Abstract
Abnormal processing of the amyloid precursor protein (APP) and β-amyloid (Aβ) plaque accumulation are defining features of Alzheimer disease (AD), a genetically complex neurodegenerative disease that is characterized by progressive synapse loss and neuronal cell death. Aβ induces synaptic dysfunction in part by altering the endocytosis and trafficking of AMPA and NMDA receptors. Reelin is a neuromodulator that increases glutamatergic neurotransmission by signaling through the postsynaptic ApoE receptors Apoer2 and Vldlr and thereby potently enhances synaptic plasticity. Here we show that Reelin can prevent the suppression of long-term potentiation and NMDA receptors, which is induced by levels of Aβ comparable to those present in an AD-afflicted brain. This reversal is dependent upon the activation of Src family tyrosine kinases. At high concentrations of Aβ peptides, Reelin can no longer overcome the Aβ induced functional suppression and this coincides with a complete blockade of the Reelin-dependent phosphorylation of NR2 subunits. We propose a model in which Aβ, Reelin, and ApoE receptors modulate neurotransmission and thus synaptic stability as opposing regulators of synaptic gain control.
Keywords: Alzheimer disease, apolipoprotein E, long-term potentiation, NMDA receptor, Aβ oligomer
Alzheimer disease (AD) is a complex genetic neurodegenerative disease that afflicts an increasing fraction of our aging population (1). A characteristic feature of AD is the accumulation of oligomeric and higher-order aggregates of the Aβ1–42 form of the amyloid-β (Aβ) peptide, which is physiologically released by sequential proteolytic cleavage from the amyloid-β precursor protein (APP) (2, 3). Abnormal, amyloidogenic Aβ processing and plaque formation progressively lead to synaptic dysfunction, synapse loss, and ultimately neuronal death.
Recent insight into the pathophysiological functions of Aβ came from studies that demonstrated a potent negative impact of Aβ oligomers on synaptic functions that underlie long-term synaptic plasticity (4–6). Incubation of hippocampal neurons and slices with Aβ oligomers leads to intracellular trapping or functional impairment of AMPA and NMDA-type glutamate receptors (7–9), thereby decreasing long-term potentiation (LTP), an enhancement of synaptic strength that is correlated with memory (4, 5, 10–13). Consistent with these in vitro findings, Palop et al. (14, 15) showed that overexpression of mutant APP forms in mice resulted in a global dysregulation of neuronal network activity in vivo.
Reelin is a signaling protein that is produced by interneurons in the brain and that has an effect on synaptic functions that is opposite to that of Aβ oligomers. Reelin addition to hippocampal slices results in enhanced LTP (16) as a result of the activation of Src family tyrosine kinases (SFKs) (17–19), which increase NMDA receptor activity by tyrosine phosphorylation of their NR2 subunits (20, 21). These effects of Reelin are mediated by a pair of homologous cell surface receptors designated apolipoprotein E receptor 2 (Apoer2) and very low-density lipoprotein receptor (Vldlr), which belong to a family of highly conserved endocytic signaling receptors. Reelin binding to both proteins in concert controls neuronal migration during development (22), as well as glutamatergic neurotransmission through differential enhancement of NMDA receptor and AMPA receptor activity in the adult nervous system (20, 21, 23, 24). Thus, Aβ and ApoE receptors converge on common molecular mechanisms, which are essential for synapse formation and maintenance. This raises the possibility that Reelin signaling could prevent or reverse the negative effect of Aβ oligomers on synaptic function.
Here we show that Reelin signaling in excitatory synapses can restore normal synaptic plasticity, which is impaired by concentrations of oligomeric Aβ peptides that lie well within the range present in the brains of AD patients. This reversal requires the ApoE receptor-dependent activation of SFKs. We propose a model in which Aβ and ApoE receptors function in tandem as opposing regulators of synaptic efficacy.
Results
Several independent studies have consistently shown that Aβ oligomers can potently suppress long-term potentiation, an electrophysiological parameter that measures activity-induced synaptic strengthening. By contrast, activation of ApoE receptor signaling by Reelin potently increases long-term potentiation, and mice that lack the Reelin receptors Apoer2 or Vldlr exhibit early LTP defects (16).
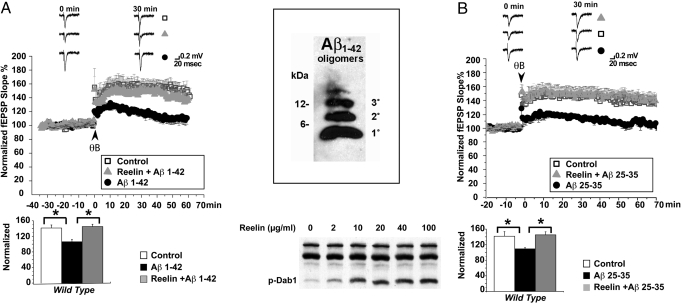
We tested whether Reelin was capable of reversing the CA1 field LTP reduction brought on by Aβ1–42 oligomers (400 nM) in WT hippocampal slices (Fig. 1A, closed circles). When Reelin was present in the perfusion medium (closed triangles), LTP was restored almost to control levels (i.e., no Aβ, no Reelin, open squares). The physical state of the Aβ1–42 oligomers used in the experiments throughout this study is shown (Fig. 1A Inset), and the potency of our purified Reelin preparations used in these studies, indicated by the ability of different Reelin concentrations to induce Dab1 tyrosine phosphorylation, is shown (Fig. 1A Lower). Consistent with the findings in other laboratories, monomeric Aβ1–42 (1°) was ineffective. A truncated form of the Aβ peptide consisting only of amino acids 25 to 35 (Aβ25–35), which form the minimal fibrillogenic region was also able to potently suppress baseline LTP at consistently lower concentrations (100 nM; Fig. 1B, closed circles). This suppression was again fully reversible by co-application of Reelin (closed triangles), indicating that LTP suppression induced by low concentrations of Aβ1–42 or Aβ25–35 was not caused by a non-specific cytotoxic effect.
Fig. 1.
Reelin rescues Aβ-induced LTP suppression in CA1. (A) Suppression of LTP by Aβ1–42 is rescued by Reelin in WT hippocampal slices. (Upper) LTP was induced by theta burst stimulation (TBS) consisting of 5 trains of 4 pulses at 100 Hz with an inter-burst interval of 20 s at 0 min. Open squares show responses from control slices receiving no treatment (n = 5). Solid black circles show responses from slices treated with 400 nM Aβ1–42 for 40 to 60 min before TBS. Solid gray triangles show responses of slices from the same animals treated with Aβ1–42 in the presence of 5 nM Reelin. Representative fEPSP traces before and 30 min after TBS for the different conditions are shown (Top). (Scale bar: 0.2 mV, 20 ms.) (Lower) Statistical analysis of average LTP responses between 30 and 35 min after TBS. Aβ1–42 significantly attenuates LTP in the absence of Reelin (from 152.99% ± 9.85% to 112.89% ± 6.35%, n = 5; P < 0.05). LTP reduction by Aβ1–42 is prevented in the presence of 5 nM Reelin and no longer significantly different from controls (147.76% ± 9.30%, n = 5; P > 0.05). Asterisk denotes significance on one-way ANOVA followed by Bonferroni post-test. (Inset, Right, Top) Representative immunoblot of synthetic Aβ1–42 oligomers used throughout the study. Monomer, dimers, trimers, and tetramers are present. (Bottom) Immunoblot of primary cortical neuronal lysates shows induction of Dab1 tyrosine phosphorylation by different concentrations of purified Reelin used throughout the study. (B) Suppression of LTP by Aβ25–35 is rescued by Reelin in WT hippocampal slices. (Upper) LTP was induced by TBS consisting of 5 trains of 4 pulses at 100 Hz with an inter-burst interval of 20 s at 0 min. Open squares show responses from control slices receiving no treatment (n = 8). Solid black circles show responses from slices treated with 100 nM Aβ25–35 for 40 to 60 min before TBS. Solid gray triangles show responses of slices from the same animals treated with Aβ25–35 in the presence of 5 nM Reelin. Representative fEPSP traces before and 30 min after TBS for the different conditions are shown (Top). (Scale bar: 0.2 mV, 20 ms.) (Lower) Statistical analysis of average LTP responses between 30 and 35 min after TBS. Aβ25–35 significantly attenuates LTP in the absence of Reelin (from 141.63% ± 11.75% to 109.21% ± 3.09%, n = 8; P < 0.05). LTP reduction by Aβ25–35 is prevented in the presence of 5 nM Reelin and no longer significantly different from controls (145.26% ± 7.80%, n = 8; P > 0.05). Asterisk denotes significance on one-way ANOVA followed by post-tests. High resolution traces are shown in the SI.
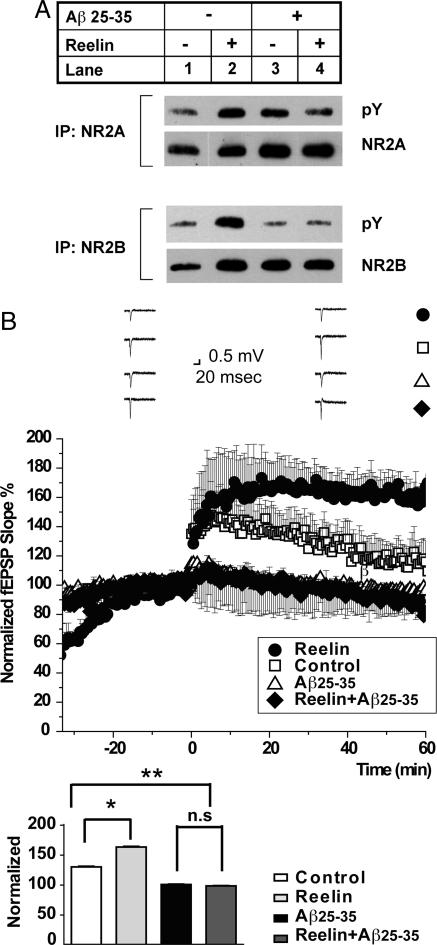
We have reported earlier (20) that Reelin increases tyrosine phosphorylation of NR2A as well as NR2B subunits (Fig. 2A, lane 2, Upper and Lower, respectively). NR2 tyrosine phosphorylation increases the expression of NMDA receptors at the cell surface by preventing their endocytosis (9). It also increases their gating (25). NR2 phosphorylation by Reelin is greatly reduced in the presence of a high concentration of 1 μM Aβ25–35 (Fig. 2A, lane 4). Similar results were obtained with Aβ1–42 (not shown). Because Reelin can no longer efficiently phosphorylate NR2 subunits in the presence of high Aβ peptide levels, we predicted that Reelin would also be unable to prevent the Aβ-mediated LTP suppression at these concentrations. As we had shown earlier (16, 20), Reelin enhances LTP in WT hippocampal slices (closed circles, Fig. 2B) over control (i.e., no Reelin, open squares). In the presence of 1 μM Aβ25–35, LTP is almost completely abolished (open triangles), and this LTP suppression can no longer be prevented by Reelin (closed diamonds). These results are consistent with the mechanism proposed by Snyder et al. in which Aβ induces the internalization of NMDA receptors (9) and thus renders them inaccessible to Reelin activated SFKs at the cell surface.
Fig. 2.
Opposing effects of Aβ and Reelin on NMDA receptor tyrosine phosphorylation. (A) High doses of Aβ peptides prevent NMDA receptor phosphorylation in response to Reelin treatment. Acute WT hippocampal slices were incubated in the presence (R) or absence of Reelin (C) and Aβ25–35. NR2A and NR2B were immunoprecipitated from the homogenized lysates of the slices and analyzed for tyrosine phosphorylation. Total amounts of immunoprecipitated NR2A and NR2B are shown (NR2A, NR2B); one independent experiment is shown (n = 3). Tyrosine phosphorylation of NR2A and NR2B in the presence of Aβ and Reelin was not significantly different from control (lane 1) by paired t test. (B) Reelin enhances LTP in the absence of Aβ, but fails to rescue LTP suppression induced by high concentrations of Aβ25–35. (Upper) In WT hippocampal slices, Reelin (closed circles) enhances and Aβ25–35 (at 1 μM, open triangles) suppresses TBS-induced LTP compared with non-treated control slices (open squares). Reelin fails to restore LTP in the presence of high concentrations of Aβ (1 μM). Application of Reelin alone (closed circles) induces an immediate shift in baseline currents as a result of its direct effect on NMDA and AMPA receptor activity (21). (Lower) Statistical analysis of average LTP responses 30 to 35 min after TBS. Control LTP (open bar), 129.30% ± 14.16%, n = 2; Reelin (gray bar), 165.65% ± 14.11%, n = 2; high-dose Αβ25–35 (black closed bar), 99.85% ± 19.19%, n = 4; Reelin with high-dose Αβ25–35, 97.56% ± 6.83%, n = 4. ANOVA was performed, followed by Bonferroni post-test. Control compared with Reelin, P < 0.05; control compared with high-dose Aβ25–35, P < 0.05; high-dose Aβ25–35 compared with Reelin and Aβ25–35 together, P > 0.05. Asterisk denotes significance.
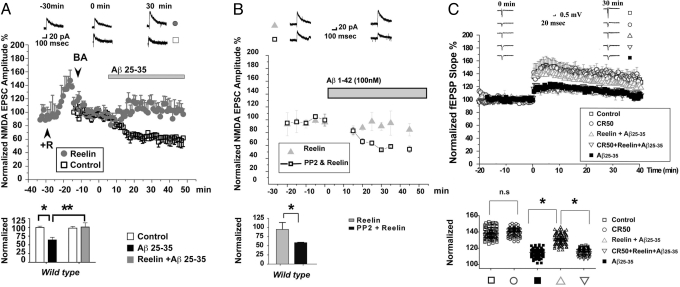
We next demonstrated that Reelin is able to compensate for the Aβ-induced reduction of NMDA currents (9) at lower—and thus thought, more physiological—Aβ concentrations (100 nM Aβ25–35), and that Reelin does this by increasing NMDA receptor activity directly. To show this, we pharmacologically isolated NMDA receptors in whole-cell recordings from WT hippocampal neurons (Fig. 3A). As expected and reported previously (21–23), Reelin application resulted in an immediate increase in NMDA currents, which was compensated by adjusting the gain back to baseline (i.e., baseline adjustment) before Aβ peptide addition (Fig. 3A, gray bar). In our hands, at 100 nM, Aβ25–35 induced a similar reduction in NMDA currents (Fig. 3A, open squares) as had been reported by Snyder et al. (9) for Aβ1–42, and this reduction was completely prevented in the presence of Reelin (Fig. 3A, filled circles). Thus, Reelin and Aβ actions antagonize each other at the synapse within their normal physiological concentration range. In the presence of low, but not high, levels of Aβ peptides, Reelin can readjust NMDA receptor activity to compensate for the Aβ-induced suppression.
Fig. 3.
Reelin rescues Aβ-induced suppression of NMDA currents in mouse hippocampus by activating SFKs. (A) Reelin prevents Aβ25–35-induced suppression of NMDA currents. (Upper) Normalized average amplitudes of evoked NMDA peak currents at +30 mV in single-cell recordings. Open squares show responses from control slices treated with 100 nM Aβ25–35 in the absence of Reelin; solid gray circles show responses in the presence of Aβ and 5 nM Reelin. Gray bar indicates the timing of Aβ application. Reelin was applied 25 to 30 min before Aβ perfusion. After stabilization, baseline levels were readjusted (by reducing the gain of the amplifier) to “pre-Reelin” levels and monitored for at least 10 min before Aβ application. The time of Aβ application is 0 min. (Top) Traces show representative whole-cell recordings 30 min before and after Reelin and Aβ treatment. (Scale bar, 20 pA, 100 ms.) (Lower) Statistical analysis comparing extent of NMDA excitatory postsynaptic current (EPSC) amplitude suppression 30 min after Aβ application in the absence and presence of Reelin. Aβ significantly suppresses NMDA currents (64% ± 7.70% of control, n = 6; P < 0.01, 2-way ANOVA). Reelin prevents this effect (101.07% ± 11.98%, n = 6; P > 0.05, 2-way ANOVA followed by post-test). Asterisks denote significance. (B) SFK activation is required for the prevention of Aβ1–42-induced NMDA receptor suppression by Reelin. (Upper) Normalized average amplitudes of evoked NMDA peak currents at +30 mV in single-cell recordings. Solid gray triangles show responses from control slices treated with 5 nM Reelin. Open squares show responses from slices treated with PP2 (10 μM) and Reelin. (Top) Traces show representative whole-cell recordings 10 min before and 30 min after Aβ1–42 application. (Scale bar, 50 pA, 100 ms.) (Lower) Statistical analysis of NMDA receptor-dependent EPSC amplitude suppression. No significant change (94.85% ± 18.84% of control) in NMDA currents was seen when 100 nM Aβ1–42 was applied in the presence of 5 nM Reelin. When the SFK inhibitor PP2 (10 μM) was added to the perfusate, application of Aβ1–42 to slices from the same animals caused a significant suppression of the NMDA currents (58.62% ± 1.05% of control, n = 4; P < 0.05, unpaired t test). (C) The function-blocking CR50 antibody prevents Reelin rescue of LTP suppression by Aβ. TBS LTP was induced as described in Fig. 1. In the presence of Reelin alone, LTP is enhanced (16). Aβ25–35 oligomers diminish LTP, and this is almost completely prevented by Reelin. Preincubation of Reelin with a threefold molar access of the function blocking CR50 antibody (30) prevents this. CR50 alone has no effect. High resolution traces are shown in the SI.
Reelin activates SFKs, mainly Fyn (17–19), through clustering of ApoE receptors (26) and transphosphorylation of Dab1 (22, 27, 28), and this does not occur if both receptors, Apoer2 and Vldlr, are genetically ablated or functionally blocked (19). Fyn is required for LTP induction, but not for baseline synaptic transmission (29). To test whether the activation of SFKs by Reelin is necessary for reversing the Aβ-mediated suppression of NMDA currents, we performed another series of single-cell recordings on hippocampal CA1 neurons treated with Reelin in the presence (Fig. 3B, open squares) or absence (Fig. 3B, gray triangles) of the SFK inhibitor PP2. In this experiment, we used oligomerized Aβ1–42 to induce NMDA receptor-dependent synaptic currents. Consistent with the results shown in Fig. 3A, Reelin was equally capable of preventing the Aβ1–42-mediated suppression of synaptic NMDA receptor currents, but this functional rescue was prevented when SFK activity was blocked with PP2 (Fig. 3B), indicating that Reelin signaling through Apoer2 and Vldlr is necessary (17–19).
To further eliminate an alternative Reelin signaling-independent mechanism for the observed reversal of the amyloid-induced suppression of field LTP and NMDA receptor activity, we also measured LTP in the presence of the Reelin function blocking CR50 monoclonal antibody (30). If the effect of Reelin were merely mediated by it binding, sequestering, and thus quenching the activity of Aβ oligomers, CR50 should have no effect on the ability of Reelin to restore normal LTP. If, conversely, activation of the Reelin signaling pathway is necessary, as also indicated by the SFK inhibitor results, CR50 should block the effect of Reelin. Fig. 3C shows that this is in fact the case. Together, these results exclude a mechanism by which Reelin neutralizes Aβ oligomers extracellularly.
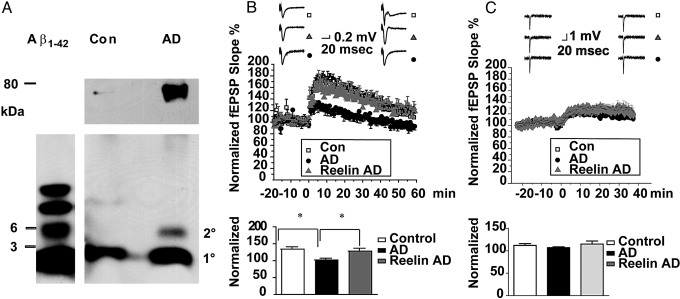
We have shown that activation of ApoE receptors with their natural ligand Reelin can prevent the impairment of synaptic functions caused by Aβ oligomers, which are present in AD but not in normal non-diseased brain (31). We therefore set out to investigate whether Reelin was equally effective in restoring normal synaptic plasticity in hippocampal slices treated with extracts from AD patients containing naturally produced and clinically demonstrated pathogenic Aβ oligomers. Human postmortem cortical brain extracts were prepared as described in Materials and Methods and by Shankar et al. (11). Aβ peptides (i.e., monomers, oligomers, and higher-order complexes) were precipitated from equal volumes of lysate, separated on lithium dodecyl sulfate (LDS) gels, and immunoblotted (Fig. 4A). Monomers and oligomers derived from synthetic Aβ1–42 aggregation were loaded (Fig. 4A Left). Cortical extracts from the control (i.e., non-AD) subject (Fig. 4A Middle) had amounts of monomeric Aβ (1°) that were comparable to those present in the extracts isolated from the AD case (Fig. 4A Right). The control sample, however, did not contain Aβ oligomers and only a trace of higher molecular weight oligomers, in contrast to the AD case extract, which contained readily detectable amounts of dimers as well as abundant multimers. We next compared the effect of control and AD brain extracts on CA1 field LTP (Fig. 4B). As reported (11), AD brain extracts (Fig. 4B, closed circles) strongly suppressed LTP compared with control (Fig. 4B, open squares) and at equivalent concentrations (50 μL extract per mL perfusion medium). Reelin almost completely prevented the LTP defect that was caused by the AD brain extract (Fig. 4B, gray triangles). The reversal of this defect by Reelin was almost entirely mediated by its effect on NMDA receptors, rather than AMPA receptors (Fig. 4C). When the same experiment was conducted in the presence of the NMDA antagonist AP5, only a small amount of NMDA receptor-independent LTP, comparable in magnitude to the amount of LTP in the presence of Aβ oligomers (Fig. 1 A and B and Fig. 4B), was detected in WT mouse CA1 (Fig. 4C, open squares). This residual NMDA receptor-independent LTP was not further reduced by the AD brain extract (Fig. 4C, black circles), nor was it increased in the presence of Reelin (Fig. 4C, gray triangles), suggesting that the AD brain extract mainly suppresses NMDA receptor activity in hippocampal CA1, and that under these conditions Reelin selectively prevents or restores NMDA, rather than AMPA receptor activity suppressed by Aβ oligomers. Taken together, these results strongly suggest that the Reelin-mediated activation of ApoE receptor signals can protect neurons from the synaptic suppression caused by Aβ oligomers that accumulate in the brains of AD patients and that the synaptic effects of synthetic and naturally produced oligomers are qualitatively equivalent.
Fig. 4.
Reelin prevents LTP suppression induced by cortical AD brain extract. (A) Human cortical brain extracts from a normal subject (Con, control) and a clinically and histopathologically confirmed AD case were prepared as described in Materials and Methods and by Shankar et al. (11). Aβ monomers, oligomers, and high molecular weight complexes (approximately 80 kDa) were immunoprecipitated and separated by LDS PAGE. Monomers and oligomers (dimers, trimers, and tetramers) derived from synthetic Aβ1–42 are shown (Left). Control brain extract (Middle) contains monomeric Aβ, but no detectable oligomers and only a trace amount of higher-order aggregates (Upper). By contrast, AD brain extract contains comparable amounts of monomeric Aβ, but Aβ dimers and higher-order multimers were also present. (B) (Top) AD brain extract (50 μL per mL perfusate, closed circles) potently suppresses LTP, compared with equivalent extracts from control brain (50 μL per mL perfusate, open squares). Reelin almost completely prevents LTP suppression by AD brain extract (gray triangles). (Lower) Statistical analysis of average LTP responses between 30 and 35 min after TBS. Control brain extract (open bar), 133.99% ± 7.14%, n = 4; AD brain extract (closed bar), 101.86% ± 5.42%, n = 8; AD brain extract plus Reelin (gray bar), 128.3% ± 8.42%, n = 8. (ANOVA followed by Bonferroni post-test.) Control compared with AD extract, P < 0.05; AD extract compared with AD extract plus Reelin, P < 0.05. Control extract compared with AD extract plus Reelin, P > 0.05. Asterisk denotes significance. (C) (Top) AD brain extract failed to suppresses NMDA receptor-independent LTP in the presence of NMDA receptor antagonist D-AP5 (50 μM, closed circles) compared with control brain extract (open squares), and Reelin did not alter NMDA receptor-independent LTP in the presence of AD brain extract (gray triangles). (Lower) Statistical analysis of average LTP response. Control (open bar), 160.49% ± 14.19%, n = 7; AD extract (closed bar), 115.79% ± 6.53%, n = 12; AD extract plus Reelin (gray bar), 156.84% ± 12.33%, n = 11. (ANOVA followed by Bonferroni post-test.) Control versus AD extract, P < 0.05; AD extract versus AD extract plus Reelin, P < 0.05. Control extract versus AD extract plus Reelin, P > 0.05. Asterisk denotes significance. High resolution traces are shown in the SI.
Discussion
Reelin is an evolutionarily highly conserved signaling protein that controls neuronal migration and positioning during brain development. In the adult brain, Reelin is expressed mainly by GABAergic interneurons, and we and others have shown that Reelin regulates the activity of excitatory synapses (16, 20, 21, 23). Here, we have demonstrated that Reelin prevents Aβ oligomer-induced suppression of synaptic NMDA receptors. Activation of SFKs by Reelin, which requires the functional ApoE receptors Apoer2 and Vldlr (17–19), is necessary for neutralizing the Aβ-mediated suppression (Fig. 3B).
The function of the CNS is dependent upon the finely tuned integration of multiple signals that continuously regulate the activity of its synapses. Some of these mechanisms involve the amyloid precursor protein APP and its product Aβ, as well as ApoE receptors and their ligand Reelin (24). Hsieh et al. (7) and Kamenetz et al. (8) have shown that γ-secretase mediated processing of APP, resulting in the release of Aβ, causes synaptic suppression through AMPA receptor removal, and that in turn synaptic activity increases APP processing through a mechanism that requires NMDA receptors. Aβ1–42 has been reported to cause the dephosphorylation of NMDA receptors by activating tyrosine phosphatases, thereby increasing NMDA receptor endocytosis (9). Application of Reelin enhances tyrosine phosphorylation of NR2A and NR2B subunits (Fig. 2A and ref. 20). We thus propose a model in which NMDA receptor activity is modulated in opposite directions by the 2 endogenous proteins, Aβ and Reelin (Fig. 5). In this model, Aβ activates tyrosine phosphatases causing NMDA receptor endocytosis and synaptic dysfunction (9), whereas Reelin activates SFKs (17–19), which serves to retain NMDA receptors at the neuronal surface but can also further increase their activity through a mechanism that involves tyrosine phosphorylation of NR2 subunits (25).
Fig. 5.
Model of the regulation of synaptic functions by Reelin. Aβ activates the striatal-enriched protein tyrosine phosphatase, leading to dephosphorylation of tyrosine residues on NMDA receptors. Dephosphorylation of the NR2B subunit correlates with increased NMDA receptor endocytosis and suppression of its synaptic function (9). Reelin activates Src family tyrosine kinases (SFK) and enhances tyrosine phosphorylation of NR2A and NR2B subunits. Reelin signaling may prevent Aβ-induced NMDA receptor endocytosis and SFK activation by Reelin restores NMDA receptor activity. We propose that Reelin signaling through the ApoE receptors Apoer2 and Vldlr counteracts the suppressive effect of Aβ at the synapse and that this is important for the maintenance of normal synaptic function.
Reelin, ApoE receptors, and Aβ oligomers are all present at synapses (4, 20, 32). Nevertheless, co-localization of Reelin and amyloid at the same synapse is actually not necessary for explaining the powerful effect of Reelin on preventing synaptic dysfunction. For example, the data in Fig. 3A clearly show that extracellular Reelin potently increases synaptic (i.e., measured as evoked responses) NMDA receptor activity and that this prevents the suppression induced by subsequently added amyloid oligomers. Although it is likely that Reelin activates the ApoE receptor-dependent signaling pathway directly at the synapse (20), it is possible that Reelin is also activating NMDA receptors extra-synaptically and that part of the effect of Reelin on antagonizing amyloid-induced synaptic dysfunction is caused by altering the synaptic dwell time of NMDA receptors (33), and not exclusively by preventing NMDA receptor endocytosis.
Our findings may have direct implications for the molecular mechanisms that underlie the pathogenesis of AD. The seminal findings by the Roses group showed that ApoE genotype predisposes to late-onset AD (34–36). ApoE isoforms differentially impair LTP in the mouse, and ApoE4 knock-in mice are particularly sensitive to LTP suppression by low concentrations of Aβ1–42 (37). These independent findings are consistent with our proposed model (Fig. 5) in which Reelin signaling through ApoE receptors counteracts the synaptic suppression induced by Aβ peptides. It is possible that these synaptic ApoE receptor functions could be differentially modulated by ApoE isoforms.
In summary, our findings show that ApoE receptor-dependent postsynaptic signals evoked by Reelin, a signaling protein that is secreted by interneurons, prevent the synaptic suppression that is induced by Aβ oligomers. Thus, Aβ and ApoE receptors form a signaling network that may serve to integrate and modulate synaptic activity. Disruption of this balance by overproduction of Aβ or by inhibition of ApoE receptors would be predicted to disturb synaptic plasticity (ref. 20 and the present study) and result in synapse and network dysfunction (6, 14, 15).
Materials and Methods
WT C57BL/6 husbandry and housing conditions are described in the SI Text. All procedures were performed in accordance with the protocols approved by the Institutional Committee for Use and Care of Laboratory Animals of the University of Texas Southwestern Medical Center at Dallas. Amyloid-β1–42 was obtained from Biosource and amyloid-β25–35 was obtained from Tocris.
Preparation and Quality Control of Protein Reagents.
Preparation of Aβ oligomers is described in the SI Text. Reelin was produced and purified and Dab1 tyrosine phosphorylation was determined as described (38).
NMDA Receptor Phosphorylation Assays.
Hippocampal slices were processed for detection of NMDA receptor phosphorylation as described in the SI Text. NR2A and NR2B subunits were immunoprecipitated from the lysate and tyrosine phosphorylation was detected by Western blot analysis as described previously (23).
Whole-Cell Patch-Clamp Recording.
Hippocampal slices were prepared from 9- to 14-d-old mice. Single-cell recordings were performed as described in detail in the SI Text. For all recordings, cells were rejected if the input resistance decreased to less than 100 MΩ or the access resistance changed by more than 20%.
Extracellular LTP Recordings.
Hippocampal slices were prepared from 2- to 3-month-old mice. LTP was induced and recorded as described in detail in the SI Text. Data pooled across slices were expressed as mean ± SEM and effects of conditioning stimulation were measured after 35 to 40 min of induction of LTP. High resolution traces are shown in Fig. S1.
NMDA Receptor-Independent LTP.
LTP was induced in the presence of the NMDA receptor antagonist D-AP5 (50 μM) with two 1-s trains, 200 Hz tetanic stimulation. Control and AD brain extracts were applied at 60 μL per mL perfusate 30 to 40 min before LTP induction and were present throughout the post-tetanic 40-min data collection period. High resolution traces are shown in Fig. S1.
Human Brain Extracts.
Frozen human AD and non-AD cortical brain samples (2–4 g) were provided by the University of Texas Southwestern Alzheimer's Disease Center and processed as described in the SI Text. Immunoprecipitation of Aβ oligomers and Western blotting were performed as described previously (11). Control and AD brain extracts were compared at 5, 25, and 50 μL per mL perfusate and yielded results that were quantitatively and qualitatively consistent with those reported by Shankar et al. (11). All experiments were performed independently at least 4 times.
Supplementary Material
Acknowledgments.
We thank Hui-Chuan Reyna, Wen-Ling Niu, and Priscilla Rodriguez for technical assistance; Mike Brown, Joe Goldstein, and Tiina Kotti for critical and constructive comments; and Drs. Nakajima and Mikoshiba for sharing CR50 antibody. This work was supported by grants from the National Institutes of Health, the American Health Assistance Foundation, and the Wolfgang-Paul Program of the Humboldt Foundation.
Footnotes
The authors declare no conflicts of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908176106/DCSupplemental.
References
- 1.Hardy J. A hundred years of Alzheimer's disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 2.De Strooper B. Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007;8:141–146. doi: 10.1038/sj.embor.7400897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selkoe DJ. The origins of Alzheimer disease: a is for amyloid. JAMA. 2000;283:1615–1617. doi: 10.1001/jama.283.12.1615. [DOI] [PubMed] [Google Scholar]
- 4.Lacor PN, et al. Synaptic targeting by Alzheimer's-related amyloid β oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert MP, et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh H, et al. AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamenetz F, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 9.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 10.Shankar GM, et al. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankar GM, et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: A potent role for trimers. J Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh DM, et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 14.Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- 15.Palop JJ, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weeber EJ, et al. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem. 2002;277:39944–39952. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- 17.Arnaud L, Ballif BA, Cooper JA. Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol Cell Biol. 2003;23:9293–9302. doi: 10.1128/MCB.23.24.9293-9302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballif BA, Arnaud L, Cooper JA. Tyrosine phosphorylation of Disabled-1 is essential for Reelin-stimulated activation of Akt and Src family kinases. Brain Res Mol Brain Res. 2003;117:152–159. doi: 10.1016/s0169-328x(03)00295-x. [DOI] [PubMed] [Google Scholar]
- 19.Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- 20.Beffert U, et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–579. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Qiu S, Zhao LF, Korwek KM, Weeber EJ. Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J Neurosci. 2006;26:12943–12955. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trommsdorff M, et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, et al. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005;25:8209–8216. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- 25.Salter MW, Kalia LV. Src kinases: A hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 26.Strasser V, et al. Receptor clustering is involved in Reelin signaling. Mol Cell Biol. 2004;24:1378–1386. doi: 10.1128/MCB.24.3.1378-1386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Arcangelo G, et al. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 28.Hiesberger T, et al. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 29.Grant SG, et al. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima K, Mikoshiba K, Miyata T, Kudo C, Ogawa M. Disruption of hippocampal development in vivo by CR-50 mAb against reelin. Proc Natl Acad Sci USA. 1997;94:8196–8201. doi: 10.1073/pnas.94.15.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer's amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 32.Groc L, et al. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci. 2007;27:10165–10175. doi: 10.1523/JNEUROSCI.1772-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campo CG, Sinagra M, Verrier D, Manzoni OJ, Chavis P. Reelin secreted by GABAergic neurons regulates glutamate receptor homeostasis. PLoS One. 2009;4:e5505. doi: 10.1371/journal.pone.0005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 35.Schmechel DE, et al. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strittmatter WJ, et al. Apolipoprotein E: High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trommer BL, et al. ApoE isoform-specific effects on LTP: Blockade by oligomeric amyloid-β1–42. Neurobiol Dis. 2005;18:75–82. doi: 10.1016/j.nbd.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Beffert U, et al. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3β. J Biol Chem. 2002;277:49958–49964. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.