda K, Pfaff DW, Kawata M. A recently identified hypothalamic nucleus expressing estrogen receptor alpha. Proc Natl Acad Sci USA. 2008;105:13632–13637. doi: 10.1073/pnas.0806503105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Pol AN, et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci. 2009;29:4622–4639. doi: 10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munzberg H, et al. Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27:69–74. doi: 10.1523/JNEUROSCI.3168-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clegg DJ, et al. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 27.Bewick GA, et al. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J. 2005;19:1680–1682. doi: 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- 28.Morton GJ, et al. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115:703–710. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merino B, Cano V, Guzman R, Somoza B, Ruiz-Gayo M. Leptin-mediated hypothalamic pathway of cholecystokinin (CCK-8) to regulate body weight in free-feeding rats. Endocrinology. 2008;149:1994–2000. doi: 10.1210/en.2007-1286. [DOI] [PubMed] [Google Scholar]
- 30.Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor alpha. Endocrinology. 2004;145:736–742. doi: 10.1210/en.2003-0894. [DOI] [PubMed] [Google Scholar]
- 31.Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) alpha to ERbeta in clonal hypothalamic neurons. Mol Endocrinol. 2006;20:2080–2092. doi: 10.1210/me.2006-0027. [DOI] [PubMed] [Google Scholar]
- 32.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 33.DeFalco J, et al. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 34.Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH → arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- 35.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 37.van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 38.Cowley MA, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 39.Lubahn DB, et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crown A, Clifton DK, Steiner RA. Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology. 2007;86:175–182. doi: 10.1159/000109095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.